Abstract
Background
Although elevated expression of NanogP8 has been detected in many human tumor tissues, its role in gastric tumorigenesis remains unclear. Therefore, this study aimed to investigate the function and regulatory mechanism of NanogP8 in gastric cancer.
Methods
In this study, NanogP8 cDNA was amplified by real time polymerase chain reaction from the human gastric cancer cell line SGC-7901. The shRNA for RNA interference was established. The NanogP8, pAkt, Akt, pERK, ERK, p-mTOR, and mTOR proteins were detected by using the Western blot assay. Cell viability was evaluated by using the CCK-8 assay. Cell migration and invasion were also examined by using the transwell assay.
Results
The results indicated that the NanogP8 overexpression promoted proliferation and migration of SGC-7901 cell line, whereas its ablation exerted opposite effects. Interestingly, NanogP8 activated Akt, a key mediator of survival signals, and without affecting total Akt protein level. The NanogP8-increased gastric cell proliferation was downregulated by Akt inhibition. Our results further showed that increasing NanogP8 expression in human gastric cancer cells promoted cell proliferation by activating the AKT/mTOR pathway and further maintained gastric cell survival.
Conclusion
Our findings extend the knowledge regarding the oncogenic functions and proved that the NanogP8 regulates cell proliferation and migration by Akt/mTOR signaling pathway in human gastric cancer SGC-7901cell line.
Keywords: NanogP8, cell proliferation, Akt, mTOR
Introduction
Gastric cancer is one of the most common malignancies worldwide with a high rate of metastasis.1 Despite enormous progress in the pathogenesis of gastric cancer,2 many issues still remain to be defined. Therefore, understanding the molecular mechanisms underlying the pathogenesis of gastric cancer will provide potential therapeutic targets for gastric cancer treatment.
Nanog is closely associated with the maintenance of self-renewal and pluripotency in embryonic stem cells.3 The regulation of Nanog and the nature of its target genes have gained prominence in the recent years.4–8 Besides embryonic Nanog gene, human Nanog has eleven pseudogenes.9 Among the pseudogenes, NanogP8, has a complete open reading frame and an ALU series in the 3′-untranslated region (3′-UTR) that is highly homologous to Nanog.3,10 Recent studies suggested that high levels of Nanog expression are positively correlated with advanced stages of cancer and a poor prognosis,11–13 indicating that Nanog is of vital importance in tumor transformation and progression. Given that only one amino acid differs between Nanog and NanogP8, the two proteins may perform similar activities in some ways.14
Growing evidences have shown that NanogP8 is expressed in many tumor cells and tumor tissues. The over-expression of NanogP8 promotes the proliferation of tumor cells in vitro.14,15 In vivo functional studies have shown that cancer-specific NanogP8 has biological (or oncogenic) functions in transgenic animals.16 However, the molecular mechanism by which NanogP8 mediates tumor development has not been investigated. Serine/threonine protein kinase, Akt, is a key mediator of survival signals in response to a range of extra- and intracellular stimuli.17 Once activated, Akt can phosphorylate many substrates to exert its functions. The best-characterized substrate of Akt is the serine/threonine kinase mTOR (mammalian target of rapamycin).18,19 Therefore, inhibition of Akt perhaps provides a potential strategy against tumor progression. It is established that the activation of the Akt/mTOR pathway can increase cell survival and apoptosis resistance.18,19 However, whether there is a connection between NanogP8 and Akt/mTOR pathway has not been elucidated in human gastric cancer.
In the present study, in order to investigate the role of NanogP8 in tumor development, the pGFP-NanogP8 and pshRNA-NanogP8 recombinant plasmids were constructed for the overexpression and inhibition of NanogP8, respectively. We found that NanogP8 regulates tumor growth and progression in human gastric cancer cells. These events were highly correlated with the activation of Akt/mTOR signal pathway. Therefore, our results extend the knowledge about the oncogenic functions of NanogP8 in human gastric cancer.
Materials and methods
Materials
Cell Counting Kit-8 (CCK-8) was purchased from (Beyotime, Haimen, Jiangsu, People’s Republic of China). Lipofectamine™ 2000 reagent was obtained from Invitrogen Company (Carlsbad, CA, USA). PD98059 (PD) and API-2 were purchased from Santa Cruz Company (Santa Cruz, CA, USA). The concentrations of PD and API-2 used in this study were 1 and 2 μM, respectively. Rapamycin was obtained from Calbiochem (Gibbstown, NJ, USA). The antibodies against NanogP8 (purchased from Abcam, Cambridge, UK), β-actin, Akt, p-Akt, ERK, p-ERK, mTOR, and p-mTOR (purchased from Cell Signaling Technology, Danvers, MA, USA) were used in Western blotting assay. The plasmids pEGFP-N1 and pGenesil-1 were kindly provided by Prof Tingxiu Xiang (The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China).
Cell culture and transfection
Human gastric cancer cell lines SGC-7901 were cultured in RPMI 1640 medium (Gibco, Grand Island, NY, USA), supplemented with 10% fetal calf serum, and maintained with 5% CO2. Transient transfection of SGC-7901 cells was performed with Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The use of the commercial human cell line, SGC-7901, was approved by the the ethical committee of Chongqing Medical University, Chongqing, People’s Republic of China.
Plasmids and RNA interference
Total RNA was extracted from SGC-7901 cells and cDNA was synthesized using reverse transcriptase. NanogP8 cDNA was amplified by real time polymerase chain reaction technique using the specific primers P1, P2, P3, and P4 as follows: the inner primers P1 – 5′-CAGGCAACTCACTTTATCC-3′, P2 – 5′-TTAGGCTCCAACCATACTC-3′; the outer primers P3 – 5′-CCGCTCGAGATGAGTGTGGATCCAGCTTG- 3′, P4 – 5′-CGGGGTACCTCAGGTTGCATGTTCATGG-3′. The DNA products were digested with KpnI and XhoI and cloned into pEGFP-N1 to generate GFP-NanogP8. All constructs were verified by sequencing. For shRNA, the PCR products were digested with BamHI and HindIII and cloned into pGenesil to generate shRNA products and the functional shRNA constructs used in the present work include the following: shNanogP8: 5′-AACAAAGCACATCTTGCCAGGA-3′; negative control: 5′-GAATCACGCACTACTCCTTACA-3′. The specificity of the oligonucleotides was confirmed by comparison with all other sequences in the National Center for Biotechnology Information (NCBI) database using Nucleotide BLAST.
Western blotting
Western blotting assay was performed as described previously.20 Cells were washed twice with ice-cold phosphate-buffered saline and prepared in radioimmunoprecipitation assay (RIPA) lysis buffer with 100 μg/mL phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktail set I for 30 minutes on ice. The proteins were loaded on SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Billerica, MA, USA), and blotted with indicated primary antibodies, followed by secondary antibodies. Immunoreactive bands were visualized with a Bio-Rad gel imaging instrument (Bio-Rad, Hercules, CA, USA).
Cell viability assays
After transfecting with indicated plasmids for 24 hours, cells were cultured in a 96-well microplate at a density of 1,000 cells/well for another 24 hours. Then the cell viability was assessed with CCK-8 according to the manufacturer’s instructions. The cell viability and proliferation were observed under the microplate reader at a wavelength of 450 nm.
Detection of cell migration and cell invasion by transwell assay
Cell migration and invasion assays were performed by transwell assay as previously described with a slight modification.21 Six millicell (Millipore) inserts with 8 μm diameter pores were placed into a 24-well plate planted with 5×104 cells in RPMI 1640 containing 1% fetal bovine serum. The lower chambers were filled with medium that contained 10% fetal bovine serum as the chemoattractant. For the invasion assays, the inserts were coated with 50 μL Matrigel (1:3 dilution; BD Biosciences, Franklin Lakes, NJ, USA). After culturing for 24 hours, the cells on the upper membrane surface were removed by scraping with a cotton swab. The cells that passed through the filter were fixed with 4% paraformaldehyde for 20 minutes, and then 0.1% crystal violet staining solution was added to stain the cells for 30 minutes. The invading cells were counted in five randomly selected high-power fields using a microscope. All the experiments were performed in triplicate with three replicates and the mean was calculated.
Statistics analysis
All assays were repeated independently for a minimum of three times. Data were represented as mean ± standard deviation (SD). Statistical analysis was performed with Student’s paired t-test. Differences were considered statistically significant at P<0.05.
Results
NanogP8 expression promotes tumor growth and progression in SGC7901 cells
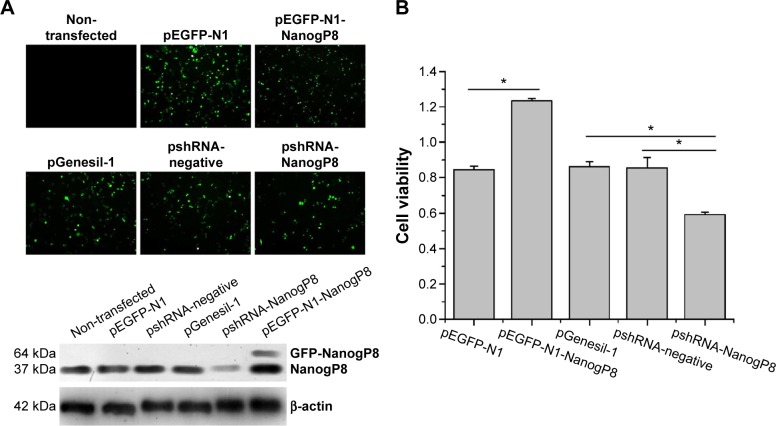
To begin dissecting the function of NanogP8, SGC7901 cells were transfected with pEGFP-N1-NanogP8 and pshRNA-NanogP8. The empty vectors pEGFP-N1, pGenesil-1, and pshRNA-negative control were used as controls. At 48 hours of post-transfection, the efficiency of transient transfection was determined by assaying green fluorescent protein (GFP) expression by fluorescence microscope and comparing with non-transfected cells. Fluorescence microscope analysis of GFP expression showed that the transfection efficiency was higher, while no green fluorescence was observed in non-transfected SGC-7901 cells (Figure 1A). Next, NanogP8 expression was detected by Western blotting. As shown in Figure 1A, compared with non-transfected and control cells, cells transfected with pEGFP-N1-NanogP8 resulted in a GFP-NanogP8 fusion protein, while shRNA-NanogP8 significantly decreased endogenous NanogP8 protein. There were no significant difference in NanogP8 protein levels between non-transfected, empty vectors, and shRNA-negative-control-transfected cells (Figure 1A).
Figure 1.
NanogP8 expression promotes SGC-7901 cell proliferation.
Notes: (A, B) SGC-7901 cells were transfected with indicated plasmids for 48 hours. (A) Fluorescence microscopy identification of green fluorescent protein expression (plasmid Genesil-1 with an enhanced green fluorescent protein [EGFP] label) in SGC-7901 cells. Western blotting was performed to determine the effect of recombinant plasmids for NanogP8 expression in SGC-7901 cells. (B) Cell viability was examined by CCK8. Data represent mean ± SD of three independent experiments. *P<0.05 vs indicated cells.
Abbreviation: SD, standard deviation.
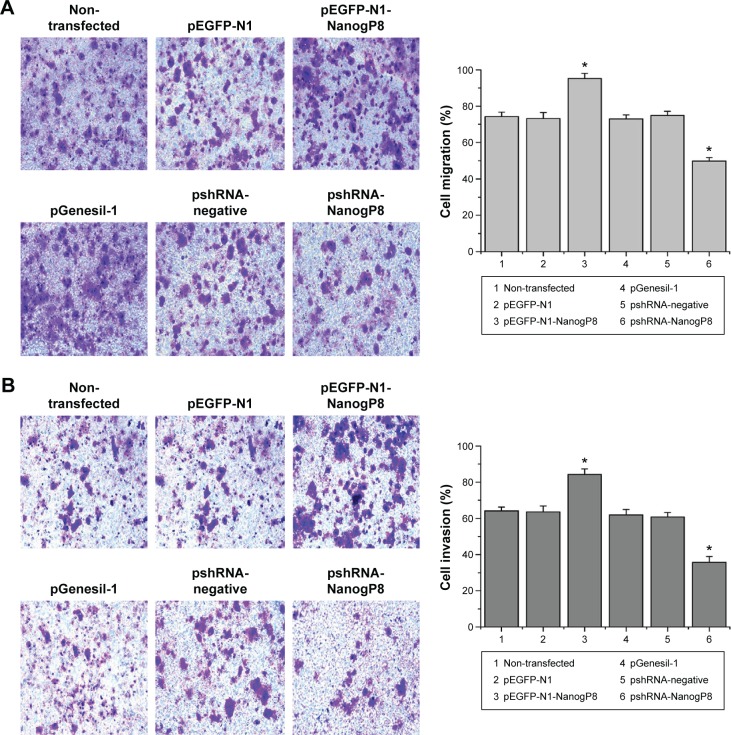
Subsequently, we examined the oncogenic functions of NanogP8 in human gastric cancer cells. To address the effects of NanogP8 in cell proliferation, SGC-7901 cells were transfected with indicated plasmids for 48 hours overexpression and cell viability was determined by CCK8. Similar to the previous findings,13,14 the result showed that cell viability significantly increased in GFP-NanogP8-transfected cells compared with GFP-transfected cells (Figure 1B). In addition, knockdown of NanogP8 by shRNA exerted opposite effects (Figure 1B). We then examined whether NanogP8 affects cell migration and invasion using a transwell plate. As shown in Figure 2A and B, overexpression of NanogP8 significantly increased SGC-7901 migration. The number of GFP-NanogP8-transfected SGC7901 cells that migrated to the lower chamber was 95.2±2.77 compared with 73.2±3.2 for the GFP-transfected SGC7901 cells. Simultaneously, pshRNA-NanogP8-transfected SGC7901 cell migration greatly decreased compared with that of pshRNA-negative-control-transfected and pGenesil-1-transfected cells. The mean number of shNanogP8 cells that migrated to the lower chamber was 49.8±1.92 compared with 74.8±2.38 for the pGenesil-transfected cells and 70.6±6.4 for the pshRNA-negative-control-transfected cells (Figure 2A).
Figure 2.
NanogP8 expression promotes SGC-7901 cell migration and cell invasion.
Notes: (A) SGC-7901 cells were transfected with indicated plasmids, and transwell migration was performed after 48 hours of transfection. Statistical analysis of migration was also performed. (B) Cell invasion assays of invasion in SGC-7901 cells were performed after 48 hours of transfection. Statistical analysis of invasion was also performed. Data represent mean ± SD of three independent experiments. *P<0.05 represents the cell migration vs non-transfected cells.
Abbreviation: SD, standard deviation.
For the invasion assay, the cell invasion of GFP-NanogP8 expressing cells was significantly enhanced compared with that of the GFP expressing cells (84.2±3.11 vs 63.6±3.20). Moreover, compared with pshRNA-negative-control-transfected (60.8±2.38) and pGenesil-1-transfected (61.8±3.11) cells, knockdown of NanogP8 showed reduced invasion (35.8±3.27) (Figure 2B). No significant differences were observed among the pEGFP-N1, pshRNA-negative control, and pGenesil-1 cell groups. These results suggested that NanogP8 plays important roles in tumor development.
NanogP8 activates Akt, but not ERK, in SGC7901 cells
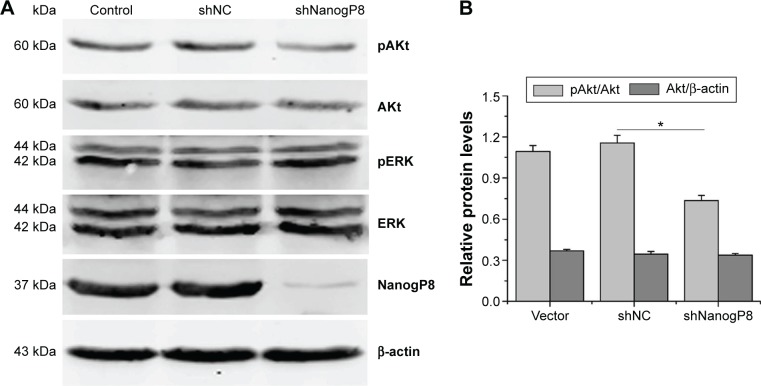
It has been shown that phosphorylation of ERK and Akt is an important cellular signaling event for cell survival and proliferation. We next sought to investigate the mechanism, by which NanogP8 confers a growth and survival advantage. SGC-7901 cells were transfected with indicated plasmids, and the effects of NanogP8 on the phosphorylation of Akt and ERK were determined by Western blot analyses. We found that knockdown of NanogP8 decreased the level of Akt phosphorylation without affecting total Akt protein level (Figure 3A, B). In addition, knockdown of NanogP8 did not affect the level of ERK phosphorylation and total ERK protein level (Figure 3A). Together, these results indicate that NanogP8 activates Akt, but not ERK, in SGC7901 cells.
Figure 3.
NanogP8 affects the phosphorylation of Aktin SGC-7901 cells.
Notes: (A) SGC-7901 cells were transfected with indicated plasmids and the levels of pAkt, total Akt, pERK, and total ERK were examined by Western blotting analysis. β-actin was used as an internal loading control. (B) Quantitative analysis of relative protein levels. Data represent mean ± SD of three independent experiments. *P<0.05 represents the relative pAkt/Akt levels in shNanogP8 group compared to shNC group.
Abbreviations: shNC, negative control shRNA; SD, standard deviation.
NanogP8 promotes SGC-7901 cell proliferation via the Akt-dependent signaling pathway
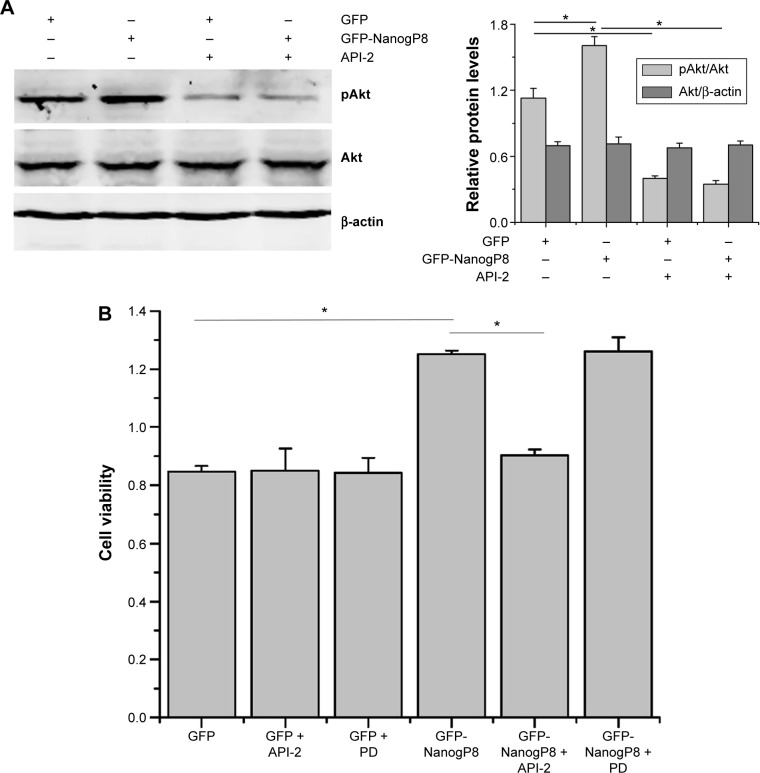
To examine the functional involvement of ERK and Akt in NanogP8-mediated cell proliferation, we examined the effect of the chemical inhibitors of ERK inhibitor PD98059 (PD) and Akt inhibitor API-2, respectively. Initially, we found that NanogP8-mediated Akt activation was inhibited by API-2 (Figure 4A). We next examined the effects of these inhibitors on SGC-7901 cell proliferation and found that NanogP8 overexpression promotes SGC7901 cell proliferation, which was reversed by treatment with API-2 but not PD (Figure 4B). This result suggests that NanogP8 mediates the prosurvival of tumor cells, at least in part, through the Akt pathway.
Figure 4.
NanogP8 promotes SGC-7901 cell proliferation via the Akt-dependent signaling pathway.
Notes: (A) SGC-7901 cells were transfected with pEGFP-N1 and pEGFP-N1-NanogP8 for 48 hours. Then the cells were treated with the Akt inhibitor (2 μM, API-2) for 30 minutes and the level of pAkt and Akt was determined by Western blot. (B) SGC-7901 cells transfected with the indicated plasmids for 36 hours and then treated with the Akt inhibitor (2 μM, API-2) and ERK inhibitor (1 μM, PD98059). After 30 minutes of incubation, the cell viability was detected by CCK8. Data represent mean ± SD of three independent experiments. *P<0.05 represents cell viability in GFP-NanogP8 group compared to GFP group and GFP-NanogP8+API-2 group, respectively.
Abbreviation: SD, standard deviation.
NanogP8 promotes SGC7901 cell proliferation through Akt/mTOR pathway
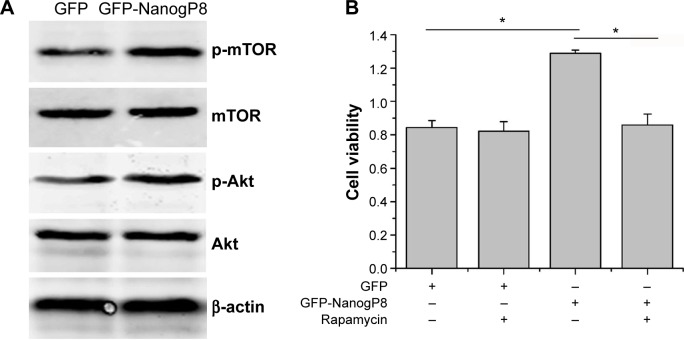
We speculated that the enhanced SGC7901 growth and survival conferred by NanogP8 may be due to its effects on proteins involved in the Akt signaling pathway. Previous study has been proposed that activated Akt can phosphorylate many substrates to exert its functions. The best-characterized substrate of Akt is the serine/threonine kinase mTOR.22 It is possible that activation of this pathway contributes to SGC-7901 cell proliferation. However, whether mTOR is involved in NanogP8-mediated cell proliferation in gastric cancer cells remains unclear. To test this possibility, we transfected GFP-NanogP8 in SGC-7901 cells for 48 hours, and then examined the activation of the Akt/mTOR signaling pathway. As shown in Figure 4A, the levels of phosphorylated Akt were significantly increased by NanogP8 overexpression. Such activation was not due to an increase in the total Akt protein. Importantly, mTOR, a downstream signaling molecule of Akt, was also activated by NanogP8 overexpression (Figure 5A). Inhibition of mTOR (by using rapamycin) suppressed NanogP8-mediated cell proliferation in SGC7901 cells (Figure 5B), which indicates a direct link between Akt/mTOR pathway and NanogP8. Therefore, our findings demonstrate that NanogP8 promotes cell proliferation through Akt/mTOR pathway in human gastric cancer cells.
Figure 5.
NanogP8 promotes SGC7901 cell proliferation through Akt/mTOR pathway.
Notes: (A) Western blot analysis of expression of indicated proteins in pEGFP-N1 and pEGFP-N1-NanogP8-transfected SGC-7901 cells. (B) SGC-7901 cells were transfected with pEGFP-N1-NanogP8 for 24 hours and then treated with mTOR inhibitor rapamycin (50 nM) for another 24 hours. The cell viability was detected by CCK8. Data represent mean ± SD of three independent experiments. *P<0.05 represents cell viability in (GFP- GFP-NanogP8+ Rapamycin-) group compared to the (GFP+ GFP-NanogP8- Rapamycin-) and (GFP-NanogP8+ Rapamycin+) group, respectively.
Abbreviation: SD, standard deviation.
Discussion
Gastric cancer is one of the most common malignancy in the world.1 Although the overall 5-year survival rate in patients can reach 50%,2 there was no apparent improvement in therapeutic effectiveness for years. Despite enormous progress in the pathogenesis of gastric cancer,2 many issues still remain to be addressed. Therefore, the identification of biomarker is of great importance in the early diagnosis and prognosis judgment, especially for a disease with poor outcome such as gastric cancer.
Previous studies have been aimed to assess the role of Nanog as a potential biomarker in several cancers.23–25 NanogP8 gene, a retro-gene of Nanog, is highly expressed in several tumor tissues and cell lines together with Nanog.14 A recent study demonstrated that NanogP8 can substitute for Nanog in directly promoting stemness in human colorectal cancer,5 suggesting that the two proteins may perform similar activities in tumor development. Although growing evidence has shown the key role of Nanog in tumorigenesis, the oncogenic functions of NanogP8 and the molecules involved in human gastric cancer are not well defined. In order to further investigate the role of Nanogp8 in tumor development, the pGFP-NanogP8 and pshRNA-NanogP8 recombinant plasmids were constructed in this study.
It has been reported that NanogP8 overexpression significantly promotes the proliferation of tumor cells in vitro and in vivo.14,15 Recent study also showed that NanogP8 knockdown retards tumor cell proliferation and clone expansion of tumor cells.26 These results are consistent with our observations. However, the mechanism by which NanogP8 confers a growth and survival advantage has not been investigated. Several studies have reported that the Akt/mTOR pathway plays a critical role in tumor growth and survival. However, whether there is a connection between Akt/mTOR pathway and NanogP8 has not been elucidated in human gastric cancer. In this study, we uncovered the Akt signaling as the downstream component of NanogP8. We found that the knockdown of NanogP8 decreased the level of Akt phosphorylation without affecting total Akt protein level. Notably, NanogP8 overexpression promotes tumor cell proliferation, which was reversed after the inhibition of Akt, but not ERK, suggesting that the Akt pathway is the key underlying the prosurvival function of NanogP8. Importantly, mTOR, a downstream signaling molecule of Akt, was also activated by NanogP8 overexpression. Inhibition of mTOR (by using rapamycin) suppressed NanogP8-mediated cell proliferation in SGC7901 cells, suggesting a direct link between Akt/mTOR pathway and NanogP8. Moreover, we did not detect any influence of NanogP8 on the expression or activation of ERK, although we cannot exclude possible contributions from other signaling cascades. However, it remained to be determined as the process by which NanogP8 triggers the Akt pathway.
The metastatic tumor remains to be the major cause of death and poor prognosis in cancer patients, and it is a process involving the invasion and migration of cells and proliferation in a new site.27 To determine the role of NanogP8 in gastric cancer cell migration and invasion, transwell migration and invasion assays were performed. Our results firstly showed that NanogP8 overexpression greatly enhanced the migration and invasion in SGC-7901 cells, whereas NanogP8 knockdown significantly reduced the migration and invasion of SGC-7901 cells. The mechanism that regulates NanogP8-mediated effects on the migration and invasion of tumor cells is unclear and needs to be further elucidated.
In conclusion, our findings extend the knowledge regarding the oncogenic functions and proved that NanogP8 regulates cell proliferation and migration by Akt/mTOR signaling pathway in human gastric cancer SGC-7901cell line.
Acknowledgments
We thank Prof Xiang Tingxiu for kindly providing plasmids pEGFP-N1 and pGenesil-1.
Footnotes
Disclosure
The authors declare no conflict of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Yuan CX, Zhou ZW, Yang YX, et al. Inhibition of mitotic aurora kinase A by alisertib induces apoptosis and autophagy of human gastric cancer AGS and NCI-N78 cells. Drug Des Devel Ther. 2015;9:487–508. doi: 10.2147/DDDT.S74127. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113(5):643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 4.Siu MK, Wong ES, Kong DS, et al. Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene. 2013;32(30):3500–3509. doi: 10.1038/onc.2012.363. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Espinoza LA, Kinders RJ, et al. NANOG modulates stemness in human colorectal cancer. Oncogene. 2013;32(37):4397–4405. doi: 10.1038/onc.2012.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Lou Y, Zheng X, et al. Wnt blockers inhibit the proliferation of lung cancer stem cells. Drug Des Devel Ther. 2015;9:2399–2407. doi: 10.2147/DDDT.S76602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji W, Jiang Z. Effect of shRNA-mediated inhibition of Nanog gene expression on the behavior of human gastric cancer cells. Oncol Lett. 2013;6(2):367–374. doi: 10.3892/ol.2013.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booth HA, Holland PW. Eleven daughters of NANOG. Genomics. 2004;84(2):229–238. doi: 10.1016/j.ygeno.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Badeaux MD, Choy G, et al. Nanog1 in NTERA-2 and recombinant NanogP8 from somatic cancer cells adopt multiple protein conformations and migrate at multiple M.W species. PLoS One. 2014;9(3):e90615. doi: 10.1371/journal.pone.0090615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herreros-Villanueva M, Bujanda L, Billadeau DD, Zhang JS. Embryonic stem cell factors and pancreatic cancer. World J Gastroenterol. 2014;20(9):2247–2254. doi: 10.3748/wjg.v20.i9.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao WY, Liaw CC, Huang YC, et al. Cyclohexylmethyl flavonoids suppress propagation of breast cancer stem cells via downregulation of NANOG. Evid Based Complement Alternat Med. 2013;2013:170261. doi: 10.1155/2013/170261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun AX, Liu CJ, Sun ZQ, Wei Z. NANOG: a promising target for digestive malignant tumors. World J Gastroenterol. 2014;20(36):13071–13078. doi: 10.3748/wjg.v20.i36.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Wang X, Li M, et al. NANOGP8 is a retrogene expressed in cancers. FEBS J. 273(8):1723–1730. doi: 10.1111/j.1742-4658.2006.05186.x. [DOI] [PubMed] [Google Scholar]
- 15.Uchino K, Hirano G, Hirahashi M, et al. Human Nanog pseudogene8 promotes the proliferation of gastrointestinal cancer cells. Exp Cell Res. 2012;318(15):1799–1807. doi: 10.1016/j.yexcr.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Badeaux MA, Jeter CR, Gong S, et al. In vivo functional studies of tumor-specific retrogene NanogP8 in transgenic animals. Cell Cycle. 2013;12(15):2395–2408. doi: 10.4161/cc.25402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29(5):233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8(3):179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Plastaras JP, Dorsey JF, Carroll K, Kim SH, Birnbaum MJ, El-Deiry WS. Role of PI3K/Akt signaling in TRAIL- and radiation-induced gastrointestinal apoptosis. Cancer Biol Ther. 2008;7(12):2047–2053. doi: 10.4161/cbt.7.12.7570. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Liu L, Xing D, Chen WR. Inhibition of the JNK/Bim pathway by Hsp70 prevents Bax activation in UV-induced apoptosis. FEBS Lett. 2010;584(22):4672–4678. doi: 10.1016/j.febslet.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Zhao ZX, Li Y, Zhou ZQ, You TG. Cortactin expression confers a more malignant phenotype to gastric cancer SGC-7901 cells. World J Gastroenterol. 2014;20(12):3287–3300. doi: 10.3748/wjg.v20.i12.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng DJ, Wang J, Zhou JY, Wu GS. Role of the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer cells. Biochem Bioph Res Co. 2010;394(3):600–605. doi: 10.1016/j.bbrc.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Y, Ma C, Wang Z, Liu Z, Liu H, Wang T. Nanog, a novel prognostic marker for lung cancer. Surg Oncol. 2013;22(4):224–229. doi: 10.1016/j.suronc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Nam EJ, Kim SW, Kim S, Kim JH, Kim YT. Prognostic impact of the cancer stem cell-related marker NANOG in ovarian serous carcinoma. Int J Gynecol Cancer. 2012;22(9):1489–1496. doi: 10.1097/IGJ.0b013e3182738307. [DOI] [PubMed] [Google Scholar]
- 25.Shan J, Shen J, Liu L, et al. Nanog regulates self-renewal of cancer stem cells through the insulin-like growth factor pathway in human hepatocellular carcinoma. Hepatology. 2012;56(3):1004–1014. doi: 10.1002/hep.25745. [DOI] [PubMed] [Google Scholar]
- 26.Jeter CR, Badeaux M, Choy G, et al. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27(5):993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottos A, Hynes NE. Cancer: staying together on the road to metastasis. Nature. 2014;514(7522):309–310. doi: 10.1038/514309a. [DOI] [PubMed] [Google Scholar]