Levels of many hormones differ between older and younger persons. This has led to a popular hypothesis that these testing differences are “abnormal” in older people and therefore should be corrected (1–5). Beyond the research community, prescriptions of anabolic hormones such as growth hormone and testosterone have skyrocketed. However, whether these endocrine changes represent “normal aging” or progressive glandular disease, and whether there would be benefit to their correction is currently unresolved.
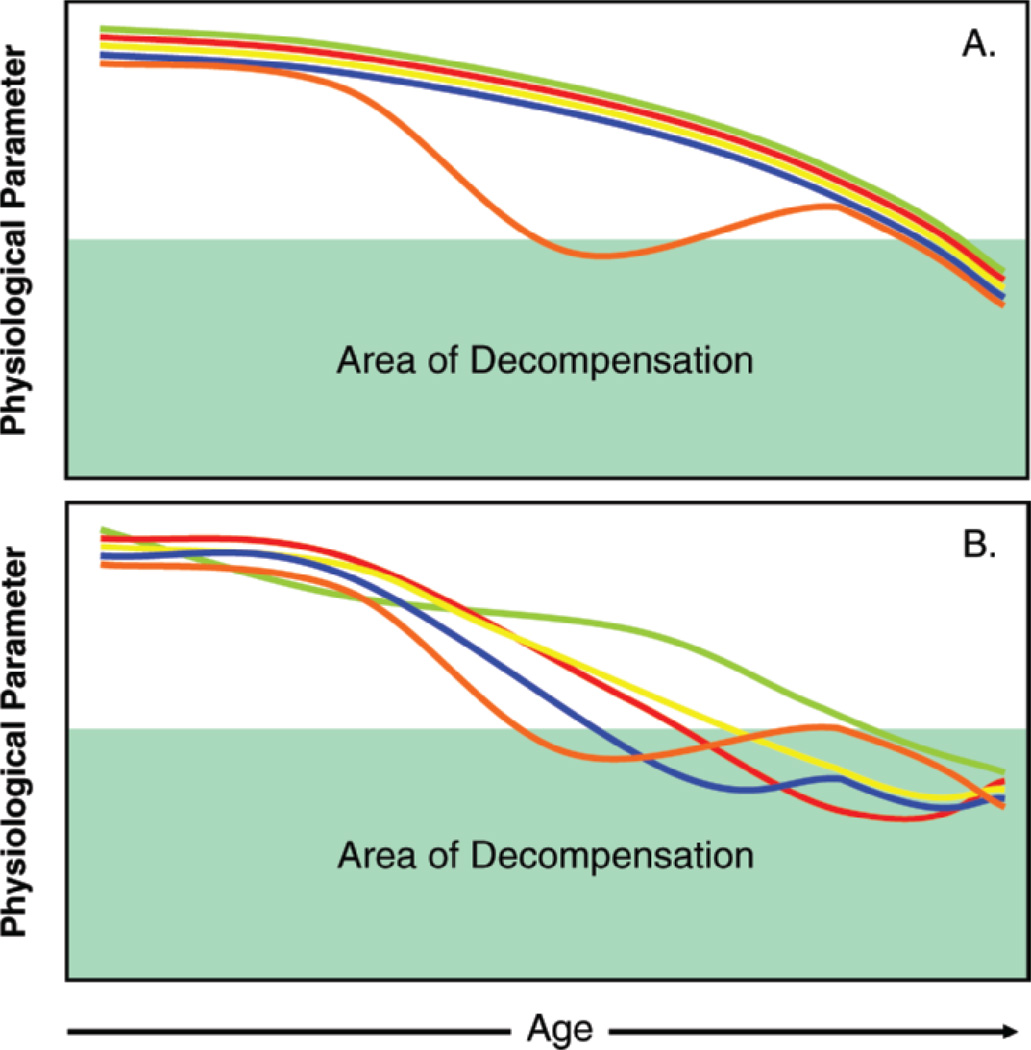
The replacement paradigm whereby a single hormonal deficiency is detected in an older person and replaced to the physiologic level of a younger adult has been attempted (Figure 1A) and has been largely disappointing. Notable examples include estrogen replacement in postmenopausal women, testosterone replacement in older men, and growth hormone (GH) and dehydroepiandrosterone (DHEA) replacement in both sexes (6–12). Explanations proposed for these disappointing results include the timing of the onset of replacement with respect to the onset of the deficiency and the need to select the right target population, based on symptoms and degree of deficiency. New trials are planned or in place that will address some of these issues (10,13).
Figure 1.
A) The one deficiency/one therapy model. In this model, one hormonal deficiency occurs that is beyond the expected age-associated decline, followed by correction of the hormonal deficiency by hormone replacement. This model has been largely disappointing to date. B) The multisystem decline model. In this model, deficiencies are occurring in multiple hormones at once, beyond the expected age-associated decline, with interactive effects between hormones. There are residual physiologic effects not addressed by correction of a single deficiency.
Or, is the single hormone replacement paradigm the wrong approach altogether?
Individual hormones do not operate independently of each other. Rather, one hormonal problem may trigger the onset of another. In a younger person, correction of the original hormonal problem may correct both, such as resolution of hypogonadism after cure of Cushing’s syndrome. In an older person, this simplistic approach is less likely to be successful for several reasons. First, the abnormalities are subtle, progressive, and not clinically discernable from the effects of nonendocrine comorbidities. Second, in the absence of overt glandular dysfunction, the inciting hormonal problem may not be apparent. Finally, the interdependence of hormones can lead to a synergistic effect, creating a vicious cycle where intervention on a single hormone has a negligible effect.
This paradigm suggests that researchers should be examining multiple hormonal axes simultaneously (Figure 1B). From the viewpoint of the gerontologist, this has great appeal, as it suggests a commonality in depletion of homeostatic reserves. Homeostasis is a fundamental mechanism for survival. To achieve and sustain homeostasis, multiple pathways within and across organ systems must interact to defend against external stressors and maintain functional integrity. The age-related decline in complexity of signaling networks, loss of redundancy, and depletion of functional reserves results in inefficiency in maintenance of homeostasis and clinical frailty (14). A key feature of homeostasis is the interrelatedness of systems, which is a departure from the one disease/one organ system approach. We believe that this is particularly applicable to the hormonal paradigm, since hormones circulate in the bloodstream to target receptors throughout the body, resulting in multisystemic effects.
Complementary or synergistic effects between hormonal axes are supported by three different studies of older men. Morley and colleagues in a cross-sectional study of 56 exceptionally healthy men found that bioavailable testosterone, dehydroepiandrosterone-sulfate (DHEAS), and insulin-like growth factor-1 (IGF-1)/GH were each associated with functional variables, though with distinctive patterns of association for the androgens compared to the GH–IGF-1 axis (15). Recent analyses from the InCHIANTI population showed that multiple hormonal deficiencies (lower levels of bioavailable testosterone, DHEAS and IGF-1), when considered in aggregate, were a strong and independent predictor of mortality, whereas deficiency in any of these hormones alone was not (16). Similar findings were presented in the report by Jankowska and colleagues, showing that, in men affected by chronic heart failure, deficiency of more than one anabolic hormone identified subgroups with a higher mortality (17).
The endocrine system and its network of hormonal pathways is one network that itself operates as part of a much broader network. This paradigm could also be further expanded to include additional networks outside the endocrine system, most notably inflammation, which can affect and be affected by multiple hormones (18–20). An example of this is the synergistic effect of the combination of low IGF-1 and high interleukin-6 (IL-6) levels in predicting progressive disability and death in older women (21).
The therapeutic implications of the paradigm of multiple hormonal dysregulation are unclear. Are there benefits to the compensatory responses? Perhaps a new steady state has been entered, albeit at a higher energetic cost. Or, are the cumulative effects across endocrine axes disadvantageous? We propose that a broader approach that extends beyond the “one deficiency, one replacement” model and into an integrated approach to multiple hormonal dysregulation is required to move research in the endocrinology of aging forward.
References
- 1.Ravaglia G, Forti P, Maioli F, et al. Endogenous sex hormones as risk factors for dementia in elderly men and women. J Gerontol A Biol Sci Med Sci. 2007;62:1035–1041. doi: 10.1093/gerona/62.9.1035. [DOI] [PubMed] [Google Scholar]
- 2.Baum LW. Review article. Sex, hormones, and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2005;60:736–743. doi: 10.1093/gerona/60.6.736. [DOI] [PubMed] [Google Scholar]
- 3.Fonda SJ, Bertrand R, O’Donnell A, Longcope C, McKinlay JB. Age, hormones, and cognitive functioning among middle-aged and elderly men: cross-sectional evidence from the Massachusetts Male Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:385–390. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- 4.Sipila S, Heikkinen E, Cheng S, et al. Endogenous hormones, muscle strength, and risk of fall-related fractures in older women. J Gerontol A Biol Sci Med Sci. 2006;61:92–96. doi: 10.1093/gerona/61.1.92. [DOI] [PubMed] [Google Scholar]
- 5.Cappola AR, Xue QL, Walston JD, et al. DHEAS levels and mortality in disabled older women: the Women’s Health and Aging Study I. J Gerontol A Biol Sci Med Sci. 2006;61:957–962. doi: 10.1093/gerona/61.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness J, Aronow WS, Newkirk E, McDanel D. Use of hormone replacement therapy by postmenopausal women after publication of the Women’s Health Initiative Trial. J Gerontol A Biol Sci Med Sci. 2005;60:460–462. doi: 10.1093/gerona/60.4.460. [DOI] [PubMed] [Google Scholar]
- 7.Perls TT. Anti-aging quackery: human growth hormone and tricks of the trade—more dangerous than ever. J Gerontol A Biol Sci Med Sci. 2004;59:682–691. doi: 10.1093/gerona/59.7.b682. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 9.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 10.Testosterone and aging: clinical research directions/Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy/Board on Health Sciences Policy. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 11.Nair KS, Rizza RA, O’Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Bravata DM, Olkin I, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Bassuk SS, Harman SM, et al. Postmenopausal hormone therapy: new questions and the case for new clinical trials. Menopause. 2006;13:139–147. doi: 10.1097/01.gme.0000177906.94515.ff. [DOI] [PubMed] [Google Scholar]
- 14.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 15.Morley JE, Kaiser F, Raum WJ, et al. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A. 1997;94:7537–7542. doi: 10.1073/pnas.94.14.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality. The Aging in the Chianti Area (InCHIANTI) Study. Arch Intern Med. 2007;167:2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankowska EA, Biel B, Majda J, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114:1829–1837. doi: 10.1161/CIRCULATIONAHA.106.649426. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Nakashima N. Dehydroepiandrosterone-sulfate inhibits nuclear factor-kappa B-dependent transcription in hepatocytes, possibly through antioxidant effect. J Clin Endocrinol Metab. 2004;89:3449–3454. doi: 10.1210/jc.2003-031441. [DOI] [PubMed] [Google Scholar]
- 19.Frost RA, Lang CH. Regulation of insulin-like growth factor-I in skeletal muscle and muscle cells. Minerva Endocrinol. 2003;28:53–73. [PubMed] [Google Scholar]
- 20.Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28:116–119. [PubMed] [Google Scholar]
- 21.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]