Abstract
Introduction
Recent studies demonstrated that erythropoietin (EPO) have a number of non-erythropoietic effects including neuroprotection and vascular protection.
Materials
Using data from a representative sample of older persons, we tested the hypothesis that EPO levels are correlated with peripheral nerve parameters (NVC and CMAP) assessed by surface ENG and with clinically diagnosed polyneuropathy. We selected 972 participants (aged 60–98 years) with complete data for the analyses.
Results
We found a significant association between EPO and age-adjusted NCV and CMAP (for NCV: 0.57 ± 0.26; p = 0.03 and for CMAP: 0.54 ± 0.23; p = 0.02). In logistic regression models adjusting for age, sex and multiple potential confounders, higher EPO levels were associated with a significantly lower probability of having a clinical diagnosis of polyneuropathy (OR = 0.43; 95% CI: 0.22–0.84).
Discussion
These findings suggest that EPO is implicated in the pathogenesis of polyneuropathy in older persons. Whether low EPO is a risk factor for polyneuropathy should be tested in future longitudinal analyses.
Keywords: EPO, Polyneuropathy, Prevention, Population-based study, InCHIANTI study
1. Introduction
Both in vivo and in vitro experimental studies have recently demonstrated that erythropoietin (EPO) has a number of non-erythropoietic effects including neuroprotection and vascular protection (Maiese et al., 2005; Sakanaka et al., 1998; Chong et al., 2002). These properties are consistent with the localization of the EPO receptors in tissue other than the bone marrow, including the kidney, the heart and the central and peripheral nervous system, and with the strong antiapoptotic activity that EPO has shown in many pre-clinical studies (Maiese et al., 2005; Sakanaka et al., 1998; Chong et al., 2002; Digicaylioglu et al., 1995).
In models of both focal and global cerebral ischemia, EPO reduced brain infarct size and safeguarded injury sensitive hippocampal neurons (Morishita et al., 1997). Systemic administration of EPO before or immediately after a retinal ischemia can protect retinal ganglion cells from apoptosis (Kilic et al., 2005). In addition, a recent phase 1–2 clinical trials suggested that erythropoietin significantly limit the extent of neural damage in patients with stroke (Ehrenreich et al., 2002).
Bianchi et al. recently shown that erythropoietin can prevent and even reverse diabetic neuropathy in rats (Bianchi et al., 2004). However, whether circulating level of EPO correlate with peripheral nerve function in humans is currently unknown. This information is critical for designing clinical trials testing therapeutic efficacy of EPO on peripheral nerve function (Lipton, 2004).
Using data from a population-based sample of older men and women enrolled in the InCHIANTI study, we tested the hypotheses that EPO levels are correlated with peripheral nerve parameters (NVC and CMAP) assessed by surface ENG and with a clinical diagnosis of polyneuropathy.
2. Methods
2.1. Study population
InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) is a longitudinal study of factors affecting mobility in late life, conducted in the Tuscany Region of Italy. The rationale, design, and data collection have been described elsewhere (Ferrucci et al., 2000). Briefly, of the 1116 persons aged 60 aged or more who participated in the blood drawing, 972 (87%) had also complete nerve conduction parameters and were considered in these analysis. The subjects who did not participate in the nerve conduction studies were generally older and had greater co-morbidity than the subjects who participated in the blood drawing.
The INRCA Institutional Review Board ratified the study protocol. Participants consented to participate and to have their blood samples analyzed for scientific purposes. For those unable to fully consent, surrogate consents were obtained from close relatives.
2.2. Definition of polyneuropathy
Standard surface electroneurographic (ENG) studies of the right peroneal nerve were conducted within 3 weeks of the home interview (Buschbacher, 1999). All studies were performed on an ENG-Neuro MYTO device (Florence, Italy) using standard ENG-Neuro disposable electrodes. A detail description of the methods is reported elsewhere (Lauretani et al., 2006). Briefly, a flexible measuring tape was used for measuring distances between stimulating and recording points. The recording electrode was placed on the skin above the extensor digitorum brevis muscle. Stimulation was done using standard supramaximal technique, proximally at the fibular head and distally over the anterior ankle. The measurements were obtained while dorsal foot skin temperature was between 30 and 34 °C (Lauretani et al., 2007).
The following parameters of nerve conduction studies were considered in the analysis: (a) the amplitude of the distal compound-muscle action potential (CMAP), which is related to the number of axons that conduct impulses from the stimulus point to the muscle and the number of functioning motor endplates; (b) the nerve conduction velocity (NCV), which is an indicator of nerve myelination, calculated by dividing the length of the nerve segment between the two stimulation points by the difference between the proximal and distal time latency to obtain the conduction velocity of the fastest motor axons (Lauretani et al., 2006). Polyneuropathy was defined by diagnostic surface electroneurography and a constellation of signs and symptoms including, sensory symptoms and reduced deep tendon reflex that the assessing physicians judged as indicative of damage of the peripheral nervous system (England et al., 2005). In particular, we established an operational diagnosis of polyneuropathy in participants with NCV less than 40 m/s or CMAP less of the 3 mV, plus at least one among the following neurological signs, that were also described in details elsewhere (Ferrucci et al., 2004): (1) Rotulea ipo-reflexia considered as a “diminished” or “absent” response to a standard stimulation using a reflex hammer; (2) impaired vibration sensitivity was tested using a shank-loaded tuning fork, set to vibrate at 128 Hz. The vibrating fork was applied and kept steady on the distal interphalangeal joints of the index finger and of the big toe. Participants were asked to report whether they felt any vibration and to indicate when the vibration disappeared. Impaired in “vibration sensitivity” was coded when the participant felt the vibration for less than 10 s; (3) reduced touch sensitivity (evaluated as the ability to sense the touch of von Frey nylon monofilaments applied to the skin of the external malleulus). Two monofilaments that require different forces to be buckled were used, namely the 4.31 and the 4.56 from the Semmes-Weinstein Aesthesiometer series. The test was repeated twice for each monofilament. Abnormal “touch sensitivity” was considered if one of the monofilaments was never sensed on at least one side. This definition, although not exhaustive, comply with the suggestion of the American Academy of Neurology (England et al., 2005).
Overall 57 (5.86%) participants, 21 men and 36 women were affected by polyneuropathy. This prevalence of polyneuropathy is similar to the prevalence reported in other epidemiological studies conducted in representative populations (ILSA, 2007).
2.3. Biological samples
Blood samples were obtained from participants after a 12-h fast, and after a 15-min rest. Aliquots of serum were stored at −80 C° and not thawed until analyzed.
Serum Creatinine levels was measured by a kinetic colorimetric assay, based on modified Jaffé method. The analytical sensitivity (lower detection limit) was 0.1 mg/dl. The intra-assay and inter-assay CV were, respectively, 0.7 and 2.3%, 0.3 and 1.7% for urine.
Serum Vitamin B12 level was detected by a RIA assay (ICN Diagnostic Division, NY, USA). The sensitivity of test was 75 pg/ml. The intra-assay CV was 11.2% and the inter-assay CV was 12.3%.
Erythropoietin serum levels were measured in duplicate using the Advantage EPO chemiluminescence immunoassay (Nichols Institute Diagnostic, San Clemente, CA), which has a sensitivity of 1.2 mU/ml, and a coefficient of variation less then 6%. The assay is referenced to the WHO Recombinant DNA-Derived Human Erythropoietin First International Standard.
Hemoglobin levels were analyzed within 6 h using the hematology autoanalyzer DASIT SE 9000 (Sysmex Corporation, Kobe, Japan).
C-reactive protein (CRP) was measured in duplicate using an ELISA and colorimetric competitive immunoassay. The minimum detectable concentration was 0.03 mg/l and the inter-assay coefficient of variation was 5%.
Free IGF-1 was measured by commercial radioimmunoassay (Diagnostic System Laboratories, Webster, TX). Inter-assay and intra-assay coefficients of variation were 6.2 and 7.3%, respectively.
2.4. Other measures
Weight and height were measured in standardized position and body mass index (BMI) was calculated as weight (kg)/height (m2).
Diseases included in the current analysis were hypertension and peripheral artery disease (PAD), defined as an ankle-arm index of 0.9 or less. Diagnostic algorithms were modified versions of those created for the Women’s Health and Aging Study (Guralnik et al., 1995).
Diabetes was defined by fasting blood glucose levels ≥ 126 mg/dl, according to new classification adopted by the American Diabetes Association (American Diabetes Association, 1998).
3. Statistical analysis
Variables are reported as means (standard deviations) for normally distributed parameters or as median and interquartile ranges, according to the polyneuropathy. Because the distribution of EPO levels was highly skewed, EPO values are reported as median and interquartile range, log-transformed to be used in analysis and then back transformed for data presentation.
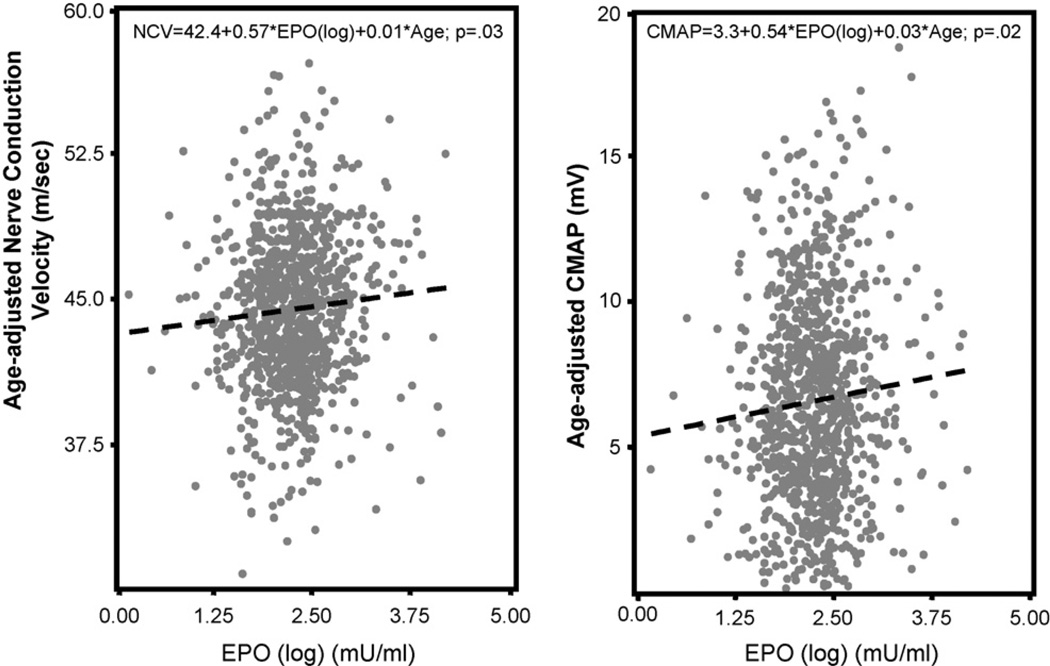
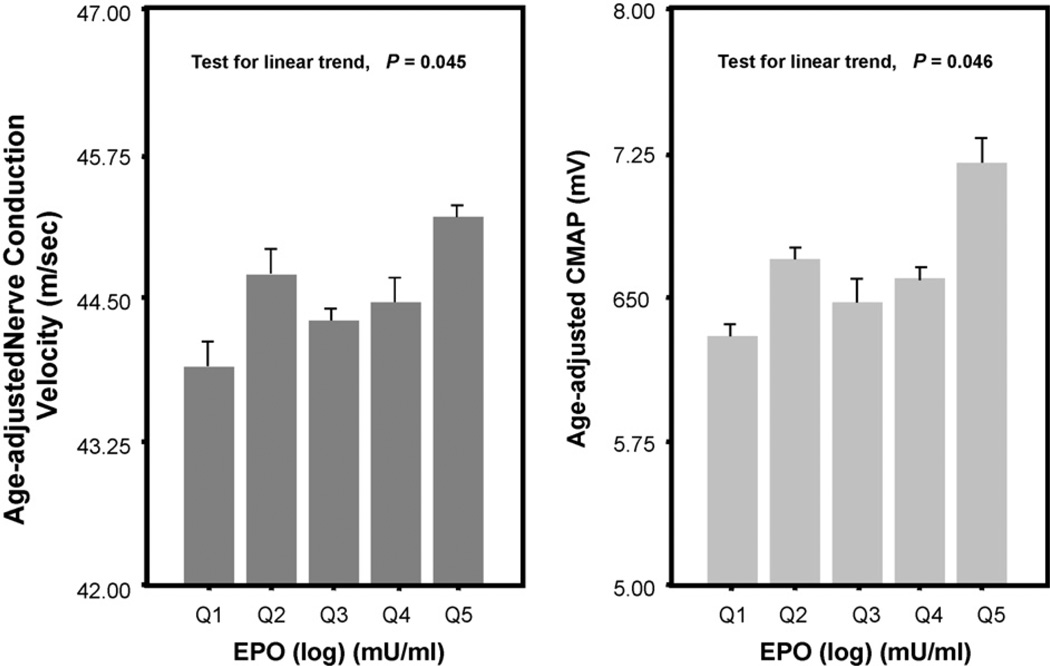
The relationship between EPO and age-adjusted NCV and CMAP were examined by scatter plots with superimposed plots of linear regression predicted values. Then, age-adjusted means of NCV and CMAP were estimated from regression models where log (EPO) was coded in quintile as dummy variables to allow for non-linear association.
A fully adjusted logistic regression analysis was used to test the hypothesis that lower EPO level was associated with a significantly higher probability of having polyneuropathy, after adjusting for potential confounders. This analysis was also performed restricting the model to non-anemic participants (n = 760).
All analyses were performed using the SAS statistical package, version 8.2 (SAS Institute Inc., Cary, North Carolina).
4. Results
Anthropometric characteristics, biological variables, and prevalence of stroke, diabetes, hypertension and peripheral artery diseases in participants with and without polyneuropathy are reported in Table 1. EPO (log) levels were significantly different in participants with polyneuropathy compared to those without polyneuropathy (Table 1).
Table 1.
Characteristics of the InCHIANTI older participants according to polyneuropathy
| Absence of polyneuropathy (n = 915) | Presence of polyneuropathy (n = 57) | p*,# | |
|---|---|---|---|
| Erythropoietin (mU/ml) (median [Q1–Q3]) | 9.9 [7.2–12.8] | 8.7 [5.9–11.6] | 0.03 |
| Age (years) (mean ± S.D.) | 75.25 ± 8.02 | 75.73 ± 6.87 | 0.65 |
| Sex, female (n, %) | 525 (57.4) | 36 (63.2) | 0.39 |
| Hemoglobin (g/dl) (mean ± S.D.) | 13.72 ± 1.34 | 13.92 ± 1.23 | 0.26 |
| Creatinine (mg/dl) (mean ± S.D.) | 0.91 ± 0.19 | 0.91 ± 0.18 | 0.95 |
| C-reactive protein (µg/ml) (median [Q1–Q3]) | 2.29 [1.10–5.13] | 2.58 [1.30–5.39] | 0.41 |
| Free IGF-1 (pg/ml) (median [Q1–Q3]) | 0.55 [0.36–0.70] | 0.56 [0.46–0.75] | 0.89 |
| Vitamin B12 (pg/ml) (median [Q1–Q3]) | 385.0 [283.0–526.0] | 407.0 [315.0–570.0] | 0.75 |
| Height (m) (mean ± S.D.) | 1.60 ± 0.09 | 1.60 ± 0.10 | 0.81 |
| BMI (kg/m2) (mean ± S.D.) | 27.06 ± 3.99 | 26.71 ± 4.44 | 0.56 |
| Diabetic (n, %) | 66 (7.2) | 9 (15.8) | 0.02 |
| Peripheral artery diseases (n, %) | 50 (5.5) | 2 (3.5) | 0.63 |
| Stroke (n, %) | 51 (5.6) | 3 (5.2) | 0.73 |
| Hypertension (n, %) | 402 (43.9) | 26 (45.6) | 0.74 |
p = Age-adjusted.
From Age-Adjusted Linear or Multinomial Logistic Regression Models as appropriate.
EPO (log) levels were significantly associated with NCV and CMAP (for NCV: 0.57 ± 0.26; p = 0.03 and for CMAP: 0.54 ± 0.24; p = 0.02). In age-adjusted analysis, we also found a significant association between both NCV and CMAP and quintiles of EPO (log) independently of age (test for linear trend: p = 0.045 for NCV and p = 0.046 for CMAP) (Figs. 1 and 2).
Fig. 1.
Fig. 2.
In logistic regression models adjusted for age and sex (Table 2, Model 1) and for multiple potential confounders (Table 2, Model 2), a lower EPO (log) level was associated with a significantly higher probability of having a clinical diagnosis of polyneuropathy (OR: 0.43 [95% CI: 0.22–0.84]). Similar findings were obtained after excluding participants with anemia from the analysis (Table 2, Model 3).
Table 2.
Relationship between erytropoietin and other risk factors with polyneuropathy in older adults
| Characteristic | OR | 95% CI | p |
|---|---|---|---|
| Model 1 | |||
| Erythropoietin (log) | 0.54 | 0.32–0.93 | 0.03 |
| Age (years) | 1.01 | 0.97–1.04 | 0.77 |
| Sex (female) | 1.27 | 0.73–2.22 | 0.40 |
| Model 2 | |||
| Erythropoietin (log) | 0.43 | 0.22–0.84 | 0.01 |
| Age (years) | 1.01 | 0.97–1.06 | 0.57 |
| Sex (female) | 1.48 | 0.78–2.82 | 0.24 |
| Body mass index (kg/m2) | 0.99 | 0.93–1.06 | 0.77 |
| Hemoglobin (g/dl) | 1.09 | 0.85–1.40 | 0.46 |
| Creatinine (mg/dl) | 1.38 | 0.28–6.77 | 0.68 |
| Diabetes | 2.47 | 1.08–15.65 | 0.03 |
| Model 3 (non-anemic population)a | |||
| Erythropoietin (log) | 0.32 | 0.15–0.68 | 0.003 |
Also adjusted for covariates of the Model 2.
5. Discussion
Using data from a representative sample of the general population, we tested the hypothesis that low EPO levels are associated with impaired peripheral nerve function in older persons. In age- and sex-adjusted analyses, we found an association between EPO level and the peripheral nerve conduction parameters. In addition, we found that a lower EPO level was associated with a significantly higher probability of having clinical diagnosis of polyneuropathy.
Our findings are consistent with previous studies showing that EPO can prevent and even reverse diabetic neuropathy in rats (Bianchi et al., 2004). Bianchi et al. have recently shown that intraperitoneal erythropoietin for up to 10 weeks improved abnormalities in nerve conduction velocity, Na+, K+-ATPase activity (a biochemical abnormality associated with diabetic neuropathy), compound-muscle action potentials, nociception (pain thresholds), and loss of cutaneous nerve fibers in rats with streptozocin-induced diabetes (Bianchi et al., 2004). Moreover, our findings are consistent with the notion that human erythropoietin (rHu-EPO), which possess potent neuroprotective properties which have been demonstrated both in central and peripheral nervous systems and in both in physiological as well as pathological conditions (Goldman and Nedergaard, 2002; Keswani et al., 2004; Scarlato et al., 2005). Interestingly, EPO and its receptor (EPO-R) are expressed in the central and peripheral nervous system, and the expression of both proteins is modulated by hypoxia (Digicaylioglu and Lipton, 2001). Accordingly, it has also been hypothesized that EPO plays a critical role in neuronal survival especially in hypoxia injury (Gorio et al., 2002). Campana et al. also demonstrated that EPO and EPO-R can be found in the sciatic nerve of adults rats in physiological state and are up-regulated in Schwann cells after painful chronic constriction injury (Campana and Myers, 2003).
Findings from recent studies showed that subcutaneous EPO therapy improved motor polyneuropathy in uremic patients, and that this non-hematopoietin EPO effect may reflect remyelination (Hassan et al., 2003). Several recent studies have outlined the mechanism by which EPO may prevent cell injury (Digicaylioglu and Lipton, 2001). There is robust experimental evidence that EPO prevents apoptosis from a number of sources, including hypoxia, exctitotoxicity, and free radical damage (Chong et al., 2002). Apoptosis in neurons is characterized by genomic DNA destruction and the loss of cell membrane asymmetry through membrane phospatidylserine (PS) externalization. There is evidence that enhanced apoptosis contributes significantly to a number of neurological disease, such as ischemic stroke, dementia, Alzheimer disease, spinal cord injury and peripheral neuropathy (Chong et al., 2002). One of the well-known mechanism by which EPO can prevent apoptotic injury is through the Janus-tirosine Kinase 2 (Jak2) signalling pathway (Digicaylioglu and Lipton, 2001). Jak2 activated by EPO triggers the PI 3-K/Akt pathway, which is essential for the maintenance of the mitochondrial membrane potential, which prevents the cellular release of cytochrome C and modulates caspase activity (Digicaylioglu and Lipton, 2001). In addition to preventing apoptotic injury, EPO also has been found to play a role in neuronal progenitor cell development, perhaps through nuclear factor-kB activation (Shingo et al., 2001).
Our study has important limitation. First, owing to the cross-sectional design, we are unable to assess the effect of progressive EPO neuroprotective effect on peripheral nerves over time. Furthermore, information on peripheral nerve function was limited to only one motor nerve. However, our findings were essentially confirmed when we combined information of the nerve conduction parameters objectively collected with a constellation of neurological sign indicative of the polyneuropathy. This is the first large population-based study of the relationship between EPO level and peripheral nerve function in older persons.
The results of this study have important clinical and speculative implications. Based on our findings, we suggest that low EPO level may be considered a potential cause of polyneuropathy dysfunction in older persons, especially when no other plausible causes can be clearly identified. The findings of this study suggest an involvement of EPO in the pathogenesis of polyneuropathy in older persons, that should be investigated and confirmed in longitudinal studies, supporting the rationale, recently reported by Lipton and other researchers, that neuroprotective drugs that are clinically well tolerated, such as erythropoietin, should be tested in clinical trials for the prevention and treatment of polyneuropaty (Lipton, 2004; Hoke, 2006).
Acknowledgments
This work was supported by National Institute on Aging Contracts N01-AG-916413, N01-AG-821336, N01-AG-5-0002, and NIA Grant R01 AG027012. This research was supported in part by the Intramural Research Program, National Institute on Aging, NIH, USA.
Sponsors’ role: None.
The authors received no financial support in relation to this manuscript.
Footnotes
Financial disclosure
The authors declare that they have no conflict of interest to disclose concerning this manuscript.
References
- American Diabetes Association. An up-to-date summary of the current classification of diabetes and standards of the care for the management of diabetes patients, including the goals of treatment. Diabetes Care. 1998;21(Suppl):1. [Google Scholar]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, Lauria G, Borgna M, Lombardi R, Cimen B, Comelekoglu U, Kanik A, Tataroglu C, Cerami A, Ghezzi P. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc. Natl. Acad. Sci. U.S.A. 2004;101:823–828. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschbacher RM. Peroneal nerve motor conduction to the extensor digitorum brevis. Am. J. Phys. Med. Rehabil. 1999;78:S26–S31. doi: 10.1097/00002060-199911001-00006. [DOI] [PubMed] [Google Scholar]
- Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur. J. Neurosci. 2003;18:1497–1506. doi: 10.1046/j.1460-9568.2003.02875.x. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich HF, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Siren AL. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ. American Academy of Neurology; American Association of Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64:199–207. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Cavazzini C, Lauretani F, Corsi A, Bartali B, Cherubini A, Launer L, Guralnik JM. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am. J. Med. 2004;116:807–815. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J. Am. Geriatr. Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nedergaard M. Erythropoietin strikes a new cord. Nat. Med. 2002;8:785–787. doi: 10.1038/nm0802-785. [DOI] [PubMed] [Google Scholar]
- Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, Di Giulio AM, Vardar E, Cerami A, Brines M. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. Bethesda, MD: National Institute on Aging; 1995. The Women’s Health and Aging Study—Health and Social Characteristics of Older Women with Disability. (NIH Pub. No. 95-4009) [Google Scholar]
- Hassan K, Simri W, Rubenchik I, Manelis J, Gross B, Shasha SM, Kristal B. Effect of erythropoietin therapy on polyneuropathy in predialytic patients. J. Nephrol. 2003;16:121–125. [PubMed] [Google Scholar]
- Hoke A. Neuroprotection in the peripheral nervous system: rationale for more effective therapies. Arch. Neurol. 2006;63:1681–1685. doi: 10.1001/archneur.63.12.1681. [DOI] [PubMed] [Google Scholar]
- Ilsa working group. Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007;68:1460–1467. doi: 10.1212/01.wnl.0000260606.36443.29. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Buldanlioglu U, Fischer A, Reed N, Polley M, Liang H, Zhou C, Jack C, Leitz GJ, Hoke A. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann. Neurol. 2004;56:815–826. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Soliz J, Bassetti CI, Gassmann M, Hermann DM. Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating ERK-1/-2. FASEB J. 2005;19:249–251. doi: 10.1096/fj.04-2493fje. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Bandinelli S, Benedetta B, Cherubini A, Iorio AD, Ble A, Giacomini V, Corsi AM, Guralnik JM, Ferrucci L. Omega-6 and omega-3 fatty acids predict accelerated decline of peripheral nerve function in older persons. Eur. J. Neurol. 2007;14:801–808. doi: 10.1111/j.1468-1331.2007.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretani F, Bandinelli S, Bartali B, Iorio AD, Giacomini V, Corsi AM, Guralnik JM, Ferrucci L. Axonal degeneration affects muscle density in older men and women. Neurobiol. Aging. 2006;27:1145–1154. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Erythropoietin for neurologic protection and diabetic neuropathy. N. Engl. J. Med. 2004;350:2516–2517. doi: 10.1056/NEJMcibr041121. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato M, Previstali SC, Carpo M, Pareyson D, Briani C, Del Bo R, Nobile-Orazio E, Quattrini A, Comi GP. Polyneuropathy in POEMS syndrome: role of angiogenic factors in the pathogenesis. Brain. 2005;128:1911–1920. doi: 10.1093/brain/awh519. [DOI] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J. Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]