Abstract
Background
The aim of this study was to investigate the effects of different statins on glucose uptake and to confirm its mechanism in primary cultured rat cardiomyocytes after administration of atorvastatin, pravastatin, and rosuvastatin.
Material/Methods
Primary cultured rat cardiomyocytes were randomly assigned to 5 groups: normal control group (OB), insulin group (S1), statin 1-μM (S2), 5-μM (S3), and 10-μM (S4) groups for 3 different statins. The 2-[3H]-DG uptake of each group was determined and the mRNA and protein expression levels of glucose transporter type 4 (GLUT4), insulin receptor substrate (IRs), and RhoA were assessed.
Results
After treatment with different concentrations of statins and insulin, the 2-[3H]-DG uptake showed a significant negative correlation with the concentration of atorvastatin (P<0.05), and no significant correlation with pravastatin and rosuvastatin. The mRNA and protein expression levels of GLUT4 and IRs-1 in primary cultured cardiomyocytes were both significantly reduced by atorvastatin treatment (P<0.05). Pravastatin and rosuvastatin showed no significant effects on GLUT4 and IRs-1 expression. The mRNA and protein expression levels of RhoA both showed no significant difference when treated with the 3 statins.
Conclusions
These results confirm that atorvastatin can inhibit insulin-induced glucose uptake in primary cultured rat cardiomyocytes by regulating the PI3K/Akt insulin signal transduction pathway.
MeSH Keywords: Glucose; Glucose Transporter Type 4; Hydroxymethylglutaryl-CoA Reductase Inhibitors; Insulin; Myocytes, Smooth Muscle
Background
Cardiovascular diseases (CVD) are the major cause of mortality in subjects with type 2 diabetes (T2D) [1,2]. Dyslipidemia is a key factor contributing to cardiovascular disease in T2D patients [3]. Previous clinical studies have shown that impairment of lipid metabolism significantly increases the risk of CVD events [3,4]. Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, can control both hypercholesterolemia and hypertriglyceridemia. The early statin trials were first reported in the 1990s, and several studies of the effects of statins showing the benefits on CVD have been published [5,6]. More recent evidence has demonstrated that statins are beneficial in reducing the risk of CVD events in people without prior evidence of CVD [7]. The effects of statins in primary and secondary prevention of CVD, especially in patients with T2D, is well established [8].
Statins can control serum glucose and lipids by regulating insulin-mediated glucose uptake. Previous studies have demonstrated that statins can reduce insulin-stimulated glucose uptake by inhibiting translocation of glucose transporter type 4 protein (GLUT4) to the membrane [9,10]. GLUT4 vesicles can translocate to the plasma membrane upon insulin stimulation via a multi-step process. In the normal status, GLUT4 is mainly located at intracellular vesicles, whereas relatively little GLUT4 resides at the plasma membrane. When stimulated by insulin, these vesicles can move to the plasma membrane, where they dock and fuse, thereby increasing the number of GLUT-4 molecules on the cell surface [11]. Insulin receptor substrate (IRs) proteins play critical roles in regulation of the insulin signaling pathway [12]. Many studies have focussed on the effect of statins on glucose uptake in adipocytes rather than myocytes [13]. However, evidence from a number of randomized clinical trials over the last 2 decades show a potential association between statin therapy and increased risk of development of diabetes [14], although this risk is low when compared with the benefits of reduction in coronary events.
In this study, the glucose uptake of cardiomyocytes was determined after administration of atorvastatin, pravastatin, and rosuvastatin to investigate the effects of various statins on the insulin-induced glucose signaling pathway and to confirm its mechanism in cardiomyocytes.
Material and Methods
Material
Neonatal Sprague-Dawley (SD) rats (age 24 h, male and female) were provided by the Experimental Animal Center of China Medical University. Atorvastatin, pravastatin, and rosuvastatin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The insulin purchased from the Sigma-Aldrich (St Louis, MO, USA). Rabbit anti-GLUT4, anti-pIRS-1, anti-RhoA, anti-β-actin, and goat anti-rabbit IgG antibodies were purchased from Sigma-Aldrich.
Cell culture
Primary cultured neonatal rat cardiomyocytes were prepared as previously described with minor modifications [15]. The heart was obtained from each neonatal SD rat with ophthalmic scissors under sterile conditions and cut into tissue blocks about l mm3. After complete digestion, the mixture was centrifuged at 1000 rpm for 8 min and the supernatant was discarded. The deposit was suspended in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum and cultured in a CO2 incubator for a differential plating period of 90 min to allow attachment of the non-myocardial cells. The cardiomyocytes were obtained from the deposit after centrifugation at 1000 rpm for 8 min and plated in 10-cm culture dishes (Falcon) at a density of 1×105 cells/ml. Following incubation for 24 h, the cultures were washed 2 times with phosphate- buffered saline (PBS) to remove the dead and non-adherent cells. The medium was changed every 3 days during incubation and the treatment factors were administrated on the fifth day.
2-[3H]-DG uptake determination
Primary cultured cardiomyocytes were randomly divided into 5 groups: normal control group, insulin group, and statin 1-μM, 5-μM, and 10-μM group, for each of the 3 different statins. The cardiomyocytes were pretreated with 0, 1, 5, and 10-μM of pravastatin, rosuvastatin, and atorvastatin for 72 h and then treated with 0.1-μM insulin for 30 min. Cells were washed twice with preheated PBS and the medium was then replaced by free-serum DMEM medium containing 2-[3H]-DG at a concentration of 1 μCi/ml. [U-14C] mannitol (0.1 μCi/ml) was also used as osmolality control [16]. The uptake of 2-[3H]-DG was determined after the cells were further incubated at 37°C and 5% CO2 in an incubator for 10 min. The medium was then replaced by pre-cooled PBS and the reaction was terminated by the addition of 10 mM phloretin. The cells were rapidly washed twice in pre-cooled PBS to remove phloretin and were dissolved in l% SDS/0.l M NaOH lysis buffer overnight at room temperature. The lysis solution (300 μl) was assayed for radioactivity by use of a liquid scintillation counter (Triathler 425-034, Hidex Oy) to determine the uptake of 2-[3H]-DG in each group [11, 17].
Protein extraction and western blotting
Primary cultured cardiomyocytes were randomly divided into 5 groups: normal control group, insulin group, statin 1-μM, 5-μM, and 10-μM group for each of the 3 different statins. The cardiomyocytes were pretreated with corresponding concentrations of statin for 72 h and then treated with 0.1-μM insulin for 30 min. The cells were collected after being washed 2 times with PBS. The membrane protein, cytoplasm protein, and total cell proteins were extracted using a cell fractionation kit (Biovision, California, USA) according to the method of previous studies [11,18–20]. The protein samples were then separated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) [21].
The expression levels of GLUT4, IRs-1, and RhoA were semi-quantified with Western blotting. The gel was transferred to a polyvinylidene fluoride (PVDF) membrane (Solvay Chemicals, Belgium) and blocked for 1 h. The rabbit anti-GLUT4, anti-IRs-1, and anti-RhoA antibody were used as primary antibody at a dilution of 1:1000. The secondary antibody was goat anti-rabbit IgG antibody at a dilution of 1:10 000. Signals were visualized with the ECL kit (Amersham International, Amersham, UK). Image J software (NIH, Bethesda, MD, USA) was used to compare the gray values between the proteins of interest and the internal control protein, as well as between the phosphorylated protein and the total protein.
RT-PCR analysis
The expression levels of GLUT4, IRs-1, and RhoA in each group were assessed by RT-PCR. Total RNA was extracted from cells using the Trizol method [22], after which cDNA was synthesized from the RNA by reverse transcription. Using specific primers, PCR amplification was performed to allow for fluorescence-based quantitation of the gene expression. PCR reaction volumes were 10 μl and composed of cDNA (1 μl), primers (0.2 μl each), 2× Premix Ex Taq (5 μl), and H2O (3.6 μL). The primer sequences used are listed in Table 1.
Table 1.
The primer sequences used in the RT-PCR.
| Gene | Upstream primer | Downstream primer |
|---|---|---|
| β-actin | CGTGCGTGACATTAAAGAG | TTGCCGATAGTGATGACCT |
| GLUT4 | CTTCATCATTGGCATGGGTTT | AGGACCGCAAATAGAAGGAAGA |
| IRs-1 | CACCCACTCCTATCCCG | CCCTACTCCGTTTGTCCAT |
| RhoA | CTGGTGATTGTTGGTGATGG | GCGATCATAATCTTCCTGCC |
Statistical analysis
All data were analyzed using SPSS 15.0 statistical software. A variance analysis and an independent t test were performed for means and data are expressed as mean ± standard deviation (χ±s). Differences with P<0.05 were considered statistically significant.
Results
The effects of statins on 2-[3H]-DG uptake
We detected the 2-[3H]-DG uptake status in primary cultured cardiomyocytes after being treated by different concentrations of statins and insulin (Figure 1). After being treated with insulin, the 2-[3H]-DG uptake in cardiomyocytes was increased significantly compared with the control cardiomyocytes. When treated with the 3 statins in 1, 5, and 10-μM, the 2-[3H]-DG uptake was decreased compared with the insulin group. For atorvastatin, the 2-[3H]-DG uptake decreased significantly with the increase in atorvastatin concentration, showing a significant negative correlation, and showing no significant correlation with pravastatin and rosuvastatin. In the 5- and 10-μM groups, the 2-[3H]-DG uptake in atorvastatin groups were significantly lower than that in pravastatin and rosuvastatin groups (P<0.05).
Figure 1.
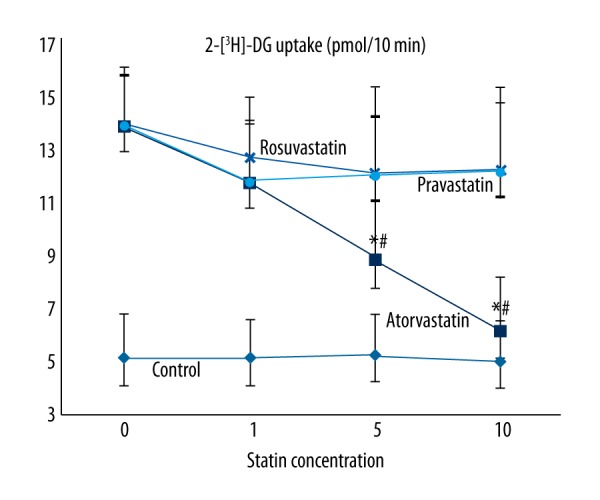
The 2-[3H]-DG uptake status in primary cultured cardiomyocytes after being treated by insulin and different statins. The 2-[3H]-DG uptake showed a significant negative correlation with the concentration of atorvastatin, but no significant correlation with pravastatin or rosuvastatin. Control – the primary cultured cardiomyocytes; Atorvastatin – the cardiomyocytes treated with insulin and atorvastatin; Pravastatin – the cardiomyocytes treated with insulin and pravastatin; Rosuvastatin – the cardiomyocytes treated with insulin and rosuvastatin. * P<0.05 compared with pravastatin and rosuvastatin groups; # P<0.05 compared with atorvastatin 1-μM group.
The expression of GLUT4 protein and mRNA
The expression of GLUT4 protein on the membrane was determined by Western blot and shown in Figure 2A. The protein expression level of GLUT4 was increased when treated by insulin (S1 group) compared with the control group (OB). The bands in Western blotting showed that the protein expression of GLUT4 was significantly reduced by atorvastatin compared with the S1 group and decreased significantly with the increase of atorvastatin concentration, showing a significant negative correlation. In pravastatin and rosuvastatin 1-, 5-, and 10-μM groups, no significant difference was observed between the groups compared with the insulin groups (Figure 2A). The mRNA expression level of GLUT4 in primary cultured cardiomyocytes treated with insulin and atorvastatin was consistent with the expression of GLUT4 protein. The GLUT4 mRNA expression in atorvastatin 1-, 5-, and 10-μM groups was all significantly lower than in the insulin group (P<0.05), and the GLUT4 mRNA expression in the atorvastatin 10-μM group was significantly lower than in the 1-μM group (P<0.05) (Figure 2B). Pravastatin and rosuvastatin showed no significant effects on the GLUT4 mRNA expression (data not shown).
Figure 2.
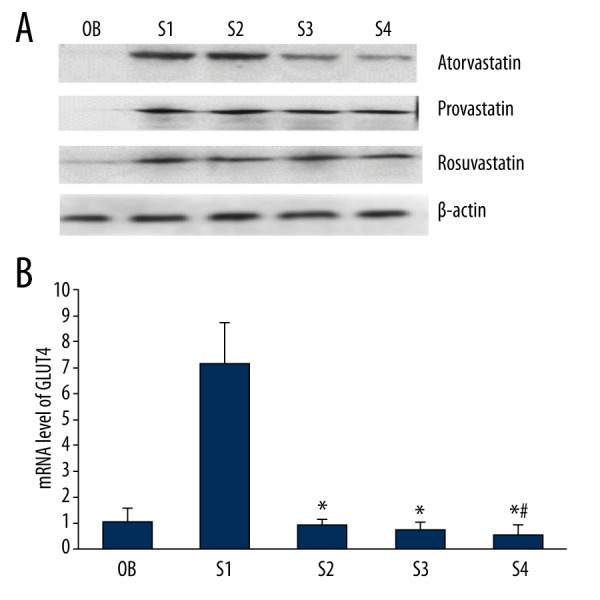
The protein and mRNA expression levels of GLUT4 in primary cultured cardiomyocytes. The protein and mRNA expression level of GLUT4 was significantly reduced by atorvastatin and showed a negative correlation with the concentration. No significant difference was observed in pravastatin and rosuvastatin groups. OB – the cardiomyocytes without treatment; S1 – the cardiomyocytes treated with insulin; S2 – the cardiomyocytes treated with insulin and 1-μM statins; S3 – statins the cardiomyocytes treated with insulin and 5-μM statins; S4 – the cardiomyocytes treated with insulin and 1-μM statins. * P<0.05 compared with S1 group; # P<0.05 compared with S2 group.
The expression of IRs-1 protein and mRNA
The mRNA and protein expression levels of IRs-1 in primary cultured cardiomyocytes were both significantly reduced by atorvastatin treatment, which was the same with the expression of GLUT4. Pravastatin and rosuvastatin showed no significant effects on the IRs-1 expression (Figure 3).
Figure 3.
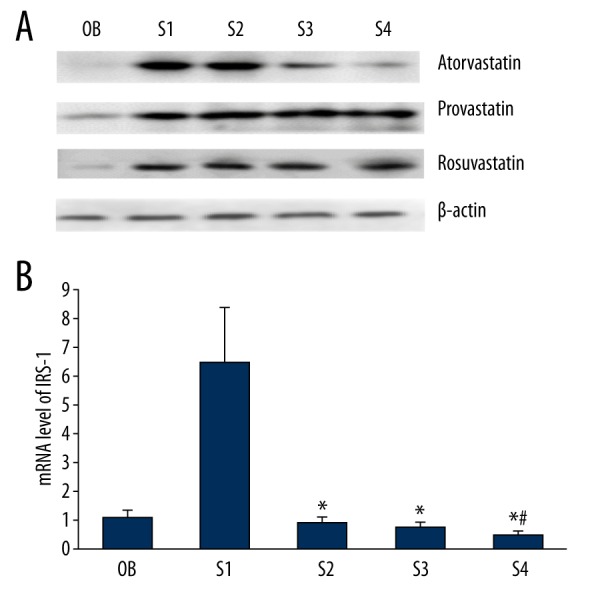
The protein and mRNA expression levels of IRs-1 in primary cultured cardiomyocytes. The protein and mRNA expression level of IRs-1 was significantly reduced by atorvastatin and showed a negative correlation with the concentration. No significant difference was observed in pravastatin and rosuvastatin groups. OB – the cardiomyocytes without treatment; S1 – the cardiomyocytes treated with insulin; S2 – the cardiomyocytes treated with insulin and 1-μM statins; S3 – statins the cardiomyocytes treated with insulin and 5-μM statins; S4 – the cardiomyocytes treated with insulin and 1-μM statins. * P<0.05 compared with S1 group; # P<0.05 compared with S2 group.
The expression of RhoA protein and mRNA
The mRNA and protein expression levels of RhoA both showed no significant difference when treated with the 3 different statins (Figure 4).
Figure 4.
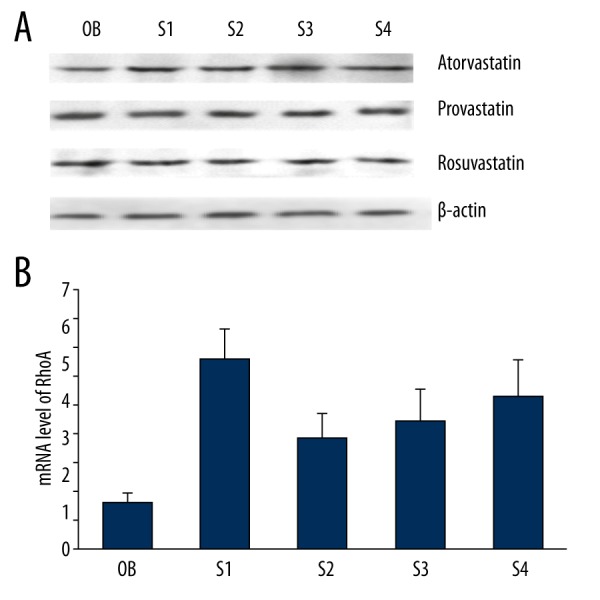
The protein and mRNA expression levels of RhoA in primary cultured cardiomyocytes. The mRNA and protein expression levels of RhoA both showed no significant difference when treated with the 3 different statins. OB – the cardiomyocytes without treatment; S1 – the cardiomyocytes treated with insulin; S2 – the cardiomyocytes treated with insulin and 1-μM statins; S3 – statins the cardiomyocytes treated with insulin and 5-μM statins; S4 – the cardiomyocytes treated with insulin and 1-μM statins.
Discussion
In our study, the 2-[3H]-DG uptake in primary cultured cardiomyocytes was inhibited by atorvastatin and showed a significant negative correlation with the concentration of atorvastatin. Statins, as the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor in the cholesterol synthesis process of hepatic cells, show different permeability to cells depending on water solubility [23]. It has been reported that lipophilic statins can more easily penetrate extrahepatic cell membranes, such as β cells, adipocytes, and skeletal muscles, while hydrophilic statins are more hepatocyte-specific and are less likely to enter β cells or adipocytes [24–26]. Because the membrane of extrahepatic cells consists of lipid bilayers, hydrophilic statins cannot penetrate it, and thus cannot reach the intracellular enzyme; however, the hepatic cell membrane contains organic anion transporters, which take hydrophilic substances into the cell [23]. Therefore, lipophilic atorvastatin can enter the cardiomyocytes and inhibit the insulin-mediated glucose uptake more easily than hydrophilic pravastatin and rosuvastatin.
To investigate the mechanism by which atorvastatin inhibits glucose uptake, we detected the expression of GLUT4, IRs-1, and RhoA. Glucose mainly depends on the glucose transporters (GLUTs) to enter into cardiomyocytes. In cardiomyocytes, the 2 most highly expressed glucose transporters are GLUT1 and GLUT4, and GLUT4 is the most abundant. GLUT1 mediates basal glucose transport, whereas GLUT4 is mainly responsible for insulin- or contraction-mediated glucose transport. In quiescent myocytes, the majority of GLUT4 protein resides in a specialized vesicle population in an intracellular compartment. Upon insulin stimulation, GLUT4 vesicles are translocated to the PM via a multiple-step process by which GLUT4 storage vesicles (GSVs) move to the PM, tether, dock, and ultimately fuse with the PM to expose GLUT4 proteins on the cell surface [9]. IRs-1 is the insulin receptor substrate protein and mainly locates in the hepatic and adipose tissue, which is sensitive to insulin [27]. IRs-1 can associate with insulin receptor (IR) as the key intermediate in the insulin phosphatidylinositol 3-kinase/ protein kinase B (PI3K/Akt) signaling pathway [9]. The blocking of phosphorylation of IRs-1 can reduce IRs-1–associated PI3K and Akt activity and ultimately decrease insulin-stimulated glucose transport activity [28]. In our study, the protein level of IRs-1 on the PM and the translocation of GLUT4 to the membrane in cardiomyocytes was reduced by atorvastatin. It is confirmed that atorvastatin can inhibit the uptake of cardiomyocytes by blocking the PI3K/Akt insulin signaling pathway. RhoA, a member of Rho family of small GTPases, is a monomeric G protein that regulates a number of cell functions, including cytoskeletal reorganization, cell motility, and gene expression [29]. There have been several reports of RhoA in the diabetic mesangial cells regulating glucose metabolism [30,31], but no effect was observed in cardiomyocytes after treatment with atorvastatin in our study.
Conclusions
In primary cultured cardiomyocytes, lipophilic atorvastatin can inhibit insulin-mediated glucose uptake by blocking the PI3K/Akt insulin signaling pathway, whereas hydrophilic pravastatin and rosuvastatin showed no effects on glucose uptake.
Footnotes
Source of support: Departmental sources
References
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular disease: The Framingham study. JAMA. 1979;241:2035–38. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 2.Turner R, Millns H, Neil H, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–28. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–8. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 4.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 5.LaRosa JC, Applegate W, Crouse JR, et al. Cholesterol lowering in the elderly: results of the Cholesterol Reduction in Seniors Program (CRISP) pilot study. Arch Intern Med. 1994;154:529–39. doi: 10.1001/archinte.154.5.529. [DOI] [PubMed] [Google Scholar]
- 6.Ward S, Jones ML, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11(14):1–160. iii–iv. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 7.de Lemos J, Braunwald E, Blazing M, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta – analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cholesterol Treatment Trialists C. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, Pereira RO, O’Neill BT, et al. Cardiac PI3K-Akt impairs insulin-stimulated glucose uptake independent of mTORC1 and GLUT4 translocation. Mol Endocrinol. 2012;27:172–84. doi: 10.1210/me.2012-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brault M, Ray J, Gomez Y-H, Mantzoros CS, Daskalopoulou SS. Statin treatment and new-onset diabetes: A review of proposed mechanisms. Metabolism. 2014;63:735–45. doi: 10.1016/j.metabol.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Yu B, Poirier LA, Nagy LE. Mobilization of GLUT-4 from intracellular vesicles by insulin and K+ depolarization in cultured H9c2 myotubes. Am J Physiol. 1999;277:E259–67. doi: 10.1152/ajpendo.1999.277.2.E259. [DOI] [PubMed] [Google Scholar]
- 12.Czech MP. Dynamics of phosphoinositides in membrane retrieval and insertion. Ann Rev Physiol. 2003;65:791–815. doi: 10.1146/annurev.physiol.65.092101.142522. [DOI] [PubMed] [Google Scholar]
- 13.Nakata M, Nagasaka S, Kusaka I, et al. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): Implications in glycaemic control. Diabetologia. 2006;49:1881–92. doi: 10.1007/s00125-006-0269-5. [DOI] [PubMed] [Google Scholar]
- 14.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Henderson S, Reynolds R, et al. Alpha 1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells. Effects on myosin light chain-2 gene expression. J Biol Chem. 1988;263:7352–58. [PubMed] [Google Scholar]
- 16.Younce CW, Burmeister MA, Ayala JE. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. Am J Physiol Cell Physiol. 2013;304:C508–18. doi: 10.1152/ajpcell.00248.2012. [DOI] [PubMed] [Google Scholar]
- 17.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–29. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman GA, Bonzelius F, Cieutat A-M, Kelly RB. A distinct class of intracellular storage vesicles, identified by expression of the glucose transporter GLUT4. Proc Natl Acad Sci. 1994;91:12750–54. doi: 10.1073/pnas.91.26.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruewer M, Hopkins AM, Hobert ME, et al. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol. 2004;287:C327–35. doi: 10.1152/ajpcell.00087.2004. [DOI] [PubMed] [Google Scholar]
- 20.Guo R, Li W, Liu B, et al. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Med Sci Monit Basic Res. 2014;20:82–92. doi: 10.12659/MSMBR.890858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamiya T, Kobayashi Y, Kanaoka K, et al. Fluorescence microscopic demonstration of cathepsin K activity as the major lysosomal cysteine proteinase in osteoclasts. J bBiochem. 1998;123:752–59. doi: 10.1093/oxfordjournals.jbchem.a022001. [DOI] [PubMed] [Google Scholar]
- 22.Simms D, Cizdziel PE, Chomczynski P. TRIzol: A new reagent for optimal single-step isolation of RNA. Focus. 1993;15:532–35. [Google Scholar]
- 23.Ichihara K, Satoh K. Disparity between angiographic regression and clinical event rates with hydrophobic statins. Lancet. 2002;359:2195–98. doi: 10.1016/S0140-6736(02)09098-0. [DOI] [PubMed] [Google Scholar]
- 24.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–25. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Chitose T, Sugiyama S, Sakamoto K, et al. Effect of a hydrophilic and a hydrophobic statin on cardiac salvage after ST-elevated acute myocardial infarction – A pilot study. Atherosclerosis. 2014;237:251–58. doi: 10.1016/j.atherosclerosis.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 26.Cho K-I, Cha T-J, Lee S-J, et al. attenuation of acetylcholine activated potassium current (IKACh) by simvastatin, not pravastatin in mouse atrial cardiomyocyte: Possible atrial fibrillation preventing effects of statin. PLoS One. 2014;9:e106570. doi: 10.1371/journal.pone.0106570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–93. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–36. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 29.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 30.Kolavennu V, Zeng L, Peng H, et al. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57:714–23. doi: 10.2337/db07-1241. [DOI] [PubMed] [Google Scholar]
- 31.Peng F, Wu D, Gao B, et al. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 2008;57:1683–92. doi: 10.2337/db07-1149. [DOI] [PubMed] [Google Scholar]


