Abstract
Objective:
The current literature has described the usefulness of elastography and diffusion-weighted MRI in patients with cancer, but to the best of our knowledge so far none of them has compared the two new methods. The tumour cell density is related to the MRI-measured apparent diffusion-weighted coefficient (ADC). The purpose of the present study was to compare quantitative elastography based on ultrasound shear wave measurements with MRI ADC.
Methods:
We prospectively examined 52 patients with histopathologically proven rectal cancer. The mean age was 67 years (range 42–90 years). Males: 39, females: 13. Tumour elasticity was measured transgluteally using the acoustic radiation force impulse (ARFI) to generate information on the mechanical properties of the tissue. The objective quantitative elastography shear wave velocity was blindly compared with the ADC measurements using a 1.5-T MRI system.
Results:
The mean tumour elasticity was 3.05 m s−1 [standard deviation (SD): 0.79], and the mean ADC was 0.69 × 10−3 mm2 s−1 (SD: 0.27). Elasticity was inversely strongly correlated with ADC, r = −0.65 (Salkin scale). ARFI = 4.392 − 1.949 × ADC, R2 = 0.43, p < 0.0001. Intercept = 4.392 (95% CI: 3.92 to 4.86), slope = −1.949 (95% CI: −1.31 to −2.59), p < 0.0001.
Conclusion:
Elasticity correlates with the estimated diffusion restriction by MRI ADC measurements in rectal tumours. The relationship between ARFI and ADC measurement was linear in our study population.
Advances in knowledge:
This work describes a correlation between tissue elasticity and diffusion in rectal cancer.
INTRODUCTION
MRI is a standard examination before treatment of rectal cancer1 and part of the staging prior to treatment planning at the multidisciplinary team conference. Diffusion-weighted imaging (DWI) is capable of non-invasively measuring water diffusivity in living tissues.2
Diffusion-weighted MRI has proven useful for the assessment of tumour response to neoadjuvant radiochemotherapy.3 Tumour cell density is related to the MRI-measured apparent diffusion-weighted coefficient (ADC); the higher the cell density, the lower the ADC value.4 Most often there is an increase in the ADC value after pre-operative treatment, indicative of the response.5
Ultrasound is widely used in the staging of early rectal cancer,6,7 and today, the combination of endorectal ultrasound (ERUS) and elastography further improves early rectal cancer staging. This is a semi-quantification of the tumour hardness using relative strain ratios; however, it seems that elastography using strain imaging may improve the staging of adenomas and early rectal cancer compared with ERUS alone.8 The advent of acoustic radiation force impulse (ARFI) imaging has enabled objective measurement of tumour elasticity. ARFI uses a short pulse <1 ms, producing shear waves in the target tissue. The shear waves are detected by the system, and the velocity is measured in m s−1. Slow shear waves indicate soft tissue and fast waves tissue with high stiffness.9 Recently, an ultrasound study10 showed that tumour tissue becomes softer after radiochemotherapy, when there is a detectable response.
Not all patients can be examined by MRI owing to contraindications such as claustrophobia, pacemaker and metal foreign bodies.11,12 These patients can only be staged by ERUS with or without elastography. The relationship between tumour cell density and tissue elasticity is not well established, and to the best of our knowledge, there are no studies comparing MRI diffusion and elastography in rectal cancer.
The main purpose of the present study was to compare quantitative elastography based on ultrasound shear wave measurements with diffusion-weighted MRI ADC values.
METHODS AND MATERIALS
Patients
The study was approved by the national data protection agency (ID: 2010-41-4392) and the Regional Scientific Ethical Committee for Southern Denmark (ID: S-20100028) and conducted in accordance with the Helsinki II Declaration. All participating patients signed the informed consent form after having received oral and written information on the study. The project was registered at ClinicalTrials.gov (NCT01379612). Patients with biopsy-verified adenocarcinoma in the rectum ≤10 cm from the anal verge were eligible for the study.
From June 2010 to January 2012, a total of 73 patients with low or mid rectal cancer referred for pre-treatment staging were screened for participation. 21 patients were not included owing to missing DW MRI, missing storage of DWI sequences or refusal to participate. The study population with both elastography ARFI and DW MRI was composed of 52 patients. The mean age was 67 years, range 42–90 years. Males: 39 (75%), females: 13 (25%).
Of the 52 patients, 16 harboured distant metastases, all diagnosed by CT. In 7 out of 16 patients, an additional positron emission tomography (PET)/CT was performed. Five patients also had an ultrasound examination of the liver, one had a hepatic MRI, and in three cases, ultrasound-guided liver biopsy was performed.
Technique
Elastography
A transgluteal ultrasound elastography scan was performed prior to treatment. We used a Siemens S2000TM ultrasound machine (Acuson Corporation, Siemens Healthcare, Mountain View, CA) with Virtual TouchTM Tissue Quantification software (Acuson Corporation, Siemens Healthcare). Tissue harmonics and a mechanical index of 1.7 were used in all patients. A 4-MHz 4V1 sector transducer was used for the examinations. The transducer was covered with an Ultracover® 44/300 (Microtek Medical B.V., Zutphen, Netherlands).10
Tumour elasticity was measured using ARFI to generate information on the mechanical properties of the tissue. With a 0.5 × 1.5-cm measure box, it was possible to measure the elasticity 0.5 cm from the surface to 8 cm in depth. The central part of the tumour was measured. The examinations were performed by one of three radiologists, all of whom have more than 10 years of experience in rectal cancer staging. All ARFI procedures were carried out via the transgluteal window with the patient in the left lateral position. The sector probe was placed a few centimetres lateral to the posterior midline and the anal verge. The sound beam was directed through the ischiorectal fat or ischial foramen, depending on tumour height. This acoustic window made it possible to axially/transversely visualize T3-4 rectal tumours from 0 to 10 cm above the anal verge.
Elastographic measurements obtained during transducer or patient movements, including peristalsis, were excluded, and new measurements were performed. If the velocity result from the equipment was XX m s−1 (error), after a short waiting time another measurement was performed. All images and data were recorded in the department's picture archive communication system (PACS).
MRI
The MRI scanning was carried out using an Ingenia 1.5-T (T) MRI unit release 4.1.3.3 with a 32-channels Philips dStream Torso coil over the pelvis (Philips Healthcare, Best, Netherlands). After localizer scans, fast T2 weighted (w) spin-echo sequences were obtained (Table 1). The scanning included 3-mm axial slices at a 90° angle to the tumour, and the axial scans were prepared by the MRI radiographer with assistance from the radiologists (SRR, CVH, TS) to ensure perpendicular images. No contrast enhancement was used. DWI was performed perpendicular to the tumour as shown in Table 1, using an echo planar imaging factor of 61. Five different b-values (strength and timing of the gradients to generate DWI) were used, applying diffusion-sensitive gradients; b = 0 s mm−2, b = 200 s mm−2, b = 400 s mm−2, b = 600 s mm−2 and b = 800 s mm−2. The first series was a set of image sequences formed by echo planar spin echo T2 weighted imaging (b = 0). The next series formed gradients at the x, y and z directions and formed isotropic images obtained by calculating diffusion vector projections of the three directions. ADC maps of the isotropic images were created automatically by the Philips Ingenia software (Philips Healthcare).
Table 1.
MRI scan examination protocol
| Parameter | Axial T2w | Sagittal T2w | AxialaT2w | Coronal T2w | DWIa |
|---|---|---|---|---|---|
| TR/TE | 4158/100 | 4608/90 | 2310/100 | 3000/100 | 3207/65 |
| WFS/BW | 1.030/210.8 | 0.626/347.2 | 1.011/214.8 | 1.074/189 | 12.917/16.8 |
| FOV (mm) | 270 | 270 | 270 | 270 | 160 |
| Slice thickness | 5 mm | 4 mm | 3 mm | 4 mm | 6 mm |
| Number of slices | 36 | 18 | 20 | 20 | 80/61 |
| Matrix | 272 × 172 | 300 × 220 | 388 × 274 | 300 × 210 | 80 × 61 |
| Acq voxel | 0.99/1.27/5.00 | 0.89/1.15/4.00 | 0.70/0.91/3.00 | 0.90/1.20/4.00 | 2.00/2.36/6.00 |
| Recon voxel | 0.80/0.80/5.00 | 0.84/0.84/4.00 | 0.56/0.56/3.00 | 0.84/0.84/4.00 | 1.25/1.24/6.00 |
| NSA | 1 | 2 | 2 | 2 | 3 |
| Scan time | 2.38 min | 3.45 min | 5.06 min | 4.18 min | 3.09 min |
Acq, acquisition; BW, bandwidth; DWI, diffusion-weighted imaging; FOV, field of view; NSA, number of averages; TE, time echo; TR, time repetition; WFS, water fat shift.
Perpendicular to the long axis of the tumour.
MRI evaluation
All patients underwent the DW MRI and ultrasound elastography examinations on the same day. All images were evaluated using an Easy Viz Impax PACS workstation (Agfa, Mortsel, Belgium; Medical Insight, Valby, Denmark) with a Coronis monitor (1600 × 1200 pixels), MegaPixels diagnostic display system (Barco, Kortijk, Belgium).
ADC maps in grayscale were automatically generated by the Philips system using a monoexponential decay model. Regions of interest (ROIs) on the ADC map were drawn manually within the solid tumour part containing slices by a gastrointestinal radiologist with more than 10 years of experience (SRR). The size and position of the ROIs were selected to cover the entire tumour area on a single section containing the largest available tumour area.
The ADC values were blindly compared with objective quantitative elastography shear wave velocities. Additionally, the radiologist was blinded to other results, the clinical data and pathology reports.
Statistical analysis
All mean velocity values were compared with the DW MRI ADC values by linear regression, and p < 0.05 was considered statistically significant. Descriptive statistics were used. Data were analysed with the Number Cruncher Statistical Systems (NCSS8 Statistical Software, Kaysville, UT, 2012).
RESULTS
The diameter of the rectal tumours ranged from 1.5 to 6.7 cm with a mean of 4.1 cm.
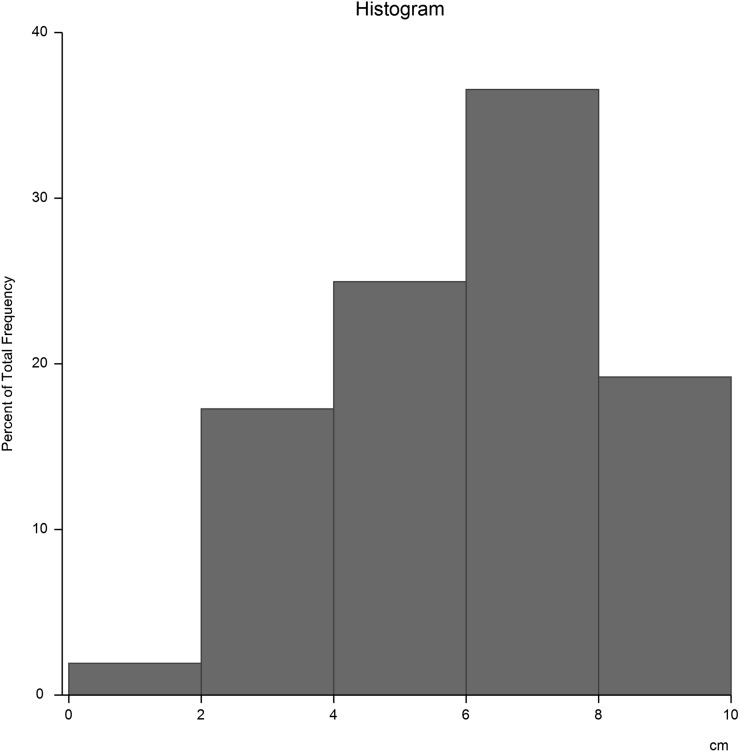
The distance of the tumour from the anal verge ranged from 1 to 10 cm (mean 6.5 cm), Figure 1.
Figure 1.
Tumour height in centimetres above the anal verge.
The mean elasticity of 52 tumours was 3.05 m s−1 (SD: 0.79), and the mean ADC was 0.69 × 10−3 mm2 s−1 (SD: 0.27). Three of the patients had a mucinous component on histology, the mean elasticity of which was 1.93 m s−1 and the mean ADC 1.11 × 10−3 mm2 s−1.
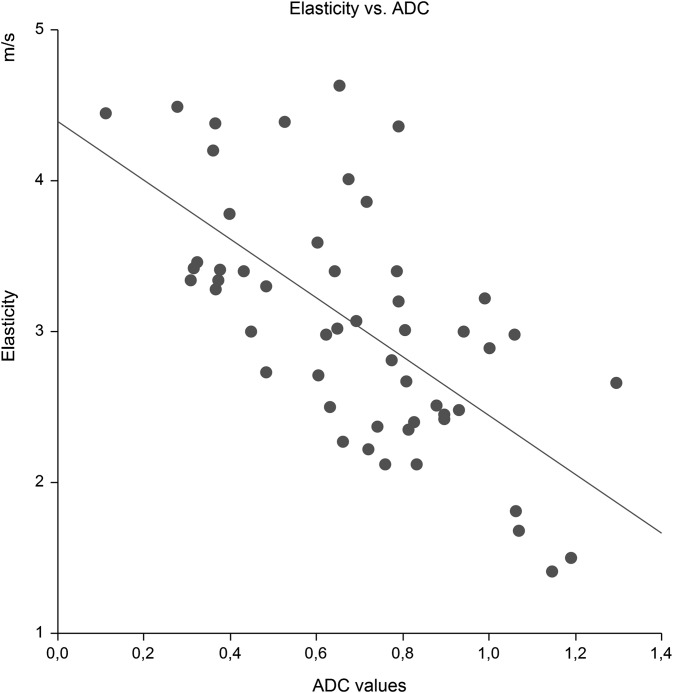
Elasticity was inversely strongly correlated with ADC, r = −0.65 (Salkin scale). ARFI = 4.392 − 1.949 × ADC, R2 = 0.43, p < 0.0001. Intercept = 4.392 (95% CI: 3.92 to 4.86), slope = −1.949 (95% CI: −1.31 to −2.59), p < 0.0001. Figure 2.
Figure 2.
The correlation between elasticity using shear wave (m s−1) and MRI apparent diffusion-weighted coefficient, ADC (×10−3mm2 s−1), p < 0.0001.
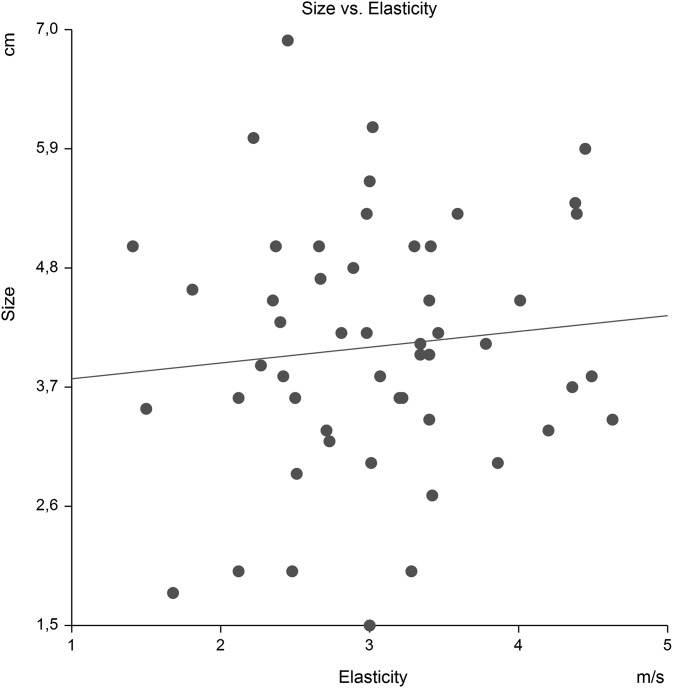
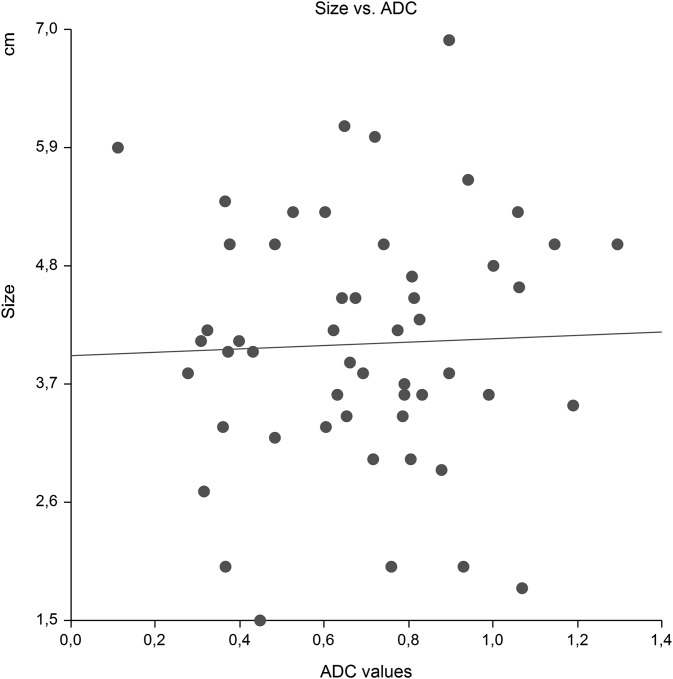
We observed no correlation between tumour size and elasticity, p = 0.48 (Figure 3), nor did we observe a correlation of size and ADC, p = 0.80 (Figure 4).
Figure 3.
Tumour size (cm) vs elasticity (m s−1). The hypothesis that the slope is zero was not rejected.
Figure 4.
Tumour size (cm) vs apparent diffusion-weighted coefficient (ADC; ×10−3 mm2 s−1). The hypothesis that the slope is zero was not rejected.
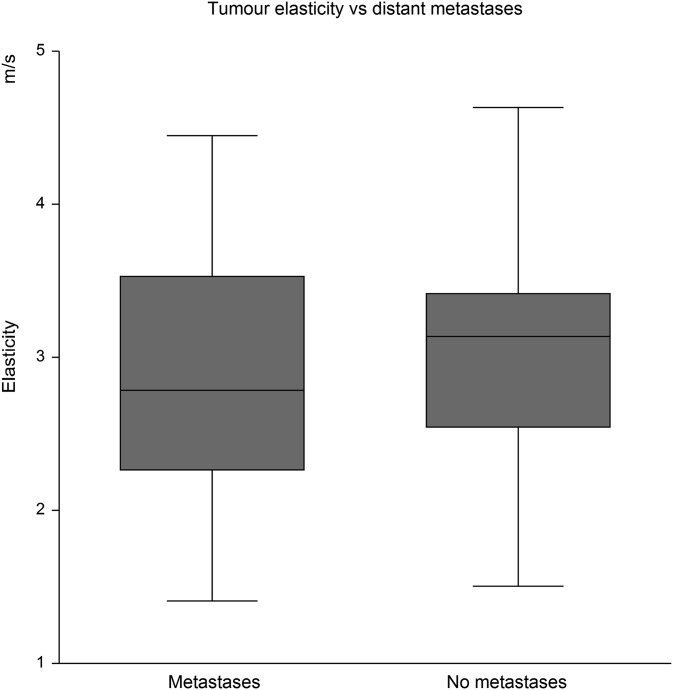
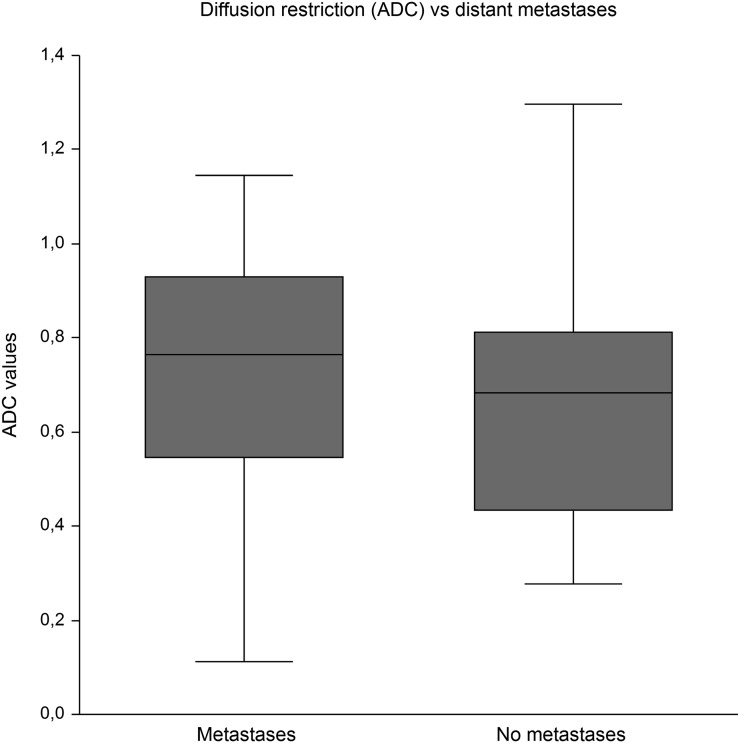
16 of the 52 patients harboured distant metastases. The metastasis status was not related to elasticity. The mean tumour hardness in patients with distant metastases was 2.89 m s−1 (95% CI: 2.42 to 3.34) vs 3.12 m s−1 (95% CI: 2.87 to 3.38) in patients without dissemination, Figure 5. The same was true for metastasis status in relation to ADC with a mean ADC of 0.72 × 10−3 mm2 s−1 (95% CI: 0.57 to 0.87) in patients with distant metastases vs 0.68 × 10−3 mm2 s−1 (95% CI: 0.59 to 0.77) in patients without dissemination, Figure 6.
Figure 5.
No difference in elastography of the primary tumours regardless of coexisting distant metastases.
Figure 6.
No difference in diffusion restriction in tumours of patients with and without distant metastases. ADC, apparent diffusion-weighted coefficient.
DISCUSSION
Our main finding of a correlation between elasticity and MRI-measured ADC in rectal cancer to the best of our knowledge has not been reported previously. We are not familiar with any single study of a correlation between tumour elasticity and diffusion restriction. However, recently, Matsubayashi et al13 found the relative ultrasonic elastographic strain score and MRI diffusion to correlate significantly with fibrotic changes in breast disease based on pathological examinations and determination degree of fibrosis in breast lesions. The finding of a correlation between elasticity and diffusion in rectal cancer is fitting well with previous independent observations that rectal tumours in good responders become softer and the diffusion restriction lower after radiochemotherapy. The well responding tumours contain more necrotic tissue and cells with a variable degree of oedema, fibrosis and inflammation. These changes are characterized by an increase in the interstitial water content where fewer barriers to diffusion exist, which leads to higher post-ADC.5
We observed a higher standard deviation in our shear wave ARFI measurements than that of the ADC values of diffusion restriction. This may be explained by the different measurement techniques; the ultrasound measurements were performed using a box with a fixed size, whereas in the ADC measurements the ROI area covers the total cross section of the tumour on the ADC map. Some tumours might be heterogeneous, and reduction of the elasticity variation could possibly be limited by using several ARFI measurements within the same tumour. The elasticity of the rectal tumours in the present study did not differ from a previous study 3.05 m s−1 vs 3.13 m s−1.10
Using MRI, we found a mean ADC value of 0.69 × 10−3 mm2 s−1, similar to pre-treatment ADC values in a new study.14 However, the ADC results may depend on the b-values. Others have reported higher basic ADC values in rectal cancer.3
Recently, Inoue et al's study of 81 patients showed that variations in ROI method (freehand, square or round) had no marked effect on interobserver variability in endometrial carcinoma. Similar to the study of Inoue et al,15 we did not measure tumour ADC by whole-volume ROI, since this is not easily available in routine clinical work, given the time-consuming nature of the method. We therefore only drew single-slice freehand ROIs. On the other hand, Lambregts et al16 found that in 64 tumours, ADC values and interobserver variability were dependent on the method of the ROI analysis, using b: 0, 500, 1000 s mm−2. They concluded that the most reproducible results were obtained when measuring ADC of the whole tumour volume.
Nasu et al found mucinous adenocarcinomas to have a higher ADC value than other types of adenocarcinoma. The mean ADC of mucinous carcinomas was 1.49 × 10−3 mm2 s−1, whereas that of tubular adenocarcinomas was 0.80 × 10−3 mm2 s−1.17 Although mucinous tumours in our study were softer and had a higher ADC level, the number of patients is too small to draw any conclusion. Mucinous tumours can occur in up to 20% of rectal cancer patients;18 but only very few (6%) of our patients harboured mucinous cancer.
According to a new phantom study by Carlsen et al,19 the lesion diameter has a significant effect on shear wave elastography, and ARFI displays limitation as to discrimination of stiffness in very small lesions. The rectal tumours in the present study had a mean diameter of 4.1 cm, which is considerably larger than the lesions in the phantom study by Carlsen et al,19 and we could not find a relation between size and stiffness in rectal cancer.
Study limitations
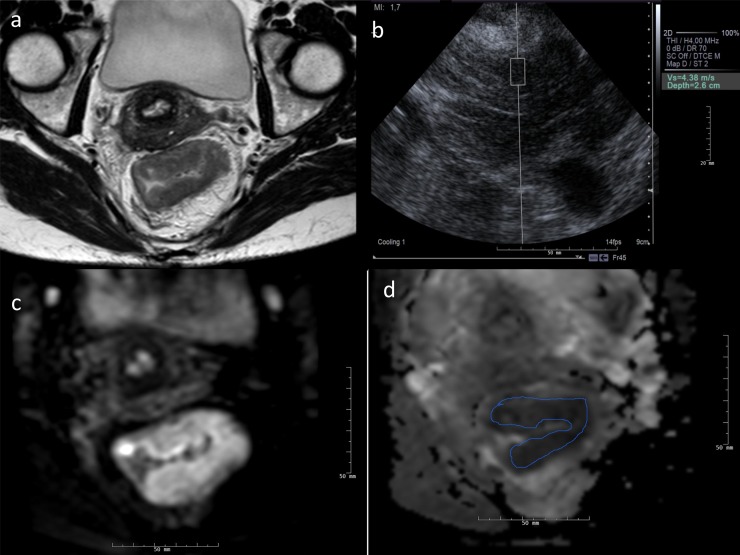
We used a 1.5-T MRI unit. Perhaps, by using a 3.0-T unit, we could have obtained more accurate ADC values. Dale et al20 found a significant difference between ADC values measured at 1.5 and 3.0 T in the liver. Others found no significant differences in ADC between repeated measurements and between ADC obtained at 1.5 T and those at 3.0 T.21−23 There could also be variations in the manually drawn ROIs on the ADC map, Figure 7.
Figure 7.
(a–d) A 44-year-old female with a 5.4-cm tumour located 8 cm above the anal verge. The distance from tumour penetration to the mesorectal fascia was 0 mm at the thin axial T2 image (a). No synchronous metastases were detected. Tumour elasticity was 4.38 cm s−1 measured transgluteally (b). MRI diffusion-weighted imaging showed increased signal at b = 800 s mm−2 (c) and diffusion restriction at the apparent diffusion-weighted coefficient (ADC) map with an ADC value of 0.518 × 10−3 mm2 s−1 (d). The patient had long-course radiochemotherapy and subsequent radical resection. The pathological classification was pT3, N0, V0, TRG3. CT of the chest and abdomen 18 months after rectal surgery detected a solitary hepatic metachronous metastasis, which was radically resected at liver surgery.
In the future, misaligned DW-MRIs and inaccurate, manually drawn ADCs can be addressed using automatic registration, thus increasing the accuracy and objectiveness of ADC measurements.24
ADC values may vary not only with different imaging parameters but also with different types of MRI systems.15
There may be some misregistration error between the ADC ROI and ARFI box measurement. Another approach could have been image fusion to ensure more exact position between the ADC and ARFI measurement, but misalignment and fusion errors may still occur. Another limitation is that shear wave elastography is affected by target depth.19 We did not use the short, direct contact elastography by ERUS, but the external transgluteal window, which gives a longer distance between the transducer and the target. Also, we did not perform an interobserver and intraobserver study of the patients. However, Waage et al25 have recently reported that rectal elastography is highly reproducible. At present, endorectal shear wave measurement is only available from one vendor, and with ERUS ARFI technique, it would also be possible to measure the elasticity of tumours >10 cm above the anal verge.
CONCLUSION
The stiffness measured by shear wave elastography correlates well with the estimated diffusion restriction by MRI ADC measurements in rectal tumours. Low ADC means increased stiffness and vice versa. The relationship between ARFI and ADC measurement was linear in our study population.
Contributor Information
Søren R Rafaelsen, Email: soeren.rafael.rafaelsen@rsyd.dk.
Chris Vagn-Hansen, Email: Chris.Aksel.Vagn-Hansen@rsyd.dk.
Torben Sørensen, Email: Torben.Soerensen@rsyd.dk.
Jan Lindebjerg, Email: Jan.Lindebjerg@rsyd.dk.
John Pløen, Email: John.Ploeen@rsyd.dk.
Anders Jakobsen, Email: Anders.Jakobsen@rsyd.dk.
REFERENCES
- 1.Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2013; 23: 2522–31. doi: 10.1007/s00330-013-2864-4 [DOI] [PubMed] [Google Scholar]
- 2.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007; 188: 1622–35. doi: 10.2214/AJR.06.1403 [DOI] [PubMed] [Google Scholar]
- 3.Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG. Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging 2012; 35: 1365–71. doi: 10.1002/jmri.23589 [DOI] [PubMed] [Google Scholar]
- 4.Intven M, Reerink O, Philippens ME. Diffusion-weighted MRI in locally advanced rectal cancer: pathological response prediction after neo-adjuvant radiochemotherapy. Strahlenther Onkol 2013; 189: 117–22. doi: 10.1007/s00066-012-0270-5 [DOI] [PubMed] [Google Scholar]
- 5.Xie H, Sun T, Chen M, Wang H, Zhou X, Zhang Y, et al. Effectiveness of the apparent diffusion coefficient for predicting the response to chemoradiation therapy in locally advanced rectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2015; 94: e517. doi: 10.1097/MD.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafaelsen SR, Kronborg O, Fenger C, Drue H. Comparison of two techniques of transrectal ultrasonography for the assessment of local extent of polypoid tumours of the rectum. Int J Colorectal Dis 1996; 11: 183–6. doi: 10.1007/s003840050040 [DOI] [PubMed] [Google Scholar]
- 7.Bruening W, Sullivan N, Paulson EC, Zafar H, Mitchell M, Treadwell J, et al. Imaging tests for the staging of colorectal cancer [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014. Available from: www.effectivehealthcare.ahrq.gov/reports/final.cfm [PubMed] [Google Scholar]
- 8.Waage JE, Bach SP, Pfeffer F, Leh S, Havre RF, Ødegaard S, et al. Combined endorectal ultrasonography and strain elastography for the staging of early rectal cancer. Colorectal Dis 2015; 17: 50–6. doi: 10.1111/codi.12764 [DOI] [PubMed] [Google Scholar]
- 9.Nightingale K. Acoustic radiation force impulse (ARFI) imaging: a review. Curr Med Imaging Rev 2011; 7: 328–39. doi: 10.2174/157340511798038657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafaelsen SR, Vagn-Hansen C, Sørensen T, Lindebjerg J, Pløen J, Jakobsen A. Ultrasound elastography in patients with rectal cancer treated with chemoradiation. Eur J Radiol 2013; 82: 913–17. doi: 10.1016/j.ejrad.2012.12.030 [DOI] [PubMed] [Google Scholar]
- 11.Giroletti E, Corbucci G. Cardiac magnetic resonance imaging: patient safety considerations. Phys Med 2005; 21: 5–13. doi: 10.1016/S1120-1797(05)80014-6 [DOI] [PubMed] [Google Scholar]
- 12.Baikoussis NG, Apostolakis E, Papakonstantinou NA, Sarantitis I, Dougenis D. Safety of magnetic resonance imaging in patients with implanted cardiac prostheses and metallic cardiovascular electronic devices. Ann Thorac Surg 2011; 91: 2006–11. doi: 10.1016/j.athoracsur.2011.02.068 [DOI] [PubMed] [Google Scholar]
- 13.Matsubayashi RN, Imanishi M, Nakagawa S, Takahashi R, Akashi M, Momosaki S, et al. Breast ultrasound elastography and magnetic resonance imaging of fibrotic changes of breast disease: correlations between elastography findings and pathologic and short Tau inversion recovery imaging results, including the enhancement ratio and apparent diffusion coefficient. J Comput Assist Tomogr 2015; 39: 94–101. doi: 10.1097/RCT.0000000000000155 [DOI] [PubMed] [Google Scholar]
- 14.Birlik B, Obuz F, Elibol FD, Celik AO, Sokmen S, Terzi C, et al. Diffusion-weighted MRI and MR-volumetry–in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Magn Reson Imaging 2015; 33: 201–12. doi: 10.1016/j.mri.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 15.Inoue C, Fujii S, Kaneda S, Fukunaga T, Kaminou T, Kigawa J, et al. Apparent diffusion coefficient (ADC) measurement in endometrial carcinoma: effect of region of interest methods on ADC values. J Magn Reson Imaging 2014; 40: 157–61. doi: 10.1002/jmri.24372 [DOI] [PubMed] [Google Scholar]
- 16.Lambregts DM, Beets GL, Maas M, Curvo-Semedo L, Kessels AG, Thywissen T, et al. Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 2011; 21: 2567–74. doi: 10.1007/s00330-011-2220-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasu K, Kuroki Y, Minami M. Diffusion-weighted imaging findings of mucinous carcinoma arising in the ano-rectal region: comparison of apparent diffusion coefficient with that of tubular adenocarcinoma. Jpn J Radiol 2012; 30: 120–7. doi: 10.1007/s11604-011-0023-x [DOI] [PubMed] [Google Scholar]
- 18.Yu SK, Chand M, Tait DM, Brown G. Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy. Eur J Cancer 2014; 50: 920–7. doi: 10.1016/j.ejca.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 19.Carlsen JF, Pedersen MR, Ewertsen C, Săftoiu A, Lönn L, Rafaelsen SR, et al. A comparative study of strain and shear-wave elastography in an elasticity phantom. AJR Am J Roentgenol 2015; 204: W236–42. doi: 10.2214/AJR.14.13076 [DOI] [PubMed] [Google Scholar]
- 20.Dale BM, Braithwaite AC, Boll DT, Merkle EM. Field strength and diffusion encoding technique affect the apparent diffusion coefficient measurements in diffusion-weighted imaging of the abdomen. Invest Radiol 2010; 45: 104–8. doi: 10.1097/RLI.0b013e3181c8ceac [DOI] [PubMed] [Google Scholar]
- 21.Rosenkrantz AB, Oei M, Babb JS, Niver BE, Taouli B. Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. J Magn Reson Imaging 2011; 33: 128–35. doi: 10.1002/jmri.22395 [DOI] [PubMed] [Google Scholar]
- 22.Rao RK, Riffel P, Meyer M, Kettnaker PJ, Lemke A, Haneder S, et al. Implementation of dual-source RF excitation in 3 T MR-scanners allows for nearly identical ADC values compared to 1.5 T MR scanners in the abdomen. PLoS One 2012; 7: e32613. doi: 10.1371/journal.pone.0032613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barral M, Soyer P, Ben Hassen W, Gayat E, Aout M, Chiaradia M, et al. Diffusion-weighted MR imaging of the normal pancreas: reproducibility and variations of apparent diffusion coefficient measurement at 1.5- and 3.0-Tesla. Diagn Interv Imaging 2013; 94: 418–27. doi: 10.1016/j.diii.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 24.Guyader JM, Bernardin L, Douglas NH, Poot DH, Niessen WJ, Klein S. Influence of image registration on apparent diffusion coefficient images computed from free-breathing diffusion MR images of the abdomen. J Magn Reson Imaging 2015; 42: 315–30. doi: 10.1002/jmri.24792 [DOI] [PubMed] [Google Scholar]
- 25.Waage JE, Rafaelsen SR, Borley NR, Havre RF, Gubberud ET, Leh S, et al. Strain elastography evaluation of rectal tumors: inter- and intraobserver reproducibility. Ultraschall Med Apr 2015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]