Abstract
Currently available anti-ulcer drugs suffer from serious side effects which limited their uses and prompted the need to search for a safe and efficient new anti-ulcer agent. Boswellia gum resin (BR) emerged as a safe, efficient, natural, and economic potential cytoprotective agent. Thus, it is of medical importance to develop gastroretentive (GR) formulations of BR to enhance its bioavailability and anti-ulcer efficacy. Early attempts involved the use of organic solvents and non-applicability to large-scale production. In this study, different tablet formulations were prepared by simple direct compression combining floating and bioadhesion mechanisms employing hydroxypropyl methylcellulose (HPMC), sodium carboxymethyl cellulose (SCMC), pectin (PC), and/or carbopol (CP) as bioadhesive polymers and sodium bicarbonate (SB) as a gas former. The prepared tablets were subjected for assessment of swelling, floating, bioadhesion, and drug release in 0.1 N HCl. The optimized GR formulation was examined for its protective effect on the gastric ulcer induced by indomethacin in albino rabbits compared with lactose tablets. The obtained results disclosed that swelling, floating, bioadhesion, and drug release of the GR tablets of BR depend mainly on the nature of the matrix and the ratio of polymer combinations. Moreover, a combination of SCMC-CP in a ratio of 2:1 (SCP21) exhibited desirable floating, bioadhesion, swelling, and extended drug release. Also, a 6-h pretreatment with SCP21 tablets decreased the severity of inflammation and number of bleeding spots among ulcer-induced rabbits in comparison to those treated with lactose tablets.
Electronic supplementary material
The online version of this article (doi:10.1208/s12249-015-0351-8) contains supplementary material, which is available to authorized users.
KEY WORDS: bioadhesion, Boswellia gum resin, cytoprotective, floating, gastroretentive
INTRODUCTION
Despite great advances in drug delivery, the oral drug administration route remains to be the most acceptable due to its economic cost, ease of administration, and patient compliance. However, the oral bioavailability is affected by many factors especially their gastric residence time. The brief gastric emptying time can result in incomplete drug release and diminished efficacy (1). Thus, the retention of oral dosage forms in the upper gastrointestinal tract (GIT) would prolong drug contact time with the gastrointestinal (GI) mucosa imparting higher bioavailability and efficacy and reduced frequency of administration (2). Since that, the development of a gastroretentive drug delivery system (GRDDS) is sometimes desirable especially for drugs locally acting in the stomach (3,4).
Various approaches have been developed to increase the retention of oral dosage forms in the stomach. Among these are bioadhesion to the gastric mucosa (5), swelling or size expansion to prevent their passage through the pylorus (6,7), high-density systems that settles down in the stomach (8), and floating drug delivery systems (FDDS) that remain buoyant above the gastric fluid (9). FDDS have been classified into two main groups: (1) effervescent formulations that produce carbon dioxide gas in contact with gastric contents and (2) non-effervescent formulations which include microporous systems, alginate beads, hydrodynamically balanced systems, and hallow microsphere-microballoons (10).
Use of one approach to provide an effective GRDD sometimes is not successful as in case of floating system that necessitates sufficient fluid in the stomach for tablet buoyancy. Hence, various combined gastroretentive mechanisms were utilized to overcome this limitation and enhance gastroretention capabilities (11). A swellable floating matrix tablet of ciprofloxacin hydrochloride using hydroxypropyl methylcellulose (HPMC), swelling agents (e.g., crospovidone, sodium starch glycolate, and croscarmellose sodium), and sodium bicarbonate (SB) showed more favorable swelling, drug release, and floating characteristics than a marketed product (CIFRAN OD®) (12). As well, a swelling-floating GRDDS of losartan based on a combination of hydroxyethyl cellulose (HEC) and sodium carboxymethyl cellulose (SCMC) offered a greater safety and an improved bioavailability relative to an immediate-release product (Cozaar®) (13).
A large number of drugs have been used for the treatment of gastric ulcers, e.g., antacids, proton pump inhibitors, and anti-histaminics. However, most of these drugs suffer from several adverse reactions which may limit their uses (14). This necessitates a rigorous search for safe, economic, and efficient anti-ulcer agents. Natural products emerge as a reasonable and affordable source to search for compounds which may be used as potential anti-ulcer agents. Badria et al. have investigated the various therapeutic applications of Boswellia including anti-inflammatory (15,16), hepaoprotective (17), immunomodulatory (18), and anti-ulcer (19). Boswellic acids are a mixture of tetra- and penta-cyclic triterpens representing 30–34% of the oleogum resin and were isolated from Boswellia carterii as presented in Fig. 1 (18).
Fig. 1.
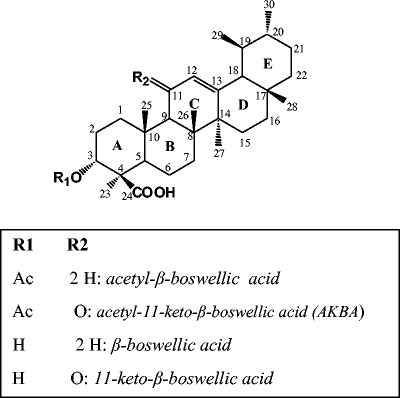
Chemical structure of major bioactive triterpenoids isolated from the oleogum resin of Boswellia carterii
The anti-ulcer activity might be attributed to the increase in the gastric mucosal resistance, local synthesis of cytoprotective prostaglandins, and/or inhibition of the leukotriene synthesis (20). Regarding the anti-ulcer potential, the total mixture of these acids showed a superior effect compared with the pure isolates as previously reported (20).
The main focus of the studies documented in the literature was to evaluate Boswellia oleogum resin (BR) as an excipient. For example, olibanum resin was utilized as a microencapsulating agent for zidovudine (21). In another study, the binding property of BR in tablet formulation was evaluated using aceclofenac as a model drug (22). However, the only attempt that has been made to prolong the gastric residence time of boswellic acid was reported by Fartyal et al. who formulated boswellic acid as a multiple unit system in the form of floating microspheres (23). In spite of the advantages offered by multiple unit floating dosage forms, they exhibited some limitations as the use of organic solvents and difficulty of large-scale production (24). From a manufacturing standpoint, a single unit dosage form can be prepared using easier techniques compared with the multiple units that demand extrusion spheronization or drug loading onto seed cores during manufacturing limiting the large-scale production (25). Moreover, these multiparticulate systems could release the drug at different sites of the GIT (26,27). On the other hand, single unit dosage forms as monolithic tablets has larger size that could hinder the fast passage via the gastric pylorus (28).
Therefore, this study was designed to formulate gastroretentive matrix (GR) of BR tablets using direct compression technique to improve both cytoprotective activity and prolong the onset of action. The effect of different synthetic and natural bioadhesive polymers on the floating, swelling ability, and in vitro drug release was investigated. Moreover, the in vivo cytoprotective effect of the selected formulation on gastric ulcers induced by indomethacin in rabbits was examined.
MATERIALS AND METHODS
Materials
HPMC (K100 LV) and pectin (PC; citrus fruit) were purchased from Fluka, Switzerland and Winlab, a division of Wilfrid Smith, UK, respectively. Carbopol 934P (CP) and magnesium stearate were supplied by Amriya Pharmaceutical Industries Co., Alexandria, Egypt. SCMC, SB, lactose monohydrate, and hydrochloric acid were obtained from El-Nasr Pharmaceutical Chemicals Co., ADWIC, Cairo, Egypt. Indomethacin meglumine was obtained from Chiesi Farmaceutici S.P.A., Parma, Italy. Eosin and hematoxylin were purchased from Merck, Germany. Oleogum resin of B. carterii Birdwood (BR) was purchased from the local herbal stores in Mansoura and authenticated with a genuine sample in the Pharmacognosy Department, Mansoura University, Egypt. All other chemicals were of analytical grade.
Preparation of BR-GR Tablets
Firstly, BR was dried and grounded into fine powder. The respective powders, namely BR, HPMC, PC, CP, and SCMC as well as a gas-forming agent (SB) were passed through sieve No. 90, separately. Tablets containing 150 mg BR were prepared by direct compression according to the design depicted in Table I. For each formulation, mixing of powders was carried out using a mortar and pestle followed by addition and mixing of lactose monohydrate and magnesium stearate. Finally, 425 mg of each mixture were weighed and fed into the die of a single punch tableting press (Type EKO, Erweka-Apparatebau, GmbH, Germany), equipped with flat-faced punches (10 mm). The compression pressure was adjusted to give tablet hardness a value between 6 and 7 kg.
Table I.
Composition of Boswellia Olegum Resin Gastroretentive (BR-GR) Tablet Formulations
| Formulaea | Formula code | HPMC | SCMC | PC | CP | Lactose |
|---|---|---|---|---|---|---|
| HPMC | HPMC | 150 | 60 | |||
| SCMC | SCMC | 150 | 60 | |||
| Pectin | PC | 150 | 60 | |||
| Carbopol | CP | 150 | 60 | |||
| HPMC-carbopol 1:2 | HCP12 | 50 | 100 | 60 | ||
| HPMC-carbopol 1:1 | HCP11 | 75 | 75 | 60 | ||
| HPMC-carbopol 2:1 | HCP21 | 100 | 50 | 60 | ||
| SCMC-carbopol 1:2 | SCP12 | 50 | 100 | 60 | ||
| SCMC-carbopol 1:1 | SCP11 | 75 | 75 | 60 | ||
| SCMC-carbopol 2:1 | SCP21 | 100 | 50 | 60 | ||
| Pectin-carbopol 1:2 | PCP12 | 50 | 100 | 60 | ||
| Pectin-carbopol 1:1 | PCP11 | 75 | 75 | 60 | ||
| Pectin-carbopol 2:1 | PCP21 | 100 | 50 | 60 | ||
| Control | C | 270 | ||||
| Control-1 | C1 | 210 |
HPMC hydroxypropyl methylcellulose, SCMC sodium carboxymethyl cellulose, PC pectin, CP carbopol
aAll formulae contain 150 mg Boswellia gum resin (BR), and 1% magnesium stearate. All formulae contain sodium bicarbonate (60 mg) except C
Evaluation of Tablets
Physical Properties of Tablets
The hardness, friability percent, and content uniformity of the prepared tablets were determined according to procedures stated in the US pharmacopoeia (29).
In Vitro Bioadhesive Strength Measurement
Bioadhesive strength of tablets was measured using a modified two-arm balance (30–32). One metal holder was used to suspend the water-collecting beaker to the balance and another to suspend a glass vial to the other side of the balance as shown in Fig. 2. A piece of rabbit stomach mucosa, 3 × 3 cm, obtained from a local slaughter house and stored in Krebs buffer at 4°C upon collection was used as the mucosal membrane. The mucosal membrane was separated by removing the underlying fat and loose tissues. The experiments were performed within 3 h of procurement of the mucosa. The rabbit gastric mucosa was tied to an inverted 100-mL beaker and placed in a large one (250 mL). Then, 0.1 N HCl was added into the large beaker up to the upper surface of the gastric mucosa to maintain mucosal viability during the experiments. Each tablet was attached to the glass vial with adhesive, and then the beaker was raised slowly until contact between rabbit mucosa and the tablet preload time were kept constant for all the formulations. A preload of 50 g was placed on the vial for 5 min (preload time) to establish adhesion bonding between tablet and rabbit stomach mucosa. After completion of the preload time, preload was removed from the vial and water was then added into the beaker from the burette in the other side. The addition of water was stopped when the tablet was detached from the rabbit mucosa. The weight of water required to detach the tablet from the mucosa was noted as mucoadhesive strength.
Fig. 2.
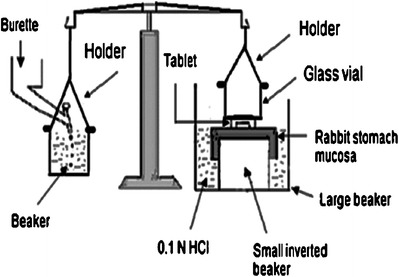
Bioadhesive strength measurement device
Degree of Tablets Swelling
The swelling degree of the tablets was determined according to the method previously adopted (33). Briefly, each tablet was individually weighed (W1) and transferred into a beaker containing 200 mL of 0.1 N HCl and maintained in a water bath at 37 ± 0.5°C. At regular time intervals, the tablet was removed and the excess surface liquid was carefully removed by a filter paper. The swollen tablet was then reweighed (W2). The mean weights of tablets were determined, and the percent swelling was calculated according to the following equation:
Floating Capacities
The floating capacities were examined as previously described (34). Glass beakers containing 100 mL of 0.1 N HCl were placed in a water bath shaker at 37 ± 0.5°C. The tablets were added separately into these beakers and observed for floating over 24 h. The time required for the tablets to rise to the surface and float (floating lag time, FLT) and the duration of floating (total floating time, TFT) were recorded.
In Vitro Drug Release
The drug release from BR-GR tablets was studied using USP apparatus II (Dissolution Apparatus USP Standards, Scientific, DA-6D, Bombay, India). The dissolution test was performed using 900 mL of 0.1 N HCl containing 0.5% (w/v) sodium lauryl sulfate to provide sink condition (23,24,33,35–37). It was maintained at temperature of 37 ± 0.5°C and stirred at 100 rpm. Aliquots (2 mL) were withdrawn at predetermined time intervals up to 12 h and replaced by fresh dissolution medium. The samples were diluted, filtered using millipore filter (0.45 μm pore size and 47 mm diameter, Gelman GN-6 Metricel membrane filter, USA) and analyzed spectrophotometrically at a wavelength of 239 nm using an UV/VIS spectrophotometer (V-550, Jasco, Japan). Plots of cumulative amount released vs. time were constructed.
Analysis of Release Data
To survey more precisely the mechanism of drug release from the investigated formulations, their in vitro release data were analyzed mathematically according to the following models: zero-order kinetics (cumulative % drug released vs. time), first-order kinetics (log % drug retained vs. time), Higuchi model (cumulative % drug released vs. square root of time) (38), and Korsmeyer–Peppas equation (log amount of drug released vs. log time) (39). The model with the highest coefficient of determination (r 2) was considered as the best fitting one.
In Vivo Evaluation of Optimized Gastroretentive BR Tablets
Induction of Gastric Erosions and Ulcers into Rabbit Model
Male New Zealand rabbits, aged 10–11 weeks and weighing 2.1–2.5 kg, were enrolled in this study. The experimental procedures conform to the ethical principles of the scientific committee of the Faculty of Pharmacy, Mansoura University, Egypt for the use of experimental animals.
The animals were deprived of food but allowed free access to water for 24 h before the day of doing the experiment. The anti-ulcerogenic effect of BR-GR tablets were investigated using indomethacin-induced gastric ulceration. The rabbits were divided into four groups (n = 6 per group) as follows: group I, received normal saline (control group); group II, received 20 mg/kg indomethacin orally (untreated); group III, received BR-lactose tablet 6 h before oral administration of indomethacin; and group IV, received orally (SCP21 formula) 6 h before oral administration of indomethacin. One hour after indomethacin administration, rabbits were sacrificed and their stomachs were excised for further macro- and microscopical examination.
Morphological Evaluation of Gastric Erosions
Immediately after rabbit's sacrifice, the freshly excised stomachs were dissected and cut along the greater curvature. The mucosa were rinsed with normal saline to remove blood contaminant, if any, and stretched on a feline board for macroscopical examination. Gross mucosal lesions were recognized as hemorrhage or linear streaks (erosions) with damage to the mucosal surface. Paul’s index was used to assess ulcerogenic effect. It is the integral indicator of the number of lesions induced per formula and is calculated by multiplying the mean number of ulcers and % of rabbits with ulcers and then divided by 100%. Moreover, the anti-ulcer activity (AA) of the preparations was calculated by dividing Paul’s index of the untreated group by that of the experimental group. The tested formulation was considered active if AA was at least of two units (40).
Histopathological Examination of Gastric Erosions
Specimens of stomach of rabbits were fixed in 10% neutral-buffered formalin for 24 h, dehydrated in ascending grades of alcohol, cleared in xylene, and embedded in paraffin. Paraffin sections were cut at 6 μm for hematoxylin and eosin stain (41). They were ranked according to the severity of the inflammatory reaction as follows: severe reaction (+++), moderate reaction (++), mild reaction (+), almost normal tissue (±), and tissue totally free from any inflammatory reaction (−).
Statistical Analysis
The resulting data are represented as mean ± SD. Statistical analysis of the data was carried out using one-way ANOVA followed by Tukey–Kramer multiple comparisons test at a level of significance of p < 0.05 with Instat Graphpad prism software (version 4.00; Graphpad software, San Diego, CA, USA).
RESULTS AND DISCUSSION
Physical Characterization of the Tablets
All formulations showed drug uniformity ranged from 98.1% to 103.51% and percentage friability values less than 1%, indicating good mechanical resistance.
The hardness of the prepared formulations was adjusted at 6–7 kg. At hardness <6 kg, the friability percent was found to be >1%. At higher hardness (>7 kg), the obtained tablets showed relatively longer FLT (>5 min) that might be attributed to the low porosity that would hinder water penetration and hydration of the outer polymer layer preventing the tablets to float (42).
In Vitro Bioadhesive Strength Measurement
Bioadhesion is a strategy to overcome the highly variable residence times at various sites in the GIT improving the efficacy. The interaction between chemical groups of the polymeric chain and in particular hydroxyl, carboxyl, amine, esteric, and amide groups of hydrophilic polymers and mucus via H-bonding or van der Waals forces can contribute to good adhesion properties (43). Moreover, polymers swelling ability and the mobility of molecules facilitate the interpenetration and interaction with the mucus layer (44–46).
The bioadhesive strength values of the prepared tablets were influenced by the nature of the bioadhesive polymers and showed the order of CP > SCMC > PC > HPMC (Table II). The lowest bioadhesive strength was observed with HPMC tablets probably due to more neutral cellulose groups and thus fewer hydrogen bonds with glycoprotein mucin leading to weaker adhesive forces (47). On the other hand, SCMC and their carboxylic groups can increase the surface charge density of the tablets and hence, form more hydrogen bonds with tissue. Moreover, SCMC tablets had initial faster hydration rate which promotes interpenetration of the polymer chain with the tissue (48). Also, pectin, as a negatively charged polymer, is known for good mucoadhesion related to a balance between available hydrogen bonding sites and an open expanded conformation (49).
Table II.
Floating Properties and Mucoadhesion of Boswellia Olegum Resin Gastroretentive (BR-GR) Tablet Formulations
| Formulae | Floating lag time (FLT; s) | Floating duration (TFT; h) | Mucoadhesion (g) |
|---|---|---|---|
| HPMC | 36.56 ± 3.40 | >24 | 21.44 ± 3.12 |
| SCMC | 28.11 ± 2.30 | >24 | 35.12 ± 2.56 |
| PC | 31.40 ± 1.24 | >24 | 31.51 ± 3.11 |
| CP | Immediately | 5.5 ± 0.50 | 40.50 ± 4.12 |
| HCP12 | immediately | 8.5 ± 0.91 | 32.50 ± 3.26 |
| HCP11 | <5 | >24 | 31.25 ± 2.67 |
| HCP21 | 9.5 ± 0.06 | >24 | 29.60 ± 2.03 |
| SCP12 | Immediately | 7.6 ± 0.63 | 39.00 ± 0.93 |
| SCP11 | <5 | >24 | 36.70 ± 2.78 |
| SCP21 | 8.3 ± 0.07 | >24 | 36.40 ± 1.65 |
| PCP12 | immediately | 6.3 ± 0.72 | 36.75 ± 2.34 |
| PCP11 | <5 | >24 | 32.90 ± 1.98 |
| PCP21 | 9.34 ± 0.04 | >24 | 31.50 ± 0.98 |
Each value represents the mean ± S.D. (n = 3)
HPMC hydroxypropyl methylcellulose, SCMC sodium carboxymethyl cellulose, PC pectin, CP carbopol
The highest mucoadhesive strength was obtained with CP tablets probably due to the numerous proton-donating carboxylic groups in CP forming hydrogen bonds with the negatively charged mucus gel (50). As well, formation of intermolecular complexes of CP with the glycoprotein could explain its high mucoadhesive strength (51). Nevertheless, the ionized part of CP has also a bioadhesive force due to the diminished intramolecular hydrogen bonds and a stretched cylindrical shape allowing higher penetration to the mucin network than the coil form of unionized CP (52). The high molecular weight, presence of strong hydrogen bond forming groups (carboxylic acid), anionic nature and sufficient chain flexibility are responsible for the high bioadhesion of CP (53). These factors together may explain the benefit of the combination of CP with each of HPMC, SCMC, and PC in different ratios. These combinations showed comparatively higher bioadhesion compared with HPMC, SCMC, and PC individually as depicted in Table II. In all these formulations, as the CP concentration increased, the mucoadhesive strength also increased. Similar results were previously obtained (35).
Degree of Tablets Swelling
Swelling percent describes the amount of water that is contained within the hydrogel at equilibrium and is a function of the network structure, hydrophilicity, and ionization of the functional groups (54). The tablet ability to hydrate influences tablet buoyancy, adhesion of swellable polymers, and drug release kinetics (55).
The obtained results highlighted strong differences among CP, SCMC, PC, and HPMC tablets regarding percent swelling (Fig. 3a). HPMC tablets exhibited less swelling ability than others with a maximum of 109.6 ± 11.6 after 7 h possibly due to the neutral cellulose groups. While, CP, SCMC, and PC are polyelectrolytes that provide hydrogel with electrostatic charges and hence, the repulsion established between similar charges forces the polymer chains to a more elongated state than that found in a neutral network, thus increasing the swelling ability (56).
Fig. 3.
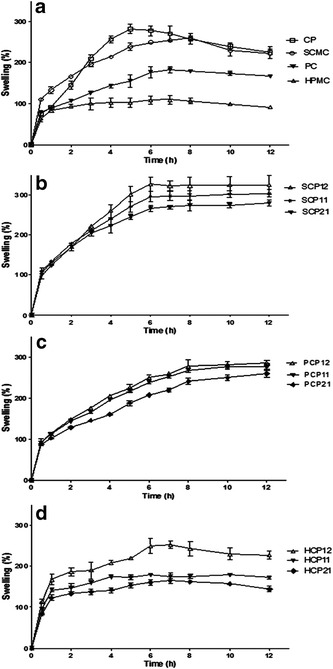
Swelling profiles of tablets containing individual polymers (a), SCMC-CP combinations (b), PC-CP combinations (c), and HPMC-CP combinations (d). Each point represents the mean ± SD (n = 3)
CP tablets exhibited the highest water uptake and a swelling profile that can be divided into two distinct phases (Fig. 3a). During rapid swelling phase (0–5 h), the swelling percentage increased till reached the maximum value (282.15 ± 17.2) after 5 h. The second phase (5–12 h) is characterized by a gradual decrease of the swelling percent till a value of (225.1 ± 21.3) after 12 h which might be due to the dissolution of the gel formed around tablets. It was previously reported that, CP is insoluble in gastric fluid (GF) and, its swelling behavior is attributed to the uncharged –COOH group that get hydrated by forming hydrogen bonds with the imbibing water and, therefore, extending the polymer chain (57). However, the basic gas generating agent, SB, could change pH of GF in the local environment of the swollen region around the tablet to a neutral or alkaline, increasing the ionization of CP which generates negative charges along their backbone, and hence, repulsion of similar charges occurred allowing uncoiling of the polymer and an extended structure (58).
SCMC tablets showed a high swelling percentage throughout the first 2 h, indicating rapid hydration and high affinity to the test medium, then reaching a maximum of 260.8 ± 4.7 within 8 h followed by a decrease that might be due to the slow and gradual erosion of the polymeric matrix (Fig. 3a). This figure also shows that the maximum swelling percentage (182.7% ± 8.1) of PC tablets was achieved after 7 h due to gel formation, uncoiling of the structure of PC molecules, and the formation of hydrogen bonds with water molecules (59). The swelling of PC tablets was significantly (p < 0.05) lower than SCMC or CP. Then, PC gel erodes, goes into solution, and hence, the swelling percentage decreased. Similar results were previously obtained (60).
In view of the above mentioned data, CP appeared to be a promising base matrix due to its high bioadhesive strength, maximum swelling percentage, and good gelling properties. Therefore, its effect on the extent of swelling was further investigated after mixing with each of HPMC, PC, or SCMC in different ratios.
The swelling profiles of SCP12, SCP11, and SCP21 tablets are shown in Fig. 3b. The higher CP content within the SCMC network increased the percentage of swelling and decreased the time for maximum swelling in comparison with those containing SCMC alone probably due to more carboxylic moieties and more expanded network. Similar behavior was also noticed with PC-CP or HPMC-CP matrices as depicted in Fig. 3c, d. However, the maximum swelling percentage of SCMC-CP formulations was significantly higher (p < 0.05) than those of the corresponding PC-CP or HPMC-CP formulations in GF that may be explained by the higher swelling of SCMC compared with PC and HPMC.
Floating Capacities
To attain in vitro buoyancy, SB was used as an effervescent base during formulation of BR-GR tablets. Upon contact of the formulated tablets with 0.1 N HCl as a dissolution medium, the acid–base reaction starts producing CO2 gas which is entrapped in the hydrocolloid gel matrix, and hence, the tablets float (61). The increase in the gas producing agent (60 vs. 30 mg) significantly shortened FLT, yet, it did not affect TFT. So, SB was added in an amount of 60 mg to all formulated tablets.
The results for FLT and TFT are illustrated in Table II. Immediate floating was observed with CP tablets upon contact with the release medium, while, the FLT values of SCMC, PC, and HPMC were between 28.11 and 36.56 s. Tablets containing SCMC, PC, or HPMC remained buoyant over the media for periods longer than 24 h possibly due to the development of a gel matrix with a higher strength to trap the air bubbles and maintain buoyancy for a longer time (24). However, those containing CP alone floated only for 6 h since CP has a high tendency to imbibe water as previously observed from the swelling study, thus, the density of this formulation increased considerably after 6 h upon contact with water inhibiting the prolonged floating (24,62).
Regarding CP combinations with each of HPMC, SCMC, and PC in different ratios revealed that increasing the amount of CP in the tablets was concomitant with a decrease in the FLT. Concerning the TFT, all CP combinations floated for periods longer than 24 h except those containing high ratio of CP (HCP12, PCP12, and SCP12) that experienced TFT of 8.7, 7.6, and 6.3 h, respectively. As mentioned above, this may be due to the higher tendency of CP to imbibe water and the subsequent increase in density inhibiting the prolonged floating.
In Vitro Drug Release
Gastroretentive drug delivery systems based on one mechanism may fail to provide an efficient prolonged release of incorporated drugs in the stomach (13). For example, bioadhesive systems encounter a challenge with the high turnover rate of gastric mucus. Similarly, the performance of floating and swelling-expanding drug delivery systems is strongly affected by the filling state of the stomach and, after predetermined time intervals; they break into smaller pieces, leaving the stomach (63). Thus, combining different gastroretentive mechanisms was considered to enhance gastroretention capabilities (13,64).
To obtain a controlled drug delivery of BR to the stomach by combining floating and bioadhesion, several matrix-gel bioadhesive polymers such as CP, HPMC, SCMC, and PC in combination with SB as a gas former were examined with respect to their effects on the drug release in GF. Lactose was used as a diluent for the formulated tablets, so the drug release from tablets containing lactose without any of these polymers (C tablets) was studied. It was found that, the dissolution rate of the drug from C tablets was slow and requires a period of 8 h for about 95% of the drug to be released as shown in Fig. 4a. This may be referred to the poor solubility of BR in water, particularly in acidic environment due to its acidic nature (65). Also, the effect of the gas former, SB, on BR release was evaluated. A complete drug release was achieved within 4 h from C1 tablets which contains SB and lactose without polymers as shown in Fig. 4a. This behavior could be due to the alkaline microenvironment provided after the dissolution of SB content within the tablet matrix resulting in increased dissolution of the acidic drug.
Fig. 4.
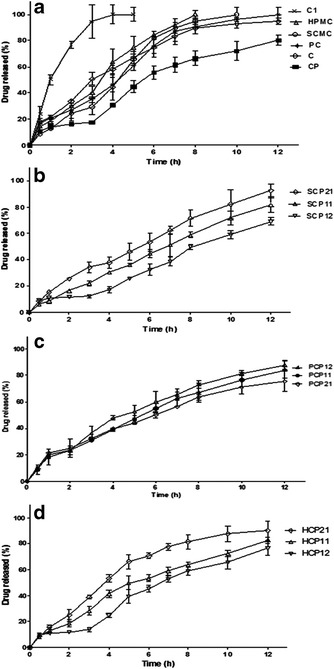
Release profiles of BR from tablets containing individual polymers (a), SCMC-CP combinations (b), PC-CP combinations (c), and HPMC-CP combinations (d). Each point represents the mean ± SD (n = 3)
The apparent drug release rate observed from PC, SCMC, and HPMC tablets, each alone, exhibited a substantial decrease compared with that from C1 tablets (Fig. 4a). Furthermore, their drug release patterns were similar with percent drug released after 8 h (Q8 h) ranging from 90.63 to 100. Thus, no considerable differences were observed among the three polymers as they seemed to behave similarly in modulating the drug release rate via formation of a gel layer around the tablets upon contact with dissolution medium followed by erosion of the swellable matrix into smaller particles exposing more surfaces for dissolution and drug release (66).
The incorporation of CP alone with SB as a matrix tablet significantly (p < 0.05) decreased the drug release rate compared with the other matrix-gel forming tablets, where Q8 h was 66.33 (Fig. 4a). This behavior may be explained on the basis that CP is a cross-linked polymer with high molecular weight and viscosity imparting a thick gel structure upon contact with the dissolution medium. Moreover, the alkaline microenvironment created by the dissolved SB within the tablet matrix could enhance this gelling effect of CP slowing further penetration of the dissolution medium (67). On the other hand, there is an inverse relationship between drug release rate and tablet dimensions, where, the increase in tablet size by swelling causes a decrease in drug release rate (68). Addition of SB to formulations containing CP was suggested to improve their retarding effect in acidic media by making the matrices form a stronger polymer network (69).
On combining SCMC with CP, the Q8 h were 71.5, 58.5, and 49.6 for SCP21, SCP11, and SCP12, respectively, as depicted in Fig. 4b. Statistical analysis revealed that SCP11 and SCP12 formulations significantly (p < 0.05) decreased the drug release compared with those containing SCMC alone. According to the results of the swelling study, both polymers showed high hydrophilicity and water uptake producing a swollen gel-like state that may substantially reduce the penetration of dissolution medium into the tablets and decrease the drug release rate (70).
Compared with tablets containing PC alone, the drug release was slower when PC was mixed with CP at different ratios, 1:1, 1:2, and 2:1 (Fig. 4c). Yet, increasing the CP content in PC-CP tablets did not significantly modify the drug release rate. In other words, the degree of retardation of drug release from GR tablets was independent of the PC-CP ratio. The obtained data may suggest that the hydration of these tablets is followed by the completion of a stable gel layer resulting in no difference in release rate from the delivery system.
The release profiles of BR from tablets containing HPMC-CP mixtures are illustrated in Fig. 4d. HCP21, HCP11, and HCP12 formulations containing HPMC-CP in respective ratios of 2:1, 1:1, and 1:2, have Q8 h values of 81.45%, 63.79%, and 58.75%, respectively. During dissolution test, it was observed that erosion of HCP21 tablets occurred resulting in higher percent of drug released. Further increase in the amount of CP in HPMC-CP mixtures (HCP11 and HCP12) maintained the integrity of the tablets and imparted a significant (p < 0.05) decrease in the drug release in comparison with HPMC alone or HCP21. As a result, the tablets remained intact over a period of 12 h probably due to the high swelling nature of CP. Similar findings were previously reported (24,62). Other investigators reported that the possible H-bonding between OH group of HPMC and carboxyl group of CP may lead to stronger cross-linking between the two polymers and formation of a thick gel structure which would retard the drug release (71,72).
SCP21 formulation was selected as the optimized one since it met the required criteria to form gel instantaneously in the pH conditions of the stomach and was able to keep its integrity. It showed excellent floating and swelling characteristics. Furthermore, it exhibited a promising initial drug release followed by a controlled behavior for a desired period of time. This initial faster hydration rate may promote the interpenetration of the tablet matrix within the gastric mucosa. Based on these findings, SCP21 was chosen for in vivo evaluation.
Drug Release Kinetics
The values of r 2 obtained from different kinetics models in Table III suggest that the drug release from the formulations may follow any one of these models. Korsmeyer and Peppas equation superposes two apparently independent mechanisms of drug transport, Fickian diffusion and a case-II transport, for the description of drug release from a swelling polymer (39). For a matrix tablet, when n equals the value of 0.45, it indicates diffusion-controlled drug release. In case of n equals 0.89, it indicates swelling-controlled drug release. Values of n between 0.45 and 0.89 can be regarded as an indicator for both the phenomena (anomalous transport). As shown in Table III, it is clear that all formulae have n values between 0.587 and 0.727, indicating anomalous transport and a drug release controlled by a coupling of diffusion and erosion.
Table III.
Kinetic Modeling of Drug Release Profiles
| Formula code | Zero order | First order | Higuchi model | Korsmeyer–Peppas | Drug transport mechanism | |
|---|---|---|---|---|---|---|
| Correlation coefficient (r 2) | r 2 | Diffusional exponent (n) | ||||
| HPMC | 0.976 | 0.841 | 0.942 | 0.997 | 0.727 | Non-Fickian |
| SCMC | 0.938 | 0.925 | 0.954 | 0.995 | 0.666 | Non-Fickian |
| PC | 0.916 | 0.947 | 0.958 | 0.997 | 0.702 | Non-Fickian |
| CP | 0.948 | 0.952 | 0.870 | 0.964 | 0.656 | Non-Fickian |
| HCP 12 | 0.961 | 0.968 | 0.932 | 0.969 | 0.626 | Non-Fickian |
| HCP 11 | 0.961 | 0.993 | 0.985 | 0.995 | 0.677 | Non-Fickian |
| HCP 21 | 0.901 | 0.990 | 0.959 | 0.998 | 0.698 | Non-Fickian |
| SCP 12 | 0.971 | 0.927 | 0.878 | 0.974 | 0.587 | Non-Fickian |
| SCP 11 | 0.997 | 0.970 | 0.975 | 0.998 | 0.636 | Non-Fickian |
| SCP 21 | 0.991 | 0.951 | 0.965 | 0.999 | 0.619 | Non-Fickian |
| PCP 12 | 0.958 | 0.990 | 0.989 | 0.998 | 0.693 | Non-Fickian |
| PCP 11 | 0.981 | 0.985 | 0.976 | 0.996 | 0.671 | Non-Fickian |
| PCP 21 | 0.980 | 0.986 | 0.979 | 0.995 | 0.667 | Non-Fickian |
HPMC hydroxypropyl methylcellulose, SCMC sodium carboxymethyl cellulose, PC pectin, CP carbopol
Macroscopical Examination of Rabbits Stomach
Stomach of group I (control) showed neither inflammation nor hemorrhage. However, the mucosa of stomach in group II which received indomethacin orally showed marked red patches of erosions. This finding was manifested by the presence of high number of ulcers, high % incidence of rabbits with ulcers (100%), and hence, the highest Paul’s index, 13.16 (Table IV). When BR-lactose formula was given 6 h before indomethacin administration (group III), high number of ulcers with high Paul’s index were found. However, the administration of BR-GR formula (SCP21), 6 h before indomethacin administration (group IV), showed significantly smaller numbers of ulcers or erosions (p < 0.05) in comparison with group II (untreated) or group III (received BR-lactose tablets). Furthermore, a smaller value of Paul’s index was obtained with group IV in comparison with group II and group III (3.32 vs. 13.16 and 11.04), respectively. They also displayed a satisfactory anti-ulcer activity (AA > 2). It is to be pointed out that, the AA of BR was prolonged for 6 h only when it was administered as GR tablets (AA = 3.59).
Table IV.
Paul’s Index, Anti-ulcer Activity, and Severity of Inflammatory Reaction of Different Rabbits Groups
| Treatment (group) | Number of ulcers (mean ± SD; n = 6) | % incidence of rabbits | Paul’s index | Anti-ulcer activity (%AA) | Severity of inflammatory reaction |
|---|---|---|---|---|---|
| I (control; no drug) | Zero | Zero | Zero | − | − |
| II (indomethacin; 20 mg/kg) | 13.16 ± 0.84 | 100 | 13.16 | − | +++ |
| III (control; BR-lactose) | 9.20 ± 0.40* | 83.33 | 11.04 | 1.21 | ++ |
| IV (GR formula; SCP 21) | 1.66 ± 0.34*, † | 50 | 3.32 | 3.59 | ± |
*p < 0.05 vs. indomethacin; † p < 0.05 vs. control (BR-lactose) tablets
Histopathological Investigation of Rabbits Stomach
Stomach of the control group showed normal structure features of the mucosa which lined by intact surface columnar epithelium (Fig. 5a). After administration of indomethacin to group II, the gastric mucosa exhibited a destruction (Fig. 5b) or erosion (Fig. 5c) of the lining epithelium with congested blood vessels in the submucosal layer. These results indicated damaged cells of gastric mucosa after administration of indomethacin. Indomethacin showed a higher ulcerogenic potential than other non-steroidal anti-inflammatory drugs (NSAIDs) possibly via inhibiting the release of protective factors; e.g. cyclooxygenase-1 (COX-1), prostaglandin E2 (PEG2), bicarbonate, mucus, and anti-oxidant parameters as well as stimulating aggressive factors; e.g. acid, and oxidant parameters (73).
Fig. 5.
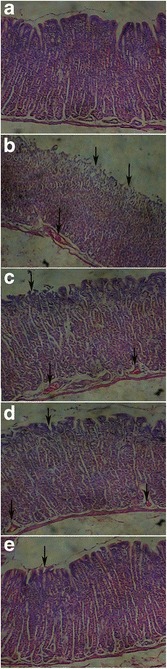
Histological examination of stomach of rabbits in group I (control) showing normal structure features (a), in group II showing erosion (b) or destruction (c) of the lining epithelium and congested blood vessels in submucosal layer (indicated by arrows), in group III showing erosion of the lining epithelium and congested blood vessels in the submucosal layer (indicated by arrows; d), and in group IV showing only minor erosion of epithelial layer with non-congested blood vessels (e)
On the other hand, the tissue of the stomach of rabbits receiving control lactose tablets (group III) 6 h before indomethacin administration showed moderate reaction where erosion of the mucous layer and congestion of the blood vessels were detected in the submucosal layer (Fig. 5d). Meanwhile, the severity of reaction in group IV was within the normal limit as the submucosal layer does not show congested blood vessels, where only minor ulceration in the lining epithelium was noticed as presented in Fig. 5e. Regarding the severity of inflammatory reaction in the rabbit’s stomach, the different rabbit’s groups could be arranged in a descending order as follows: group II (no treatment) > group III > group VI > group I (control group) (Table IV). Thus, formulation of BR into GR tablets prolonged its residence in the stomach and increased its solubility due to SB content leading to higher bioavailability with prominent prolonged gastroprotective effect. So, maintenance of a local concentration of BR for a longer time in the rabbit stomach could increase the gastric mucosal resistance and local synthesis of cytoprotective prostaglandins, thus, protect the rabbits from occurrence of ulcer more effectively than its control formula. The greater gastric cytoprotective effect of GR floating tablets of pantoprazole and nizatidine than their conventional dosage forms has been confirmed (74,75).
CONCLUSION
Using simple compression, different GR tablets of the natural safe anti-ulcer BR were formulated. Among these, tablets containing combination of SCMC-CP in ratios of 2:1(SCP21) showed satisfactory results with respect to FLT, TFT, swelling, bioadhesion, and extended drug release up to 12 h. The in vivo study proved the superiority of SCP21 formula over control BR-lactose tablets due to its prolonged cytoprotective effect against gastric ulceration induced by indomethacin.
ELECTRONIC SUPPLEMENTARY MATERIAL
Below is the link to the electronic supplementary material.
(DOCX 4324 kb)
References
- 1.Rouge N, Buio P, Doelker E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int J Pharm. 1996;136:117–39. doi: 10.1016/0378-5173(96)85200-8. [DOI] [Google Scholar]
- 2.Fell JT. Targeting of drugs and delivery systems to specific sites in the gastrointestinal tract. J Anat. 1996;189:517–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy CS, Pillay V, Choonara YE, Du Toit LC. Gastroretentive drug delivery systems: current developments in novel system design and evaluation. Curr Drug Deliv. 2009;6:451–60. doi: 10.2174/156720109789941687. [DOI] [PubMed] [Google Scholar]
- 4.Nayak AK, Maji R, Das B. Gastroretentive drug delivery systems: a review. Asian J Pharm Clin Res. 2010;3:2–10. [Google Scholar]
- 5.Bravo-Osuna I, Vauthier C, Farabollini A, Palmieri GF, Ponchel G. Mucoadhesion mechanism of chitosan and thiolated chitosan-poly(isobutyl cyanoacrylate) core-shell nanoparticles. Biomaterials. 2007;28:2233–43. doi: 10.1016/j.biomaterials.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 6.El-Zahaby SA, Kassem AA, El-Kamel AH. Formulation and in vitro evaluation of size expanding gastro-retentive systems of levofloxacin hemihydrates. Int J Pharm. 2014;464:10–8. doi: 10.1016/j.ijpharm.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Sugihara H, Matsui Y, Takeuchi H, Wilding I, Connor A, Abe K, et al. Development of a gastric retentive system as a sustained release formulation of pranlukast hydrate and its subsequent in vivo verification in human studies. Eur J Pharm Sci. 2014;53:62–8. doi: 10.1016/j.ejps.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Tuleu C, Andrieux C, Boy P, Chaumeil JC. Gastrointestinal transit of pellets in rats: effect of size and density. Int J Pharm. 1999;180:123–31. doi: 10.1016/S0378-5173(98)00400-1. [DOI] [PubMed] [Google Scholar]
- 9.Stops F, Fell JT, Collett JH, Martini LG. Floating dosage forms to prolong gastro-retention-the characterisation of calcium alginate beads. Int J Pharm. 2008;350:301–11. doi: 10.1016/j.ijpharm.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Garg R, Gupta GD. Progress in controlled gastroretentive delivery systems. Trop J Pharm Res. 2008;7:1055–66. doi: 10.4314/tjpr.v7i3.14691. [DOI] [Google Scholar]
- 11.Chitnis VS, Malshe VS, Lalla JK. Bioadhesive polymers-synthesis, evaluation and application in controlled release tablets. Drug Dev Ind Pharm. 1991;17:879–92. doi: 10.3109/03639049109040824. [DOI] [Google Scholar]
- 12.Arza RAK, Gonugunta CSR, Veerareddy PR. Formulation and evaluation of swellable and floating gastroretentive ciprofloxacin hydrochloride tablets. AAPS PharmSciTech. 2009;10:220–6. doi: 10.1208/s12249-009-9200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen RN, Ho HO, Yu CY, Sheu MT. Development of swelling/floating gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose for Losartan and its clinical relevance in healthy volunteers with CYP2C9 polymorphism. Eur J Pharm Sci. 2010;39:82–9. doi: 10.1016/j.ejps.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Bruntan LL, Lazo JS, Parker KL. Goodman and Gilman’s: the pharmacological basis of therapeutics. 11. New York: McGraw Hill Companies; 2006. [Google Scholar]
- 15.Badria FA, El-Farahaty T, Shabana AA, Hawas SA, El-Batoty MF. Boswellia-curcumin preparation for treating knee osteoarthritis: a clinical evaluation. Alt Complement Ther. 2002;8:341–8. doi: 10.1089/107628002761574635. [DOI] [Google Scholar]
- 16.Badria FA, Mohammed E, El-Badrawy M, El-Desouky M. Natural leukotriene inhibitor from Boswellia: a potential new alternative for treating bronchial asthma. Alt Complement Ther. 2004;10:257–65. doi: 10.1089/act.2004.10.257. [DOI] [Google Scholar]
- 17.Badria FA, Houssen WE, El-Nashar EM, Saaed SA. Effect of glycyrrhizin and Boswellia carterii extract on liver injury: biochemical and histopathological evaluation. Biosci Biotech Res Asia. 2003;1:93–6. [Google Scholar]
- 18.Badria FA, Mikhaeil BR, Maatooq GT, Amer MMA. Immunomodulatory triterpenoids from the oleogum resin of Boswellia carterii Birdwood. Z Naturforsch C. 2003;58:505–16. doi: 10.1515/znc-2003-7-811. [DOI] [PubMed] [Google Scholar]
- 19.Badria FA. Preparation a new product of natural origin for treatment of hyperacidity and colitis. Egyptian Patent # 23376. 2001 (April 30, 2001 expires April 29, 2021, Egypt).
- 20.Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Johri RK, et al. The gastric ulcer protective effect of boswellic acids, a leukotriene inhibitor from Boswellia serrata, in rats. Phytomedicine. 2008;15:408–15. doi: 10.1016/j.phymed.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Panda S, Pattnaik S, Maharana L, Botta GB, Mohapatra P. Formulation and evaluation of zidovudine loaded olibanum resin microcapsules: exploring the use of natural resins as biodegradable polymeric materials for controlled release. Asian J Pharm Clin Res. 2013;6:191–6. [Google Scholar]
- 22.Chaudhari SP, Patil PR, Deshmukh TA, Tekade BW, Patil VR. Evaluation of binding properties of Boswellia serrata Roxb. gum in tablet Formulation. J Pharm Educ Res. 2011;2:61–5. [Google Scholar]
- 23.Fartyal S, Jha SK, Karchuli MS, Gupta R, Vajpayee A. Formulation and evaluation of floating microspheres of Boswellic acid. Int J Pharm Tech Res. 2011;3:76–81. [Google Scholar]
- 24.Tavakoli N, Varshosaz J, Dorkoosh F, Motaghi S, Tamaddon L. Development and evaluation of a monolithic floating drug delivery system for acyclovir. Chem Pharm Bull. 2012;60:172–7. doi: 10.1248/cpb.60.172. [DOI] [PubMed] [Google Scholar]
- 25.Mcconnell EI, Basit AW. Modified release oral drug delivery. In Aulton ME, Taylor KMG, editors. Aulton’s pharmaceutics the design and manufacture of medicines. Fourth edition; 2013. p. 555.
- 26.Srinatha A, Pandit JK. Multi-unit floating alginate system: effect of additives on ciprofloxacin release. Drug Deliv. 2008;15:471–6. doi: 10.1080/10717540802329282. [DOI] [PubMed] [Google Scholar]
- 27.Sahoo SK, Mohapatra S, Dhal SK, Behera BC, Barik BB. Formulation of floating microspheres of ciprofloxacin hydrochloride by crosslinking technique. Ind Pharm. 2007;6:65–8. [Google Scholar]
- 28.Davis SS, Stockwell AF, Taylor MJ, Hardy JG, Whalley DR, Wilson CG, et al. The effect of density on the gastric emptying of single-and multiple-unit dosage forms. Pharm Res. 1986;3:208–13. doi: 10.1023/A:1016334629169. [DOI] [PubMed] [Google Scholar]
- 29.USP28-NF23. “The United States Pharmacopeia” 28th, The National Formulary 23rd: The United States Pharmacopoeial Convention, Inc., Washington, 2005.
- 30.Desai KGH, Kumar TMP. Preparation and evaluation of a noval buccal adhesive system. AAPS PharmSciTech. 2004;5:1–9. doi: 10.1208/pt050335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Jain S, Muthu MS, Tiwari S, Tilak R. Preparation and evaluation of buccal bioadhesive films containing clotrimazole. AAPS PharmSciTech. 2008;9:660–7. doi: 10.1208/s12249-008-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Soni R, Rawat MK, Jain A, Deshpande SB, Singh SK, et al. In vitro and in vivo evaluation of buccal bioadhesive films containing salbutamol sulphate. Chem Pharm Bull. 2010;58:307–11. doi: 10.1248/cpb.58.307. [DOI] [PubMed] [Google Scholar]
- 33.Tadros MI. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur J Pharm Biopharm. 2010;74:332–9. doi: 10.1016/j.ejpb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Jaimini M, Rana AC, Tanwar YS. Formulation and evaluation of famotidine floating tablets. Curr Drug Deliv. 2007;4:51–5. doi: 10.2174/156720107779314730. [DOI] [PubMed] [Google Scholar]
- 35.Darandale SS, Vavian PR. Design of a gastroretentive mucoadhesive dosage form of furosemide for controlled release. APSB. 2012;2:509–17. [Google Scholar]
- 36.Chun MK, Bhusal P, Choi HK. Application of carbopol/PVP interpolymer complex to prepare mucoadhesive floating granule. Arch Pharm Res. 2013;36:745–51. doi: 10.1007/s12272-013-0035-4. [DOI] [PubMed] [Google Scholar]
- 37.USP31-NF26. “The United States Pharmacopeia” 31st, The National Formulary 26th: The United States Pharmacopoeial Convention, Inc., Washington, 2008, pp. 1303–1306.
- 38.Higuchi T. Mechanism of sustained action medication. J Pharm Sci. 1963;52:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 39.Korsmeyer RW, Gurny R, Docler E, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [Google Scholar]
- 40.Zueva EP, Reikhart DV, Krylova SG. 2003. Medicinal plants in therapy of gastroduodenal ulcer. In Krylova SG, Khotimchenko YS, Zueva EP, Amosova EN, Razina TG, Efimova LA. Gastroprotective effect of natural non-starch polysaccharides. Bull Exp Biol Med. 2006; 142:454–7. [DOI] [PubMed]
- 41.Bancroft JD, Gamble M. Theory and practice of histological techniques. 5. London: Churchill Livingstone; 2002. p. 130. [Google Scholar]
- 42.Hasçiçek C, Yüksel-Tilkan G, Türkmen B, Özdemir N. Effect of formulation parameters on the drug release and floating properties of gastric floating two-layer tablets with acetylsalicylic acid. Acta Pharm. 2011;61:303–12. doi: 10.2478/v10007-011-0028-0. [DOI] [PubMed] [Google Scholar]
- 43.Bigucci F, Luppi B, Cerchiara T, Sorrenti M, Bettinetti G, Rodriguez L, et al. Chitosan/pectin polyelectrolyte complexes: selection of suitable preparative conditions for colon-specific delivery of vancomycin. Eur J Pharm Sci. 2008;35:435–41. doi: 10.1016/j.ejps.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Duchene D, Touchard F, Peppas NA. Pharmaceutical and medical aspects of bioadhesive systems for drug administration. Drug Dev Ind Pharm. 1988;14:283–318. doi: 10.3109/03639048809151972. [DOI] [Google Scholar]
- 45.Gurny R, Meyer JM, Peppas NA. Bioadhesive intraoral release systems: design, testing and analysis. Biomaterials. 1984;5:336–40. doi: 10.1016/0142-9612(84)90031-0. [DOI] [PubMed] [Google Scholar]
- 46.Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Rel. 1985;2:257–75. doi: 10.1016/0168-3659(85)90050-1. [DOI] [Google Scholar]
- 47.Choi MK, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in-situ gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm. 1998;165:33–44. doi: 10.1016/S0378-5173(97)00386-4. [DOI] [Google Scholar]
- 48.Peh KK, Wong CF. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J Pharm Pharm Sci. 1999;2:53–61. [PubMed] [Google Scholar]
- 49.Madsen F, Eberth K, Smart JD. A rheological examination of the mucoadhesive/mucus interaction: the effect of mucoadhesive type and concentration. J Control Release. 1998;50:167–78. doi: 10.1016/S0168-3659(97)00138-7. [DOI] [PubMed] [Google Scholar]
- 50.Gu JM, Robinson JR, Leung SHS. Binding of acrylic polymers to mucin/epithelial surface: structure property relationships. Crit Rev Ther Drug Carrier Syst. 1988;5:21–67. [PubMed] [Google Scholar]
- 51.Mortazavi SA, Smart JD. Factors influencing gel strengthening at the mucoadhesive mucus interface. J Pharm Pharmacol. 1994;46:86–90. doi: 10.1111/j.2042-7158.1994.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 52.Hassan EE, Gallo JM. Simple rheological method for the in vitro assessment of mucin polymer bioadhesive bond strength. Pharm Res. 1990;7:491–5. doi: 10.1023/A:1015812615635. [DOI] [PubMed] [Google Scholar]
- 53.Singla AK, Chawla M, Singh A. Potential applications of carbomer in oral mucoadhesive controlled drug delivery system: a review. Drug Dev Ind Pharm. 2000;26:913–24. doi: 10.1081/DDC-100101318. [DOI] [PubMed] [Google Scholar]
- 54.Nanjwade BK, Adichwal SA, Nanjwade VK, Gaikwad KR, Thakare SA, Manvi FV. Development and evaluation of gastroretentive floating tablets of glipizide based on effervescent technology. J Drug MetabToxicol. 2012;3:1–6. [Google Scholar]
- 55.Mitchell K, Ford JL, Armstrong DJ, Elliott PNC, Rostron C, Hogan JE. The influence of concentration on the release of drugs from gels and matrices containing Methocel®. Int J Pharm. 1993;100:155–63. doi: 10.1016/0378-5173(93)90086-U. [DOI] [Google Scholar]
- 56.Sannino A, Demitri C, Madaghiele M. Biodegradable cellulose-based hydrogels: design and applications. Materials. 2009;2:353–73. doi: 10.3390/ma2020353. [DOI] [Google Scholar]
- 57.Mortazavi S. In vitro assessment of mucus/mucoadhesive interactions. Int J Pharm. 1995;124:173–82. doi: 10.1016/0378-5173(95)00073-R. [DOI] [Google Scholar]
- 58.Praveen G. Development and in vitro evaluation of buccoadhesive tablets of losartan potassium. Pharma Innov. 2012;1:63–70. [Google Scholar]
- 59.Kaur A, Kaur G. Mucoadhesive buccal patches based on interpolymer complexes of chitosan–pectin for delivery of carvedilol. SPJ. 2012;20:21–7. doi: 10.1016/j.jsps.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talukdar MM, Kinget R. Swelling and drug release behavior of xanthan gum matrix tablets. Int J Pharm. 1995;120:63–72. doi: 10.1016/0378-5173(94)00410-7. [DOI] [Google Scholar]
- 61.Goole J, Deleuze P, Vanderbist F, Amighi K. New levodopa sustained-release floating minitablets coated with insoluble acrylic polymer. Eur J Pharm Biopharm. 2008;68:310–8. doi: 10.1016/j.ejpb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 62.Li S, Lin S, Daggy BP, Mirchandani HL, Chien YW. Effect of HPMC and carbopol on the release and floating properties of gastric floating drug delivery system using factorial design. Int J Pharm. 2003;253:13–22. doi: 10.1016/S0378-5173(02)00642-7. [DOI] [PubMed] [Google Scholar]
- 63.Streubel A, Siepmann J, Bodmeier R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr Opin Pharmacol. 2006;6:501–8. doi: 10.1016/j.coph.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Nur AO, Zhang JS. Captopril floating and/or bioadhesive tablets: design and release kinetics. Drug Dev Ind Pharm. 2000;26:965–9. doi: 10.1081/DDC-100101323. [DOI] [PubMed] [Google Scholar]
- 65.Karlina M, Pozharitskaya O, Kosman V, Ivanova S. Molecular-biological problems of drug design and mechanism of drug action, bioavailability of boswellic acids: in vitro/in vivo correlation. Pharm Chem J. 2007;41:569–72. doi: 10.1007/s11094-008-0017-x. [DOI] [Google Scholar]
- 66.Omidian H, Park K. Swelling agents and devices in oral drug delivery. J Drug Delivery Sci Technol. 2008;18:83–93. doi: 10.1016/S1773-2247(08)50016-5. [DOI] [Google Scholar]
- 67.Kar R, Mohapatraa S, Bhanjaa S, Das D, Barik B. Formulation and in vitro characterization of xanthan gum-based sustained release matrix tablets of isosorbide-5-mononitrate. Iran J Pharm Res. 2010;9:13–9. [PMC free article] [PubMed] [Google Scholar]
- 68.Salve P. Development and in vitro evaluation of gas generating floating tablets of metformin hydrochloride. Asian J Res Pharm Sci. 2011;1:105–12. [Google Scholar]
- 69.Bravo SA, Lamas MC, Salomon CJ. Swellable matrices for the controlled-release of diclofenac sodium: formulation and in vitro studies. Pharm Dev Technol. 2004;9:75–83. doi: 10.1081/PDT-120027420. [DOI] [PubMed] [Google Scholar]
- 70.Emami J, Varshosaz J, Saljoughian N. Development and evaluation of controlled-release buccoadhesive verapamil hydrochloride tablets. DARU. 2008;16:60–9. [Google Scholar]
- 71.Kumar R, Patil MB, Patil SR, Paschapur MS. Formulation and evaluation of effervescent floating tablet of famotidine. Int J Pharm Tech Res. 2009;1:754–63. [Google Scholar]
- 72.Samani SM, Montaseri H, Kazemi A. The effect of polymer blends on release profiles of diclofenac sodium from matrices. Eur J Pharm Biopharm. 2003;55:351–5. doi: 10.1016/S0939-6411(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 73.Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33:224–34. doi: 10.1007/s10753-009-9176-5. [DOI] [PubMed] [Google Scholar]
- 74.Balata G. Design and evaluation of gastroretentive floating tablet of nizatidine: a trial to improve its efficacy. Int J Pharm Pharm Sci. 2014;6:423–9. [Google Scholar]
- 75.Sarangapani S, Bangaru J, Rajappan M. In vitro and in vivo evaluation of the gastroretentive floating dosage form. Int Res J Pharm. 2014;5:695–700. doi: 10.7897/2230-8407.0509142. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 4324 kb)


