Abstract
Study design
Randomized clinical trial.
Objectives
To investigate the immediate effects of soft tissue mobilization (STM) versus therapeutic ultrasound (US) in patients with neck and arm pain who demonstrate neural mechanical sensitivity.
Background
While experts have suggested that individuals with neck and arm pain associated with neural tissue mechanical sensitivity may benefit from STM, there has been little research to investigate this hypothesis.
Methods
Twenty-three patients with neck and arm pain and a positive upper limb neurodynamic test (ULNT) were randomly assigned to receive STM or therapeutic US during a single session. Outcome measures were collected immediately before and after treatment, and at 2–4 day follow-up. Primary outcomes were the Global Rating of Change (GROC), range of motion (ROM) during the ULNT, and pain rating during the ULNT. Secondary measures included the Neck Disability Index (NDI), Patient-Specific Functional Scale (PSFS), Numeric Pain Rating Scale (NPRS), and active range of shoulder abduction motion combined with the wrist neutral or wrist extension.
Results
A greater proportion of patients in the STM group reported a significant improvement on the GROC immediately after treatment (P = 0·003, STM = 75%, US = 9%), and at 2–4 day follow-up (P = 0·027, STM = 58%, US = 9%). Patients who received STM demonstrated greater improvements in ROM during ULNT (P = 0·026), PSFS (P = 0·007), and shoulder active ROM combined with wrist extension (P = 0·028). Improvements in Numeric Pain Rating Scale and pain during the ULNT were observed only in the STM group. There was no difference between groups for the NDI or shoulder abduction ROM with wrist neutral.
Conclusion
Patients with neck and arm pain demonstrated greater improvements in ULNT ROM, GROC, and PSFS, and pain following STM than after receiving therapeutic US.
Level of evidence
Therapy, level 1b.
Keywords: Cervical radiculopathy, Whiplash, Upper limb neurodynamic test, Soft tissue mobilization
Introduction
Neck and arm pain is common in the general population.1,2 Individuals with neck and upper extremity symptoms demonstrate greater levels of disability than do individuals with neck pain alone.3 While individuals with cervical radiculopathy often report neck pain, they most frequently seek treatment due to arm pain.3–5 Individuals with whiplash associated disorders who demonstrate signs of irritation of the brachial plexus and subsequent neck and arm pain reported more severe symptoms and achieved worse outcomes than those without brachial plexus irritation.6 Some authors have suggested that patients with neck and arm pain should be treated more expeditiously in order to avoid the further negative impact on mental health status associated with chronic symptoms.3
Neck and arm pain may be associated with several factors including cervical disc disease,7 osteophyte formation,8 neural tissue mechanical sensitivity,9–11 and soft tissue dysfunction.12–14 Neural tissue mechanosensitivity is a condition where there is an elevated painful response (hyperalgesia) to mechanical stimuli, i.e. changes in tension and/or compression of the neural tissues.9–11,15,16 Mechanical sensitivity of the neural structures of the upper limb may be related to impaired movement of the nerves as they glide past adjacent structures such as joints, discs, ligaments, muscles, and other soft tissue structures such as the intermuscular septum.9,17,18 For example, patients with non-specific arm pain and carpal tunnel syndrome demonstrate decreased movement of the median nerve at the wrist where it travels past the carpal bones and flexor retinaculum.17
It has been suggested that neural mobilization, a treatment approach focusing on facilitating movement of the nerve and surrounding structures, may be beneficial for those who present with cervicobrachial syndrome.9,10,15,19–21 The goal of interventions is either to encourage gliding of the nerve by controlled angular movements or to allow more space for the nerve to move by improving mobility of the structures that surround the nerve (the neural container).9,11,15,19–23 An example of such an intervention includes the cervical lateral glide mobilization with the involved upper extremity placed in a position designed to take up the slack in the brachial plexus.16,21,24,25 However, several authors have advised against applying cervical spine mobilization techniques to patients who demonstrate either normal or excessive mobility of the cervical spine,26,27 which may include some patients with cervicobrachial syndrome.17 Hypermobility is a frequent finding in patients with whiplash-associated disorder,28 a group who commonly reports neck and arm pain.6,29–31 Furthermore, joint mobilization techniques may be contra-indicated in those who have recently undergone cervical surgery.27 For these patients, interventions other than cervical joint mobilization may be indicated.
Just as the bony structures may restrict movement of the neural tissues, restriction of the surrounding soft tissue may also impair movement of the nerve and compress the nerves as they course through the neural container.9 Patients with decreased upper limb neural extensibility, as indicated by decreased range in the upper limb neurodynamic test (ULNT), demonstrated a reduced amount of length of the upper trapezii compared to those with greater extensibility.32 Greening et al.17 hypothesized that reduced sliding of the median nerve observed in patients with whiplash injury or non-specific arm pain (NSAP) may be related to shortening of the scalene muscle which may elevate the first rib and restrict sliding of the medial cord of the brachial plexus.
Dysfunction of the soft tissue structures of the upper quarter (muscle and connective tissue) may also provide nociceptive input to the nervous system, contributing to the pain perceived by the patient. Tenderness on palpation, shortened length, and hyperirritable tender points within a palpable taut band of the upper quarter soft tissues are common findings in patients with neck pain.33,34 Letchuman et al.13 reported that cervical radiculopathy was associated with increased tender points in the muscles innervated by the involved nerve root.
There is preliminary evidence that soft tissue techniques may be beneficial for patients with carpal tunnel syndrome35,36 and with cervical radiculopathy.20 Burke et al.35 investigated the effects of two different soft tissue mobilization (STM) techniques designed to address soft tissue restrictions in the forearm and hand of patients with carpal tunnel syndrome. Although clinical improvements were not different between them, both manually applied and instrument assisted STM techniques improved pain, range of motion (ROM), nerve conduction latencies, and function. De-la-Llave Rincon et al.36 recently reported clinically important reduction in hand pain in a case series of patients with carpal tunnel syndrome after a single application of STM followed by manual nerve sliders directed at the median nerve.
Both STM techniques and therapeutic ultrasound (US) are used in the management of upper quadrant conditions.37,38 A recent systematic review concluded that there is moderate evidence for the use of US in the management of carpal tunnel syndrome,39 a condition of localized neural mechanosensitivity. Animal research suggests that US may influence spinal nociceptive processing in models of peripheral inflammation.40
The purpose of this study was to investigate the immediate and short term treatment effects of STM versus therapeutic US in a group of patients with neck and arm pain who demonstrate signs of mechanosensitivity of the neural structures of the upper limb. It was hypothesized that patients who received soft tissue mobilization would demonstrate greater improvements in ratings of pain and function, and in measures of ROM, than did patients who received therapeutic US. Additionally, it was hypothesized that a greater proportion of patients who received soft tissue mobilization would report a clinically important change in patient perceived improvement, decreased pain, and would demonstrate increased ROM than those who received therapeutic US.
Methods
Study design
The study was a randomized clinical trial with two groups: a treatment group who received STM and a comparison group that received therapeutic US. The protocol was approved by the Institutional Review Board at Rocky Mountain University of Health Professions (Provo, UT, USA) and Cayuga Medical Center (Ithaca, NY, USA) and all patients provided written informed consent. The ClinicalTrials.gov identifier is NCT02081456.
Patients
Between November 2009 and June 2012, consecutive patients with neck and arm pain presenting to Cayuga Medical Center’s Physical Therapy Department were screened for eligibility criteria. Patients were between 18 and 65 years old. Patients were eligible if the examination revealed the following:
-
1.
active movement dysfunction that could be related to mechanical sensitivity of the neural structures of the upper limb (i.e. painful shoulder abduction with elbow extension that is limited more when the wrist is extended than when the wrist is in neutral);
-
2.
positive response to upper limb neural provocation testing (ULNT 1) (see description below for details)
-
3.
tenderness to palpation over the cervical nerve trunks, brachial plexus, or along the median nerve;
-
4.
tender points or taut bands in the muscles of the upper quadrant including the scalenes, cervical paraspinals, trapezius, deltoid, pectoralis major or minor, rotator cuff, biceps, triceps, coracobrachialis, brachialis, radiobrachialis, pronator teres, supinator, forearm extensor, forearm flexor, pronator quadratus, and hand intrinsic muscles.
Patients were excluded based on the following criteria:
-
1.
red flags noted in the medical screening questionnaire such as tumor, fracture, history of metabolic disease, and prolonged history of corticosteroid use;
-
2.
signs of central nervous system involvement such as hyper-reflexia, unsteadiness during gait, ataxia, disturbed vision, nystagmus, altered taste, and positive Babinski’s or Hoffman’s reflexes;
-
3.
cervical spine surgery within the last 3 months;
-
4.
litigation associated with their neck and/or upper limb pain;
-
5.
insufficient English language skills to complete the questionnaires and follow-up instructions;
-
6.
inability to complete the treatment and follow-up schedule
-
7.
current pregnancy.
Sample size calculation
To determine sample size, an estimate of effect size was calculated from the data provided by Coppieters et al.21 This study examined the effects of a neuromobilization technique in a patient population similar to the current study. Using the values for each group provided by Coppieters et al., Cohen’s d was estimated as d = 2·02 for improvement in elbow ROM during the ULNT, and d = 1·1 for the NPRS, both large effects. Since an ANOVA was used to analyze the effects of interventions, an estimate of effect size index f was made. The information needed to calculate f to determine sample size for an ANOVA was not provided by Coppieters et al.21 Cohen41 states that a value of f = 0·5 may be used to estimate a large effect. Considering that the calculated d for elbow ROM is 2·02 and a ‘large’ d can be estimated at 0·80, using f = 0·5 is reasonable. Using f = 0·5 and power = 80% produced a sample size of 17 patients per group.
Examination procedures
Patients completed the medical history form and self-report measures and underwent a standardized evaluation by a licensed physical therapist. This process included reviewing medical history and self-report measures, collecting a subjective history, and performing a physical examination. All patients who satisfied the eligibility criteria were invited to participate in the study.
Consecutive patients who met the inclusion criteria and who consented to randomization were assigned to receive either STM or therapeutic US. Self-report questionnaires were completed and physical impairment measures (described below) were taken before and after the patient received the intervention according to group assignment. Patients returned for follow-up measures within 2–4 days. After all follow-up measures were taken, the study procedures were completed and the patient continued to receive treatment as deemed appropriate by the treating physical therapist.
Treating therapists
All interventions were provided by one of eight licensed physical therapists with experience ranging from one to 15 (mean = 8·4, SD = 6·25) years of practice. Five therapists were Orthopedic Certified Specialists by the American Board of Physical Therapy Specialties, and two therapists were enrolled in an APTA-credentialed orthopedic residency program. All therapists attended a 3-hour initial training session and received a written manual covering the study protocol including recruitment, and examination and intervention procedures. It was not possible to blind treating therapists due to the nature of the interventions. However, one therapist was assigned to collect all outcome measures and was kept blind to group assignment.
Outcome measures
Global Rating of Change (GROC)
The GROC was the primary outcome measure and was collected immediately post-treatment and at the follow-up. The GROC is a self-report measure used to measure the patients' overall perception of improvement since the beginning of treatment. The scale ranges from −7 (a very great deal worse) to +7 (a very great deal better) with 0 representing no change. Scores between ±4 and ±5 represent moderate changes, while scores of ±6 or ±7 represent large changes.42 The GROC has been used to determine the effectiveness of physical therapy interventions for patients with cervical radiculopathy.43,44
Neck Disability Index (NDI)
The NDI is a self-report measure containing 10 items that assesses a patient’s disability due to neck pain.45 Test-retest reliability for the NDI in patients with cervical radiculopathy has been reported to be fair (ICC = 0·55)39 to moderate (ICC = 0·68),46 and the minimal clinically important change has been reported to be range from 7·0 points or 14%46 to 8·5 points or 17%.47
Patient Specific Functional Scale (PSFS)
The PSFS is a self-report measure used to measure the patient’s perceived level of disability. The patient rates three activities that are difficult due to their condition, on a 0–10 scale, with 0 representing inability to perform the activity and 10 representing the ability to perform the activity as well as they could before the onset of symptoms. The PSFS has demonstrated variable test–retest reliability in patients with cervical radiculopathy ranging from slight (ICC = 0·17)47 to high (ICC = 0·82)46 and the minimally clinically important change ranges from 2·046 to 2·2.47
Numeric Pain Rating Scale (24-hour NPRS)
The NPRS was used to measure pain intensity. The patient rated ‘average pain’, ‘least pain’, and ‘worst pain’ over the last 24 hours on a 0–10 scale, 0 representing no pain and 10 representing the worst pain imaginable. The average of these three scores was used for data analysis of ‘daily pain’.48 The NPRS has demonstrated acceptable levels of test–retest reliability (ICC = 0·5947 to 0·6346) and a 2·2 point change in the NPRS has been reported to be clinically meaningful.47
Upper Limb Neurodynamic Test (ULNT ROM and ULNT Pain)
The ULNT was performed as described by Wainner et al.5 The initial amount of scapular depression was standardized with the use of a pressure sensor (Stabiliser, Chattanooga, Australia) in a manner described by Edgar et al.32 The pressure cuff was folded in 3 to fit the superior region of the patient’s shoulder. It was be pre-inflated to 20 mmHg, and the therapist depressed the shoulder until an increase of 40 mmHg was recorded (Fig. 1). The test was taken to the point of ‘submaximal pain’ during elbow extension, defined as the point in the range where the patient experienced ‘a substantial discomfort, which corresponds to the greatest level of pain which the patient was prepared to tolerate, knowing that the test had to be performed repeatedly’.49 The degree of elbow flexion was measured with a standard goniometer (ULNT ROM). Positive test criteria included one or more of the following:
Figure 1.
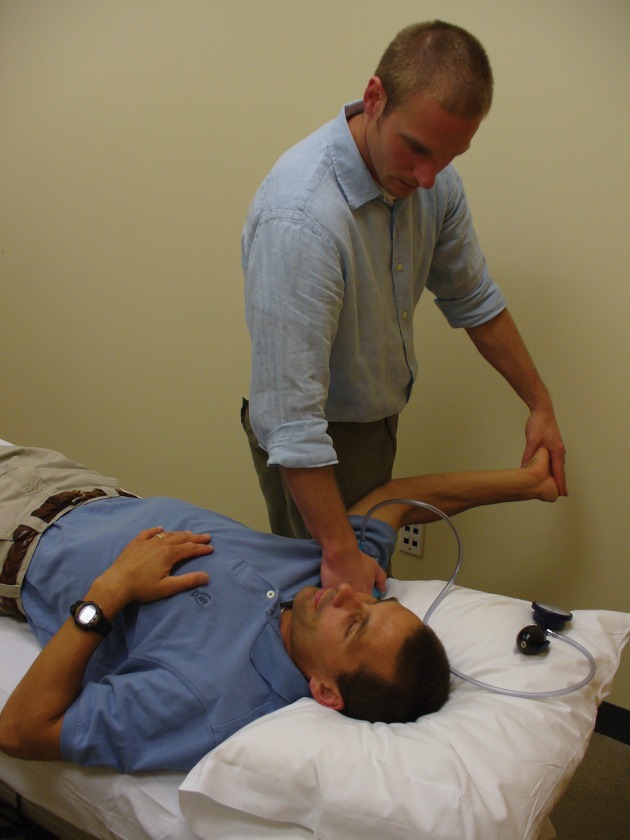
Measuring the upper limb neurodynamic test.
Source: Author
-
1.
patient’s symptoms are reproduced;
-
2.
side-to-side differences (>10°) in elbow extension upon completion of all motion sequences;
-
3.
symptomatic limb side: contralateral cervical sidebending increases symptoms or ipsilateral sidebending decreases symptoms (structural differentiation).7
Reliability for the ULNT in patients with cervicobrachial syndrome is excellent (ICC = 0·98). The minimally statistical meaningful change is 7·5° of elbow ROM.49
Additionally, a single numeric rating of pain on a scale of 0–10 was used to describe the intensity of pain experienced during the ULNT (ULNT PAIN).
Shoulder Abduction Active Range of Motion (SHLD ABD AROM)
Shoulder abduction ROM was measured in two conditions with varying amounts of tension on the median nerve: with the elbow extended and the wrist in neutral (SHLD ABD) and with the elbow extended and the wrist extended (SHLD ABD+WE).9,50 Shoulder AROM measurements were taken with a bubble goniometer as described by Lewis et al.51 (Fig. 2). They reported the reliability of this method to be high for both asymptomatic individuals and patients with shoulder pain (ICC = 0·98). The therapist collecting these outcome measures was blind to group assignment.
Figure 2.
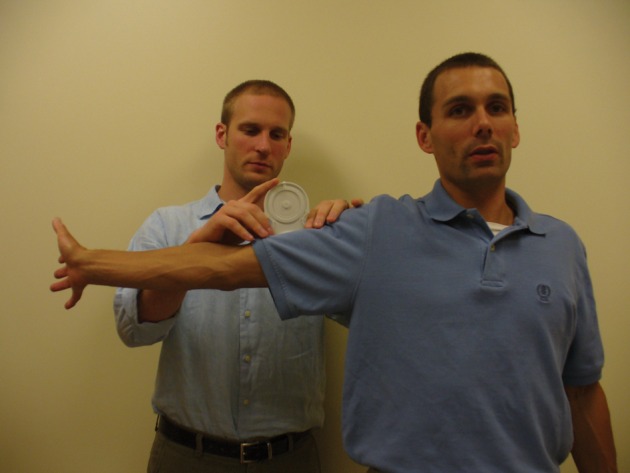
Measuring shoulder abduction with wrist extension.
Source: Author
The ULNT ROM, ULNT NPRS, SHLD ABD, and SHLD ABD+WE were collected at baseline, immediately following treatment, and at the follow-up session. The NDI, PSFS, and 24-hour NPRS were collected only at baseline and at the follow-up session as they were not expected to change within the treatment session.
Randomization
Following examination and completion of baseline measures patients were randomly assigned to receive either STM or therapeutic US by having the treating therapist open an opaque, sealed envelope with the patients’ group assignment immediately before the start of treatment.
Interventions
STM
Patients in the STM group received treatment in supine, with their head resting on one pillow and the involved UE positioned in abduction and external rotation to preload the neural structures of the upper limb in a manner similar to that used by Coppieters et al.21
Manual pressure was applied to the soft tissues of the upper quadrant in a deep, stroking manner with the intention to improve the mobility of the soft tissues surrounding the pathway of the neural structures of the upper limb as well as any tender or tight tissues. Treatment began proximally and proceeded distally. The therapist was free to concentrate on any tissues of the cervical and scapular region and upper extremity that he/she deemed relevant. The therapist modified the intensity of the technique according to the patient’s response. If the patient reported any increase in their symptoms other than a sensation of local tenderness, pressure, pull, or stretch in the region that the soft tissue mobilization was being applied, the therapist either decreased the amount of pressure used or reduced the amount of abduction and/or external rotation of the patient’s arm to decrease the amount of tension on the peripheral nerves. The therapist spent approximately 7 minutes on the neck and scapular region, 4 minutes on the upper arm, and 4 minutes on the forearm and hand. The therapist was allowed to vary the time spent on each region according to his/her assessment of the patient’s condition. The procedure lasted a total of 15 minutes.
Therapeutic US
Patients received therapeutic US applied for a period of 5 minutes to the most painful region of the neck, then a second 5-minute dose at the most painful region of the upper extremity. The US dose was 0·5 w/cm2, with sonation time 50% and frequency 1 MHz.40,52 The patient lay supine with the hand of the involved upper extremity placed on the abdomen and the elbow supported on a pillow. The two US doses and interaction time with the patient lasted a total of 15 minutes in an attempt to have equal patient/therapist contact between the two groups.
Data analysis
Descriptive statistics was calculated to summarize the data. These statistics included measures of central tendency and dispersion for continuous data and frequency counts for categorical data. SPSS 19·0 for Windows (IBM Corporation, Armonk, NY, USA) was used for all analyses. Baseline demographic and outcome measures were compared between groups using unpaired t-test for continuous data and the Fisher’s exact test for categorical data. The Fisher’s exact test was used instead of the Chi-square test due to the small sample size. The Fisher’s exact test is recommended when sample sizes are small and when one of the expected frequencies is less than 5.53
A two-way mixed design repeated-measures ANOVA with one between-group factor (group, with two levels: STM and US), and one repeated measures factor (time, with three levels: pre-treatment, post-treatment, and follow-up) was used to analyze the effects of treatment within groups and between groups for measures taken at all three time intervals. A two-way mixed design repeated measures ANOVA was used to analyze effect within and between groups for measures taken only pre-treatment and at follow-up. Interaction and main effects were calculated. Tukey’s honestly significant difference (HSD) was calculated post hoc to assess the significance of differences between and within groups.
The Fisher’s exact test was used to determine if the proportion of patients who respond positively to treatment was different between groups. A positive response to treatment was based on any one of three reference criteria determined a priori: (1) a GROC score of at least +3; or (2) a reduction in pain during the ULNT of at least 2·2; or 3) an improvement in elbow ROM during the ULNT of >7·5°. Jaeshke et al.42 considered a one- to three-point increase on the GROC to be the minimally clinically important difference. A GROC score of +3 has previously been used as the criterion to determine a favorable response to manual physical therapy interventions.54 A 2·0-point change in the NPRS has been reported to be clinically meaningful.55 While there is currently no estimate of the minimal clinically meaningful difference for elbow extension ROM during the ULNT, it has been reported that differences greater than 7·5° represent true (non-error) differences.49 Additionally, the proportion of patients who achieved the minimally clinically important difference (MCID) of 2·2 points on the PSFS was compared with the Fisher exact test. Then the Number Needed to Treat (NNT) with 95% confidence intervals (CIs) was calculated for the GROC, PSFS and having achieved the combined positive response on the GROC, ULNT NPRS, and ULNT ROM. The level of significance for all analyses was set at P<0·05.
Results
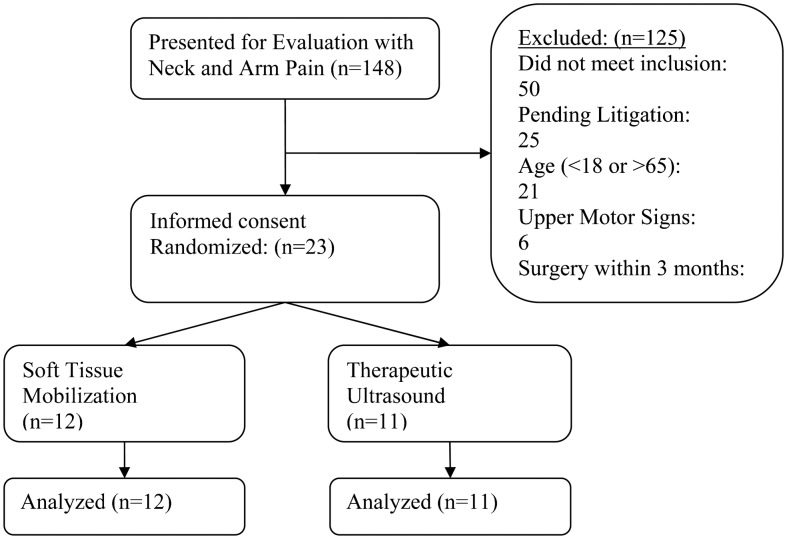
One-hundred and forty-eight patients were screened for inclusion from November 2009 through December 2011, and 23 patients (21 women, 2 men) participated. One-hundred and twenty-five patients were excluded (see Fig. 3 for flow diagram of subject recruitment and retention). Twelve patients were randomized to the STM group and 11 to the US group. All participating patients received allocated treatment and completed the study protocol. Table 1 indicates the baseline scores for patient characteristics (age, duration of symptoms) and specific dependent variables. As indicated in Table 1, there were no differences between groups at baseline with respect to any variable. The change scores for all outcome measures are displayed in Table 2.
Figure 3.
CONSORT flow diagram of patients through the trial.
Table 1.
Study baseline characteristics
| All patients (n = 23) | STM group (n = 12) | US group (n = 11) | P value | |
|---|---|---|---|---|
| Age (years) | 44·22±10·24 | 46·25±10·42 | 42·00±10·42 | 0·332 |
| Duration (days) | 77·52±66·65 | 61·17±45·02 | 95·36±82·89 | 0·227 |
| 24-hour NPRS (0–10) | 4·73±1·43 | 4·23±2·17 | 5·00±2·00 | 0·759 |
| NDI (0–50) | 25·65±11·50 | 27·69±11·69 | 23·40±11·00 | 0·223 |
| PSFS (0–10) | 4·21±2·03 | 4·60±1·44 | 4·11±2·30 | 0·957 |
| ULNT NPRS (0–10) | 6·39±1·67 | 6·00±1·83 | 6·60±1·43 | 0·368 |
| UNLT ROM (°) | 127·1±21·16 | 128·38±17·72 | 124·80±25·58 | 0·850 |
| SHLD ABD (°) | 84·74±50·21 | 85·62±50·78 | 88·30±50·91 | 0·994 |
| SHLD ABD+WE (°) | 57·17±44·59 | 56·77±41·26 | 59·70±50·50 | 0·941 |
Note: Values are mean±standard deviation.
NDI = Neck Disability Index; 24-hour NPRS = Numeric Pain Rating Scale average over previous 24 hours; PSFS = Patient-Specific Functional Scale; ULNT ROM = Upper Limb Neurodynamic Test range of motion; ULNT NPRS = Upper Limb Neurodynamic Test Numeric Pain Rating Scale; SHLD ABD = shoulder abduction range of motion; SHLD ADB+WE = shoulder abduction with wrist extension range of motion.
Table 2.
Change scores from baseline to immediate follow up and baseline to 2–4 day follow-up
| Baseline to Immediate post-treatment | Baseline to 2–4 day follow-up | Main effects P value | Interaction P value | |
|---|---|---|---|---|
| NDI (0–50) | ||||
| US | NA | −1·20±6·20 (−5·63, 3·23) | 0·075 | 0·225 |
| STM | NA | −6·00±10·83 (−12·55, 0·55) | ||
| PSFS (0–10) | ||||
| US | NA | 0·30±0·64 (−0·16, 0·76) | 0·000* | 0·007* |
| STM | NA | 1·67±1·29 (0·80, 2·53) | ||
| 24-hour NPRS (0–10) | ||||
| US | NA | −0·80±1·93 (−2·18, 0·58) | 0·005* | 0·461 |
| STM | NA | −1·31±1·32 (−2·10, −0·51) | ||
| ULNT ROM (°) | ||||
| US | 4·60±7·28 (−0·60, 9·80) | 1·40±16·93 (−10·71, 13·51) | 0·003* | 0·026* |
| STM | 16·00±10·12 (9·88, 22·12) | 15·85±14·282 (7·22, 24·48) | ||
| ULNT NPRS | ||||
| US | −0·40±0·70 (−0·90, 0·10) | 0·00±1·16 (−0·83, 0·83) | 0·030* | 0·087 |
| STM | −1·08±1·44 (−1·95, −0·21) | −1·35±1·89 (−2·49, −0·21) | ||
| SHLD ABD (°) | ||||
| US | 2·60±13·85 (−7·31, 12·51) | 1·20±19·80 (−12·96, 15·36) | 0·368 | 0·447 |
| STM | 13·15±36·78 (−9·07, 35·38) | 12·85±37·62 (−9·89, 35·58) | ||
| SHLD ABD+WE (°) | ||||
| US | 6·50±17·98 (−6·36, 19·36) | 2·50±15·27 (−8·43, 13·43) | 0·000* | 0·028* |
| STM | 25·92±22·74 (12·18, 39·67) | 16·23±13·20 (8·26, 24·21) |
Note: Values are mean±standard deviation (95% CI).
P value from repeated measures ANOVA.
STM = soft tissue mobilization; US = ultrasound; NDI = Neck Disability Index; PSFS = Patient-Specific Functional Scale; 24-hour NPRS = Numeric Pain Rating Scale average over previous 24 hours; ULNT ROM = Upper Limb Neurodynamic Test range of motion; ULNT NPRS = Upper Limb Neurodynamic Test Numeric Pain Rating Scale; SHLD ABD = shoulder abduction range of motion; SHLD ADB+WE = shoulder abduction with wrist extension range of motion.
Indicates statistical significance.
Comparison between pre-treatment and 2–4 day follow-up
Analysis of the NDI scores did not reveal any differences within or between groups (P = 0·075). That is, NDI scores were similar between the two groups at pre-treatment (baseline), and at the 2–4 day follow-up. Likewise, NDI scores did not change significantly within either group between the baseline and 2–4 day follow-up periods.
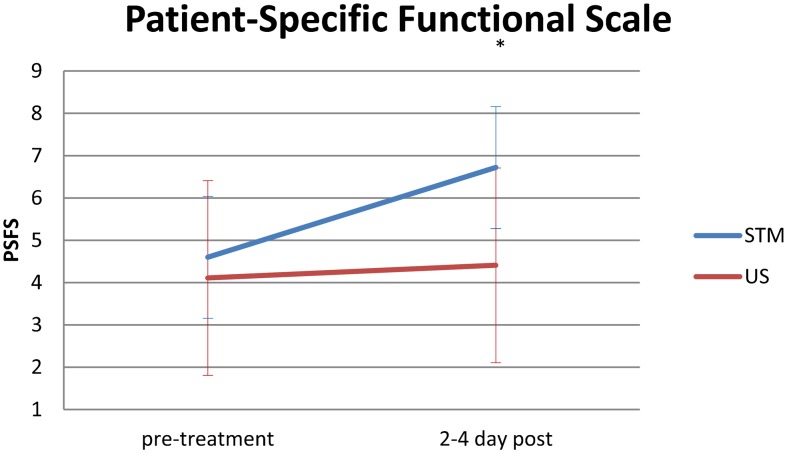
The analysis for the PSFS indicated both significant interaction effects (PSFS*group, P = 0·007) and main effects (P<0·001). Tukey’s HSD for the PSFS was 0·898. Therefore, at 2–4 day follow-up, the STM group demonstrated a significant 1·67-point increase in PSFS scores from pre-treatment to 2–4 day follow-up, whereas there were no significant changes within the US group. Additionally, while scores were similar at baseline, at 2–4 day follow-up, the STM group reached significantly higher values than did the US group (Fig. 4).
Figure 4.
Pre-treatment and 2–4 day post-treatment mean values for the Patient-Specific Functional Scale in the soft tissue mobilization (STM) and ultrasound (US) groups. *Interaction P = 0·007.
In the STM group, NPRS scores decreased significantly from 4·23 at baseline to 2·92 at the 2–4 day follow-up (P = 0·005, Tukey’s HSD = 0·987). No significant changes occurred in the NPRS scores in the US group during the same time period, and the interaction (NPRS*group) was not significant (P = 0·461).
Comparison between pre-treatment, immediate post-treatment, and 2–4 day follow-up
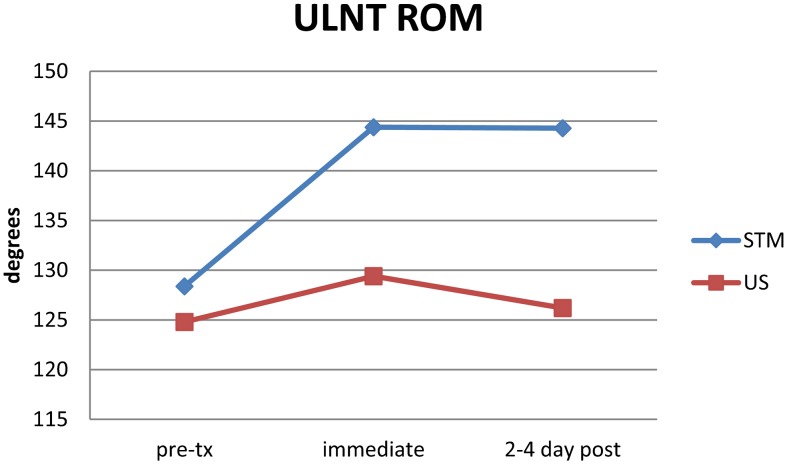
The repeated-measures ANOVA for ULNT ROM indicated significant differences existed for main effects (P = 0·003) and the between-group interaction (ULNT*group, P = 0·026). Tukey’s HSD was 11·28. Therefore, ULNT ROM values for the STM group were significantly greater than the pre-treatment scores at both immediate post-treatment and at 2–4 day follow-up, while ULNT ROM values within the US group were similar across all time periods. There was no significant change from immediate follow-up to 2–4 day follow-up in the STM group. STM ULNT ROM was significantly greater than in the US group at both follow-up periods (Fig. 5).
Figure 5.
Pre-treatment, immediate post-treatment and 2–4 day follow-up scores (mean) for the Upper Limb Neurodynamic Test Range of Motion (ULNT ROM) in the soft tissue mobilization (STM) and ultrasound (US) groups. Interaction P = 0·026.
The proportion of patients who demonstrated an improvement of at least 7·5° of elbow extension during the ULNT was 83% for the STM group and 27% in the US group immediately post-treatment, and 58% and 18% for the STM and US groups, respectively, at 2–4 day follow-up. The proportion of patients who demonstrated this minimally statistically important difference was significantly different between groups immediately post-treatment (P = 0·012), but not at 2–4 day follow-up (P = 0·089).
The repeated-measures ANOVA for pain ratings during the ULNT was significant (P = 0·030) and Tukey’s HSD was 1·241. These analyses indicated that 2–4 day follow-up values, but not immediate follow-up values, in the STM group, were less than at pre-treatment. Additionally, pain scores during the ULNT for the STM group were significantly less than those in the US group at both immediate and 2–4 day follow-up. The interaction between groups was not significant (ULNT NPRS*group, P = 0·087). During the immediate post-treatment measurement and at 2–4 day follow-up, the proportion of patients who demonstrated an improvement in pain during the ULNT of 2·0 or greater was 25% for the STM group and 9% for the US group which was not statistically different (P = 0·590).
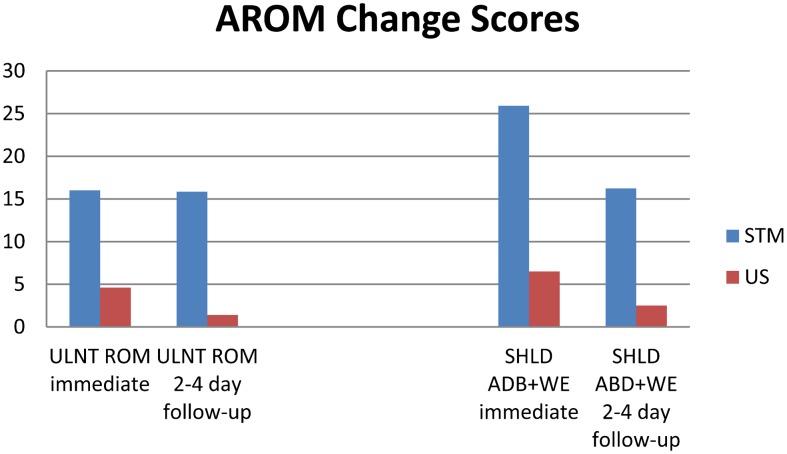
There were no significant differences within or between groups for SHLD ABD across all time periods (P = 0·368). For SHLD ABD+WE, there was a significant difference (P<0·001), and the between-group interaction was also significant (SHLD ABD+WE*group, P = 0·028). Tukey’s HSD for SHLD ABD+WE was 14·99. While there were no changes in SHLD ABD+WE in the US group, the STM group demonstrated significant improvement both at immediate post-treatment and at 2–4 day follow-up compared to baseline. SHLD ABD+WE values were significantly greater in the SMT group compared to the US group at both post-treatment periods. Changes in ROM from baseline to each post-treatment period for ULNT ROM and SHLD ABD+WE AROM can be found in Fig. 6.
Figure 6.
Change scores compared to pre-treatment for Upper Limb Neurodynamic Test Range of Motion (ULNT ROM) and Shoulder Abduction with Wrist Extension Range of Motion (SHLD ABD+WE) at immediate post-treatment and 2–4 day follow-up in the soft tissue mobilization (STM) and ultrasound (US) groups.
The proportion of patients who scored ≧+3 of the GROC was significantly different at both the immediate (P = 0·003) and 2–4 day follow-up (P = 0·027) periods. Immediately post-treatment, 75% in the STM group compared to only 9% for the US group reached this cutoff. At 2–4 day follow-up, the proportion of those remaining above this cutoff declined to 58% in the STM, while remaining at 9% for the US group.
The NNT for the GROC immediately post-treatment was 1·52 (95% CI: 1·0–2·8) and at 2–4 day follow-up was 2·03 (95% CI: 1·2–6·0). For the PSFS at follow-up, the NNT was 3·07 (95% CI: number needed to harm = 1183·8 and number needed to help = 1·53).55 The NNT for the combined response on the GROC, ULNT ROM, and ULNT NPRS was 4·0 (95% CI: 2·0–199·8).
Discussion
Results of this study suggest that STM may yield greater immediate improvements in ROM, disability, and patient perceived improvement as compared to US. While the improvement in PSFS for the STM group did not surpass the MCID of 2·2 points,47 75% of those receiving STM reported a meaningful global improvement as indicated by the GROC score of ‘moderately better’ immediately post-treatment, declining to 58% at 2–4 day follow-up. This finding is in contrast to the 9% who received US that reported perceived improvement at both immediate- and short-term follow-up. NDI scores did not change significantly in either group in this study. Given the relatively short time-frame of this study, large changes in NDI or PSFS might not be expected. Cleland et al.43 reported that the PSFS is more sensitive to change than the NDI in a cohort of patients with cervical radiculopathy, which might account for the different responses in functional outcomes between the PSFS and NDI observed over the short timeframe in this study.
The NNT for the GROC indicated that one in every two patients will benefit from treatment both immediately and at 2–4 day follow-up with relatively small 95% CIs. Given the wide 95% CI for the NNT for combined improvement on the GROC, ULNT ROM, and ULNT NPRS, this value should be interpreted with caution. Interpretation of the NNT for the PSFS is more complex. Considering that the 95% CI for the absolute risk reduction is from −0·08% to 65·24%, Altman56 recommends that one state that either the number needed to help is 1·5, or the number needed to harm is 1183·8. Considering the overall positive results from STM, and that no adverse events were reported during the trial, the likelihood that STM is harmful is low.
Patients who received STM demonstrated improvements in ROM measured both during the ULNT and during active motion of shoulder abduction combined with wrist extension, a maneuver designed to actively test the compliance of the median nerve.9,11,21 Improvements observed in the STM group for ULNT ROM immediately post-treatment (16·0°) and at 2–4 day follow-up (15·85°) both exceed the MCID of 7·5°.49 Interestingly, these improvements in ULNT ROM were accompanied by increased SHLD ABD+WE ROM immediately post-treatment (25·92°) and at 2–4 day follow-up (16·23°). Decreased mechanical sensitivity of the median nerve and related structures might allow more tolerance to the sliding and tensioning movements that are required to allow greater ROM of the upper limb during each of these movement patterns.50,57
STM in comparison to other interventions for neck and arm pain
Results from this study can best be compared to those reported by Coppieters et al.21 who investigated the immediate effects of cervical lateral glide mobilization in a similar population of patients with neck and arm pain. The immediate improvement of 16·0° of elbow extension during the ULNT in this study is comparable to the 19·4° change demonstrated by Coppieters et al.21 Additionally, this study appears to demonstrate that this improvement in ROM was maintained over the 2–4 day follow-up period. While NPRS scores improved significantly for patients in the STM group, pain measured during the UNLT did not. This finding is in contrast to the results reported by Coppieters et al,21 who reported a 1·5-point reduction in pain intensity during the ULNT, despite similar increases in ROM following cervical lateral glide mobilizations. Cervical lateral glide mobilizations have been shown to have immediate hypoalgesic effects in patients with neurodynamic impairments.58 The immediate hypoalgesic effects of STM have not yet been investigated in this population.
Improvements in ROM, daily pain, and functional measures following STM are likely due to a wide variety of mechanical and neurophysiologic factors. Reductions in muscle stretch pain and mechanical hyperalgesia have been demonstrated after massage in a model of experimental muscle pain.59 Reductions in upper trapezius EMG activity during maximal isometric contraction, and upper motor neuron pool excitability of the flexor carpi radialis coincided with increased neck ROM following massage of the neck and shoulders.60 Decreased cortisol levels and increased dopamine and serotonin levels have been reported immediately after a single session of massage, as well as after longer-term follow-up.61 Animal studies suggest that massage may exert its analgesic effects via activation of periaqueductal gray-opioid systems due to the release of oxytocin.62
Both this study and the trial by Coppieters et al.21 found no significant improvement in any outcome measure following the application of US, despite the use of different parameters. It is possible that different applications of therapeutic US may yield different results for this population. As each of these studies only investigated immediate and short term effects following a single treatment session, one cannot conclude that US has no effect for patients with neurogenic neck and arm pain over longer timeframes. Therapeutic US is effective in the management of more localized peripheral neurogenic dysfunction, specifically carpal tunnel syndrome (CTS).39,63 In a systematic review, Huisstede et al.39 reported that there was no evidence for the benefit of US over placebo at 2-week follow-up, but that there was moderate evidence for symptom improvement at 7 weeks. They found no evidence to suggest that a particular intensity or frequency was more effective.39 One possible explanation for the difference in responses between patients with CTS and those with neck and arm pain who demonstrate mechanical sensitivity of the median nerve may be that the primary dysfunction in CTS is localized to a relatively small and superficial region, and therefore amenable to focused interventions. In contrast, therapeutic US may not address the more diffuse source of nociceptive input that may be at play in neurogenic neck and arm pain. Additionally, patients with neurogenic neck and arm pain have demonstrated signs of generalized sensory hypersensitivity hypoesthetic changes that may be indicative of central sensitization and well as peripheral nerve dysfunction.64 Manual therapy interventions may affect not only peripheral sources of nociceptive input, but also descending inhibitory pathways,65 which may explain the greater effect of manual therapy in this population.
Study limitations
There are several limitations to this study. The first is the short follow-up period. While immediate within-session and between-session changes are often utilized in clinical reasoning,66,67 the effect of either STM or therapeutic US may differ over several sessions as commonly used in clinical practice. The intent of this study was to investigate the isolated effects of each intervention. Considering that manual therapy interventions are utilized as a component of care in combination with other treatments such as education and exercise, future research should employ designs which would be able to assess the addition of STM to a multimodal package over longer timeframes.
For this study, patient recruitment was challenging, and the small sample size is a limitation of the study. While the a priori power analysis suggested that 34 patients would be required, after 148 patients were screened for inclusion only 23 (15·5%) were randomized. Other trials using similar inclusion criteria reported similar recruitment rates. Allison et al.19 recruited 18·8% of screened patients, while Nee and Butler16 recruited 10·2% of screened volunteers, and also stopped enrollment shy of their a priori sample size due to time constraints. To improve recruitment, future research should widen inclusion criteria without sacrificing homogeneity. Results from a recent case series provide preliminary evidence that patients over the age of 65 with cervical radiculopathy may benefit from manual therapy including STM.68 The exclusion of this older population may have eliminated patients with neural mechanical sensitivity that might be likely to benefit from STM. Utilizing multiple clinics across a wide geographic region would also not only improve recruitment, but improve external validity as well.
Future research should investigate the clinical effects of the STM protocol used in this study over a longer timeframe. While it will be important to select a homogeneous population with evidence of neural mechanical sensitivity, selection criteria should include a wider age range and multiple medical diagnoses in order to capture an adequate sample size. It is likely that a specific subgroup of individuals with neurodynamic impairments may benefit from STM, and efforts should be designed to identify patient characteristics that correspond with a positive outcome.
Conclusions
Patients with neck and arm pain demonstrated greater improvements in ULNT ROM, GROC, and PSFS following a single session of STM compared to therapeutic US at both immediate- and short-term follow-up. These preliminary results suggest that STM may be a valuable intervention in the management of patients with neck and arm pain. Future research should investigate the effects of STM within multimodal management for neck and arm pain with evidence of neural mechanical sensitivity on measures such as long-term clinical outcome and cost of care.
Disclaimer Statements
Contributors M. Costello: initial proposal, study design, study management, data collection, data analysis, and discussion; J. Cleland: study design, data analysis, discussion, and manuscript editing; E. Puentedura: study design, discussion, and manuscript editing; C. Ciccone: study design, data analysis, and manuscript editing.
Funding None.
Conflicts of interest The authors have no conflict of interest.
Ethics approval RB approval from Rocky Mountain University of Health Professions Protocol #080737-03 and Cayuga Medical Center at Ithaca Protocol #08-08.
References
- 1.Cote P, Cassidy JD, Carroll L. The factors associated with neck pain and its related disability in the Saskatchewan population. Spine. 2000;25:1109–17. [DOI] [PubMed] [Google Scholar]
- 2.Makela M, Heliovaara M, Sievers K, Impivaara O, Knekt P, Aromaa A. Prevalence, determinants, and consequences of chronic neck pain in Finland. Am J Epidemiol. 1991;134:1356–67. [DOI] [PubMed] [Google Scholar]
- 3.Daffner SD, Hilibrand AS, Hanscom BS, Brislin BT, Vaccaro AR, Albert TJ. Impact of neck and arm pain on overall health status. Spine. 2003;28:2030–5. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JA, Whitman JM, Fritz JM, Palmer JA. Manual physical therapy, cervical traction, and strengthening exercises in patients with cervical radiculopathy: a case series. J Orthop Sports Phys Ther. 2005;35:802–11. [DOI] [PubMed] [Google Scholar]
- 5.Wainner RS, Fritz JM, Irrgang JJ, Boninger ML, Delitto A, Allison S. Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine. 2003;28:52–62. [DOI] [PubMed] [Google Scholar]
- 6.Ide M, Ide J, Yamaga M, Takagi K. Symptoms and signs of irritation of the brachial plexus in whiplash injuries. J Bone Joint Surg Br. 2001;83:226–9. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka N, Fujimoto Y, An HS, Ikuta Y, Yasuda M. The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine. 2000;25:286–91. [DOI] [PubMed] [Google Scholar]
- 8.Muhle C, Bischoff L, Weinert D, et al. Exacerbated pain in cervical radiculopathy at axial rotation, flexion, extension, and coupled motions of the cervical spine: evaluation by kinematic magnetic resonance imaging. Invest Radiol. 1998;33:279–88. [DOI] [PubMed] [Google Scholar]
- 9.Butler DS. The Sensitive nervous system. Adelaide, SA: NOI Group Publications; 2000. [Google Scholar]
- 10.Elvey RL. Physical evaluation of the peripheral nervous system in disorders of pain and dysfunction. J Hand Ther. 1997;10:122–9. [DOI] [PubMed] [Google Scholar]
- 11.Shacklock MO. Clinical neurodynamics: a new system of musculoskeletal treatment. Sydney: Elsevier Butterworth-Heinemann; 2005. [Google Scholar]
- 12.Fernández-de-las-Peñas C, Palomeque-del-Cerro L, Fernández-Carnero J. Manual treatment of post-whiplash injury. J Movement Bodywork Ther. 2005;9:109–19. [Google Scholar]
- 13.Letchuman R, Gay RE, Shelerud RA, VanOstrand LA. Are tender points associated with cervical radiculopathy? Arch Phys Med Rehabil. 2005;86:1333–7. [DOI] [PubMed] [Google Scholar]
- 14.Travell JG, Simons DG. Myofascial pain and dysfunction, the trigger point manual. Vol. 1. The upper extremities. Baltimore, MD: Williams & Wilkins; 1983. [Google Scholar]
- 15.Hall TM, Elvey RL. Nerve trunk pain: physical diagnosis and treatment. Man Ther. 1999;4:63–73. [DOI] [PubMed] [Google Scholar]
- 16.Nee RJ, Butler D. Management of peripheral neuropathic pain: interpreting neurobiology, neurodynamics, and clinical evidence. Phys Ther Sport. 2006;7:36–49. [Google Scholar]
- 17.Greening J, Dilley A, Lynn B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain. 2005;115:248–53. [DOI] [PubMed] [Google Scholar]
- 18.Monsivais JJ, Sun Y, Rajeshekhar TP. The scalene reflex: relationship between increase median or ulnar nerve pressure and scalene muscle activity. J Reconstr Microsurg. 1995;11(4):271–5. [DOI] [PubMed] [Google Scholar]
- 19.Allison GT, Nagy BM, Hall T. A randomized clinical trial of manual therapy for cervico-brachial pain syndrome – a pilot study. Man Ther. 2002;7:95–102. [DOI] [PubMed] [Google Scholar]
- 20.Costello M. Treatment of a patient with cervical radiculopathy using thoracic spine thrust manipulation, soft tissue mobilization, and exercise. J Manual Manipulative Ther. 2008;16:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppieters MW, Stappaerts KH, Wouters LL, Janssens K. The immediate effects of a cervical lateral glide treatment technique in patients with neurogenic cervicobrachial pain. J Orthop Sports Phys Ther. 2003;33:369–78. [DOI] [PubMed] [Google Scholar]
- 22.Elvey RL. Treatment of arm pain associated with abnormal brachial plexus tension. Aust J Physiother. 1986;31:225–30. [DOI] [PubMed] [Google Scholar]
- 23.Ellis RF, Hing WA. Neural mobilization: a systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J Manual Manipulative Ther. 2008;16:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain. 1996;68:69–74. [DOI] [PubMed] [Google Scholar]
- 25.Nee RJ, Vincenzino B, Jull GA, Cleland JA, Coppieters MW. Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve-related neck and arm pain; a randomised trial. J Physiother. 2012;58:23–51. [DOI] [PubMed] [Google Scholar]
- 26.Greenman P. Principles of manual therapy. 2nd ed. Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- 27.Paris SV, Loubert PV. Foundations of clinical orthopaedics. St Augustine, FL: Institute Press; 1999. [Google Scholar]
- 28.Kristjansson E, Leivseth G, Brinckmann P, Frobin W. Increased sagittal plane segmental motion in the lower cervical spine in women with chronic whiplash-associated disorders, grades I–II: a case–control study using a new measurement protocol. Spine. 2003;28:2215–21. [DOI] [PubMed] [Google Scholar]
- 29.Squires B, Gargan MF, Bannister GC. Soft-tissue injuries of the cervical spine. 15-year follow-up. J Bone Joint Surg Br. 1996;78:955–7. [DOI] [PubMed] [Google Scholar]
- 30.Sterling M, Kenardy J, Jull G, Vicenzino B. The development of psychological changes following whiplash injury. Pain. 2003;106:481–9. [DOI] [PubMed] [Google Scholar]
- 31.Sterling M, Treleaven J, Jull G. Responses to a clinical test of mechanical provocation of nerve tissue in whiplash associated disorder. Man Ther. 2002;7:89–94. [DOI] [PubMed] [Google Scholar]
- 32.Edgar D, Jull G, Sutton S. The relationship between upper trapezius muscle length and upper quadrant neural tissue extensibility. Aust J Physiother. 1994;40:99–103. [DOI] [PubMed] [Google Scholar]
- 33.Cleland JA, Childs JD, Fritz JM, Whitman JM. Interrater reliability of the history and physical examination in patients with mechanical neck pain. Arch Phys Med Rehabil. 2006;87:1388–95. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-de-las-Penas C, Alonso-Blanco C, Miangolarra JC. Myofascial trigger points in subjects presenting with mechanical neck pain: a blinded, controlled study. Man Ther. 2007;12:29–33. [DOI] [PubMed] [Google Scholar]
- 35.Burke J, Buchberger DJ, Carey-Loghmani MT, Dougherty PE, Greco DS, Dishman JD. A pilot study comparing two manual therapy interventions for carpal tunnel syndrome. J Manipulative Physiol Ther. 2007;30:50–61. [DOI] [PubMed] [Google Scholar]
- 36.De-la-Llave-Rincon AI, Ortega-Santiago R, Ambite-Quesada S, et al. Response of pain intensity to soft tissue mobilization and neurodynamic technique: a series of 18 patients with chronic carpal tunnel syndrome. J Manipulative Physiol Ther. 2012;35:420–7. [DOI] [PubMed] [Google Scholar]
- 37.Karels CH, Polling W, Bierma-Zeinstra SM, Burdorf A, Verhagen AP, Koes BW. Treatment of arm, neck, and/or shoulder complaints in physical therapy practice. Spine. 2006;31:E584–9. [DOI] [PubMed] [Google Scholar]
- 38.MacDermid JC, Wojkowski S, Kargus C, Marley M, Stevenson E. Hand therapist management of the lateral epicondylosis: a survey of expert opinion and practice patterns. J Hand Ther. 2010;23:18–29. [DOI] [PubMed] [Google Scholar]
- 39.Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, van Middelkoop M, Koes BW. Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments — a systematic review. Arch Phys Med Rehabil. 2010;91:981–1004. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh YL. Peripheral therapeutic ultrasound stimulation alters the distribution of spinal C-fos immunoreactivity induced by early or late phase of inflammation. Ultrasound Med Biol. 2008;34:475–86. [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 42.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–15. [DOI] [PubMed] [Google Scholar]
- 43.Cleland JA, Fritz JM, Whitman JM, Heath R. Predictors of short-term outcome in people with a clinical diagnosis of cervical radiculopathy. Phys Ther. 2007;87:1619–32. [DOI] [PubMed] [Google Scholar]
- 44.Young IA, Michener LA, Cleland JA, Aguilera AJ, Snyder AR. Manual therapy, exercise, and traction for patients with cervical radiculopathy: a randomized clinical trial. Phys Ther. 2009;89:632–42. [DOI] [PubMed] [Google Scholar]
- 45.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–15. [PubMed] [Google Scholar]
- 46.Cleland JA, Fritz JM, Whitman JM, Palmer JA. The reliability and construct validity of the Neck Disability Index and patient specific functional scale in patients with cervical radiculopathy. Spine. 2006;31:598–602. [DOI] [PubMed] [Google Scholar]
- 47.Young IA, Cleland JA, Michener LA, Brown C. Reliability, construct validity, and responsiveness of the neck disability index, patient-specific functional scale, and numeric pain rating scale in patients with cervical radiculopathy. Am J Phys Med Rehabil. 2010;89:831–9. [DOI] [PubMed] [Google Scholar]
- 48.Jensen MP, Miller L, Fisher LD. Assessment of pain during medical procedures: a comparison of three scales. Clin J Pain. 1998;14:343–9. [DOI] [PubMed] [Google Scholar]
- 49.Coppieters M, Stappaerts K, Janssens K, Jull G. Reliability of detecting ‘onset of pain’ and ‘submaximal pain’ during neural provocation testing of the upper quadrant. Physiother Res Int. 2002;7:146–56. [DOI] [PubMed] [Google Scholar]
- 50.Topp KS, Boyd BS. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys Ther. 2006;86:92–109. [DOI] [PubMed] [Google Scholar]
- 51.Lewis JS, Wright C, Green A. Subacromial impingement syndrome: the effect of changing posture on shoulder range of movement. J Orthop Sports Phys Ther. 2005;35:72–87. [DOI] [PubMed] [Google Scholar]
- 52.Robertson VJ. Dosage and treatment response in randomized clinical trials of therapeutic ultrasound. Phys Ther Sport. 2002;3:124–33. [Google Scholar]
- 53.Daniel WW. Biostatistics: a foundation for analysis in the health sciences. 7th ed. New York: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 54.Currier LL, Froehlich PJ, Carow SD, et al. Development of a clinical prediction rule to identify patients with knee pain and clinical evidence of knee osteoarthritis who demonstrate a favorable short-term response to hip mobilization. Phys Ther. 2007;87:1106–19. [DOI] [PubMed] [Google Scholar]
- 55.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–4. [DOI] [PubMed] [Google Scholar]
- 56.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coppieters MW, Hough AD, Dilley A. Different nerve-gliding exercises induce different magnitudes of median nerve longitudinal excursion: an in vivo study using dynamic ultrasound imaging. J Orthop Sports Phys Ther. 2009;39:164–71. [DOI] [PubMed] [Google Scholar]
- 58.Vicenzino B, Collins D, Benson H, Wright A. An investigation of the interrelationship between manipulative therapy-induced hypoalgesia and sympathoexcitation. J Manipulative Physiol Ther. 1998;21:448–53. [PubMed] [Google Scholar]
- 59.Frey Law LA, Evans S, Knudtson J, Nus S, Scholl K, Sluka KA. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008;9:714–21. [DOI] [PubMed] [Google Scholar]
- 60.Sefton JM, Yarar C, Carpenter DM, Berry JW. Physiological and clinical changes after therapeutic massage of the neck and shoulders. Man Ther. 2011;16:487–94. [DOI] [PubMed] [Google Scholar]
- 61.Field T, Hernandez-Reif M, Diego M, Schanberg S, Kuhn C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Intern J Neurosci. 2005;115:1397–413. [DOI] [PubMed] [Google Scholar]
- 62.Lund I, Ge Y, Yu LC, et al. Repeated massage-like stimulation induces long-term effects on nociception: contribution of oxytocinergic mechanisms. Eur J Neurosci. 2002;16:330–8. [DOI] [PubMed] [Google Scholar]
- 63.Piazzini DB, Aprile I, Ferrara PE, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil. 2007;21:299–314. [DOI] [PubMed] [Google Scholar]
- 64.Chien A, Eliav E, Sterling M. Whiplash (grade II) and cervical radiculopathy share a similar sensory presentation: an investigation using quantitative sensory testing. Clin J Pain. 2008;24:595–603. [DOI] [PubMed] [Google Scholar]
- 65.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tuttle N. Do changes within a manual therapy treatment session predict between-session changes for patients with cervical spine pain? Aust J Physiother. 2005;51:43–8. [DOI] [PubMed] [Google Scholar]
- 67.Tuttle N, Laakso L, Barrett R. Change in impairments in the first two treatments predicts outcome in impairments, but not in activity limitations, in subacute neck pain: an observational study. Aust J Physiother. 2006;52:281–5. [DOI] [PubMed] [Google Scholar]
- 68.Forbush SW, Cox T, Wilson E. Treatment of patients with degenerative cervical radiculopathy using a multimodal conservative approach in a geriatric population: a case series. J Orthop Sports Phys Ther. 2011;41:723–33. [DOI] [PubMed] [Google Scholar]