Abstract
Objectives:
To investigate the diagnostic performance of advanced modelled iterative reconstruction (ADMIRE) to filtered back projection (FBP) when using an ultralow-dose protocol for the detection of solid and subsolid pulmonary nodules.
Methods:
Single-energy CT was performed at 100 kVp with tin filtration in an anthropomorphic chest phantom with solid and subsolid pulmonary nodules (2–10 mm, attenuation, 20 to −800 HU at 120 kVp). The mean volume CT dose index (CTDIvol) of the standard chest protocol was 2.2 mGy. Subsequent scans were obtained at 1/8 (0.28 mGy), 1/20 (0.10 mGy) and 1/70 (0.03 mGy) dose levels by lowering tube voltage and tube current. Images were reconstructed with FBP and ADMIRE. One reader measured image noise; two readers determined image quality and assessed nodule localization.
Results:
Image noise was significantly reduced using ADMIRE compared with FBP (ADMIRE at a strength level of 5 : 70.4% for 1/20; 71.6% for 1/8; p < 0.001). Interobserver agreement for image quality was excellent (k = 0.88). Image quality was considered diagnostic for all images at 1/20 dose using ADMIRE. Sensitivity of nodule detection was 97.1% (100% for solid, 93.8% for subsolid nodules) at 1/20 dose and 100% for both nodule entities at 1/8 dose using ADMIRE 5. Images obtained with 1/70 dose had moderate sensitivity (overall 85.7%; solid 95%; subsolid 73.3%).
Conclusion:
Our study suggests that with a combination of tin filtration and ADMIRE, the CTDIvol of chest CT can be lowered considerably, while sensitivity for nodule detection remains high. For solid nodules, CTDIvol was 0.10 mGy, while subsolid nodules required a slightly higher CTDIvol of 0.28 mGy.
Advances in knowledge:
Detection of subsolid nodules is feasible with ultralow-dose protocols.
INTRODUCTION
Pulmonary nodules are a common finding in chest CT. While most of the pulmonary nodules are benign and harmless to the patient, some represent lung cancer1,2 or, in patients with underlying malignancy, indicate metastatic spread of cancer to the lung. This makes the detection and follow-up of lung nodules essential.1
The debates about the assumed radiation-associated risk of cancer development from ionizing radiation urged the radiological community to lower the radiation exposure of the patient to a level that is “as low as reasonably achievable” (the so-called ALARA principle).3 Owing to the cancer-associated risk of CT examinations, stated by the linear no-threshold4 dose–response model and the BEIR VII report,5 radiation dose optimization is of particular interest in patients who undergo CT examinations for screening purposes and who undergo repeated follow-up CT. A number of CT dose-reduction techniques have been developed using different approaches.6–9 Currently used radiation dose-reduction techniques include the optimization of tube current while keeping tube voltage constant,10 automated attenuation-based tube voltage selection,6 reduction of the scan length,7 increase in pitch and rotation time.11
A reduction of radiation dose usually leads to increased quantum noise. Image reconstruction techniques and filters are used to minimize this effect and to maintain diagnostic image quality.9,12 Today, different methods are clinically implemented.
An important nodule entity in lung cancer screening is subsolid pulmonary nodules,11 which include semi-solid and pure ground-glass nodules. Subsolid pulmonary nodules are associated with a wide range of differential diagnoses and are known to have a significantly higher risk of malignancy than do solid pulmonary nodules.12,13 In the Early Lung Cancer Action Project, Henschke et al12 reported that 69% of semi-solid nodules, 18% of ground-glass nodules and 7% of solid nodules were malignant. Since subsolid nodules have a lower density than solid nodules, the limit of the maximal tolerable noise level is lower and hence the required radiation dose level is higher than that for solid nodules.
The purpose of this phantom study was to investigate the diagnostic performance of advanced modelled iterative reconstruction (ADMIRE 3 and 5) to filtered back projection (FBP) when using an ultralow-dose protocol in the detection of solid and subsolid pulmonary nodules.
METHODS AND MATERIALS
Phantom
A custom-made anthropomorphic chest phantom (serial number QSA-452; Quality Assurance in Radiology and Medicine, Moehrendorf, Germany) simulating an adult male (lateral diameter, 35 cm; anteroposterior diameter, 25 cm; extension ring of 2.5-cm thickness) containing 19 solid (2–10 mm; attenuation, 20–80 HU at 120 kVp) and 16 subsolid pulmonary nodules of various sizes (2–10 mm; attenuation −700 to −800 HU with semi-solid nodule of 50 HU at 120 kVp) was used in this study. The chest phantom contained microspheres representing solid and subsolid pulmonary nodules and was filled with cork granulate material (−800 HU at 120 kVp) simulating lung parenchyma. Additionally, resin, calcium carbonate, magnesium oxide and hydroxyapatite were used to simulate soft tissue, lung- and bone-equivalent structures.
CT scan protocol
Single-energy CT was performed on a third-generation dual-source CT (SOMATOM® Force; Siemens Healthcare; Forchheim, Germany) equipped with an integrated high-resolution circuit detector (Stellar Technology; Siemens). The phantom was scanned with a collimation of 96 × 1.5 mm and a slice acquisition of 240 × 1.5 mm, gantry rotation time of 0.25 s and pitch of 1.2. Scans included the entire phantom with a constant scan length of 30 cm in the z-axis, simulating the dimensions of an adult lung. Standard chest CT was performed at 120 kVp with 100 mAs. Subsequent image acquisition was performed at a tube voltage of 100 kVp using tin filtration, and tube current–time products were adjusted in order to obtain three radiation dose levels (CT volume dose index CTDIvol): 1/8, 1/20 and 1/70 of our standard dose protocol (Table 1).
Table 1.
Scan protocols with radiation dose parameters
| Protocol | Parameter | CTDIvol (mGy) | DLP (mGy cm) | ED (mSv) |
|---|---|---|---|---|
| Standard dose | 120 kVp/100 mAs ref | 2.20 | 65.3 | 1.11 |
| 1/8 standard dose | 100 Sn kVp/80 mAs | 0.28 | 8 | 0.14 |
| 1/20 standard dose | 100 Sn kVp/30 mAs | 0.10 | 3 | 0.05 |
| 1/70 standard dose | 100 Sn kVp/10 mAs | 0.03 | 1 | 0.02 |
CTDIvol, CT volume dose index; DLP, dose-length product; ED, effective dose for different dose levels.
According to Eckermann et al,14 the effective radiation dose of chest CT was calculated by multiplying the dose-length product with a body-specific conversion coefficient EDLP of 0.017 mSv mGy−1 cm−1.
Data reconstruction
All CT images were reconstructed with FBP and ADMIRE at a strength level of 3 or 5, using a slice thickness of 1.5 mm, an increment of 1 mm and a sharp tissue convolution kernel (Br64). The reconstructed field of view was 350 mm, and the image matrix was 512 × 512 pixels.
ADMIRE combines statistical data modelling in the raw data domain and model-based noise detection in the image domain.3 In up to two iterations, ADMIRE removes geometric imperfections such as cone beam artefacts. Subsequently, statistical optimization of the data is performed, reaching a target noise reduction which depends on the selected ADMIRE strength level.15,16 Compared with the antecessor SAFIRE (sinogram affirmed iterative reconstruction), ADMIRE offers a higher resolution at organ borders, improving delineation of edges and thus is beneficial also for pulmonary nodule detection.15
Subsequent analyses were performed using a picture archiving and communication system (Impax, V. 6.5.5.1033; Agfa-Gevaert, Mortsel, Belgium) on a high-definition liquid crystal display monitor (Barco™; Medical Imaging Systems, Kortrijk, Belgium).
Radiation dose
The mean CTDIvol and the dose–length product (DLP) were obtained from the electronically logged patient protocol for each CT acquisition. The effective radiation dose of chest CT was calculated by multiplying the dose–length product with a body-specific conversion coefficient EDLP of 0.017 mSv mGy−1 cm.14
CT data analysis
Quantitative analysis
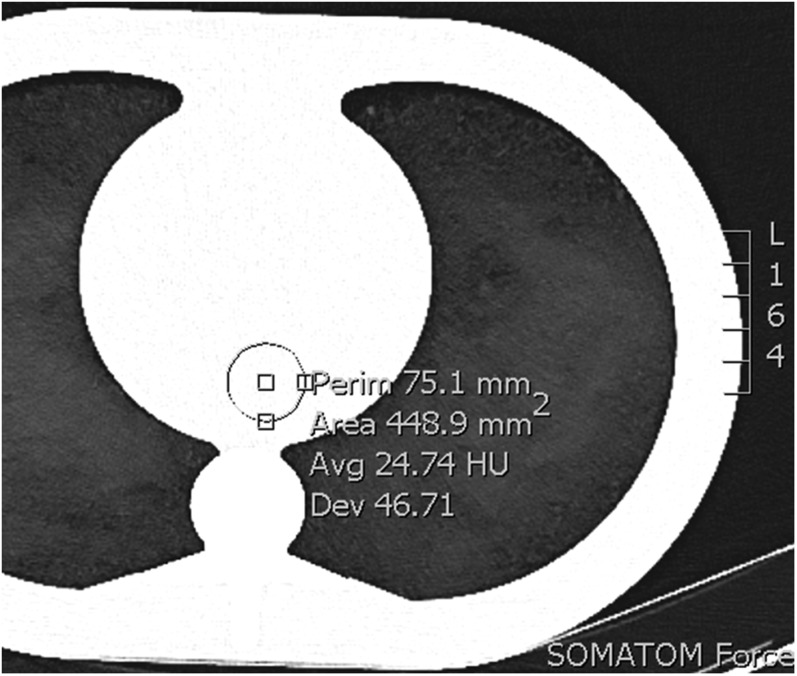
Image noise was measured by one blinded reader by placing a circular region of interest (ROI) in a homogenous area of the phantom representing the central scan field of view. The ROI size was fixed at 449 mm2 (Figure 1). Mean image noise was defined as the average of the standard deviation of the attenuation in five consecutive ROI measurements at different slice positions.3
Figure 1.
Region of interest placement, size 449 mm2. Avg, average; Dev, standard deviation; Perim, perimeter.
Qualitative analysis
The obtained images were presented to two independent readers (both with 3 years' experience in chest radiology). Readers could modify the window width and level after the initial presentation with a lung tissue window (window level, −600 HU; width, 1200 HU). First, both readers rated the overall image quality according to Gordic et al3 on a 5-point Likert scale (1 = non-diagnostic image quality, strong artefacts; 2 = severe blurring with uncertain evaluation; 3 = moderate blurring; 4 = slight blurring with unrestricted diagnostic image assessment; 5 = excellent image quality, no artefacts). An image quality from 3 to 5 was considered as diagnostic. The same two readers also independently evaluated the number and type (i.e., solid or subsolid) of pulmonary nodules.
A total of 12 data sets were reviewed by both readers. Between each reading session, the reading pause was at least 5 days. To reduce the recall bias regarding the position of the nodules, the series were in addition mirrored either horizontally or vertically between two reading sessions.
Statistical analysis
Statistical analyses were conducted using commercially available software (SPSS®, release 21.0; IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± standard deviation, while categorical variables were expressed as frequencies or percentages. Sensitivity of nodule detection was calculated for each protocol. The constructional drawing of the phantom served as the standard of reference. All data sets were included into the analysis of sensitivity, even if the image quality was considered non-diagnostic. Cohen's Kappa (κ) was used to assess interreader and intrareader agreement for subjective image quality and interreader agreement for nodule detection. κ-results were stratified qualitatively by score (slight agreement, 0.01–0.20; fair agreement, 0.21–0.40; moderate agreement, 0.41–0.60; substantial agreement, 0.61–0.80; excellent agreement, 0.81–0.99.17 Friedman analysis of variance was used to assess image noise of the scans for significant differences among the different tube current levels. A two-sided p-value <0.05 was considered to indicate statistical significance.
RESULTS
Image noise
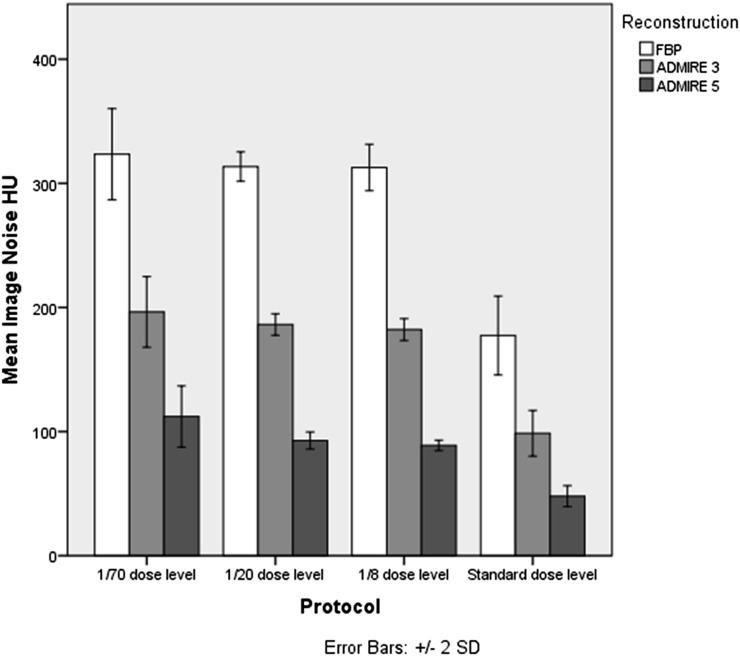
Image noise was highest in images acquired at 1/70 dose level of standard dose reconstructed with FBP (mean 323 HU) and was lowest in images at standard dose reconstructed with ADMIRE at a strength level of 5 (mean 48 HU). When comparing image noise between FBP and ADMIRE 5, we achieved a noise reduction of 71.6% for 1/8, 70.4% for 1/20 and 65.2% for 1/70 dose protocol when using ADMIRE 5 (p < 0.001) (Figure 2).
Figure 2.
Image noise stratified by dose protocol: filtered back projection (FBP), advanced modelled iterative reconstruction (ADMIRE). SD, standard deviation.
Subjective image quality
Interobserver agreement for image quality assessment was excellent (k = 0.88). Intrareader agreement for image quality assessment was excellent for both readers (Reader 1: k = 0.845; Reader 2: k = 0.896). Images acquired with 1/70 and 1/20 dose level using FBP were rated by both readers with an image quality score of 2 (severe blurring with uncertain evaluation). The image quality was diagnostic for all images scanned with 1/20 dose (3 for ADMIRE 3 and 5) and 1/8 dose (4 for ADMIRE 3 and 5 for ADMIRE 5) using IR (Table 2, Figures 3, 4).
Table 2.
Image noise and subjective image quality
| Protocol | Reconstruction | Image noise | p-value | Subjective image quality |
|
|---|---|---|---|---|---|
| Reader 1 | Reader 2 | ||||
| Standard | FBP | 177 (155–193) | p < 0.001 | 3 | 3 |
| ADMIRE 3 | 99 (86–109) | 4 | 4 | ||
| ADMIRE 5 | 48 (42–52) | 5 | 5 | ||
| 1/8 standard dose | FBP | 313 (297–320) | p < 0.001 | 4 | 4 |
| ADMIRE 3 | 182 (176–187) | 4 | 4 | ||
| ADMIRE 5 | 89 (88–91) | 5 | 5 | ||
| 1/20 standard dose | FBP | 314 (305–321) | p < 0.001 | 2 | 2 |
| ADMIRE 3 | 186 (181–192) | 3 | 3 | ||
| ADMIRE 5 | 93 (88–97) | 3 | 3 | ||
| 1/70 standard dose | FBP | 314 (305–321) | p < 0.001 | 2 | 2 |
| ADMIRE 3 | 196 (181–218) | 3 | 3 | ||
| ADMIRE 5 | 113 (100–133) | 3 | 3 | ||
ADMIRE, advanced modelled iterative reconstruction; FBP, filtered back projection.
Image quality rated on a 5-point Likert scale.
Ranges are displayed in parentheses.
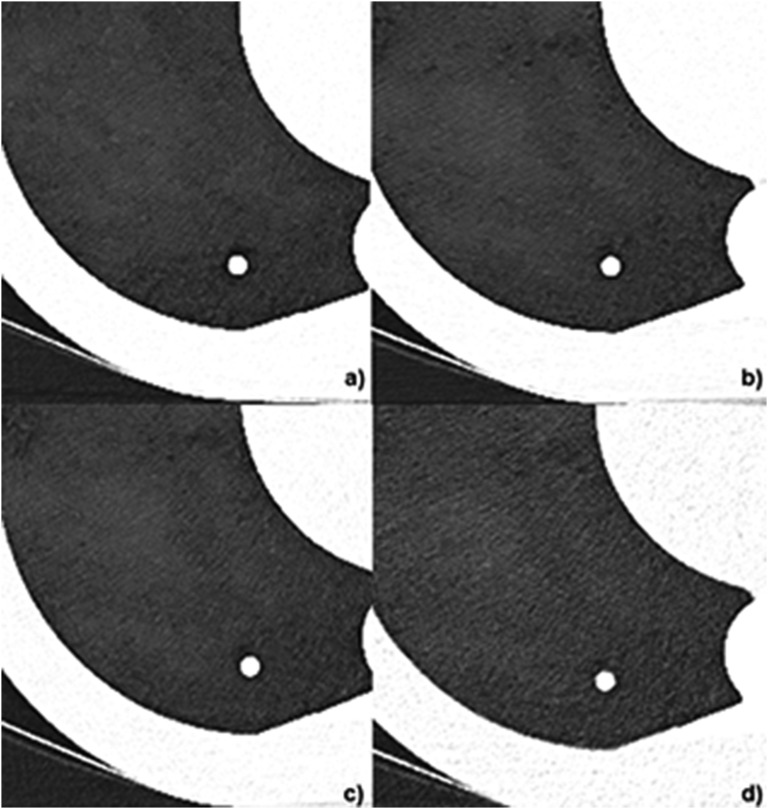
Figure 3.
Representative images of a solid nodule (8 mm diameter) obtained with different CT dose levels: (a) standard dose 120 kVp, 100 mAs; (b) 1/8 dose level 100 kVp, 80 mAs; (c) 1/20 dose level 100 kVp, 30 mAs; (d) 1/70 dose level 100 kVp, 10 mAs; all images were reconstructed with advanced modelled iterative reconstruction (ADMIRE) at a strength level of 5.
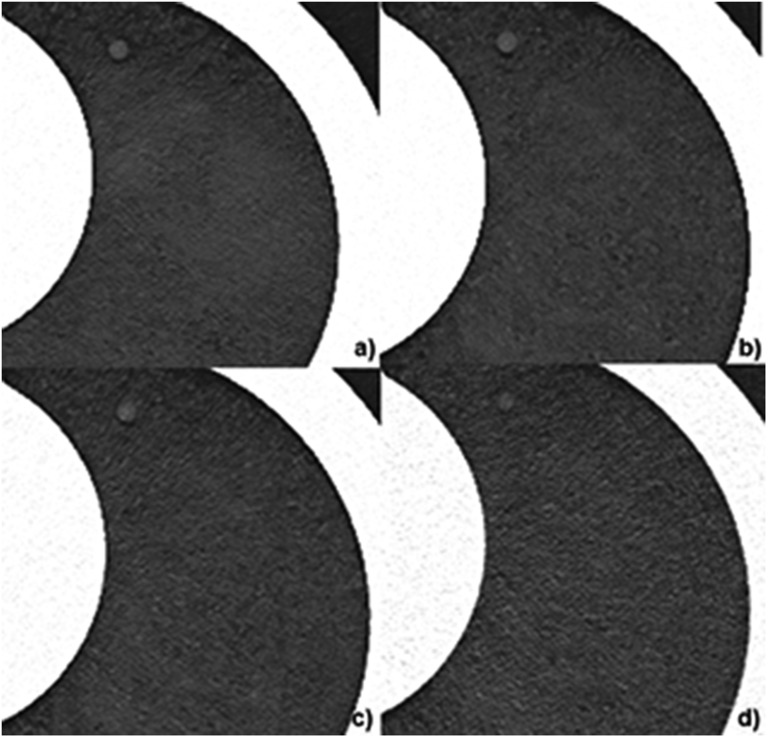
Figure 4.
Representative images of a subsolid nodule (6 mm diameter) obtained with different CT dose levels: (a) standard dose 120 kVp, 100 mAs; (b) 1/8 dose level 100 kVp, 80 mAs; (c) 1/20 dose level 100 kVp, 30 mAs; (d) 1/70 dose level 100 kVp, 10 mAs; all images were reconstructed with advanced modelled iterative reconstruction (ADMIRE) at a strength level of 5. Note the decreasing conspicuity of the nodule at increasing noise levels.
Diagnostic accuracy
Interobserver agreement for nodule localization was excellent for images acquired with the standard dose protocol as well as for images acquired with the 1/8 dose protocol, independent from used IR (standard dose: kFBP = 0.941; kADMIRE3 = 1.000; kADMIRE5 = 0.941; 1/8 dose: kFBP = 0.880; kADMIRE3 = 0.880; kADMIRE5 = 0.941). Interobserver agreement for nodule localization acquired with the 1/8 dose protocol was moderate (kFBP = 0.581) for images reconstructed with FBP and substantial to excellent for images reconstructed with ADMIRE at strength levels of 3 and 5 (kADMIRE3 = 0.696 and kADMIRE5 = 0.88). Images obtained with the 1/70 dose protocol had moderate sensitivity for images reconstructed with FBP and ADMIRE 5 and excellent agreement for images acquired at ADMIRE 5 (kFBP = 0.581; kADMIRE3 = 0.88; kADMIRE5 = 0.581).
For better understanding in the following paragraph, only the sensitivities of Reader 1 are cited. For sensitivities of Reader 2, please refer to Table 3.
Table 3.
Sensitivity for pulmonary nodule detection
| Protocol | IR | Reader | Sensitivity |
FP | ||
|---|---|---|---|---|---|---|
| Overall | Solid | Subsolid | ||||
| Standard | 0 | R1 | 100 | 100 | 100 | 0 |
| 0 | R2 | 97.14 | 100 | 93.75 | 0 | |
| 3 | R1 | 100 | 100 | 100 | 0 | |
| 3 | R2 | 100 | 100 | 100 | 0 | |
| 5 | R1 | 97.14 | 100 | 93.75 | 0 | |
| 5 | R2 | 100 | 100 | 100 | 0 | |
| 1/8 standard dose | 0 | R1 | 88.57 | 94.74 | 81.25 | 0 |
| 0 | R2 | 88.57 | 100 | 75.00 | 1 | |
| 3 | R1 | 91.43 | 100 | 81.25 | 0 | |
| 3 | R2 | 97.14 | 100 | 93.75 | 1 | |
| 5 | R1 | 100 | 100 | 100 | 0 | |
| 5 | R2 | 97.14 | 100 | 94.74 | 0 | |
| 1/20 standard dose | 0 | R1 | 85.71 | 100 | 68.75 | 2 |
| 0 | R2 | 62.50 | 62.50 | 62.50 | 3 | |
| 3 | R1 | 97.14 | 100 | 93.75 | 2 | |
| 3 | R2 | 97.14 | 100 | 93.75 | 2 | |
| 5 | R1 | 97.14 | 100 | 93.75 | 2 | |
| 5 | R2 | 97.14 | 100 | 93.75 | 1 | |
| 1/70 standard dose | 0 | R1 | 65.71 | 89.47 | 62.50 | 3 |
| 0 | R2 | 77.14 | 68.42 | 62.50 | 4 | |
| 3 | R1 | 85.71 | 94.74 | 75.00 | 3 | |
| 3 | R2 | 82.86 | 78.95 | 87.50 | 2 | |
| 5 | R1 | 85.71 | 95.00 | 73.33 | 2 | |
| 5 | R2 | 67.50 | 84.21 | 52.38 | 1 | |
FP, false positive; IR, Iterative reconstruction.
IR=0 indicates that FBP was used, IR=3 indicates that ADMIRE 3 was used, IR=5 indicates that ADMIRE 5 was used.
Sensitivity for detection of pulmonary nodules at 1/20 dose level was 93.8% for subsolid nodules and 100% for solid nodules using ADMIRE at a strength level of 5. At 1/8 dose level, the sensitivity was 100% for both nodule types using ADMIRE 5. Images obtained with 1/70 dose level and reconstructed with ADMIRE 5 had a moderate sensitivity for subsolid nodule detection (73.3%), while sensitivity for solid nodule detection was still of 95% (Table 3).
Regarding the categorization of nodules into solid or subsolid, there was only a moderate agreement throughout the different dose protocols with the standard of reference. While in the standard dose protocol, the rate of correctly classified nodules varied between 66% and 77%, in the 1/8 dose protocol, the rate of correctly classified nodules ranged between 58% and 78%. Using IR in this query did not show to have an impact in nodule classification (Table 4).
Table 4.
Concordance in nodule classification with the standard of reference
| Protocol | IR | Reader | Concordance in nodule classification (%) |
|---|---|---|---|
| Standard | 0 | R1 | 65.71 |
| 0 | R2 | 77.14 | |
| 3 | R1 | 73.53 | |
| 3 | R2 | 73.53 | |
| 5 | R1 | 77.14 | |
| 5 | R2 | 77.14 | |
| 1/8 standard dose | 0 | R1 | 67.74 |
| 0 | R2 | 70.97 | |
| 3 | R1 | 72.00 | |
| 3 | R2 | 70.59 | |
| 5 | R1 | 68.57 | |
| 5 | R2 | 70.59 | |
| 1/20 standard dose | 0 | R1 | 73.33 |
| 0 | R2 | 59.09 | |
| 3 | R1 | 58.80 | |
| 3 | R2 | 61.77 | |
| 5 | R1 | 61.76 | |
| 5 | R2 | 61.76 | |
| 1/70 standard dose | 0 | R1 | 65.22 |
| 0 | R2 | 77.78 | |
| 3 | R1 | 66.67 | |
| 3 | R2 | 58.62 | |
| 5 | R1 | 66.67 | |
| 5 | R2 | 68.00 |
Iterative reconstruction (IR).
IR=0 indicates that FBP was used, IR=3 indicates that ADMIRE 3 was used and IR=5 indicates that ADMIRE 5 was used.
DISCUSSION
Optimization of radiation dose in CT imaging remains a continuous endeavour. To our knowledge, this phantom study is the first to evaluate the image quality and sensitivity for the detection of subsolid nodules for such ultralow radiation doses. Our results show that with the use of tin filtration for single-energy CT in combination with IR, the radiation dose can be lowered to 0.10 mGy (1/20 dose level) while maintaining diagnostic image quality and high sensitivity for the detection of solid pulmonary nodules. Our results show further that for the detection of subsolid nodules higher radiation doses are needed (0.28 mGy; 1/8 dose level) in order to obtain similar nodule detection sensitivity as for solid nodules.
Reducing radiation dose in CT normally goes along with increased quantum noise. This leads to a reduction in image quality and sensitivity for the detection of pulmonary nodules. These negative effects of dose reduction were compensated in this study with different approaches: first, by adding a tin filter to narrow the energy spectrum and second, by using advanced IR at the high strength Levels 3 and 5. Using these methods, we were able to lower the effective doses down to levels from conventional chest X-ray examinations, which are reported to be in the range of 0.02 mSv for posteroanterior studies and in the range of 0.1 mSv for anteroposterior and lateral studies of the chest.18
The tin filter absorbs primarily low-energy photons that contribute little to image quality but increase the dose of radiation that a patient receives.12 Thus, the detector receives mostly higher energy spectra of the X-ray beam. This results in a higher image quality and a reduction of radiation dose for low tube current protocols because less photons are traversing the patient.3,15
In our study, image noise was significantly reduced up to 71.6% in images obtained with 1/8 of the standard dose using ADMIRE 5 compared with FBP and led to an improvement of the subjective image quality.
To date, some studies showed that IR enables a significant reduction of image noise and artefacts, improving the detection of solid pulmonary nodules.6,7,16 Yamashiro et al16 investigated the value of three-dimensional processing (AIDR3D) in chest CT imaging and showed that such image processing improves image quality enabling a reduction of radiation exposure of 50%. Newell et al19 evaluated, in a phantom study, the impact of ultralow radiation dose single-energy CT with tin filtration and third-generation IR on density-based quantitative measures in chronic obstructive lung disease. The authors showed that it might be possible to acquire accurate quantitative CT images with acceptable image noise at a very low CTDIvol of 0.15 mGy.19 Chen et al10 evaluated automated tube current modulation in combination with adaptive statistical IR and found that it was possible to reduce the effective radiation dose of chest CT down to 0.74 mSv while maintaining a reasonable image quality.10 Gordic et al3 used a single-energy protocol with tin filtration and ADMIRE ex vivo for the detection of solid pulmonary nodules and were able to lower effective radiation doses further down to 0.06 mSv. By contrast, our study indicates that reduction of effective dose is possible down to 0.14 mSv while high detection of both nodule entities is guaranteed. A further reduction showed an impairment of sensitivity for subsolid nodule detection.
The studies mentioned above3,10,19 altogether conclude that IR techniques have the potential to significantly reduce radiation dose while maintaining image quality. However, IR techniques have also been criticized by some authors owing to the artificial texture of the reconstructed images and the appearance changes of images reconstructed with IR.20,21 Also, noise originating from the electronic circuit of the detector unit might become more significant where statistical- or model-based IR is used.20 These above-mentioned problems were not noted in our study, even in images reconstructed with high ADMIRE strength levels (3 and 5). The discordance in classifying pulmonary nodules was already described by van Riel et al22 in a recent work who stated that there is a substantial interobserver variability for nodule classification on low-radiation dose scans.
An important issue in chest CT represents the quantitative assessment of pulmonary nodules. Volumetric measurements are important in the follow-up of lung nodules, because malignant nodules may grow asymmetrically and their growth may remain unnoticed with manual measurements of the diameter only.23 Doo et al23 showed that IR significantly improved the accuracy of lung nodule volumetry in low-dose CT compared with FBP, particularly for ground-glass nodules (−630 HU). Also, Siegelmann et al24 in a recent work state that pulmonary nodules with ground-glass opacity can be reliably measured with low-dose techniques down to a CTDIvol of 0.8 mGy regardless of IR. Willemink et al25 found that with IR no clinically significant differences between lung nodule volumes are evident.
Limitations of this study are as follows: first, the protocols were tested on a phantom. Scanning with various CT protocols precludes application in humans for ethical reasons, and CT phantoms have the benefit that no separate diagnostic image modality is required to obtain a standard of reference. Second, since the phantom represented a standard-sized adult our findings might not be applicable for overweight or obese patients. Third, we investigated solid and subsolid nodules and did not discriminate further between semi-solid and ground-glass nodules. One of the most important questions related to detection sensitivity for these nodules is agreement regarding the presence or absence of a solid component, with disagreement reaching as much as 12% in conventional low-dose CT.26
Fourth, measurements of attenuation and size have not been performed. Increased variation of diameters and mass is a real concern when decreasing dose levels to the levels attempted. However, measurement variation was not a primary aim of this study.
Fifth, changing the tube voltage between standard and reduced dose protocols could also have had an important influence on the conspicuity of the lesions. However, between the reduced dose protocols only tube current was changed.
In conclusion, our study indicates that with a combination of tin filtration and ADMIRE, the mean CTDIvol of chest CT can be lowered considerably, while sensitivity for nodule detection remains high. For solid nodules, CTDIvol was 0.10 mGy, while subsolid nodules required a slightly higher CTDIvol of 0.28 mGy.
Contributor Information
Katharina Martini, Email: Katharina.Martini@usz.ch.
Kai Higashigaito, Email: Kai.Higashigaito@usz.ch.
Borna K Barth, Email: Borna.Barth@usz.ch.
Stephan Baumueller, Email: Stephan.Baumueller@usz.ch.
Hatem Alkadhi, Email: Hatem.Alkadhi@usz.ch.
Thomas Frauenfelder, Email: ThomasFrauenfelder@usz.ch.
REFERENCES
- 1.Theros EG. 1976 Caldwell Lecture: varying manifestation of peripheral pulmonary neoplasms: a radiologic-pathologic correlative study. AJR Am J Roentgenol 1977; 128: 893–914. doi: 10.2214/ajr.128.6.893 [DOI] [PubMed] [Google Scholar]
- 2.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237: 395–400. doi: 10.1148/radiol.2372041887 [DOI] [PubMed] [Google Scholar]
- 3.Gordic S, Morsbach F, Schmidt B, Allmendinger T, Flohr T, Husarik D, et al. Ultralow-dose chest computed tomography for pulmonary nodule detection: first performance evaluation of single energy scanning with spectral shaping. Invest Radiol 2014; 49: 465–73. doi: 10.1097/RLI.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 4.Calabrese EJ. Origin of the linearity no threshold (LNT) dose–response concept. Arch Toxicol 2013; 87: 1621–33. doi: 10.1007/s00204-013-1104-7 [DOI] [PubMed] [Google Scholar]
- 5.National Research Council (US) Board on Radiation Effects Research. Health Risks Expo Low Levels Ionizing Radiat BEIR VII, Phase Lett Rep. Washington, DC: National Academies Press (US); 1998. Available from: http://www.ncbi.nlm.nih.gov/books/NBK224187/. [PubMed] [Google Scholar]
- 6.Kalra MK, Maher MM, Toth TL, Schmidt B, Westerman BL, Morgan HT, et al. Techniques and applications of automatic tube current modulation for CT. Radiology 2004; 233: 649–57. doi: 10.1148/radiol.2333031150 [DOI] [PubMed] [Google Scholar]
- 7.Campbell J, Kalra MK, Rizzo S, Maher MM, Shepard JA. Scanning beyond anatomic limits of the thorax in chest CT: findings, radiation dose, and automatic tube current modulation. AJR Am J Roentgenol 2005; 185: 1525–30. doi: 10.2214/AJR.04.1512 [DOI] [PubMed] [Google Scholar]
- 8.Kalender WA, Buchenau S, Deak P, Kellermeier M, Langner O, van Straten M, et al. Technical approaches to the optimisation of CT. Phys Med 2008; 24: 71–9. doi: 10.1016/j.ejmp.2008.01.012 [DOI] [PubMed] [Google Scholar]
- 9.Kubo T, Ohno Y, Kauczor HU, Hatabu H. Radiation dose reduction in chest CT—review of available options. Eur J Radiol 2014; 83: 1953–61. doi: 10.1016/j.ejrad.2014.06.033 [DOI] [PubMed] [Google Scholar]
- 10.Chen JH, Jin EH, He W, Zhao LQ. Combining automatic tube current modulation with adaptive statistical iterative reconstruction for low-dose chest CT screening. PloS One 2014; 9: e92414. doi: 10.1371/journal.pone.0092414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013; 266: 304–17. doi: 10.1148/radiol.12120628 [DOI] [PubMed] [Google Scholar]
- 12.Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS; I-ELCAP Group. CT Screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002; 178: 1053–7. doi: 10.2214/ajr.178.5.1781053 [DOI] [PubMed] [Google Scholar]
- 13.Eisenhuber E, Mostbeck G, Prosch H, Schaefer-Prokop C. Management subsolider pulmonaler Rundherde. [In German.] Radiologe 2014; 54: 427–35. doi: 10.1007/s00117-013-2602-6 [DOI] [PubMed] [Google Scholar]
- 14.Eckermann K, Harrison J, Menzel H-G, Clement CH. Compendium of dose coefficients based on ICRP Publication 60. ICRP Publication 119. Ann ICRP 2012; 41(Suppl.). [DOI] [PubMed] [Google Scholar]
- 15.Euler A, Heye T, Kekelidze M, Bongartz G, Szucs-Farkas Z, Sommer C, et al. Assessment of image quality and low-contrast detectability in abdominal CT of obese patients: comparison of a novel integrated circuit with a conventional discrete circuit detector at different tube voltages. Eur Radiol 2015; 25: 687–93. doi: 10.1007/s00330-014-3459-4 [DOI] [PubMed] [Google Scholar]
- 16.Yamashiro T, Miyara T, Honda O, Kamiya H, Murata K, Ohno Y, et al. Adaptive iterative dose reduction using three dimensional processing (AIDR3D) improves chest CT image quality and reduces radiation exposure. PLoS One 2014; 9: e105735. doi: 10.1371/journal.pone.0105735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- 18.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248: 254–63. doi: 10.1148/radiol.2481071451 [DOI] [PubMed] [Google Scholar]
- 19.Newell JD, Jr, Fuld MK, Allmendinger T, Sieren JP, Chan KS, Guo J, et al. Very low-dose (0.15 mGy) chest CT protocols using the COPDGene 2 test object and a third-generation dual-source CT scanner with corresponding third-generation iterative reconstruction software. Invest Radiol 2015; 50: 40–5. doi: 10.1097/RLI.0000000000000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khawaja RD, Singh S, Madan R, Sharma A, Padole A, Pourjabbar S, et al. Ultra low-dose chest CT using filtered back projection: comparison of 80-, 100- and 120 kVp protocols in a prospective randomized study. Eur J Radiol 2014; 83: 1934–44. doi: 10.1016/j.ejrad.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Kalra MK, Gilman MD, Hsieh J, Pien HH, Digumarthy SR, et al. Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology 2011; 259: 565–73. doi: 10.1148/radiol.11101450 [DOI] [PubMed] [Google Scholar]
- 22.van Riel SJ, Sánchez CI, Bankier AA, Naidich DP, Verschaketen J, Scholten ET, et al. Observer variability for classification of pulmonary nodules on low-dose CT images and its effect on nodule management. Radiology 2015; 22: 142700. [DOI] [PubMed] [Google Scholar]
- 23.Doo KW, Kang EY, Yong HS, Woo OH, Lee KY, Oh YW. Accuracy of lung nodule volumetry in low-dose CT with iterative reconstruction: an anthropomorphic thoracic phantom study. Br J Radiol 2014; 87: 20130644. doi: 10.1259/bjr.20130644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegelman JW, Supanich MP, Gavrielides MA. Pulmonary nodules with ground-glass opacity can be reliably measured with low-dose techniques regardless of iterative reconstruction: results of a phantom study. AJR AM J Roentgenol 2015; 204: 1247–7. [DOI] [PubMed] [Google Scholar]
- 25.Willemink MJ, Leiner T, Budde RP, de Kort FP, Vligenhart R, van Ooijien PM, et al. Systematic error in lung nodule volumetry: effect of iterative reconstruction versus filtered back projection at different CT parameters. AJR AM J Roentgenol 2012; 199: 1241–6. [DOI] [PubMed] [Google Scholar]
- 26.Scholten ET, Jacobs C, van Ginneken B, van Riel S, Vliegenthart R, Oudkerk M, et al. Detection and quantification of the solid component in pulmonary subsolid nodules by semiautomatic segmentation. Eur Radiol 2015; 25: 488–96. doi: 10.1007/s00330-014-3427-z [DOI] [PubMed] [Google Scholar]