Abstract
Chemotherapy via oral route of anticancer drugs offers much convenience and compliance to patients. However, oral chemotherapy has been challenged by limited absorption due to poor drug solubility and intestinal efflux. In this study, we aimed to develop a nanosuspension formulation of oridonin (Odn) using its cyclodextrin inclusion complexes to enhance oral bioavailability. Nanosuspensions containing Odn/2 hydroxypropyl-β-cyclodextrin inclusion complexes (Odn-CICs) were prepared by a solvent evaporation followed by wet media milling technique. The nanosuspensions were characterized by scanning electron microscopy (SEM), differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), and dissolution. The resulting nanosuspensions were approximately 313.8 nm in particle size and presented a microcrystal morphology. Nanosuspensions loading Odn-CICs dramatically enhanced the dissolution of Odn. Further, the intestinal effective permeability of Odn was markedly enhanced in the presence of 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) and poloxamer. Bioavailability studies showed that nanosuspensions with Odn-CICs can significantly promote the oral absorption of Odn with a relative bioavailability of 213.99% (Odn suspensions as reference). Odn itself possesses a moderate permeability and marginal intestinal metabolism. Thus, the enhanced bioavailability for Odn-CIC nanosuspensions can be attributed to improved dissolution and permeability by interaction with absorptive epithelia and anti-drug efflux. Nanosuspensions prepared from inclusion complexes may be a promising approach for the oral delivery of anticancer agents.
KEY WORDS: bioavailability, cyclodextrin inclusion complexes, dissolution, nanosuspensions, oridonin
INTRODUCTION
Modern chemistry and high-throughput screening largely accelerate the progress of drug discovery. Unfortunately, the majority of drug candidates, especially anticancer agents, possess unsatisfactory druggability due to poor aqueous solubility and membrane permeability (1). It is a formidable obstacle to develop injectable formulations for systemic delivery of anticancer drugs. Depending on convenience, compliance, and low cost, oral administration has been the most popular route that people take medications. Although oral delivery of anticancer drugs has gained increasing interest in recent years, the oral route is often impeded by limited bioavailability associated with multiple factors, such as aqueous solubility, drug permeability, susceptibility to efflux pump, and first-pass metabolism (2).
Pharmaceutical nanotechnology greatly advanced the development of drug delivery systems in the past decades. Likewise, great progress in oral drug delivery has been achieved, resulting in several successful products on the market, e.g., Triglide® (fenofibrate nanocrystals) (3) and Sandimmune Neoral® (cyclosporin A self-microemulsions) (4). These nanosystems significantly enhance the dissolution or bioavailability of poorly water-soluble drugs. However, to date, no oral nanoproduct of anticancer drugs has been commercially available. The bioavailability of Biopharmaceutics Classification System (BCS) II drugs, to a great extent, is limited by their solubility and/or dissolution rate in the aqueous intestinal milieu. It can be improved by the enhancement of drug’s dissolution. In the case of anticancer drugs (mostly identified as BCS IV drug), the bioavailability is not only dominated by dissolution but also by drug efflux and first-pass effect. Thus, there is an intrinsic interest to explore innovative or combined technique to orally deliver anticancer drugs.
Drug–cyclodextrin conjugates are pharmaceutically defined as cyclodextrin inclusion complexes (CICs) where one drug (guest molecule) is incorporated into the cavity of cyclodextrin (host molecule) via non-covalent interaction. CICs can be prepared by precipitation from saturated aqueous solution, kneading, freeze/spray-drying, and melting techniques (5). Generally, CICs are intermediate products. In most cases, they need to be reprocessed for the final application. One-step processing should be a promising approach to produce CIC-loaded preparations, e.g., co-precipitation of drug–cyclodextrin solution onto pellets by fluid bed (6). It is difficult to harvest the product from the container where drug–CICs are fabricated from the conventional solvent evaporation. Although CICs are competent in drug solubilization and dissolution enhancement, their role in inhibiting the drug efflux is inadequate. Nanosuspensions containing CICs can offset the shortcoming of CICs alone due to the participation of surfactants used to stabilize the drug nanocrystals. Nanosuspensions prepared by wet media milling are a simple and convenient technique to obtain nanoscale drug intermediates (7,8). The combination of nanocrystallization and cyclodextrin complexation techniques is a new attempt to formulate poorly water-soluble drugs. It is also a workable technique to harvest the resulting CICs. However, nanosuspensions containing CICs prepared by wet media milling has not been investigated.
Oridonin (Odn), a diterpenoid extracted from Rabdosia rubescens, has been demonstrated to possess various pharmacological activities, such as anti-inflammation, anti-bacterial, and anti-neoplastic effects. Recently, more attention has been paid on its anticancer effects. Odn can trigger cell cycle arrest, apoptosis, and autophagy in a wide spectrum of cancer cell lines (9). Oral bioavailability of Odn is fairly limited due to poor water solubility and absorption barrier. There are considerable difficulties in systemic delivery of Odn owing to low drug load and lack of registered injectable excipients. Various strategies have been attempted to orally deliver Odn, including self-microemulsifying drug delivery systems (10), solid dispersions (11), and nanosuspensions (12). However, these systems paid less attention to the physiological barriers that affect the oral bioavailability of Odn, such as intestinal metabolism and drug efflux (2).
In this study, we engineered nanosuspensions of Odn/2-hydroxypropyl-β-cyclodextrin inclusion complexes (Odn-CICs) with poloxamer 407 as stabilizer (Scheme 1), aiming to enhance the oral bioavailability of Odn by improving the dissolution and inhibiting the drug efflux through cyclodextrin and poloxamer. Nanosuspensions of Odn-CICs were prepared by the solvent evaporation/media milling method and characterized by particle size, morphology, dissolution, etc. Intestinal metabolism and permeability of Odn and Odn-CICs were evaluated by rat intestine microsome incubation and single-pass intestinal perfusion techniques, respectively. Finally, the oral bioavailability of nanosuspensions of Odn-CICs was evaluated in rats with Odn suspensions as reference.
Scheme 1.
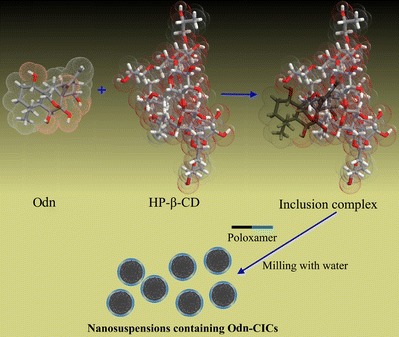
Illustrations of inclusion and preparation processes of Odn/HP-β-CD inclusion complexes
MATERIALS AND METHODS
Materials
Oridonin was purchased from Baoji Herbest Bio-Tech Co., Ltd (Baoji, China). 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD) was obtained from Maxdragon Biochemical Technology Co. Ltd (Guangzhou, China). Poloxamer 407 was supplied by BASF (Ludwigshafen, Germany). Cyclosporin A was bought from Sigma–Aldrich (Shanghai, China). HPLC-grade methanol was provided by Merck (Darmstadt, Germany). Deionized water was prepared with a water purifying system (Woter, Chengdu, China). All other chemicals or reagents were of analytical grade.
Phase Solubility Study
Phase solubility diagram was drawn according to Higuchi and Connors (13). Briefly, excessive Odn was added to 5 mL of distilled water that contains different concentrations of HP-β-CD (0, 10, 20, 30, 40, 50, 60, and 80 mg/mL). Samples were agitated with a magnetic stirrer for 48 h in a sealed bottle at 600 rpm and 25°C. Then, the samples were centrifuged against Centrifuge 5424 (Eppendorf, Hauppauge, NY, USA) at 12,000g for 10 min. Odn concentration in the supernatant was analyzed by the established HPLC method below. The phase solubility diagram was plotted using HP-β-CD concentration as independent variable and Odn concentration as variance. The complex formation constant (K f) was calculated based on the following equation: K f = S / S 0(1 − S), where S and S 0 denote the slope of linear equation (if applicable) and solubility of drug in the absence of HP-β-CD, respectively.
Preparation of Odn-CIC Nanosuspensions
Nanosuspensions of Odn-CICs were prepared by the solvent evaporation/media milling technique. Typically, Odn and HP-β-CD (at a stoichiometric molar ratio of 1:1.2) were dissolved in ethanol and then the solvent was removed under reduced pressure using a rotatory evaporator until no residual ethanol can be smelled out. Drug and HP-β-CD were spontaneously assembled into CICs upon the solvent elimination. Subsequently, poloxamer 407 and an appropriate amount of water were introduced into the dried CICs. The system was subjected to abrasion with 2 g milling pearls (zirconium oxide, 1.2 mm) stirred at 1000 rpm for 24 h to form nanosuspensions. Particle size as index was adopted to optimize the formulation of Odn-CIC nanosuspensions with variable poloxamer 407 amount and invariable CICs (100 mg).
Characterization of Odn-CIC Nanosuspensions
Particle size of Odn-CIC nanosuspensions was determined by Zetasizer Nano ZS (Malvern, Worcestershire, UK) based on dynamic light scattering at 25°C. An aliquot sample (0.1 mL) was diluted with deionized water to 1 mL and then subjected to laser diffraction. The data were collected with the built-in software for the analysis of particle size and distribution. In addition, the zeta potential of particle was measured using the potential module.
The morphology of Odn-CIC nanosuspensions was observed by scanning electron microscopy (SEM). The sample for SEM detection was first diluted to five times around with water. Then, the nanoparticles were fixed to the supporter by evaporating the residual water under ambient atmospheres. After the procedure of sputter coating with gold, the surface of nanoparticles was scanned with SEM (Philips XL-30E, Amsterdam, Netherlands) for image gathering.
Quantification of Odn
Oridonin concentration of in vitro samples was determined by HPLC (Dionex UltiMate 3000, Thermo Scientific, MA, USA). The HPLC system was equipped with a quaternary pump, a degasser, an autosampler, a column heater, and a multichannel rapid scanning UV–Vis detector. Odn was separated by a Thermo Syncronis C18 column (5 μm, 4.6 mm × 250 mm) guarded with a precolumn at 40°C and detected at 240 nm. The injection volume was 20 μL. A mobile phase of methanol–water (60:40, v/v) pumped at a flow rate of 1.0 mL/min was utilized to elute the analytes.
All in vivo samples of Odn (unless specified otherwise) were quantified by Waters UPLC-QTOF/MS that comprises the ACQUITY UPLC system and Xevo G2 QTOF (Milford, MA, USA). Chromatographic elution was performed on an ACQUITY UPLC BEH column (2.1 × 100 mm, 1.7 μm; Waters) using a gradient of 0.1% formic acid in water (mobile phase A) versus 0.1% formic acid in acetonitrile (mobile phase B) at a flow rate of 0.45 mL/min. The gradient elution program was 5% B at 0 to 1 min, 5 to 95% B at 1 to 3 min, 95% B at 3 to 3.5 min, and 95 to 5% B at 3.5 to 4 min. Quantitation was processed based on the full scan analysis and extracted ion chromatograms using MassLynx version 4.1. The configuration and parameter settings of instrument were consistent with the published literature (14).
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was performed on DSC 204A/G phoenix instrument (Netzsch, Baveria, Germany). Samples of pure drug, HP-β-CD, physical mixture, and Odn-CICs (equivalent to 5 mg Odn) were placed in a non-hermetically sealed aluminum pan and proceeded to thermal analysis. The samples were heated from 25°C to 300°C at a heating rate of 10°C/min. The instrument was calibrated with a standard material of indium. All the DSC processes were performed in nitrogen atmosphere at a flow rate of 100 mL/min.
Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) was traced to further estimate the possible interactions between Odn and HP-β-CD in Odn-CICs. In brief, samples of pure drug, HP-β-CD, physical mixture, and Odn-CICs were ground thoroughly with KBr to obtain an infrared transparent matrix. FTIR scanning was run on an FTIR-8400S spectrometer (Shimadzu, Toyota, Japan). Spectra were recorded from 3500 to 600 cm−1 with a resolution of 0.1 cm−1.
Dissolution Studies
Dissolution studies of Odn were carried out in 900 mL water using ZRS-8G dissolution tester (Tianjin, China), according to the Chinese Pharmacopeia Method II (the paddle method). Samples (raw material, Odn suspensions, and Odn-CICs nanosuspensions) containing 50 mg Odn were put into the dissolution cup and thermostatically maintained at 37°C at a rotation speed of 75 rpm. At predetermined time points, 5 mL of sample was withdrawn and immediately replaced by the same volume of fresh medium to keep a constant volume. The samples were filtered with Millex® AP membrane (Millipore, 0.22 μm), and Odn concentration in filtrates was determined by HPLC.
In Situ Single-Pass Intestinal Perfusion
In situ single-pass intestinal perfusion was employed to determine the intestinal permeability of free Odn as well as Odn-CICs as described (15). Briefly, Sprague–Dawley (SD) rats weighing 220 ± 20 g were fasted overnight but freely accessible to water before perfusion. Surgical procedures were performed on rats after anesthesia with an intraperitoneal injection of 20% urethane (1.0 g/kg). A midline longitudinal incision was made to expose the abdomen. The jejunum segment ∼10 cm was cannulated with silicone tubes (Φ 2.5 × 4 mm). Perfusates were prepared in Krebs Ringer buffer (pH 7.4) containing 100 μg/mL of Odn or Odn-CICs. Moreover, co-perfusion of Odn and cyclosporin A (a drug efflux transporter inhibitor, 20 mM) was performed to evaluate the efflux effect. After pre-perfusion for 30 min to reach a steady state, the effluents were collected every 15 min up to 120 min. Finally, the radius and length of intestinal segment were measured. The effective permeability coefficient (P eff) was calculated based on the following equation:
where Q is the flow rate (0.2 mL/min), r is the radius of the intestine (cm), L is the length of the perfused intestinal segment (cm), and C in and C out are the inlet and outlet concentration of Odn, respectively. For accurate calculation of C out, net water flux in the perfusion experiment was calibrated by weight.
Intestinal Metabolism Study
Intestinal stability of Odn was evaluated through a microsomal metabolism technique. Rat intestinal microsomes (RIM) were prepared from adult male SD rats as described (16). RIM were obtained from the whole small intestine. The resulting microsomes were suspended in 250 mM sucrose solution and stored at −80°C ready for use. To quantify RIM, the protein concentration in microsomes was determined using a protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as a standard.
Oridonin was incubated with RIM in the absence or presence of HP-β-CD at 37°C as reported procedure (17). Briefly, the reactive medium comprised 0.26 mg/mL microsomal protein, 1.0 mM NADP+, 3.3 mM UDPGA, 3.3 mM MgCl2, 0.4 UI/mL glucose-6-phosphate dehydrogenase, and 100 μg/mL Odn in pH 7.2 phosphate buffer. The reactive system was terminated after 2 h incubation by adding ice-cold acetonitrile, followed by vortex and centrifugation (10 min, 12,000g). The supernatants were collected and subjected to HPLC analysis. All experiments were performed in triplicate. The metabolic stability of Odn was assessed based on the percentage of parent drug vs time plot.
Bioavailability Studies
All animal experiments were conducted conforming to the Guidelines on the Care and Use of Animals for Scientific Purposes. Meanwhile, the protocols for the animal studies were reviewed and approved by the Experimental Animal Ethical Committee of Jinan University. SD rats (250 ± 20 g) were randomly divided into two groups (n = 6). Rats were fasted for 24 h prior to the experiment but allowed free access to water. The rats were administrated by gavage with nanosuspensions of Odn-CICs or the reference (conventional suspensions of Odn, suspended with carboxymethylcellulose sodium) at a dose of 50 mg/kg. Blood samples (about 0.25 mL) were withdrawn from the tail vein at specified time points (0.25, 0.5, 1.0, 2.0, 4, 6, and 8 h) after administration. Plasma was separated by centrifugation at 5000g for 5 min.
Oridonin in rat plasma was retrieved by a liquid–liquid extraction procedure with ethyl acetate. Typically, five-fold volume of ethyl acetate was added into 100 μL plasma supplemented with 10 μL of 10 μg/mL SNX-2112 as internal standard. The samples were then vortexed vigorously for 5 min and centrifuged at 12,000g for 10 min. The supernatant was transferred to centrifuge tubes followed by vacuum evaporation at 35°C using a Concentrator Plus (Eppendorf, Hauppauge, NY, USA). The residues were reconstituted in 100 μL acetonitrile and analyzed by UPLC-QTOF/MS after centrifugation. Non-compartmental analysis was used to process the data and extract the pharmacokinetic parameters with PKSolver 2.0 software.
RESULTS AND DISCUSSION
Solubility Diagram of Odn/HP-β-CD
Figure 1 shows the phase solubility diagram of Odn vs HP-β-CD with increasing concentration in water. The complexation between Odn and HP-β-CD was confirmed to be an AL-type by linear fitting (correlation coefficient 0.9802). This type of plot indicated the formation of soluble CICs at a stoichiometric rate of 1:1. The aqueous solubility of Odn determined in our laboratory was 0.524 mg/mL (1.44 × 10−3 mol/L, Odn molecular weight 364.4). Thus, the calculated complex formation constant (K f) was 42.37 L/mol, demonstrating a stable complexation. Phase solubility study suggested that it was feasible to use HP-β-CD to include and solubilize Odn.
Fig. 1.
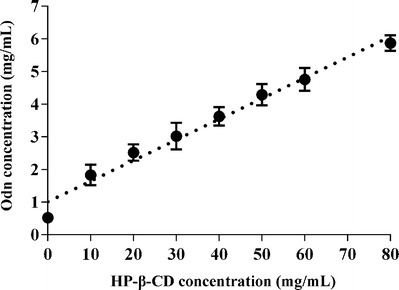
Phase solubility diagram plotted with concentration of Odn against increasing concentrations of HP-β-CD in water
Preparation and Characterization of Odn-CICs Nanosuspensions
In the preliminary experiment, we found that it was difficult to obtain nanosuspensions <500 nm by a direct wet media milling toward Odn-CICs. The addition of surfactants (poloxamer series) largely reduced the particle size of Odn-CIC suspensions up to nanoscale. Poloxamer 407 not only can facilitate the formation of nanosuspensions of Odn-CICs but also has the ability to overcome multidrug efflux via P-glycoprotein (18). Accordingly, poloxamer 407 was used to prepare Odn-CIC nanosuspensions. The effect of poloxamer 407 rate on particle size as well as distribution is showed in Fig. 2. Particle size together with polydispersity index (PDI) of nanosuspensions decreased as poloxamer 407 amount increased. Although CICs significantly enhanced the apparent solubility of Odn (from 0.524 to 5.87 mg/mL), the solid Odn-CICs were still unable to dissolve in a limited volume of water. Preparation of Odn-CIC nanosuspensions either could improve the dissolution of Odn or reduce the binding by intestinal free mucins, which was advantageous to the enhancement of bioavailability.
Fig. 2.
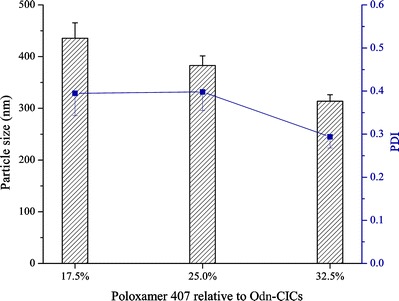
The effect of poloxamer 407 rate relative to Odn-CICs on the particle size and PDI of Odn-CICs nanosuspensions
The particle size of Odn-CICs nanosuspensions prepared using 32.5% poloxamer 407 relative to Odn-CICs was 313.8 nm and presented a unimodel distribution (PDI = 0.294) (Fig. 3a). The particle of nanosuspensions possessed a zeta potential of 27.6 mv, implying an acceptable physical stability. The resulting nanosuspension particles were microcrystal structure in morphology as observed by SEM (Fig. 3b). The particle size was estimated to be 500 nm around from the scale bar, which was a little larger than the hydrodynamic size given by nanoparticle analyzer. This may be associated with crystal growth upon dehydration in sample preparation. Additionally, an obvious poloxamer lining was underneath the particles. SEM photograph clearly indicated that the nanosuspensions were made up of Odn-CICs nanocrystals stabilized by poloxamer. A 2-week investigation for nanosuspension stability showed that slight sedimentation on particles would take place upon storage in ambient conditions. However, the particles could be readily re-dispersed by shaking with no significant change in particle size, showing a satisfactory physical stability.
Fig. 3.
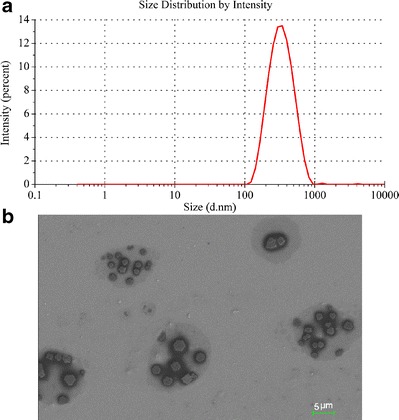
Particle size distribution (a) and SEM micrograph (b) of Odn-CIC nanosuspensions
DSC
DSC thermograms of Odn, HP-β-CD, physical mixture, and Odn-CICs are shown in Fig. 4. The endothermic peak at 253°C corresponded to the melting point of Odn (Fig. 4a). There was no any endothermic event for HP-β-CD (Fig. 4b), revealing the amorphous nature of this excipient. The physical mixture showed an identical endothermic peak to that of pure Odn (Fig. 4c) at 250°C around. However, the sharp peak of Odn vanished in the DSC curve of Odn-CICs (Fig. 4d), suggesting that the encapsulated Odn by cyclodextrin was amorphous or molecular state. HP-β-CD is a highly water-soluble, hydroxyl-rich, and low-molecular (ca. 1400) compound, though its effect on suppression of recrystallization was insignificant in comparison with commonly used solid dispersion carriers, such as PVP. The disappearance of crystalline Odn in Odn-CICs lent a strong support to the notion that Odn has been encapsulated into the cavity of HP-β-CD.
Fig. 4.
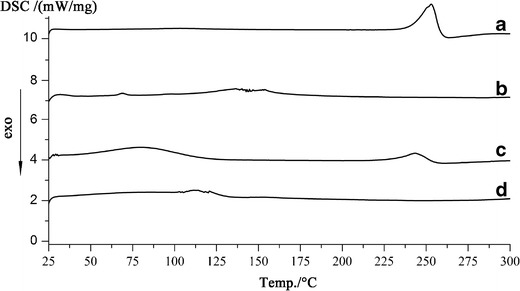
DSC thermograms: pure drug (a), HP-β-CD (b), physical mixture (c), and Odn-CICs (d)
FTIR
FTIR spectra of Odn, HP-β-CD, physical mixture, and Odn-CICs are shown in Fig. 5. Changes in the characteristic bands of the FTIR spectra were distinctly observed, revealing an alteration of molecule micromilieu. The diagnostic peaks of Odn appeared at 3381.6, 2866.9, 1710.9, 1647.3, and 1169.7 cm−1, corresponding to the stretching vibrations of –OH (νs), ═CH2 (νs), –CO– (νs), and C–O–C (νs), respectively. The Odn-associated peaks for pure drug and physical mixture were nearly identical. However, those diagnostic peaks, as marked by yellow lines, disappeared or shifted in position in the FTIR spectrum of Odn-CICs. Alternations in characteristic peaks indicated that there were intense molecular interactions between Odn and HP-β-CD. This may be ascribable to the effect of inclusion and formation of hydrogen bonds.
Fig. 5.
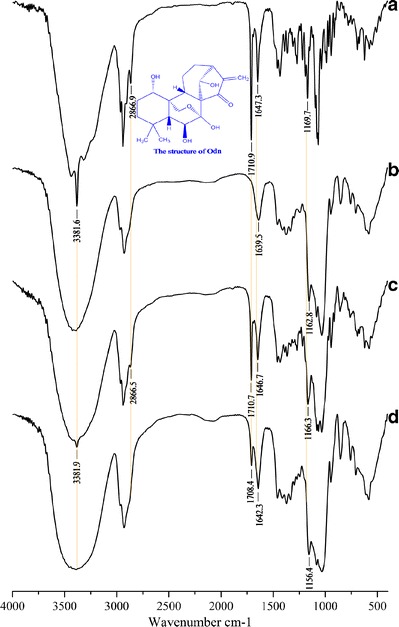
FTIR spectra: pure drug (a), HP-β-CD (b), physical mixture (c), and Odn-CICs (d)
In addition, it could be inferred that the cycloheptane and partial adjacent cyclohexane moiety was incorporated into the cavity of HP-β-CD from the FTIR changes of corresponding functional groups. The FTIR absorption of methylene (═CH2) and carbonyl (–CO–) located in the cycloheptane ring underwent significant modifications. The former disappeared and the latter shifted down in the FTIR spectrum of Odn-CICs. It was suggested that the cycloheptane moiety was included by HP-β-CD. Also, the variation of peak position for the ether bond (C–O–C) on the cyclohexane ring implied an incorporation of this part. FTIR is one of the most commonly used techniques to explore the complexation process of drug and cyclodextrin (19,20). In the present experiment, the FTIR spectra have provided definite information on the intermolecular interaction and inclusion pattern as illustrated in Scheme 1.
Dissolution
The dissolution profiles of pure Odn, conventional suspensions of Odn, and nanosuspensions of Odn-CICs in water are shown in Fig. 6. It can be seen that the raw material possessed a slow dissolution, and the accumulative dissolution was just 69.28% at 60 min. The dissolution rate of pure Odn was limited to a great extent by its aqueous solubility. Conventional suspensions of Odn exhibited a rapid dissolution in the first 5 min, though the dissolution seemed to arrive at a standstill afterwards. The dissolutions of pure Odn and conventional Odn suspensions were incomplete, though the intrinsic solubility of Odn was more than 500 μg/mL. The phenomenon suggested that Odn dissolution was either dominated by the intrinsic solubility or by the dispersity and wettability. Compared to conventional Odn suspensions, the dissolution of Odn-CICs nanosuspensions was strikingly fast and hundred-percent. It only spent 2.5 min to accomplish the dissolution.
Fig. 6.
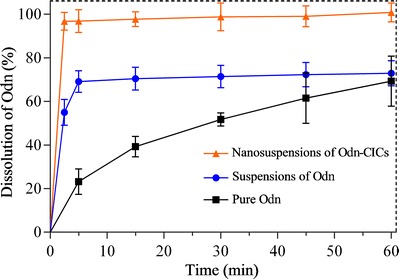
Dissolution profiles of pure Odn, suspensions of Odn, and nanosuspensions of Odn-CICs in water
Crude drugs are readily captured by mucins and rapidly removed from the GI tract, resulting in reduced oral bioavailability. Nano-drug delivery systems have shown the potential to orally deliver various anticancer molecules for the enhancement of bioavailability through nanoscale effect (21). Reduction in particle size improves the dissolution of drug, and it further increases the contact area of the drug with the absorptive epithelia. Besides improved dissolution and reduced mucin entrapment, nanosuspensions containing Odn-CICs possess various features of CICs, e.g., improved stability, reduced GI irritation, and enhanced absorption (22).
Improved Intestinal Permeability
As shown in Table I, the effective permeability (P eff) of Odn solution in the jejunum was 4.81 × 10−5 cm/s. The value indicated that the intestinal permeability of free Odn was moderate, because drugs with P eff < 5.0 × 10−5 cm/s were predicted to be partially absorbed for humans (23). The P eff of free Odn significantly increased to 7.41 × 10−5 cm/s when cyclosporin A, an inhibitor of P-gp and CYP 3A4 (24), was introduced into the perfusate. This showed that a transmembrane efflux mediated by P-gp and/or CYP 3A was involved in the transport of Odn. However, the P eff was markedly improved for Odn in the presence of HP-β-CD and poloxamer 407, which were used to prepare nanosuspensions of Odn-CICs, as compared with free Odn or Odn plus cyclosporin A. The P eff value was up to 1.25 × 10−4 cm/s. It can be attributed to the interaction of cyclodextrin with the lipid components of biomembrane and the inhibitory effects of both HP-β-CD and poloxamer 407 on efflux transporters. It has been reported that methylated cyclodextrin reduced the activation energy required to incorporate cholesterol in the hydrophobic cavity, thereby modifying the fluidity and permeability of biomembrane (25,26). Poloxamers are also reported capable of altering the lipid bilayer (resulting in significant decrease in the ATPase activity) by depleting the ATP pool of the cells (27).
Table I.
The Intestinal Effective Permeability (P eff) of Odn and Odn-CICs Determined by In Situ Single-Pass Intestinal Perfusion Using the Jejunum (n = 5)
| Intestinal segment | P eff (cm/s) |
|---|---|
| Odn | 4.81 ± 1.67 × 10−5 |
| Odn + cyclosporin A | 7.41 ± 1.29 × 10−5* |
| Odn-CICs | 1.25 ± 0.36 × 10−4** |
Odn: oridonin, CICs: cyclodextrin inclusion complexes
Paired t test, *P = 0.05; **P = 0.01, significantly different from Odn
It is noted that the complexation of drug by cyclodextrin not only result in not only increased solubility but also decreased permeability (28–30). There are two causes accounting for the phenomenon: (1) drug–CD complex is provided with a higher solubility than free drug that reduces the drug’s lipophilicity toward biomembrane; (2) enhanced molecular mass due to complexation degrades the permeability of drug according to Lipinski’s rule of five. However, the tradeoff of solubility–permeability on drug–cyclodextrin complexes is highly correlated with the nature of drug. The contradiction of solubility increase and permeability decrease seems more evident in the case of high-lipophilic drugs. In our study, Odn is a mid-lipophilic compound which can dissociate from the cavity of cyclodextrin upon dilution in the GI tract. In addition, the apparent permeability of drug is involved in multiple factors, such as intrinsic solubility of drug, contact area with absorptive epithelium, dissociation of drug in the specific medium, and effects of excipients. Accordingly, the enhancement both in solubility and permeability of Odn as complexing with HP-β-CD may be a synergetic consequence of drug property and excipients’ effects.
Intestinal Metabolism of Odn and Odn-CICs
One factor reducing bioavailability is the intestinal metabolism of drug after oral administration. Figure 7 shows the metabolic profiles of Odn and Odn-CICs in the simulated medium containing rat intestinal microsomes. Odn shared the same metabolism with Odn-CICs. Both Odn and Odn-CICs underwent marginal intestinal metabolism, and the metabolic rates after 120-min incubation were all less than 5%. The results demonstrated that Odn is relatively stable in the GI tract. The effect of intestinal metabolism on oral bioavailability of Odn is insignificant.
Fig. 7.
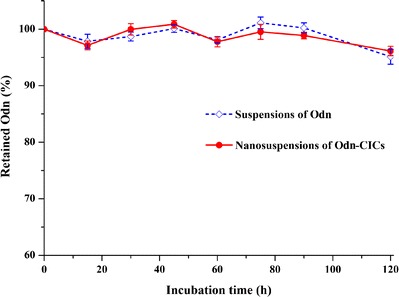
Assessment of Odn metabolism in the intestine through microsomal incubation: coenzymes containing phase I (NADP+) and phase II (UDPGA) were used to launch the metabolic reaction
Enhanced Bioavailability of Odn
Plasma Odn concentration vs time plots after oral administration of Odn suspensions and Odn-CICs nanosuspensions are presented in Fig. 8. Main pharmacokinetic parameters computed based on non-compartmental model are shown in Table II. The peak times (T max) of two preparations were approximately 0.62–0.75 h, for which there was no significant difference between them. However, significant differences in peak concentration (C max), elimination half-life (T 1/2), and area under the concentration–time curve (AUC 0-t) were yielded for two distinct preparations. The C max of Odn-CICs nanosuspensions reached 1.565 μg/mL, whereas it was just 0.589 μg/mL for Odn suspensions. Conventional Odn suspensions exhibited a relatively long T 1/2 in comparison with Odn-CICs nanosuspensions. This was connected with the lower plasma Odn concentrations and slower later elimination. Nanosuspensions consisting of Odn-CICs demonstrated increased blood drug levels at each time point. The relative bioavailability of Odn-CICs nanosuspensions to Odn suspensions was 213.99%. These results turned out that nanosuspensions, simply prepared from drug–CICs, can significantly enhance the oral bioavailability of anticancer drugs.
Fig. 8.
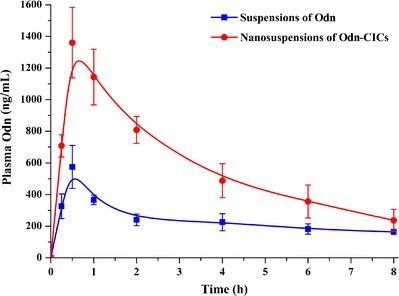
Plasma Odn concentration–time profiles after oral administration of Odn suspensions and Odn-CICs nanosuspensions to rats at a dose of 50 mg/kg (mean ± SD, n = 6)
Table II.
Pharmacokinetic Parameters of Odn Suspensions and Odn-CICs Nanosuspensions After Oral Administration to Rats at a Dose of 50 mg/kg (n = 6, mean ± SD)
| Parameters | Odn suspensions | Odn-CICs nanosuspensions |
|---|---|---|
| C max (μg/mL) | 0.589 ± 0.253 | 1.565 ± 0.406** |
| T max (h) | 0.625 ± 0.250 | 0.750 ± 0.288 |
| T 1/2 (h) | 13.405 ± 3.867 | 5.035 ± 1.639** |
| AUC0-t (μg/h/mL) | 2.609 ± 4.84 | 5.557 ± 1.321** |
| RBA (%) | / | 213.99 |
ODN oridonin, CICs cyclodextrin inclusion complexes, AUC area under the plasma Odn concentration–time curve, RBA relative bioavailability
One-way ANOVA, **P < 0.01, compared with the reference of Odn suspensions
The effective concentration of Odn was estimated to be approximately 5 μM/mL (1.57 μg/mL) using 562 and HL 60 cancer cell lines (31). This therapeutic concentration might be readily achieved by repeated administration of Odn-CICs nanosuspensions via the oral route. Nanosuspensions (e.g., nanocrystals suspended in water) have shown potential in the enhancement of oral bioavailability of poorly soluble drugs (3). Likewise, the ability of cyclodextrin in promoting drug absorption has been verified (32). However, the nanocrystal technique combined with cyclodextrin complexation has not been explored. Nanosuspensions of Odn-CICs should be a mixture containing soluble Odn, Odn-CICs, and nanocrystals of Odn-CICs, since the Odn-CICs quantity exceeded the solvent power of water used in nanosuspensions. Nanosuspensions containing Odn-CICs possessed high drug dispersity and additional formulation advantages. Thus, the mechanisms responsible to enhanced bioavailability of Odn were proposed: (1) the nanosuspensions of Odn-CICs dramatically improved the dissolution of the drug and (2) the excipients involved in nanosuspensions significantly enhanced the intestinal permeability through interaction with biomembrane and anti-drug efflux effect.
CONCLUSIONS
This work demonstrates that the combination of cyclodextrin inclusion and nanocrystallization can significantly enhance the dissolution and bioavailability of Odn. Poor water solubility, unfavorable permeability, and potential drug efflux mediated by efflux transporters constitute the formidable carrier of drug absorption. Nanosuspensions containing drug–cyclodextrin complexes are able not only to improve the dissolution of drug but also to promote the oral absorption through an anti-efflux effect by virtue of cyclodextrin and poloxamer. Our study would provide valuable information for oral chemotherapy of anticancer drugs using a combined formulation strategy.
ACKNOWLEDGMENTS
This work was partially supported by the China Postdoctoral Science Foundation (2014M562253). The authors should be thankful to Chenghua Li from Jinan University (Guangzhou, China) for assistance in DSC measurement.
Contributor Information
Baojian Wu, Phone: +86 20 85220482, Email: bj.wu@hotmail.com.
Zhihai Shi, Phone: +86 371 65714522, Email: szhvet@163.com.
REFERENCES
- 1.Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: basic approaches and practical applications. Int J Pharm. 2011;420(1):1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: challenges and opportunities. J Control Release. 2013;170(1):15–40. doi: 10.1016/j.jconrel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Moschwitzer JP. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453(1):142–56. doi: 10.1016/j.ijpharm.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Fatouros DG, Karpf DM, Nielsen FS, Mullertz A. Clinical studies with oral lipid based formulations of poorly soluble compounds. Ther Clin Risk Manag. 2007;3(4):591–604. [PMC free article] [PubMed] [Google Scholar]
- 5.Miller LA, Carrier RL, Ahmed I. Practical considerations in development of solid dosage forms that contain cyclodextrin. J Pharm Sci. 2007;96(7):1691–707. doi: 10.1002/jps.20831. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Wu D, Lai J, Lu Y, Yin Z, Wu W. Piroxicam/2-hydroxypropyl-beta-cyclodextrin inclusion complex prepared by a new fluid-bed coating technique. J Pharm Sci. 2009;98(2):665–75. doi: 10.1002/jps.21453. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Rong X, Laru J, van Veen B, Kiesvaara J, Hirvonen J, et al. Nanosuspensions of poorly soluble drugs: preparation and development by wet milling. Int J Pharm. 2011;411(1–2):215–22. doi: 10.1016/j.ijpharm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Tuomela A, Liu P, Puranen J, Ronkko S, Laaksonen T, Kalesnykas G, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467(1–2):34–41. doi: 10.1016/j.ijpharm.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Tian W, Chen SY. Recent advances in the molecular basis of anti-neoplastic mechanisms of oridonin. Chin J Integr Med. 2013;19(4):315–20. doi: 10.1007/s11655-013-1437-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Zhang P, Feng N, Zhang X, Wu S, Zhao J. Optimization and in situ intestinal absorption of self-microemulsifying drug delivery system of oridonin. Int J Pharm. 2009;365(1–2):136–42. doi: 10.1016/j.ijpharm.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Liu Y, Liu T, Zhao L, Zhao J, Feng N. Development and in-vivo assessment of the bioavailability of oridonin solid dispersions by the gas anti-solvent technique. Int J Pharm. 2011;411(1–2):172–7. doi: 10.1016/j.ijpharm.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Zhang D, Chen M, Zheng T, Wang S. Preparation and characterization of an oridonin nanosuspension for solubility and dissolution velocity enhancement. Drug Dev Ind Pharm. 2007;33(12):1332–9. doi: 10.1080/03639040701741810. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi T, Connors KA. Phase solubility techniques. Adv Anal Chem Instrum. 1965;4:117–22. [Google Scholar]
- 14.Liu H, Sun H, Lu D, Zhang Y, Zhang X, Ma Z, et al. Identification of glucuronidation and biliary excretion as the main mechanisms for gossypol clearance: in vivo and in vitro evidence. Xenobiotica. 2014;44(8):696–707. doi: 10.3109/00498254.2014.891780. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Zhang T, Zhou X, Liu H, Sun H, Ma Z, et al. Enhancement of oral bioavailability of tripterine through lipid nanospheres: preparation, characterization, and absorption evaluation. J Pharm Sci. 2014;103(6):1711–9. doi: 10.1002/jps.23967. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. 2003;304(3):1228–35. doi: 10.1124/jpet.102.046409. [DOI] [PubMed] [Google Scholar]
- 17.Tang L, Singh R, Liu Z, Hu M. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol Pharm. 2009;6(5):1466–82. doi: 10.1021/mp8002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahmani FZ, Yang H, Zhou J, Yao J, Zhang T, Zhang Q. Enhanced oral bioavailability of paclitaxel in pluronic/LHR mixed polymeric micelles: preparation, in vitro and in vivo evaluation. Eur J Pharm Sci. 2012;47(1):179–89. doi: 10.1016/j.ejps.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Aloisio C, Gomes de Oliveira A, Longhi M. Characterization, inclusion mode, phase-solubility and in vitro release studies of inclusion binary complexes with cyclodextrins and meglumine using sulfamerazine as model drug. Drug Dev Ind Pharm. 2014;40(7):919–28. doi: 10.3109/03639045.2013.790408. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Guo T, Qi J, Zhang J, Wu W. Enhanced dissolution and stability of lansoprazole by cyclodextrin inclusion complexation: preparation, characterization, and molecular modeling. AAPS PharmSciTech. 2012;13(4):1222–9. doi: 10.1208/s12249-012-9842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzaferro S, Bouchemal K, Ponchel G. Oral delivery of anticancer drugs III: formulation using drug delivery systems. Drug Discov Today. 2013;18(1–2):99–104. doi: 10.1016/j.drudis.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Tiwari G, Tiwari R, Rai AK. Cyclodextrins in delivery systems: applications. J Pharm Bioallied Sci. 2010;2(2):72–9. doi: 10.4103/0975-7406.67003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakeri-Milani P, Valizadeh H, Tajerzadeh H, Azarmi Y, Islambolchilar Z, Barzegar S, et al. Predicting human intestinal permeability using single-pass intestinal perfusion in rat. J Pharm Pharm Sci. 2007;10(3):368–79. [PubMed] [Google Scholar]
- 24.Asperen J, Tellingen O, Valk MA, Rozenhart M, Beijnen JH. Enhanced oral absorption and decreased elimination of paclitaxel in mice cotreated with cyclosporin A. Clin Cancer Res. 1998;4(10):2293–7. [PubMed] [Google Scholar]
- 25.Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270(29):17250–6. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 26.Yunomae K, Arima H, Hirayama F, Uekama K. Involvement of cholesterol in the inhibitory effect of dimethyl-beta-cyclodextrin on P-glycoprotein and MRP2 function in Caco-2 cells. FEBS Lett. 2003;536(1–3):225–31. doi: 10.1016/S0014-5793(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 27.Batrakova EV, Li S, Li Y, Alakhov VY, Kabanov AV. Effect of pluronic P85 on ATPase activity of drug efflux transporters. Pharm Res. 2004;21(12):2226–33. doi: 10.1007/s11095-004-7675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL. The solubility-permeability interplay in using cyclodextrins as pharmaceutical solubilizers: mechanistic modeling and application to progesterone. J Pharm Sci. 2010;99(6):2739–49. doi: 10.1002/jps.22033. [DOI] [PubMed] [Google Scholar]
- 29.Dahan A, Miller JM. The solubility-permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012;14(2):244–51. doi: 10.1208/s12248-012-9337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beig A, Miller JM, Dahan A. The interaction of nifedipine with selected cyclodextrins and the subsequent solubility-permeability trade-off. Eur J Pharm Biopharm. 2013;85(3 Pt B):1293–9. doi: 10.1016/j.ejpb.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Weng H, Huang H, Dong B, Zhao P, Zhou H, Qu L. Inhibition of miR-17 and miR-20a by oridonin triggers apoptosis and reverses chemoresistance by derepressing BIM-S. Cancer Res. 2014;74(16):4409–19. doi: 10.1158/0008-5472.CAN-13-1748. [DOI] [PubMed] [Google Scholar]
- 32.Salústio PJ, Pontes P, Conduto C, Sanches I, Carvalho C, Arrais J, et al. Advanced technologies for oral controlled release: cyclodextrins for oral controlled release. AAPS PharmSciTech. 2011;12(4):1276–92. doi: 10.1208/s12249-011-9690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


