Abstract
Objective:
To evaluate the efficacy of intraoperative three-dimensional (3D) Iso-C C-arm-navigated percutaneous radiofrequency ablation (RFA) of osteoid osteomas.
Methods:
35 patients (20 males and 15 females) with osteoid osteomas underwent treatment with intraoperative 3D Iso-C C-arm navigation-guided RFA. The tumour was first biopsied for pathological examination, the core needle was removed and the RFA needle was inserted into the nidus. Post-operative X-rays and CT scans were performed to evaluate the degree of ablation and to assess for recurrence at 3-month follow-up. Patients also completed a visual analogue scale (VAS) both pre-operatively and 3 days post-operatively to subjectively assess pain.
Results:
Pathological diagnosis confirmed osteoid osteoma in 19 cases. The other 16 cases were not pathologically diagnosed owing to inadequate biopsy specimens. In all cases, localized pain was immediately relieved following RFA. Patients reported significantly decreased pain, with mean pre-operative VAS scores of 3.4 reducing to 0.80 at 3 days post-operatively and further to 0.06 at 3-month follow-up (p < 0.05). The mean follow-up time was 15.5 months (range: 3–38 months).
Conclusion:
3D Iso-C C-arm navigation-guided RFA is a safe and effective option for the treatment of osteoid osteomas and may be considered in place of intraoperative CT-guided and open resection.
Advances in knowledge:
C-arm image-guided percutaneous RFA mitigates the need for pre-operative CT as well as intraoperative scintigraphy, provides real-time imaging of the anatomy, facilitates accurate resection of the tumour and enables immediate confirmation of excision.
INTRODUCTION
Osteoid osteoma is a small, benign osteoblastic bone tumour most commonly found in long bones including the femur or tibia.1 Osteoid osteomas account for roughly 10% of all benign bone tumours and characteristically present with dull aching pain that may be relieved with non-steroidal anti-inflammatory drugs (NSAIDs).2 They typically present in young individuals, most commonly between 7 and 25 years of age, with a male-to-female ratio of 3 : 1.2 Traditionally, treatment involves open surgical resection of the tumour with complete intralesional excision or destruction of the nidus.3–6 However, surgical resection of osteoid osteomas without accurate localization of the nidus can often result in overexcision of bone, which may lead to instability and/or pathological fractures. In addition, inaccurate localization of the lesion may result in recurrence and/or secondary surgery.7–9 In order to reduce the risk of recurrence, it is often necessary to unroof and directly identify the nidus, as direct visualization facilitates complete removal.
Percutaneous radiofrequency ablation (RFA) is a minimally invasive treatment that has shown good efficacy in the management of osteoid osteomas and can be performed using CT guidance.10,11 Intraoperative three-dimensional (3D) Iso-C C-arm navigation has proved to be a reliable tool for localizing lesions and has been previously used in the treatment of osteoid osteomas but has yet to be proposed for use with RFA.12–15 We present a series of 35 cases of osteoid osteomas treated at Beijing Jishuitan Hospital of Peking University with intraoperative 3D Iso-C C-arm navigation-guided RFA and assess the clinical efficacy of this procedure.
METHODS AND MATERIALS
Patient selection
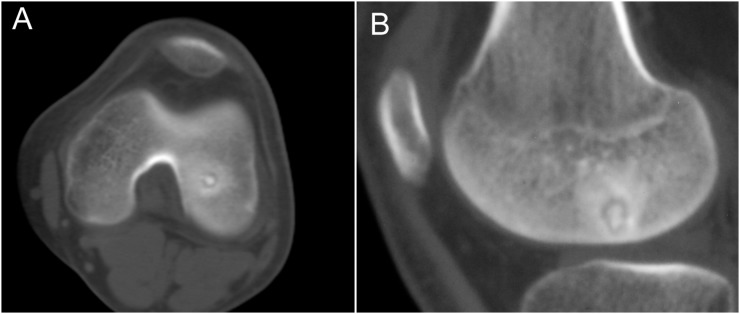
35 patients with osteoid osteomas underwent treatment with intraoperative 3D Iso-C C-arm navigation-guided RFA between June 2011 and March 2014 in the Department of Orthopedic Oncology of Beijing Jishuitan Hospital, Peking University, Beijing, China. All lesions were pre-operatively diagnosed radiologically using X-ray, CT and MRI (Figure 1). All activities pertaining to this study and its data collection were approved by the Institutional Review Board and Ethics Committee of our institution.
Figure 1.
Pre-operative CT images of osteoid osteoma in the epiphysis of the lower end of the left femur in a 16-year-old male. (a) Axial plane view; (b) sagittal view.
C-arm-guided navigation
After patients were administered epidural anaesthetics, a minimally invasive reference array was attached to the involved bone and registered with the 3D intraoperative Iso-C C-arm navigation using the Spine Navigation 1.2 system (Stryker; Kalamazoo, MI). The motorized fluoroscope rotated continuously through a 190° arc and obtained 100 fluoroscopic images of the involved bone. The acquired images were transferred to the Spine Navigation 1.2 system, and real-time intraoperative multiplanar images of the affected bone and the nidus were reconstructed for surgical navigation.
Surgical procedure
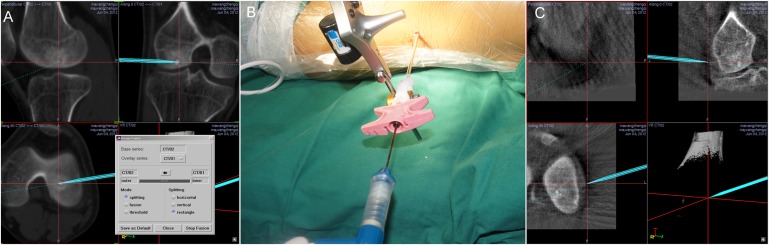
The planning feature of the navigation software was used to determine a percutaneous route that avoided neurovascular structures. The tumour was first biopsied for pathological examination, the core needle was removed and the RFA needle was inserted into the nidus. RFA was performed at 90 °C for two sessions of 3 min with an interval of 3 min between sessions. For tumours <5 mm, additional localization using a navigation instrument was performed in order to ensure correct placement of the RFA needle (Figure 2).
Figure 2.
(a) A core needle was introduced into the nidus with computer navigation. (b) The core needle was removed and a radiofrequency ablation needle was inserted. (c) Ordinary C-arm scanning images can also show the nidus, but the resolution is much less than that of a CT scan. After fusion with pre-operative CT, the nidus is much more easily identifiable.
Post-operative evaluation
Post-operative X-rays and CT scans were performed to evaluate the degree of ablation and to assess for recurrence at 3-month follow-up. Patients also completed a visual analogue scale (VAS) both pre-operatively and 3 days post-operatively to subjectively assess pain.
Statistical analysis
Statistical analysis was performed using SPSS® v. 21.0 software (IBM Corporation; Armonk, NY; formerly SPSS Inc., Chicago, IL). A paired t-test was used to compare the VAS data. p-values of <0.05 were considered statistically significant.
RESULTS
The evaluated patient population included 20 males and 15 females with a mean age of 17 years (range: 7–38 years). Lesions were located on the femur in 25 cases, on the tibia in 7 cases, on the calcaneus in 1 case, on the acetabulum in 1 case and on the humerus in 1 case (Table 1). All patients presented with localized pain for a mean duration of 9 months (range: 2–36 months).
Table 1.
Lesions characteristics
| Lesion location | Number of patients | Mean age (years) | Outcomes | Complications | Mean time until resolution (months) |
|---|---|---|---|---|---|
| Femur | 25 | 18.6 | Successful RFA | None observed | 16 |
| Tibia | 7 | 13.4 | Successful RFA | None observed | 12 |
| Calcaneus | 1 | 8 | Successful RFA | None observed | 34 |
| Acetabulum | 1 | 10 | Successful RFA | None observed | 12 |
| Humerus | 1 | 19 | Successful RFA | None observed | 11 |
RFA, radiofrequency ablation.
C-arm-guided RFA was successfully performed on all 35 patients without any intraoperative or post-operative complications observed. All patients were able to ambulate on post-operative Day 2, and the mean follow-up time was 15.5 months (range: 3–38 months).
Localized pain was significantly reduced in all cases post-operatively. At 3-month follow-up, only two patients reported mild pain. Both of these patients experienced tenderness with palpation at 2-month follow-up, which was absent at 6-month follow-up, and thus considered this pain to be ablation related. The VAS results decreased significantly from a mean of 3.4 pre-operatively to 0.8 at 3 days post-operatively and 0.06 at 3-month follow-up (p < 0.05).
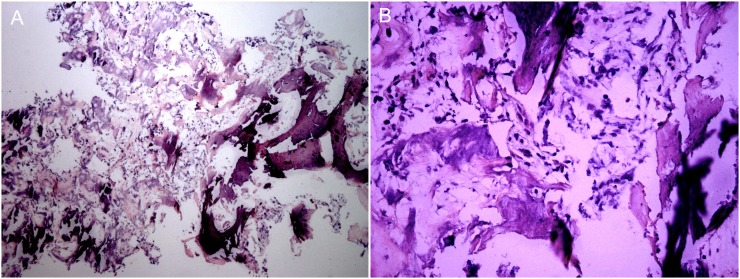
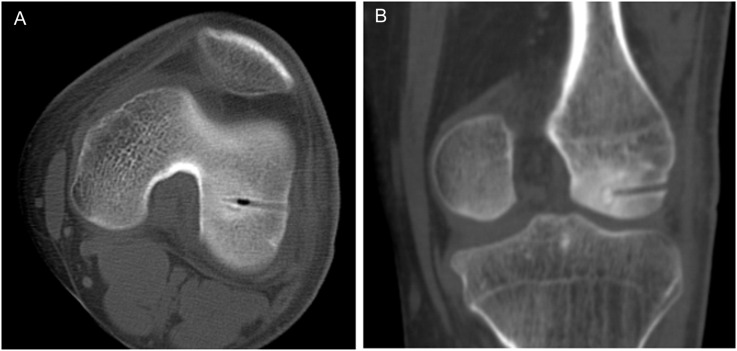
Pathological diagnosis of osteoid osteoma was confirmed only in 19 cases as there was inadequate biopsy material in the remaining 16 cases (Figure 3). CT scans showed significant resolution of the tumour as early as post-operative Day 3 (Figure 4), though in some cases, tumour was visualized on CT up to 24 months post-operatively. Although all the treated lesions did eventually resolve, some cases took over 6 months to completely resolve.
Figure 3.
Haematoxylin–eosin staining of the bone biopsy specimen. (a) ×100; (b) ×400.
Figure 4.
CT scan of the nidus at post-operative Day 3 (a, axial view) and 1 year (b, sagittal view).
DISCUSSION
Traditional treatment of osteoid osteoma involves open excision with complete intralesional excision or destruction of the nidus.3–6 These methods have proven less useful when there is inaccurate localization of the nidus and, in such cases, can result in instability and pathological fractures from overexcision of bone. Several minimally invasive techniques for the management of osteoid osteoma have been reported including percutaneous core drilling, RFA, laser photocoagulation, intraoperative scintigraphy and, recently, CT-based navigation.6,12,16–18 In addition to these techniques, percutaneous RFA provides another effective and accurate minimally invasive option.10,11
Percutaneous RFA allows for accurate targeting of heat to the intended area under real-time image guidance. In practice, high-frequency alternating current is transmitted through the skin, which induces tissue damage, resulting in coagulative necrosis.9,19 Additionally, this technique does not require hospitalization, has good recovery times and has a very low complication rate.20 A limitation of RFA, however, is its use of thermal ablation—the amount of alternating current applied to the tumour must be very strictly regulated, as to avoid damaging surrounding normal tissue.21
Osteoid osteomas are characterized by having a central circular nidus of varying size, with varying amounts of sclerosis present—in some cases, sclerosis may be completely absent, whereas in others, it may be present in considerable amounts. CT scans are the golden standard for visualizing the nidus and for guiding treatments; however, their usage bears drawbacks including radiation exposure.
C-arm-guided percutaneous RFA mitigates the need for pre-operative CT and relatively high doses of radiation.22 Using C-arm-guided RFA, we are able to overcome these drawbacks and can therefore offer patients an alternative treatment that is both safe and effective. Moreover, intraoperative scintigraphy can also be used to localize the nidus; however, the tumour will not be clearly visible. Therefore, wide surgical resection of the affected bony structure is needed to ensure complete removal of the tumour.23,24 C-arm based navigation has the advantages of real-time imaging of the anatomy, accurate resection of the tumour and convenient confirmation of the procedure results. In comparison with CT-guided navigation, C-arm navigation has no need for pre-operative CT scanning.
In our study, C-arm-guided RFA was successfully performed on all 35 patients without any observed perioperative complications. In comparison, perioperative complications were reported in a previous CT-guided study.2 Localized pain felt by patients was greatly reduced in all cases post-operatively, and only two patients reported mild pain at 3-month follow-up. Since osteoid osteomas are characteristic for their painful progression, the reduction of pain is significant. Furthermore, subjectively evaluated pain scores significantly decreased from a mean of 3.4 pre-operatively to 0.8 at 3 days and further decreased to 0.06 at 3-month follow-up (p < 0.05). The intraoperative images were similar to conventional CT scan images, and in all cases, excellent 3D visualizations of the tumour were achieved from the Iso-C C-arm.
Despite the benefits of this procedure, our patients had a low rate of pathological diagnosis—only 54% of patients were pathologically diagnosed (19 of 35). The remaining 16 patients had inadequate biopsy specimens insufficient for pathological diagnosis. The importance of histological diagnosis has been shown in previous studies. In a series of 38 patients with osteoid osteomas diagnosed clinically and radiologically, 6 cases were found to have different histological diagnoses.8,9,25 Additionally, some tumours treated herein took up to 24 months to fully resolve, highlighting a potentially long time for full resolution. Thus, in cases where pre-operative diagnosis is not completely certain, a more conventional technique that provides adequate pathology samples should be selected. In cases of osteoid osteoma with severe pain, surgeons may consider a treatment with a quicker period for full resolution. On the contrary, as osteoid osteoma is a benign bone tumour, metastatic disease is not a consideration.
CONCLUSION
3D Iso-C C-arm navigation-guided RFA is a safe and effective option for the treatment of osteoid osteomas and may be considered in place of intraoperative CT-guided and open resection. Despite the demonstrated potential of this technique, additional clinical studies with more patients are necessary to achieve a more complete understanding of complication rates and resolution time.
Contributor Information
Feng Yu, Email: fengyu1261@126.com.
Xiao-hui Niu, Email: xiaohuiniu1@126.com.
Qing Zhang, Email: QingZhang126@126.com.
Hai-tao Zhao, Email: HaitaoZhao1@126.com.
Li-hui Xu, Email: LihuiXu1@126.com.
Zhi-ping Deng, Email: zhipingdeng1@126.com.
REFERENCES
- 1.de Palma L, Candelari R, Antico E, Politano R, Luniew E, Giordanengo M, et al. Treatment of osteoid osteoma with CT-guided percutaneous radiofrequency thermoablation. Orthopedics 2013; 36: E581–7. doi: 10.3928/01477447-20130426-19 [DOI] [PubMed] [Google Scholar]
- 2.Jankharia B, Burute N. Percutaneous radiofrequency ablation for osteoid osteoma: how we do it. Indian J Radiol Imaging 2009; 19: 36. doi: 10.4103/0971-3026.44523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozaki T, Liljenqvist U, Hillmann A, Halm H, Lindner N, Gosheger G, et al. Osteoid osteoma and osteoblastoma of the spine: experiences with 22 patients. Clin Orthop Relat Res 2002; 397: 394–402. doi: 10.1097/00003086-200204000-00046 [DOI] [PubMed] [Google Scholar]
- 4.Frassica FJ, Waltrip RL, Sponseller PD, Ma LD, McCarthy EF, Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am 1996; 27: 559–74. [PubMed] [Google Scholar]
- 5.Donkol RH, Al-Nammi A, Moghazi K. Efficacy of percutaneous radiofrequency ablation of osteoid osteoma in children. Pediatr Radiol 2008; 38: 180–5. doi: 10.1007/s00247-007-0690-z [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology 2003; 229: 171–5. doi: 10.1148/radiol.2291021053 [DOI] [PubMed] [Google Scholar]
- 7.Ghanem I. The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr 2006; 18: 36–41. doi: 10.1097/01.mop.0000193277.47119.15 [DOI] [PubMed] [Google Scholar]
- 8.Hadjipavlou AG, Lander PH, Marchesi D, Katonis PG, Gaitanis IN. Minimally invasive surgery for ablation of osteoid osteoma of the spine. Spine (Phila Pa 1976) 2005; 5: S72–3. doi: 10.1097/01.BRS.0000092386.96824.DB [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am 1998; 80: 815–21. [DOI] [PubMed] [Google Scholar]
- 10.Cioni R, Armillotta N, Bargellini I, Zampa V, Cappelli C, Vagli P, et al. CT-guided radiofrequency ablation of osteoid osteoma: long-term results. Eur Radiol 2004; 14: 1203–8. doi: 10.1007/s00330-004-2276-6 [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology 1992; 183: 29–33. doi: 10.1148/radiology.183.1.1549690 [DOI] [PubMed] [Google Scholar]
- 12.Kendoff D, Hufner T, Citak M, Geerling J, Mössinger E, Bastian L, et al. Navigated Iso-C 3D-based percutaneous osteoid osteoma resection: a preliminary clinical report. Comput Aided Surg 2005; 10: 157–63. doi: 10.3109/10929080500229868 [DOI] [PubMed] [Google Scholar]
- 13.Rajasekaran S, Kamath V, Shetty AP. Intraoperative Iso-C three-dimensional navigation in excision of spinal osteoid osteomas. Spine (Phila Pa 1976) 2008; 33: E25–9. doi: 10.1097/BRS.0b013e31815e6308 [DOI] [PubMed] [Google Scholar]
- 14.Rajasekaran S, Karthik K, Chandra VR, Rajkumar N, Dheenadhayalan J. Role of intraoperative 3D C-arm-based navigation in percutaneous excision of osteoid osteoma of long bones in children. J Pediatr Orthop B 2010; 19: 195–200. doi: 10.1097/BPB.0b013e328333997a [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Zhang Q, Niu XH, Yu F, Li Y, Zhao HT, et al. Computer navigation-guided excision of osteoid osteomas. [In Chinese.] Zhonghua Wai Ke Za Zhi 2011; 49: 808–11. [PubMed] [Google Scholar]
- 16.Gangi A, Alizadeh H, Wong L, Buy X, Dietemann JL, Roy C. Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology 2007; 242: 293–301. doi: 10.1148/radiol.2421041404 [DOI] [PubMed] [Google Scholar]
- 17.Osebold WR, Lester EL, Hurley JH, Vincent RL. Intraoperative use of the mobile gamma camera in localizing and excising osteoid osteomas of the spine. Spine (Phila Pa 1976) 1993; 18: 1816–28. doi: 10.1097/00007632-199310000-00018 [DOI] [PubMed] [Google Scholar]
- 18.Van Royen BJ, Baayen JC, Pijpers R, Noske DP, Schakenraad D, Wuisman PI. Osteoid osteoma of the spine: a novel technique using combined computer-assisted and gamma probe-guided high-speed intralesional drill excision. Spine (Phila Pa 1976) 2005; 30: 369–73. doi: 10.1097/01.brs.0000152531.49095.34 [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Choi SH, Park HS, Lee MW, Han CJ, Choi JI, et al. Radiofrequency thermal ablation in canine femur: evaluation of coagulation necrosis reproducibility and MRI-histopathologic correlation. AJR Am J Roentgenol 2005; 185: 661–7. doi: 10.2214/ajr.185.3.01850661 [DOI] [PubMed] [Google Scholar]
- 20.Singh C, Shankar A. Osteoid osteoma of L4 pedical: a novel technique of intralesional excision without creating instability. Int J Spine Surg 2007; 3: 2. [Google Scholar]
- 21.Künzli BM, Abitabile P, Maurer CA. Radiofrequency ablation of liver tumors: actual limitations and potential solutions in the future. World J Hepatol 2011; 3: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehani MM, Berry M. Radiation doses in computed tomography. The increasing doses of radiation need to be controlled. BMJ 2000; 320: 593–4. doi: 10.1136/bmj.320.7235.593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinski LA, Goris M, Bleck EE, Halpern A, Hirshman P. Intraoperative skeletal scintigraphy for localization of osteoid osteoma in the spine. Case report. J Bone Joint Surg Am 1980; 62: 143–4. [PubMed] [Google Scholar]
- 24.Wioland M, Sergent-Alaoui A. Didactic review of 175 radionuclide-guided excisions of osteoid osteomas. Eur J Nucl Med 1996; 23: 1003–11. doi: 10.1007/BF01084380 [DOI] [PubMed] [Google Scholar]
- 25.Moberg E. The natural course of osteoid osteoma. J Bone Joint Surg Am 1951; 33: 166–70. [PubMed] [Google Scholar]