Abstract
Delivering diclofenac diethylamine transdermally by means of a hydrogel is an approach to reduce or avoid systemic toxicity of the drug while providing local action for a prolonged period. In the present investigation, a process was developed to produce nanosize particles (about 10 nm) of diclofenac diethylamine in situ during the development of hydrogel, using simple mixing technique. Hydrogel was developed with polyvinyl alcohol (PVA) (5.8% w/w) and carbopol 71G (1.5% w/w). The formulations were evaluated on the basis of field emission scanning electron microscopy, texture analysis, and the assessment of various physiochemical properties. Viscosity (163–165 cps for hydrogel containing microsize drug particles and 171–173 cps for hydrogel containing nanosize drug particles, respectively) and swelling index (varied between 0.62 and 0.68) data favor the hydrogels for satisfactory topical applications. The measured hardness of the different hydrogels was uniform indicating a uniform spreadability. Data of in vitro skin (cadaver) permeation for 10 h showed that the enhancement ratios of the flux of the formulation containing nanosize drug (without the permeation enhancer) were 9.72 and 1.30 compared to the formulation containing microsized drug and the marketed formulations, respectively. In vivo plasma level of the drug increased predominantly for the hydrogel containing nanosize drug-clusters. The study depicts a simple technique for preparing hydrogel containing nanosize diclofenac diethylamine particles in situ, which can be commercially viable. The study also shows the advantage of the experimental transdermal hydrogel with nanosize drug particles over the hydrogel with microsize drug particles.
KEY WORDS: anti-inflammatory, cadaver skin, nanosize dispersion, permeation enhancement, transdermal
INTRODUCTION
Hydrogels are composed of cross-linked, three-dimensional hydrophilic polymer networks, which swell, but do not dissolve in contact with water [1–3]. Hydrogels have been considered for use in a wide range of biomedical and pharmaceutical applications, mainly due to their high water content and rubbery nature [4,5]. Because of those properties, hydrogel materials resemble natural living tissue more than any other class of synthetic biomaterials [6–10]. This water-based gel provides better patient compliance since it is easily washable by water, and medication can be stopped at any time. Moreover, it does not produce any untoward stickiness as seen in the case of many ointments and creams. The attraction of the hydrogel formulation over the conventional cream and ointment has been engaging more and more researchers to work in the field to bring cost-effective and more efficacious topical formulations. The ammonium salt of diclofenac, i.e., diclofenac diethylamine (DDA), is now widely used for topical applications [11,12]. Diclofenac is an acidic non-steroidal anti-inflammatory compound. Diclofenac has a log P of 4.75 and is less easily permeable to the skin due to its scanty partitioning between the lipophilic stratum corneum and the hydrophilic dermis [13]. To resolve solubility problems, diethylamine salt of diclofenac has been prepared [14]. This salt can better partition towards a lipid phase [15]. Hence, diclofenac diethylamine is the most preferable salt of diclofenac for skin permeation. Delivering DDA transdermally in a nanosize form is an approach for faster skin permeation of drug, reducing or avoidance of systemic toxicity of it, while providing local action for a prolonged period. Most of the available methods of gel containing drug nanoparticles are to convert the drug into nanosize, and then it is dispersed in the gel. The novelty of the present hydrogel system is that the drug precipitates in situ in nanosize clusters and dispersed homogeneously in the formulation. No nanoparticle has been developed separately. This makes the formulation very simple and cost-effective in terms of scalability. Further, we wanted to evaluate the effects of drug particle size in the formulation on skin permeation. The hypothesis was that due to the nanosize of DDA, the drug could have better local action because of better skin permeation and could even provide systemic effects simultaneously. In contrast, most of the marketed formulations fail to provide systemic effects due to low skin permeability of drug micro particles [11].
MATERIALS AND METHODS
Materials
DDA was obtained as a gift sample from Kothari Labs (Saugor, Madhya Pradesh, India). PVA (M.W. 125000, S.D. Fine-Chem Pvt. Ltd., Mumbai, Maharashtra, India), carbopol 71G (Noveon, Cleveland, OH, USA), and Voveran gel (batch no. 77072 T; Novartis India Limited, Bangalore, Karnataka, India), containing 1.1% (w/w) diclofenac diethylamine, were purchased. Cadaver skin was obtained from R.G.Kar Medical College and Hospital, Kolkata, West Bengal, India. All other materials were of analytical grade.
Preparation of Hydrogel with Dispersed Nano-Size Drug Particles With or Without Skin Permeation Enhancer
Required amount of DDA (Table I) was dissolved in minimum possible amount (~2–3 mL) of 95% ethanol. PVA solution (12% w/v) was prepared by continuous stirring of the required amount of PVA in hot water (60°C) followed by cooling at room temperature. Required amount of triethanolamine (1.9% w/w) was added in PVA solution at this stage, whenever applicable. The drug solution was slowly added to PVA solution containing triethanolamine and homogenized with a homogenizer (Daihan Scientific Co. Ltd., Seoul, South Korea) at 3000 rpm for 1 min. PVA acts as a surface stabilizer. Due to its degree of polarity, PVA has high aqueous solubility but it demonstrates a non-ideal solution behavior in water [16]. We have used a homogenization speed of 3000 rpm for 1 min to ensure efficient mixing of drug solution with PVA solution. Triethanolamine is a known skin permeation enhancer (hence called enhancer) [17] used in various topical formulations. Reports are available where triethanolamine has been used in topical formulation from 0.5 to 1.7% w/w [18] and from 0 to 5% w/w [19]. Initially, different concentrations of triethanolamine from 0.5 to 2.5% w/w have been tried and the optimum concentration of 1.9% w/w has been chosen based on skin permeation data of the drug. The requisite amount of carbopol 71G was dispersed in 50 mL of water, and drug-PVA mixture/drug-PVA mixture with triethanolamine was added to it at a ratio of 1:1 v/v and mixed well. Finally, few drops of 2% w/v sodium hydroxide were added to it and mixed thoroughly. The preparation was allowed to stand overnight and pH was adjusted to 7.0.
Table I.
Composition of Hydrogel
| Ingredients | % of ingredients (w/w) | |||
|---|---|---|---|---|
| Hydrogel with nanosize drug without enhancer | Hydrogel with nanosize drug with enhancer | Hydrogel with microsize drug without enhancer | Hydrogel with microsize drug with enhancer | |
| Polyvinyl alcohol | 5.8 | 5.8 | 5.8 | 5.8 |
| Carbopol 71G | 1.5 | 1.5 | 1.5 | 1.5 |
| Diclofenac diethylamine | 1.1 | 1.1 | 1.1 | 1.1 |
| Triethanolamine | – | 1.9 | – | 1.9 |
| Ethanol (95%) | 2 mL | 2 mL | – | – |
| Water | 98 mL | 98 mL | 100 mL | 100 mL |
| Sodium hydroxide solution (2% w/v) | Few drops | Few drops | Few drops | Few drops |
Batch size 100 g
Preparation of Hydrogels With Drug Dispersion in Microsize With or Without Skin Permeation Enhancer
Hydrogel formulations with microsize drug particles were obtained by dispersing coarse drug particles (Table I) in an aqueous PVA (12%) solution (with or without triethanolamine) using a homogenizer as mentioned above. The rest of the process was also similar as mentioned before.
Physicochemical Characterization of Hydrogels
Microscopic Study for Observation of Particles
High-resolution field emission scanning electron microscope (FESEM) (JSM 6700F, JEOL, Tokyo, Japan) was used to observe drug particles in the hydrogel. The hydrogel was spread on a stub, coated using platinum by ion sputtering technique and observed under FESEM. Hydrogel with the microsize drug particles was observed in an optical microscope (Axiostar Plus, Carl Zeiss, Jena, Germany).
Viscosity Study
The viscosity of hydrogels was measured using Viscometer TV-10 (Toki Sangyo Co. Ltd., Tokyo, Japan). The spindle number M4 was used. The length and diameter of the cylinders were 10.5 and 3 cm, respectively, and those of the spindle were 6.4 and 1.8 cm, respectively. Viscosities of the different formulations were determined at 25°C following the guideline of the manufacturer.
Study of Swelling Index
Measured amount (W 0) of hydrogel was taken and allowed to swell on a Petri dish in water at 25 ± 0.5°C. After removal of excess water by brief soaking with a blotting paper, weight (wet weight) was noted at the predetermined time intervals (1–10 h) (at every half an hour), till it became constant (W t). When the weight became constant (W t), swelling index was calculated in terms of water uptake [20].
Skin Irritation Test
PVA-carbopol 71G-DDA (nanosize) hydrogel with triethanolamine was applied to the skin of the forehands of ten individuals (five males, five females, age 22–28 years) once daily (kept for 10 h) for seven consecutive days to test any kind of skin irritation. Gel (0.5 g) was applied on 10 cm2 area on the dorsal surface of the forehand. The individuals were monitored for any kind of skin irritations including itching, rashes, redness, swelling, inflammations, allergic manifestations, and any discomfort at the application site till the next 7 days after the completion of application. The Institutional Ethical Committee (IEC) of Jadavpur University has approved to carry out the work on human material (ref. no. IEC/JU/PHARMTECH/12/07/2007).
The skin irritation test was also conducted on Sprague-Dawley male rats (130–150 g body weight). Five rats were taken. They had free access to food and water. The experiment was conducted for 7 days with necessary, humane care of the animals after obtaining permission from Jadavpur University Animal Ethics Committee. After removal of hair (18 h before hydrogel application) from the backside of all the rats with a depilatory cream (Anne French), 0.5 g (wet weight) of the hydrogel (containing nanosize drug particles with permeation enhancer) was applied on 10 cm2 area marked earlier. Before the application of gel, photograph of the skin was taken. After 10 h, the gel was washed and skin photograph was taken and compared with the initial photograph.
Test of Hardness of Hydrogel With Texture Analyzer
Hardness was measured using texture analyzer (Brookfield engineering, Middleboro, MA, USA). Only hydrogels without the permeation enhancer were analyzed by this test as it was assumed that the addition of permeation enhancer would not change the texture property to a great extent. The test was carried out by using the fixture having male and female attachments. The hydrogel was taken into the female donor compartment up to a specific mark, and the surface was leveled with a sharp knife. The male probe was then allowed to come down just to touch the surface with the maneuver of the load cell. The experiment was then started and the movement of the male probe was guided by the preset parameters. The male probe was moved through the hydrogel at a speed of 30 mm/min up to a specified distance of 15 mm and then retracted backward to its original position. The trigger load was set to 5 g and two cycles was completed for each set of test, following the manufacturer’s guidelines.
Drug Release
Drug release patterns from the various hydrogels were determined in vitro through a dialysis membrane at 37°C in a water bath, with a modification of the earlier reported method [21]. Briefly, 2 g of each of the prepared hydrogels was separately taken inside a dialysis sac of approximately 5 mL volume each made from dialysis cellulose membrane having a molecular cutoff value of 12–14 kD (Sigma-Aldrich Corporation, Bangalore, India). Drug release was determined in phosphate buffer pH 7.4 following the reported method [21]. The cumulative percentage drug release was plotted against time. Drug release data were tested for zero-order, first-order, and Higuchi kinetic models. The kinetic model which generates the best fit line was considered.
In Vitro Skin Permeation Study
In vitro skin permeation study was carried out in a diffusion (modified Keshary-Chien) cell. The diffusion cell has been designed incorporating all the designing aspects of Keshary-Chien cell except the diameter and length of the cell. The cell had the capacity of 68 mL with a cross-sectional area of 1.68 cm2. The permeation studies were performed using the abdominal cadaver skin. The skin was processed as mentioned elsewhere [22]. The stripped skin was tied at the donor compartment with the dorsal side facing upwards, and a measured quantity (~1 g wet weight) of gel was placed in the donor compartment. This donor compartment containing skin and gel was then placed on the dorsal side of the skin in the receiver compartment of diffusion cell containing phosphate buffer pH 7.4. For both the marketed formulation and the prepared hydrogel, the effective dosage of drug applied was calculated to be approximately 6.5 mg/cm2. The temperature of the diffusion cell was maintained at 37°C by circulating warm water through the jacketed portion of the cell. This whole assembly was kept on a magnetic stirrer, and the solution in the receiver compartment was continuously stirred during the entire experiment using magnetic bead. The samples were appropriately diluted and absorbance was determined at 275 nm against phosphate buffer, pH 7.4, as blank using a spectrophotometer (Varian Inc., Palo Alto, CA, USA).
In Vivo Experiment in Rats
Animals used for the in vivo experiments were adult Wister rats of either sex (at a ratio 1:1) weighing 130–150 g. The animals were housed in polypropylene cages individually and were fed with the standard pellet diets and water ad libitum. They were kept in 12-h light/dark cycle at 25 ± 2°C and 55% ± 5% relative humidity (RH). In vivo experimental protocol used here was approved by the Jadavpur University Animal Ethics Committee (AEC) and procedures followed well in accordance with the guidelines of AEC. Necessary humane care of the animals was always considered. Four rats (two of either sex) were used for each group studied. The hair on the backside of the rats was removed with a depilatory cream (Anne French) on the previous day of the day of application of hydrogels. After 24 h, 1 g (wet weight) of each different hydrogels (containing nanosize drug with or without skin permeation enhancer/microsize drug particles with skin permeation enhancer/the commercial formulation) was applied on 10 cm2 depleted skin area marked earlier of rats of the abovementioned formulation groups. At definite time intervals, 250 μL of blood was collected from the tail vein; plasma was separated; plasma protein was precipitated using acidified acetonitrile; and drug content was analyzed by HPLC principally according to the method described earlier [23] and mentioned below.
HPLC Analysis of Drug from Plasma Samples
The mobile phase used for the analysis of DDA in the plasma samples was 0.01 M sodium acetate, pH 4.2, and acetonitrile (at a ratio 1:1 v/v). Sodium acetate (205.075 mg) was weighed and dissolved in 200 mL HPLC grade water. The pH of the solution was adjusted to 4.2 with glacial acetic acid and the volume was made up to 250 mL. Throughout the HPLC analysis, Milli-Q water (Millipore, MA, USA) was used. The column used for the analysis was Novapak C18 reversed phase (Waters Corporation, MA, USA) having dimensions of 3.9 mm × 150 mm and particle size of 5 μm. The mobile phase flow rate was maintained at 1.5-mL/min and run time for each sample was 10 min. The sample to be analyzed was injected using a Rheodyne injector (50 μL), and the detection was carried out at 280 nm in a photodiode array detector (Waters Corporation, MA, USA). Analysis and processing of the results were performed by Waters Millennium software.
Stability Study
The stability of the hydrogel containing nanosize drug particles was evaluated by a short-term stability study for 3 months [24]. The stability study was carried out at three different conditions, at refrigerated condition at 4°C (±2°C), at 25°C (±2°C)/60% (±5%) RH, and 40°C (±2°C)/75% (±5%) RH in a stability chamber (Darwin Chambers Company, St. Louis, USA). Upon completion of 3 months, various parameters such as viscosity, pH, spreadability, particle size (as evaluated by FESEM), and drug content (by HPLC) were evaluated using same respective procedure as mentioned above in the experimental formulations and compared with those of the freshly prepared formulation.
Statistical Calculations
All statistical calculations were performed with GraphPad Instat version 3.0 (GraphPad Software, Inc., San Diego, CA, USA). The data were analyzed by one-way ANOVA followed by Dunnett multiple ranges test to determine significant differences. Statistical significance was based on the probability value of less than 0.05.
RESULTS
In the present study, using a simple technique, nanosize particles (~10 nm) of DDA were produced in situ and dispersed in the experimentally developed hydrogels. As a reference formulation, microsized DDA was dispersed in the experimentally developed hydrogel. The effect of drug particle size of the formulations on skin permeation was evaluated. Various physico-chemical studies such as hydrogel morphology, hardness test, skin irritation test, gel viscosity and swelling index, drug release, in vitro skin permeation study, and in vivo drug plasma profile in rats were performed on the prepared hydrogels.
Hydrogel Morphology
Drug particles dispersed in the hydrogels at the different experimental parameters were depicted in Fig. 1. The field emission scanning electron microscope (FESEM) photograph of the hydrogel dispersed with the nanosize drug particles (without enhancer) is shown in Fig. 1a. The drug particles of approximately 10 nm size were dispersed uniformly throughout the polymer gel matrix. The hydrogel with microsize dispersion (Fig. 1b) had drug particles of various sizes (approximately from 10 to 100 μm) dispersed in the hydrogel matrix.
Fig. 1.
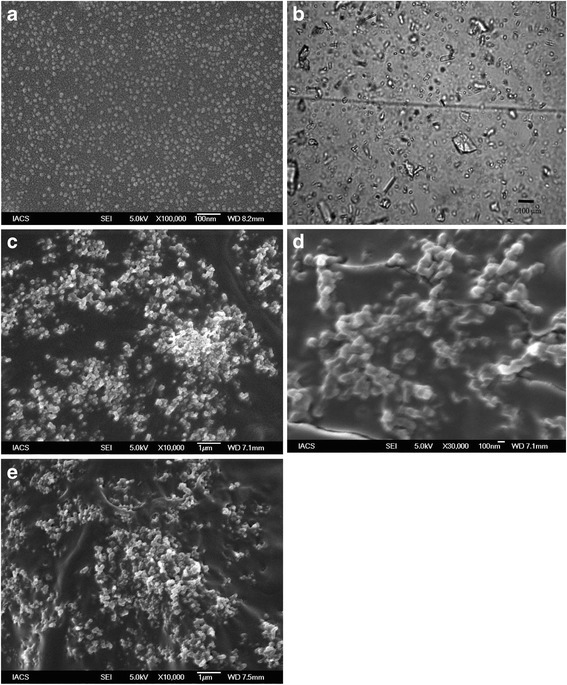
Drug particles in the hydrogels. a FESEM photograph of freshly prepared nanosize drug-loaded gel (with triethanolamine) showing uniformly distributed drug particles of size, 10 nm. b Light microscope picture of freshly prepared hydrogel with microsized drug particle (with triethanolamine). c FESEM photograph of nanosize drug loaded hydrogel (with triethanolamine) upon storage of the samples at 4°C. d FESEM photograph of nanosize drug loaded hydrogel (with triethanolamine) upon storage of the samples at 25°C and 60% RH. e SEM of nanosize drug loaded hydrogel (with triethanolamine) upon storage of the samples at 40°C and 75% RH
Skin Irritation Test
Skin irritation test of the hydrogels was conducted on rats and humans. No allergic responses were observed in rats after seven consecutive days of application, neither from the hydrogel containing nanosize drug particles nor from the hydrogel containing microsize particles. No skin irritation was detected in human volunteers as well. There was no change on the rat skin morphology before and after the application of the gel (data not shown).
Viscosity and Swelling Study
Both the hydrogel formulations containing microsize drug particles (with or without permeation enhancer) had the average viscosity in the range of 163–165 cps. The average viscosity value of the hydrogels with the nanosize drug particles (with or without enhancer) was approximately 171–173 cps. However, the difference in viscosity of four hydrogels was statistically not significant (p, 0.25).
Swelling indices of the hydrogels (with microsize/nanosize drug particles) were found to vary little. Maximum swelling was found to occur between 6 and 8 h and the values varied between 0.62 and 0.68.
Texture Analyzer Study
Figure 2 graphically represents the load versus time profile during the movement of the male probe through the hydrogel sample kept in the female holder. Figure 2 is an overlay of the two different hydrogels containing microsize/nanosize particles (both the hydrogels were without permeation enhancer). Each plot represents two cycles of experiments with the same sample. The presence of drug nanoparticle in the hydrogel did not alter spreadability of the gel as compared to the gel containing microparticulate drug dispersion.
Fig. 2.
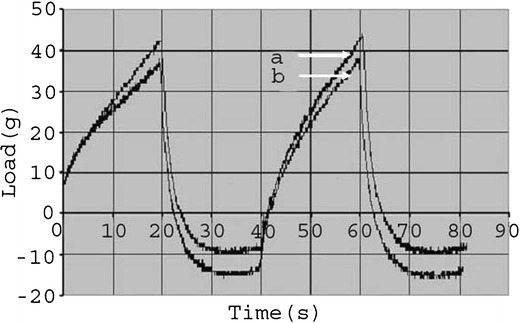
Analysis of load vs. time profile of hydrogel formulation using texture analyzer (a hydrogel with nanosize particles without enhancer, b hydrogel with microsize particles without enhancer)
Drug Release
Drug release from the two formulations with or without skin permeation enhancer and with nanosize-drug particles was approximately two times higher than the two formulations with or without skin permeation enhancer with microsize drug and the commercial hydrogel formulation. The drug release data (Fig. 3) from both the formulations with nanosize drug clusters were statistically compared with the other formulations. The difference in drug release from the two formulations with nanosize drug particles was statistically significant when compared to that of the marketed formulation (p < 0.05 for both the sets), the formulation with microsize drug without enhancer (p < 0.05 for both the sets), and the formulation with microsize drug with enhancer (p < 0.05 for both the sets). However, no significant difference in drug release was found between the two formulations with microsize drug (p, 0.7782) and between those two formulations and the commercial hydrogel (p, 0.85). No statistically significant difference in drug release was found between the two formulations with nanosize drug with or without the skin permeation enhancer (p, 0.1075). When drug release data were analyzed by various kinetic models, the best fit line was obtained with the Higuchi kinetic model for all the formulations tested.
Fig. 3.
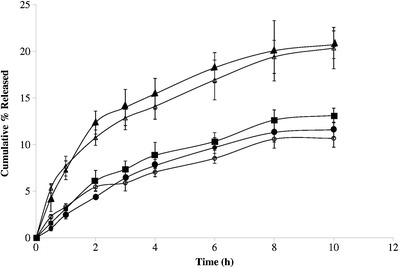
Drug release from hydrogel formulations of diclofenac diethylamine (white triangle hydrogel with nanosize drug without enhancer, black triangle hydrogel with nanosize drug with enhancer, white circle hydrogel with microsize drug without enhancer, black circle hydrogel with microsize drug with enhancer, black square marketed hydrogel formulation). Data show mean ± SD (n = 5)
Skin Permeation Study
Skin permeation profiles of DDA from the hydrogel formulations are shown in Fig. 4. The flux (J) referred as dose/hour was calculated from the slope of the linear portion of the cumulative amount permeated versus time curve (Fig. 4) [25–27]. The permeability coefficient (Kp) was calculated from the equation J = Kp.C where C is the initial drug concentration. However, ideally, the Kp should be calculated from the steady-state flux which is achieved when the flux reached a plateau level [28,29]. There are multiple reports which suggest that Kp can be calculated from the abovementioned equation even if the steady state is not reached [26,27]. In our investigation, the curves showing the skin permeation profile of drug released from the hydrogels with microsized drug particles (with or without enhancer) and the marketed hydrogel showed convincing linearity where from Kp was calculated. However, the skin permeation profile of the drug from the hydrogels with nanosize drug particle (with or without enhancer) had not enough convincing linearity where the curves were drawn by point to point joining. In those cases, best fits of the individual linear graphics were used to determine the respective Kp for further calculation. The calculated flux and Kp values are given for all the formulations in Table II. Skin permeation study was conducted for 10-h time period. Hydrogel formulation with microsize drug particles and without the skin permeation enhancer showed the least amount of drug permeation through cadaver skin with a flux (J) value of 3.89 μg/cm2/h. The addition of 1.9% (w/w) triethanolamine to this hydrogel formulation increased the permeation to a significant extent (p < 0.05) with a flux (J) of 25.16 μg/cm2/h. The amount of drug permeated at the initial time point from the formulation with nanosize drug and the permeation enhancer was nearly equal to the maximum flux achieved with the hydrogel containing microsize drug with the permeation enhancer (Fig. 4). Reduction of particle size to nanometer size (~10 nm) increased the permeation and the flux obtained was 37.83 μg/cm2/h which was significantly (p < 0.05) higher than those of the two hydrogel formulations with microsize drug particles. The addition of permeation enhancer in the hydrogel with nanosize drug particles enhanced the flux to 44.77 μg/cm2/h which was again significantly (p < 0.05) higher than the two formulations having microsize drug distribution. The commercial hydrogel formulation was found to show a comparable profile with the hydrogel containing microsize drug particles with the permeation enhancer but significantly lower than the formulation having nanosize drug particles with (p < 0.05) or without (p < 0.05) the enhancer. Enhancement ratio, as calculated by the ratio of drug permeability coefficients with or without enhancer, varied predominantly. Drug permeability coefficient (Table II) was calculated by dividing flux value with the concentration of the solution, and the enhancement ratio was calculated from the ratio of permeability coefficient value. Hydrogel having nanosize drug particles with the skin permeation enhancer showed the highest permeation coefficient value followed by the hydrogel with nanosize drug particles, the commercial gel and the gel with microsize drug dispersion and skin permeation enhancer. Based on the enhancement ratio, the formulations can be arranged in descending order of permeation: nanosize + permeation enhancer > nanosize > marketed > microsize + permeation enhancer > microsize. Drug permeation from the commercial formulation exceeded the flux of the hydrogel with microsize particle with the permeation enhancer except the initial time point which was slightly lower.
Fig. 4.
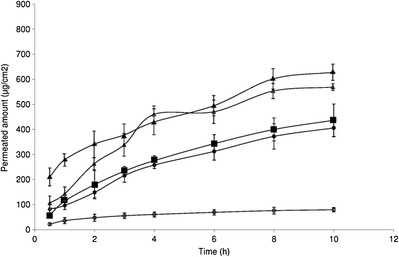
Permeation of hydrogel formulations of diclofenac diethylamine through cadaver skin (white triangle hydrogel with nanosize drug without enhancer, black triangle hydrogel with nanosize drug with enhancer, white circle hydrogel with microsize drug without enhancer, black circle hydrogel with microsize drug with enhancer, black square marketed hydrogel formulation). Data show mean ± SD (n = 5)
Table II.
Calculated Value of Flux (J), Permeability Coefficient (Kp), and Swelling Index
| Formulation name | Flux (J) μg/cm2/h | Permeability coefficient (Kp) cm/h | Enhancement ratio (in comparison to microsized formulation) | Enhancement ratio (in comparison to marketed formulation) | Swelling index |
|---|---|---|---|---|---|
| Microsized | 3.89 | 0.229 × 10−3 | 1.0 | – | 0.66 ± 0.11 |
| Microsized + permeation enhancer | 25.16 | 1.48 × 10−3 | 6.46 | – | 0.64 ± 0.10 |
| Nanosize | 37.83 | 2.225 × 10−3 | 9.72 | 1.30 | 0.62 ± 0.12 |
| Nanosize + permeation enhancer | 44.77 | 2.633 × 10−3 | 11.50 | 1.54 | 0.65 ± 0.08 |
| Marketed formulation | 29.17 | 1.716 × 10−3 | 7.49 | 1.0 | Not done |
Drug Plasma Levels from Hydrogels
The blood level of the drug from the hydrogel containing microsized drug particle was unexpectedly low in the first few hours and could not be detected in many cases. Thus, the data has not been included in Fig. 5. However, for the rest of the experimental samples and the commercial formulation, variable drug plasma levels were detected. Hydrogel containing microsized drug with the skin permeation enhancer showed the minimum plasma levels of the drug whereas the hydrogel with nanosize drug with the skin permeation enhancer and that without the skin permeation enhancer markedly improved plasma level of the drug. Hydrogel formulations containing nanosize drug particles (with or without skin permeation enhancer) showed the higher plasma level compared to the marketed formulation and the variation was statistically significant (p < 0.05 for both the sets) in both the cases. Again, when hydrogels with nanosize drug with or without the skin permeation enhancer were compared, the hydrogel with nanosize drug with the enhancer showed a steadier drug plasma level, but that without the skin permeation enhancer showed an eventual drop of plasma level of the drug after 3 h. However, the variation of data between those two formulations was found to be statistically insignificant (p, 0.24).
Fig. 5.
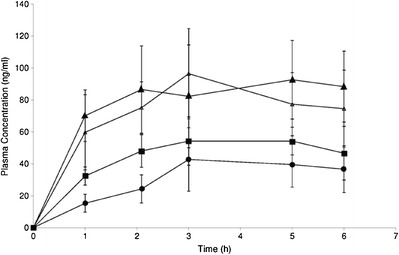
Plasma profile of diclofenac diethylamine from hydrogels in rats (white triangle hydrogel with nanosize drug without enhancer, black triangle hydrogel with nanosize drug with enhancer, black circle hydrogel with microsize drug with enhancer, black square marketed hydrogel formulation). Data show mean ± SD (n = 4)
Stability Study
No predominant changes were detected in spreadability, viscosity, pH values, and particle size of the experimental formulations containing nanosize drug particle stored for 3 months as compared to the control formulations (respective formulation prepared freshly). However, drug content was found to vary in the stored samples: 1.72, 1.67. and 2.12% (for the hydrogels with nanosize drug particles with permeation enhancer stored at 4°C, 25°C/60% RH, 40°C/75% RH, respectively); 0.67, 2.35, and 1.92% (for the hydrogel with nanosize drug particles without permeation enhancer stored at 4°C, 25°C/60% RH, 40°C/75% RH, respectively). The values were within the limit (5%) stipulated by ICH guideline [30]. FTIR findings showed that there were no predominant changes in the FTIR spectra (Fig. 6) of the hydrogels stored at various conditions compared to the freshly prepared samples. Upon storage, the drug particles were found to have a tendency to agglomerate (Fig. 1c–e).
Fig. 6.
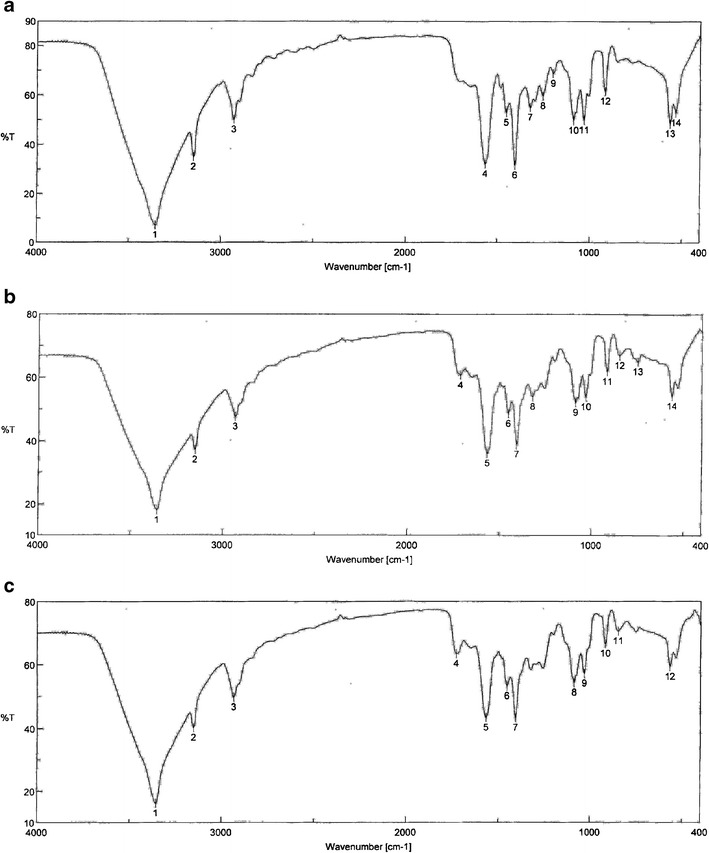
FTIR spectra of hydrogels with nano size drug dispersion with skin permeation enhancer a freshly prepared, b stored at 25°C/60% RH, and c stored at 40°C/75% RH
DISCUSSION
Morphology of Hydrogel
Addition of drug-in-ethanol solution in the polymer mixture of the hydrogel generated nanosize drug particles in situ. Nanosize drug particles (~10 nm) thus developed were probably stabilized by PVA that acts as surface stabilizer. The phenomenon in this study is somewhat similar to our previously reported work [31]. Strong intermolecular ionic forces exist between drug molecules whereas weak physical interaction like electrostatic forces [32,33] occurs between the ionic drug and the slightly polar polymers. This drug-polymer interaction might form soluble polyion drug complex [34] with an individual diameter of about 10 nm in this case. Drug polyion complexes of nano dimension surrounded by polymer molecules prevented the complexes to form larger aggregates while they were dispersed in the polymer matrix. During solvent evaporation, those nanoaggregates settled down in the polymeric base.
Viscosity Study
Drug contents in all the experimental hydrogels were the same. Increase in viscosity of hydrogel with nanosize particles might be due to an increased bond chain entanglement between the drug particles (due to nanosize, they were more in number in the same amount as compared to the gel containing microsize drug particles) and the polymeric chains of hydrogels by weak bonds such as weak hydrogen bond, van der Waals force of attraction, dipole moments, etc. This leads to reduction of polymeric chain mobility and increased viscosity. Enhanced chain entanglement suggests that the formulations took a longer period to swell maximally. Further, reasonable swelling of the gel favors patient compliance [35,36]. Viscosity is the force of inherent resistance against the flow of a liquid. In our hydrogel, viscosity was around 160–170 cps. In the reported findings [37,38], viscosity of hydrogels was found to vary between 30 and 200 cps. The viscosity in this range was found to be satisfactory enough to apply on to the skin.
Texture Analyzer Study
Texture analyzer has primarily been used for assessment of rheological properties of gels used in food industry [39–42]. Several researchers have used it to characterize the rheological properties of hydrogel for drug delivery as well [43–45]. The hardness was determined to assess the spreadability of hydrogel formulations as therapeutic efficacy of hydrogel depends upon its spreadability [46]. Spreadability is the ease with which a spread can be applied in a thin even layer to a surface. As spreadability and hardness are well correlated [47], the results obtained from this study may be a support for an indirect indication of spreadability. In the present study, during the forward movement of the male probe, the load increased proportionately with the time up to the preset movement of a distance of 15 mm. At the tip break point, the movement was stopped and the probe was started to retract and thereby the load fell. During the retraction, the hydrogel sticking to the male probe caused the load to be applied in the opposite direction and thereby a negative movement of the curve was obtained. The hardness profile of the two drug-loaded batches of the same hydrogel formulation shows overlapping curves. This suggests that they had similar hardness profile. Thus, dispersing the drug in nanosize form did not change the spreadability of the hydrogel compared to its microsize form. Further, spreadability of the experimental gels was found to be good enough for topical application. The hardness profile data thus obtained from the study may lead to assess inter and intrabatch uniformity during manufacturing.
Drug Release
The saturation solubility of the drug in the hydrogel formulation containing nanosize drug particles increased and hence higher drug release was obtained as compared to the other formulations. Drug release patterns from all the hydrogels were found to follow Higuchi kinetics. This is also corroborated with the earlier reports that drug release kinetics from carbopol hydrogel often follows Higuchi kinetics (diffusion controlled release from matrix) [48,49] unless some agents (e.g., glycerol) [50] or techniques (e.g., iontophoresis) are used to modify the drug release kinetics [51]. Any similar agent/process to modify the gel matrix was not used in our study. Hence, the present findings mostly support the findings of Banga and Chien [49].
Skin Permeation Study
Hydrogel with nanosize drug particles offered a significant enhancement of skin permeation in terms of both the initial flux value and the maximum flux than the hydrogels with microsize particles and the commercial hydrogel formulation. The flux values obtained from the hydrogels (with or without the enhancer) with nanosize drug particles were quite higher than those obtained from the two hydrogels with microsized drug particles with or without the enhancer.
Enhancement of skin permeation of drug from the hydrogel containing nanosize DDA dispersion might be due to one or more reasons. Comparative studies of gels with microsize drug particles and nanosize particles on skin occlusivity showed that nanosize particles have a distinct advantage over microsize particles [52,53]. Researchers have demonstrated that particles of less than 200 nm, in general, have a distinct advantage in providing high occlusivity to the skin leading to better hydration of the skin and thereby improved permeation [54–56]. DDA belongs to the poorly water-soluble class of drugs. It has a variable water solubility of 0.41% w/w to 1.3% w/v in a microsize state. Saturation solubility of the nanosize drug particles (formed in situ) in the hydrogel could not be established since the drug particles in this size range from the experimental polymer matrix were not possible to separate. However, reduction of drug particle sizes in nanometer range has been reported to increase drug permeation through the skin, primarily due to increase in skin hydration, enhanced solubility in the biphasic fluid (mixture of sebum and sweat), and increased partitioning in stratum corneum [55,57,58]. The above studies support our findings that distribution of nanosize drug particles in hydrogels enhanced skin permeation of drug much more as compared to the gels containing microsize drug particles. Inclusion of permeation enhancer in the hydrogel formulation with nanosize drug particles was found to enhance skin permeation of DDA. Figure 4 describes that the best skin permeation-enhancing effect was obtained from the hydrogel containing nanosize drug and the permeation enhancer (triethanolamine). When the skin permeation of nanosize DDA from the hydrogel formulation was compared to that of the formulation containing nanosize DDA along with triethanolamine, enhancement of drug permeation (higher flux) was observed in the second case. Drug permeability coefficients were much higher (~10 times) for both the nanosize drug formulations (with or without enhancer) compared to the hydrogels with the microsize drug. Again, when skin permeation of drug from the hydrogel containing nanosize drug particles was compared with that of the hydrogel containing microsize DDA, there was about 4 times enhancement of cumulative drug skin permeation at the initial hour of study and the value was 6 times more than that of the hydrogel with microsize drug at the 10th hour. The findings are further supported by the earlier reports describing the enhancement of skin delivery of the drug due to nanosize [55,56,59]. The hydrogel with nanosize particles showed better permeation (statistically significant; p, 0.02) as compared to the commercial reference formulation, which suggests that the hydrogel with nanosize drug particles might provide better therapeutic efficacy.
Gel with nanosize drug particles and the skin permeation enhancer showed the highest permeation coefficient value, followed by the gel with nanosize drug particles, the commercial gel, and the gel with microsize drug dispersion and the skin permeation enhancer.
Blood Levels of Drug from Hydrogels in Rats
Reduction of particle size in the nanometer range increases saturation solubility of the drug and hence enhances its skin permeation [18]. In addition, reports also suggest that particle size below 50 nm diameter can directly penetrate the skin [60,61], additionally enhancing the skin permeation. In the present study, both the hydrogel formulations (with or without the skin permeation enhancer) with nanosize drug particles showed a superior plasma drug level than the commercial hydrogel formulation and the experimental hydrogel containing microsized particles. This is corroborated with the fact that the hydrogel formulations with nanosize drug particles had a better in vitro drug release and skin permeation profile than the other formulations.
Stability Study
The hydrogel containing nanosize drug particles undergone a stability study (in different conditions) for 3 months did not show any stability related issues except the sample which was stored at higher temperature and humidity condition. For the samples which were stored at room temperature and refrigerated condition, physicochemical properties such as spreadability, viscosity, and pH value of the experimental formulations remained unchanged as compared to the freshly prepared formulation (data not shown). The drug contents as assessed in those hydrogels were within the recommended limit as well. However, agglomeration of drug particles was observed in the hydrogel, which was stored at higher temperature and humidity conditions. Softening of the gel matrices at a higher temperature might lead to such phenomenon. It is therefore recommended to store the hydrogels at room temperature.
CONCLUSIONS
In conclusion, a simple technique for in situ development of nanosize DDA during hydrogel preparation was established. The study depicts a predominantly improved skin permeation of drug from a transdermal hydrogel (based on PVA and carbopol 71G) with uniformly distributed nanosize DDA particles as compared to the hydrogel with microsized drug particles and a commercial hydrogel formulation. The effect of triethanolamine on the experimental concentration was not much beneficial when added to hydrogel with the nanosize drug. In vivo studies showed that systemic drug availability from the experimental hydrogel containing nanosize drug particles markedly improved over the commercial hydrogel formulation, as well. Further, for commercial purpose, this technique may be easily scaled up to prepare hydrogel with nanosize DDA for better skin permeation. Preparing 10 nm particle sizes and distributing the same uniformly in the hydrogel formulation system is not an easy task and it is costly as well. Here, we have developed an in situ preparation of nanosize particles which distributes the particle in the hydrogel uniformly. This makes the process cost-effective and easily scalable from the commercial point of view.
Acknowledgments
We are indebted to the financial support provided by the Department of Biotechnology (Government of India) twinning project, grant no. BT/504/NE/TBP/2013, to conduct the work.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Pooley SA, Rivas BL, Lillo FE, Pizarro GDC. Hydrogels from acrylic acid with n, n dimethylacrylamide: synthesis, characterization, and water absorption properties. J Chil Chem Soc. 2010;55:19–24. doi: 10.4067/S0717-97072010000100006. [DOI] [Google Scholar]
- 2.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/S0169-409X(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Shin SR, Lee YM, Kim SI. Swelling characterizations of chitosan and polyacrylonitrile semi interpenetrating polymer network hydrogels. J Appl Polym Sci. 2003;87:2011–5. doi: 10.1002/app.11699. [DOI] [Google Scholar]
- 4.Kadajji VG, Betageri GV. Water soluble polymers for pharmaceuticral applications. Polym. 2011;3:1972–2009. doi: 10.3390/polym3041972. [DOI] [Google Scholar]
- 5.Park J, Huh K, Ye M, Park K. Hydrogels. In: Sunggyu L, editor. Encyclopedia of chemical processing. Florida: CRC Press; 2005. pp. 1307–17. [Google Scholar]
- 6.Simões S, Ana Figueiras A, Francisco VF. Modular hydrogels for drug delivery. J Biomater Nanobiotech. 2012;3:185–99. doi: 10.4236/jbnb.2012.32025. [DOI] [Google Scholar]
- 7.Peppas N, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/S0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 8.Risbud MV, Hardikar AA, Bhat SV, Bhonde RR. pH-sensitive freeze-dried chitosan-polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J Control Release. 2000;68:23–30. doi: 10.1016/S0168-3659(00)00208-X. [DOI] [PubMed] [Google Scholar]
- 9.Kishida A, Ikada Y. Hydrogels for biomedical and pharmaceutical applications. In: Dumitriu S, editor. Polymeric biomaterials. Florida: CRC Press; 2002. pp. 133–45. [Google Scholar]
- 10.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–14. doi: 10.1016/S0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 11.Hasler-Nguyen N, Fotopoulos G. Effect of rubbing on the in vitro skin permeation of diclofenac-diethylamine 1.16% gel. BMC Res Notes. 2012;5:321–5. doi: 10.1186/1756-0500-5-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baboota S, Shakeel F, Kohli K. Formulation and evaluation of once-a-day transdermal gels of diclofenac diethylamine. Method Find Exp Clin. 2006;28:109–14. doi: 10.1358/mf.2006.28.2.977842. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Fang L. Percutaneous absorption of diclofenac acid and its salts from emulgel. Asian J Pharm Sci. 2008;3:131–41. [Google Scholar]
- 14.Fini A, Bassini G, Monastero A, Cavallari C. Diclofenac salts, viii. Effect of the counterions on the permeation through porcine membrane from aqueous saturated solutions. Pharmaceutics. 2012;4:413–29. doi: 10.3390/pharmaceutics4030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fini A, Fazio G, Gonzalez-Rodriguez M, Cavallari C, Passerini N, Rodriguez L. Formation of ion-pairs in aqueous solutions of diclofenac salts. Int J Pharm. 1999;187:163–73. doi: 10.1016/S0378-5173(99)00180-5. [DOI] [PubMed] [Google Scholar]
- 16.Aina A, Morris A, Gupta M, Billa N, Madhvani N, Sharma R, et al. Dissolution behavior of poly vinyl alcohol in water and its effect on the physical morphologies of PLGA scaffolds. UK JPB. 2014;2:1–6. [Google Scholar]
- 17.Whang J, Gwak H. Effects of amines on percutaneous absorption of alendronate. Drug Dev Ind Pharm. 2011;37:491–7. doi: 10.3109/03639045.2010.525237. [DOI] [PubMed] [Google Scholar]
- 18.Mauludin R, Müller RH, Keck CM. Kinetic solubility and dissolution velocity of rutin nanocrystals. Eur J Pharm Sci. 2009;36:502–10. doi: 10.1016/j.ejps.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh D. Transdermal and trans-membrane delivery compositions. US Patent 1998;5,731,303.
- 20.Patel VM, Prajapati BG, Patel HV, Patel KM. Mucoadhesive bilayer tablets of propranolol hydrochloride. AAPS PharmSciTech. 2007;8:203–8. doi: 10.1208/pt0803077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joung YK, Choi JH, Park KM, Park KD. PLGA microparticle-embedded thermosensitive hydrogels for sustained release of hydrophobic drugs. Biomed Mat. 2007;2:269–73. doi: 10.1088/1748-6041/2/4/010. [DOI] [PubMed] [Google Scholar]
- 22.Shahiwala A, Misra A. Studies in topical application of niosomally entrapped nimesulide. J Pharm Sci. 2002;5:220–5. [PubMed] [Google Scholar]
- 23.Mukherjee B, Mahapatra S, Das S, Roy G, Dey S. HPLC detection of plasma concentrations of diclofenac in human volunteers administered with povidone-ethylcellulose based experimental transdermal matrix-type patches. Methods Find Exp Clin Pharmacol. 2006;28:301–6. doi: 10.1358/mf.2006.28.5.1000338. [DOI] [PubMed] [Google Scholar]
- 24.Ekblad T, Bergstro MG, Ederth T, Conlan SL, Mutton R, Clare AS, et al. Poly (ethylene glycol)-containing hydrogel surfaces for antifouling applications in marine and freshwater environments. Biomacromolecules. 2008;9:2775–83. doi: 10.1021/bm800547m. [DOI] [PubMed] [Google Scholar]
- 25.Dhawan B, Aggarwal G, Harikumar SL. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int J Pharm Investig. 2014;4:65–76. doi: 10.4103/2230-973X.133053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panigrahi L, Pattnaik S, Ghosal SK. The effect of pH and organic ester penetration enhancers on skin permeation kinetics of terbutaline sulfate from pseudolatex-type transdermal delivery systems through mouse and human cadaver skins. AAPS PharmSciTech. 2005;6:167–73. doi: 10.1208/pt060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özgüney IS, Karasulu HY, Kantarci G, Sözer S, Güneri T, Ertan G. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS PharmSciTech. 2006;7:39–45. doi: 10.1208/pt070488. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta S, Ghosh SK, Ray S, Kaurav SS, Mazumder B. In vitro & in vivo studies on lornoxicam loaded nanoemulsion gels for topical application. Curr Drug Del. 2014;11:132–8. doi: 10.2174/15672018113106660063. [DOI] [PubMed] [Google Scholar]
- 29.Franz TJ, Lehman PA, Franz SF, North-Root H, Demetrulias JL, Kelling CK, et al. Percutaneous penetration of N-nitrosodiethanolamine through human skin (in vitro): comparison of finite and infinite dose applications from cosmetic vehicles. Toxicol Sci. 1993;21:213–21. doi: 10.1093/toxsci/21.2.213. [DOI] [PubMed] [Google Scholar]
- 30.Stability Testing of New Drug Substances and Products Q1A (R2). In:ICH Harmonised Tripartite Guideline. 2003. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf. Accessed 5 May 2015.
- 31.Arora P, Mukherjee B. Design, development, physicochemical, and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J Pharm Sci. 2002;91:2076–89. doi: 10.1002/jps.10200. [DOI] [PubMed] [Google Scholar]
- 32.Merkle HP, Knoch A, Gienger G. Release kinetics of polymeric laminates for transdermal delivery: experimental evaluation and physical modelling. J Control Release. 1985;2:99–110. doi: 10.1016/0168-3659(85)90036-7. [DOI] [Google Scholar]
- 33.Kapsi SG, Ayres JW. Processing factors in development of solid solution formulation of itraconazole for enhancement of drug dissolution and bioavailability. Int J Pharm. 2001;229:193–203. doi: 10.1016/S0378-5173(01)00867-5. [DOI] [PubMed] [Google Scholar]
- 34.Bhalerao S, Lalla J, Rane M. Study of processing parameters influencing the properties of diltiazem hydrochloride microspheres. J Microencapsul. 2001;18:299–307. doi: 10.1080/02652040010019488. [DOI] [PubMed] [Google Scholar]
- 35.Sun DD, Lee PI. Crosslinked hydrogels—a promising class of insoluble solid molecular dispersion carriers for enhancing the delivery of poorly soluble drugs. Acta Pharm Sinica B. 2014;4:26–36. doi: 10.1016/j.apsb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Kamel AH, Sokar MS, Naggar VF, Al-Gamal SS. Bioadhesive controlled release metronidazole vaginal tablets. Acta Pharm. 2002;52:171–9. [Google Scholar]
- 37.Dubey A, Prabhu P. Formulation and evaluation of stimuli-sensitive hydrogels of timolol maleate and brimonidine tartrate for the treatment of glaucoma. Int J Pharm Investig. 2014;4:112–8. doi: 10.4103/2230-973X.138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta NV, Shivakumar HG. Interpenetrating network superporous hydrogels for gastroretentive application-preparation, swelling and mechanical properties. Turk J Pharm Sci. 2012;9:127–38. [Google Scholar]
- 39.Chambal B, Bergenståh B, Dejmek P. Heat induced gels from coconut press cake proteins. Food and Nutrition Sciences. 2014;5:562–70. doi: 10.4236/fns.2014.56066. [DOI] [Google Scholar]
- 40.Glibowski P, Wasko A. Effect of thermochemical treatment on the structure of inulin and its gelling properties. Int J Food Sci Tech. 2008;43:2075–82. doi: 10.1111/j.1365-2621.2008.01825.x. [DOI] [Google Scholar]
- 41.Yang H, Wang Y, Jiang M, Oh JH, Herring J, Zhou P. 2-step optimization of the extraction and subsequent physical properties of channel catfish (ictalurus punctatus) skin gelatin. J Food Sci. 2007;72:C188–95. doi: 10.1111/j.1750-3841.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 42.Corrales M, Han JH, Tauscher B. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int J Food Sci Tech. 2009;44:425–33. doi: 10.1111/j.1365-2621.2008.01790.x. [DOI] [Google Scholar]
- 43.Vashist A, Ahmad S. Hydrogels: smart materials for drug delivery. Orient J Chem. 2013;29:861–70. doi: 10.13005/ojc/290303. [DOI] [Google Scholar]
- 44.Martin L, Wilson CG, Koosha F, Uchegbu IF. Sustained buccal delivery of the hydrophobic drug denbufylline using physically cross-linked palmitoyl glycol chitosan hydrogels. Eur J Pharm Biopharm. 2003;55:35–45. doi: 10.1016/S0939-6411(02)00118-2. [DOI] [PubMed] [Google Scholar]
- 45.Khutoryanskaya OV, Mayeva ZA, Mun GA, Khutoryanskiy VV. Designing temperature-responsive biocompatible copolymers and hydrogels based on 2-hydroxyethyl (meth) acrylates. Biomacromolecules. 2008;9:3353–61. doi: 10.1021/bm8006242. [DOI] [PubMed] [Google Scholar]
- 46.Rashmi M, Garg R, Kumar S, Gupta GD. Topical gel: a review. In:Pharmainfo.net. 2008. http://www.pharmainfo.net/reviews/topical-gel-review. Accessed 4 Oct 2014.
- 47.Glibowski P, Zarzycki P, Krzepkowska M. The rheological and instrumental textural properties of selected table fats. Int J Food Prop. 2008;11:678–86. doi: 10.1080/10942910701622599. [DOI] [Google Scholar]
- 48.Ortan A, Ferdes M, Rodino S, Pirvu CD, Draganescu D. Topical delivery system of liposomally encapsulated volatile oil of anethum graveolens. Farmacia. 2013;61:361–70. [Google Scholar]
- 49.Banga AK, Chien YW. Hydrogel-based lontotherapeutic delivery devices for transdermal delivery of peptide/protein drugs. Pharm Res. 1993;10:697–702. doi: 10.1023/A:1018955631835. [DOI] [PubMed] [Google Scholar]
- 50.GarcíGonzález N, Kellaway I, Blanco Fuente H, Anguiano Igea S, Delgado Charro B, Otero Espinar F, et al. Influence of glycerol concentration and carbopol molecular weight on swelling and drug release characteristics of metoclopramide hydrogels. Int J Pharm. 1994;104:107–13. doi: 10.1016/0378-5173(94)90185-6. [DOI] [Google Scholar]
- 51.Bannon Y, Corish J, Corrigan O. Iontophoretic transport of model compounds from a gel matrix across a cellophane membrane. Drug Dev Ind Pharm. 1987;13:2617–30. doi: 10.3109/03639048709020606. [DOI] [Google Scholar]
- 52.Müller R, Radtke M, Wissing S. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–55. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 53.Müller R, Dingler A. The next generation after the liposomes: solid lipid nanoparticles (SLN, Lipopearls) as dermal carrier in cosmetics. Eurocosmetics. 1998;7:19–26. [Google Scholar]
- 54.Kumbhar D, Wavikar P, Vavia P. Niosomal gel of lornoxicam for topical delivery: in vitro assessment and pharmacodynamic activity. AAPS PharmSciTech. 2013;14:1072–82. doi: 10.1208/s12249-013-9986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhai H, Maibach HI. Effects of skin occlusion on percutaneous absorption: an overview skin. Pharmacol Physiol. 2001;14:1–10. doi: 10.1159/000056328. [DOI] [PubMed] [Google Scholar]
- 56.Wissing S, Lippacher A, Müller R. Investigations on the occlusive properties of solid lipid nanoparticles (SLN) J Cosmet Sci. 2001;52:313–24. [PubMed] [Google Scholar]
- 57.Sloan KB. Prodrugs: topical and ocular drug delivery vol 53. New York: Marcel Dekker; 1992. [Google Scholar]
- 58.Benson HAE. Transdermal drug delivery: penetration enhancement techniques. Curr Drug Del. 2005;2:23–33. doi: 10.2174/1567201052772915. [DOI] [PubMed] [Google Scholar]
- 59.Chourasia MK, Kang L, Chan SY. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results Pharma Sci. 2011;1:60–7. doi: 10.1016/j.rinphs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider M, Stracke F, Hansen S, Schaefer UF. Nanoparticles and their interactions with the dermal barrier. Dermato-Endocrinol. 2009;1:197–206. doi: 10.4161/derm.1.4.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581–7. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]


