Abstract
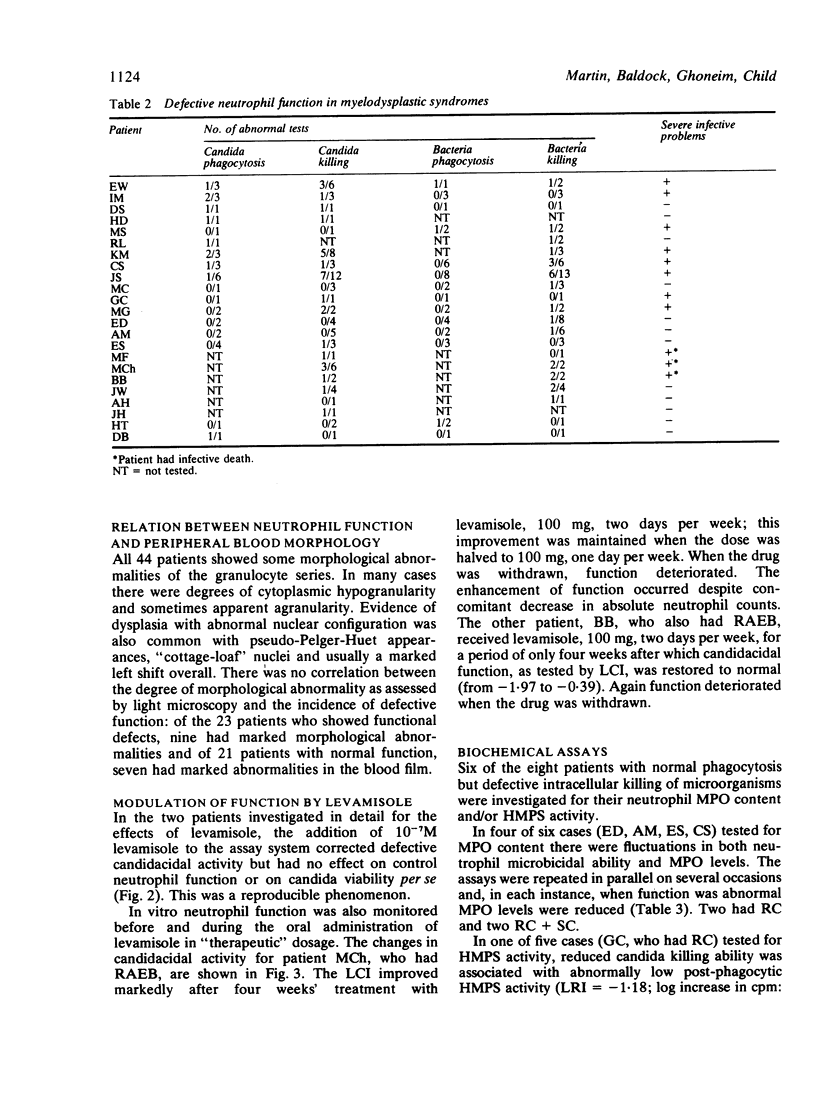
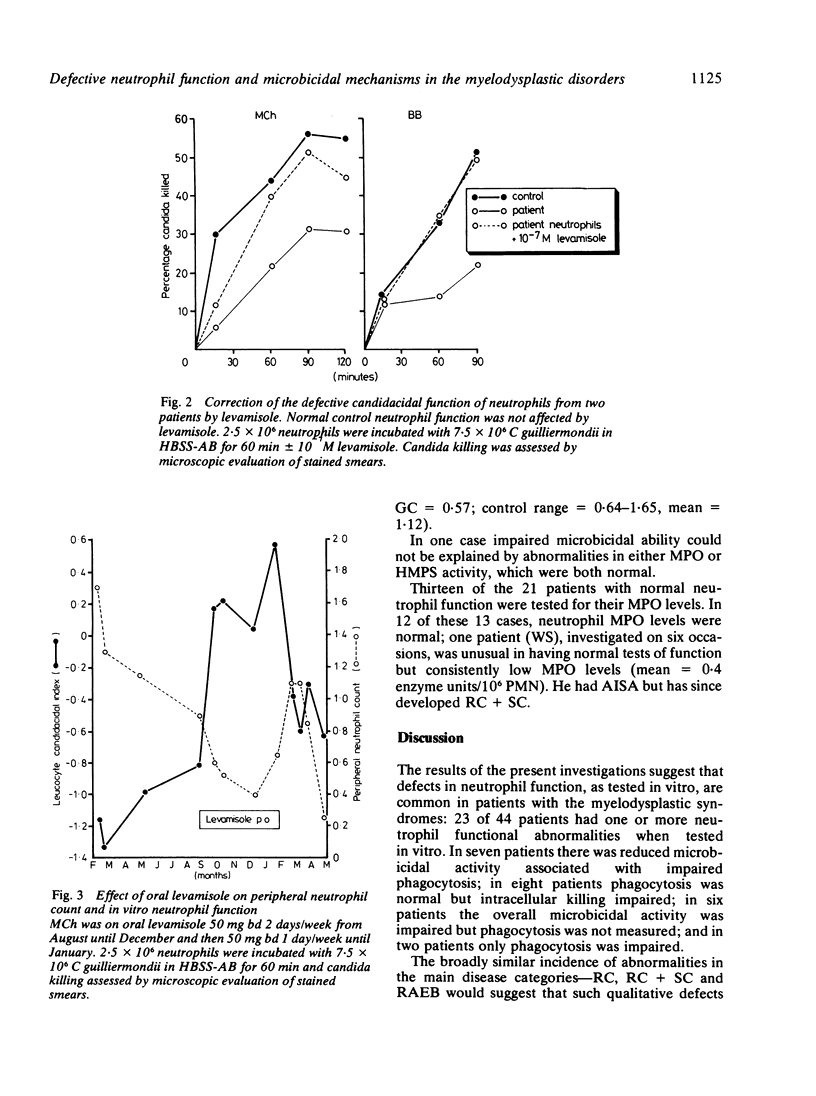
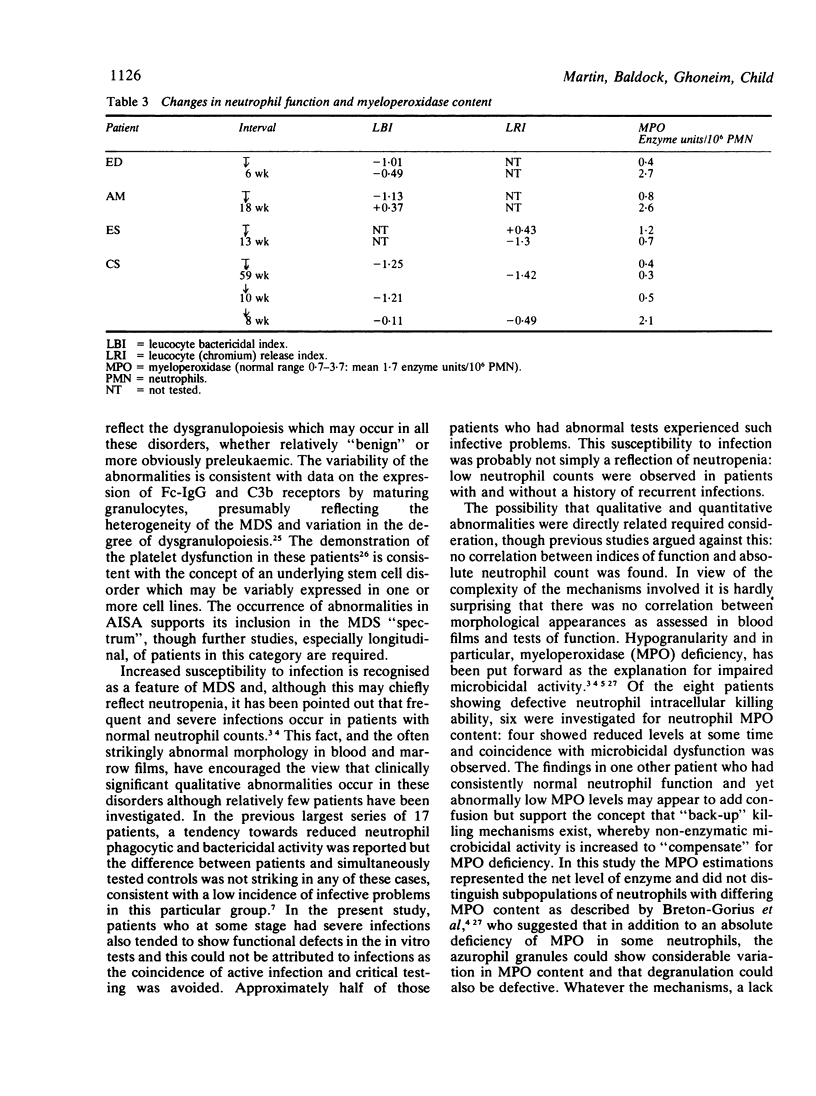
Neutrophil function studies have been carried out in a series of 44 patients with primary myelodysplastic syndromes (MDS). In vitro tests of phagocytosis and killing of Candida guilliermondii and Staphylococcus aureus identified 13 patients with abnormal neutrophil function at presentation and a further 10 who developed abnormalities during the course of their disease. The incidence of defective function in the five disease categories in this series was: refractory cytopenia (RC) 8/17; refractory cytopenia with sideroblastic change (RC + SC) 5/8; acquired idiopathic sideroblastic anaemia (AISA) 2/4; refractory anaemia with excess blasts (RAEB) 7/11; chronic myelomonocytic leukaemia (CMML) 1/4. Eleven of 23 patients with defective neutrophil function experienced severe infective complications; in only three of these patients were neutrophil counts less than 1 X 10(9)/l and susceptibility to infection was considered to reflect, at least partially, qualitative neutrophil abnormalities. There was no correlation between absolute neutrophil count and defective function. Abnormal overall neutrophil microbicidal activity was equally associated with impaired and normal phagocytosis. Some patients with intracellular killing defects had reduced myeloperoxidase (MPO) activities and one had reduced hexose monophosphate shunt (HMPS) activity. In two patients, whose neutrophils showed markedly impaired candidacidal activity, levamisole corrected function when added in vitro at 10(-7) M and also when administered in therapeutic dosage. It is suggested that deranged function, probably reflecting abnormalities in maturation of the granulocyte series, occurs across the myelodysplastic spectrum and that several microbicidal mechanisms may be defective.
Full text
PDF


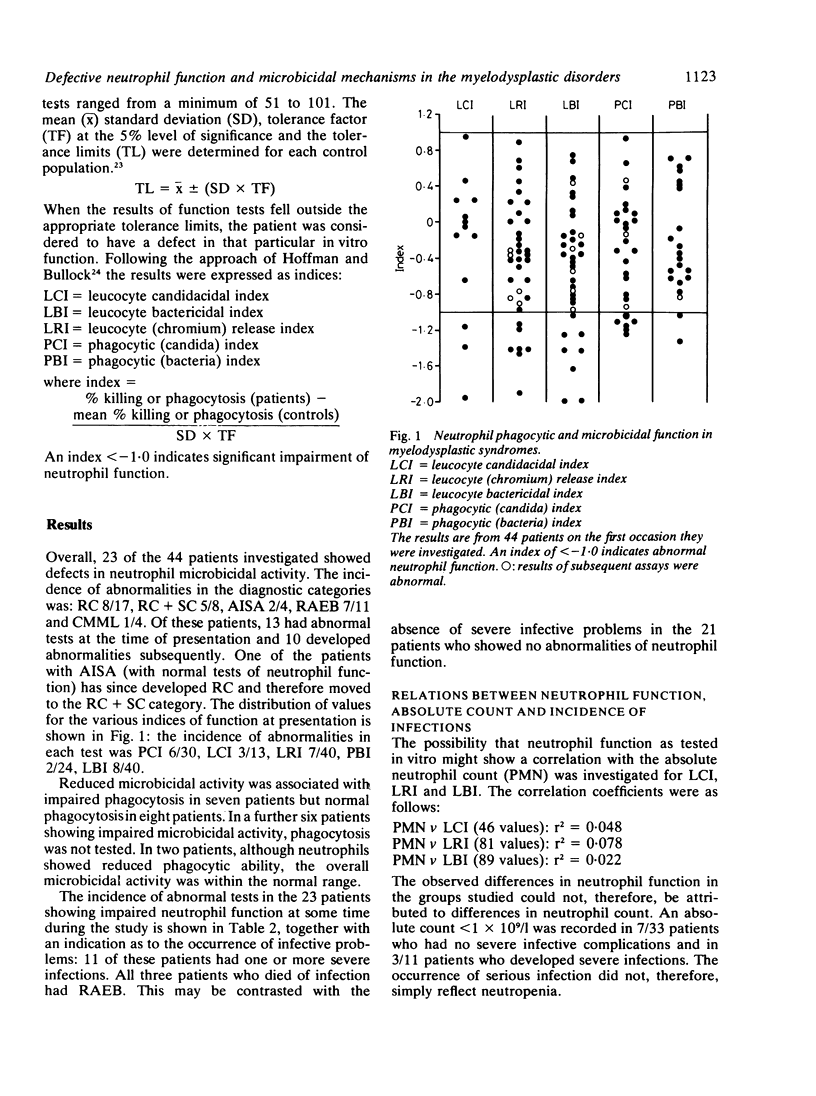


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Glover A., Koornhof H. J., Rabson A. R. In vitro stimulation of neutrophil motility by levamisole: maintenance of cgmp levels in chemotactically stimulated levamisole-treated neutrophils. J Immunol. 1976 Aug;117(2):428–432. [PubMed] [Google Scholar]
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982 Jun;51(2):189–199. [PubMed] [Google Scholar]
- Breton-Gorius J., Coquin Y., Vilde J. L., Dreyfus B. Cytochemical and ultrastructural studies of aberrant granules in the neutrophils of two patients with myeloperoxidase deficiency during a preleukemic state: relationship to abnormal bactericidal activity. Nouv Rev Fr Hematol Blood Cells. 1976;17(1-2):187–209. doi: 10.1007/978-3-642-66312-3_13. [DOI] [PubMed] [Google Scholar]
- Breton-Gorius J., Houssay D., Dreyfus B. Partial myeloperoxidase deficiency in a case of preleukaemia. I. Studies of fine structure and peroxidase synthesis of promyelocytes. Br J Haematol. 1975 Jul;30(3):273–278. doi: 10.1111/j.1365-2141.1975.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Breton-Gorius J., Houssay D., Vilde J. L., Dreyfus B. Partial myeloperoxidase deficiency in a case of preleukaemia. II. Defects of degranulation and abnormal bactericidal activity of blood neutrophils. Br J Haematol. 1975 Jul;30(3):279–288. doi: 10.1111/j.1365-2141.1975.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Child J. A., Martin S., Cawley J. C., Ghoneim A. T. Defective microbicidal function of neutrophils in haematological malignancies and lymphomas: correction by levamisole in vitro. Biomedicine. 1978 Jul;29(5):159–161. [PubMed] [Google Scholar]
- Cline M. J. Defective mononuclear phagocyte function in patients with myelomonocytic leukemia and in some patients with lymphoma. J Clin Invest. 1973 Sep;52(9):2185–2190. doi: 10.1172/JCI107403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DACIE J. V., SMITH M. D., WHITE J. C., MOLLIN D. L. Refractory normoblastic anaemia: a clinical and haematological study of seven cases. Br J Haematol. 1959 Jan;5(1):56–82. doi: 10.1111/j.1365-2141.1959.tb04013.x. [DOI] [PubMed] [Google Scholar]
- El-Maalem H., Fletcher J. Defective neutrophil function in chronic granulocytic leukaemia. Br J Haematol. 1976 Sep;34(1):95–103. doi: 10.1111/j.1365-2141.1976.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Hoffman T. A., Bullock W. E. A statistical approach to the polymorphonuclear leukocyte bactericidal assay. J Lab Clin Med. 1973 Jan;81(1):148–156. [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J. B. Neutrophil function and diagnosis of pre-leukaemic states. Postgrad Med J. 1979 Jun;55(644):396–399. doi: 10.1136/pgmj.55.644.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEBANOFF S. J. INACTIVATION OF ESTROGEN BY RAT UTERINE PREPARATIONS. Endocrinology. 1965 Feb;76:301–311. doi: 10.1210/endo-76-2-301. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Goldberg L. S., Apple M. A., Rosenthal N. P. Refractory megaloblastic anemia with myeloperoxidase-deficient neutrophils. Ann Intern Med. 1972 Mar;76(3):447–453. doi: 10.7326/0003-4819-76-3-447. [DOI] [PubMed] [Google Scholar]
- Linman J. W., Bagby G. C., Jr The preleukemic syndrome (hemopoietic dysplasia). Cancer. 1978 Aug;42(2 Suppl):854–864. doi: 10.1002/1097-0142(197808)42:2+<854::aid-cncr2820420707>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Martin S., Ghoneim A. T., Child J. A. A new neutrophil candida killing test: chromium-51 release from Candida guilliermondii. J Clin Pathol. 1980 Aug;33(8):757–761. doi: 10.1136/jcp.33.8.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olofsson T., Olsson I. Granulocyte function in chronic granulocytic leukaemia. I. Bactericidal and metabolic capabilities during phagocytosis in isolated granulocytes. Br J Haematol. 1975 Mar;29(3):427–441. doi: 10.1111/j.1365-2141.1975.tb01840.x. [DOI] [PubMed] [Google Scholar]
- Ruutu P., Ruutu T., Vuopio P., Kosunen T. U., de la Chapelle A. Function of neutrophils in preleukaemia. Scand J Haematol. 1977 Apr;18(4):317–325. doi: 10.1111/j.1600-0609.1977.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Absence of cytochrome b reduction in stimulated neutrophils from both female and male patients with chronic granulomatous disease. FEBS Lett. 1980 Jan 28;110(1):111–114. doi: 10.1016/0014-5793(80)80035-4. [DOI] [PubMed] [Google Scholar]
- Solberg C. O., Schreiner K., Hellum K. B., Hamre E. Neutrophil granulocyte function in the early diagnosis of acute myelomonocytic and myeloblastic leukaemia. Acta Med Scand. 1975 Mar;197(3):147–151. doi: 10.1111/j.0954-6820.1975.tb04896.x. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Boler J., Valdimarsson H. A chromium release assay for phagocytic killing of Candida albicans. J Immunol Methods. 1976;13(3-4):227–233. doi: 10.1016/0022-1759(76)90069-7. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Boler J., Valdimarsson H. Phagocytosis measured as inhibition of uridine uptake by Candida albicans. J Immunol Methods. 1977;14(1):19–24. doi: 10.1016/s0022-1759(97)90016-8. [DOI] [PubMed] [Google Scholar]


