Abstract
Objective:
IMPORT HIGH is a multicentre randomized UK trial testing dose-escalated intensity-modulated radiotherapy (IMRT) after tumour excision in females with early breast cancer and higher than average local recurrence risk. A survey was carried out to investigate the impact of this trial on the adoption of advanced breast radiotherapy (RT) techniques in the UK.
Methods:
A questionnaire was sent to all 26 IMPORT HIGH recruiting RT centres to determine whether the trial has influenced non-trial breast RT techniques in terms of volume delineation, dosimetry, treatment delivery and verification. In order to compare the clinical practice of breast RT between IMPORT HIGH and non–IMPORT HIGH centres, parts of the Royal College of Radiologists (RCR) breast RT audit result were used in this study.
Results:
26/26 participating centres completed the questionnaire. After joining the trial, the number of centres routinely using tumour bed clips to guide whole-breast RT rose from 5 (19%) to 21 (81%). 20/26 (77%) centres now contour target volumes and organs at risk (OARs) in some or all patients compared with 14 (54%) before the trial. 14/26 (54%) centres offer inverse-planned IMRT for selected non-trial patients with breast cancer, and 10/14 (71%) have adopted the IMPORT HIGH trial protocol for target volume and OARs dose constraints. Only 2/26 (8%) centres used clip information routinely for breast treatment verification prior to IMPORT HIGH, a minority that has since risen to 7/26 (27%). Data on 1386 patients was included from the RCR audit. This suggested that more cases from IMPORT HIGH centres had surgical clips implanted (83 vs 67%), were treated using CT guided planning with full three-dimensional dose compensation (100 vs 75%), and were treated with photon boost RT (30 vs 8%).
Conclusion:
The study suggests that participation in the IMPORT HIGH trial has played an important part in providing the guidance and support networks needed for the safe integration of advanced RT techniques, where appropriate, as a standard of care for breast cancer patients treated at participating cancer centres.
Advances in knowledge:
We investigated the impact of the IMPORT HIGH trial on the adoption of advanced breast RT techniques in the UK and the trial has influenced non-trial breast RT techniques in terms of volume delineation, dosimetry, treatment delivery and verification.
INTRODUCTION
The UK National Cancer Research Institute established the National Radiotherapy Trials Quality Assurance (RTTQA) group to co-ordinate clinical trial quality assurance (QA) work. The RTTQA group is a multidisciplinary team which undertakes a programme of activities in relation to individual clinical trials, necessary to ensure adherence to RT components of a trial protocol.1 For the past decade, the RTTQA group has carried out QA for breast RT trials, including START, FAST, SUPREMO, IMPORT LOW, IMPORT HIGH and FAST FORWARD.2
The process of conducting a clinical trial can have positive spin-off effects for participating centres. For example, the National Cancer Research Institute START trials led to improvements in breast RT by standardizing the techniques used in trials, such as definition of the dose prescription point.1,2 The scrutiny provided by a radiotherapy (RT) QA programme has the potential to benefit all patients, not just those in clinical trials, through the impact of research activities on staff, facilities and culture. Venables et al2 suggested that collaboration in clinical trials allows centres to introduce new techniques in a safe environment through generating a strong co-operative network of trial centres under the supervision of the RT QA team.
Intensity Modulated and Partial Organ Radiotherapy (IMPORT) HIGH (CRUK/06/003) is a Phase III UK multicentre randomized trial testing dose escalated intensity-modulated RT (IMRT) for females treated by breast conservation surgery and appropriate systemic therapy for early breast cancer.3 The trial was developed in partnership with and is centrally managed by The Institute of Cancer Research Clinical Trials and Statistics Unit and sponsored by The Institute of Cancer Research. It is hypothesized that the expertise and experience gained by the process of site participation in trials have greatly facilitated the incorporation of advanced breast RT techniques into routine clinical practice. Against this background, a questionnaire-based survey investigated the clinical impact of the IMPORT HIGH trial on current non-trial breast RT practice in the UK.
METHODS AND MATERIALS
IMPORT HIGH trial
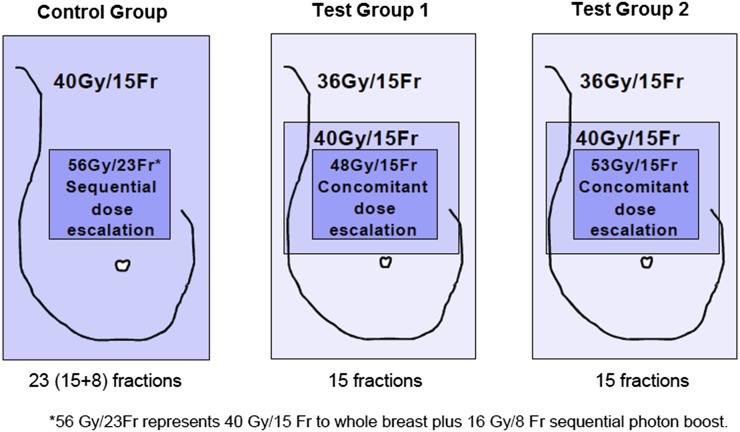
IMPORT HIGH tests synchronous vs standard sequential tumour bed boost dose, localized using titanium clips in females with early breast cancer at higher than average risk of local tumour recurrence after tumour excision (Figure 1).3 The trial requires all patients to have titanium clips or gold seed markers inserted to the wall of the tumour bed during breast conservation surgery.
Figure 1.
IMPORT HIGH trial schema.
In the test regimens, the trial requires a planned dose distribution incorporating three dose levels across the breast within strict dose constraints. Participating sites are required to use either forward- or inverse-planned IMRT and image-guided RT (IGRT) in order to deliver the planned dose gradient to the tumour bed and whole breast accurately.
Study questionnaire
In August 2013, a bespoke questionnaire (Table 1) was sent to the lead clinical oncologist, physicist and radiographer of 26 IMPORT HIGH active recruiting RT centres with the aim of estimating the clinical impact of IMPORT HIGH in terms of changing practices in relation to volume delineation, RT planning, treatment delivery and verification. All 26 centres had been entering patients into the IMPORT HIGH trials for a minimum of 12 months.
Table 1.
Questionnaire sent to all IMPORT HIGH centres
| Questions | Answers |
|---|---|
|
Volume delineation | |
| Do you use titanium clip markers to guide whole breast radiotherapy planning (placement of whole breast treatment fields) for non trial patients with breast cancer? | i) Yes |
| ii) No | |
| If the answer is yes: | |
| Before joining IMPORT HIGH | |
| a) None | |
| b) <50% of patients | |
| c) ≥50% of patients | |
| d) All patients | |
| After joining IMPORT HIGH | |
| a) None | |
| b) <50% of patients | |
| c) ≥50% of patients | |
| d) All patients | |
| Do you do any target volumes and organs at risk (OAR) contouring on non trial patients with breast cancer? | i) Yes |
| ii) No | |
| If the answer is yes: | |
| Before joining IMPORT HIGH | |
| a) None | |
| b) <50% of patients | |
| c) ≥50% of patients | |
| d) All patients | |
| After joining IMPORT HIGH | |
| a) None | |
| b) <50% of patients | |
| c) ≥50% of patients | |
| d) All patients | |
| How do you do localise post-operative tumour bed for non trial patients with breast cancer in breast boost radiotherapy planning? Please choose all that apply. | Before joining IMPORT HIGH |
| a) Clinical markup | |
| b) CT only | |
| c) CT with implanted markers | |
| d) Others. Please specify | |
| After joining IMPORT HIGH | |
| a) Clinical markup | |
| b) CT only | |
| c) CT with implanted markers | |
| d) Others. Please specify | |
| Radiotherapy planning and treatment delivery | |
| Do you use inverse planned or forward planned IMRT in IMPORT HIGH trial? | i) Forward planned IMRT |
| ii) Inverse planned IMRT | |
| For inverse planned IMRT centres, do you offer IMRT (either forward planned or inverse planned) to non trial patients with breast cancer? If yes, please specify the reasons. | If the answer is inverse planned IMRT: |
| Before joining IMPORT HIGH | |
| a) None | |
| b) <50% of patients | |
| c) ≥50% of patients | |
| d) All patients | |
| After joining IMPORT HIGH | |
| a) None | |
| b) <50% of patients | |
| c) ≥50% of patients | |
| d) All patients | |
| Do you use the IMPORT HIGH trial protocol for target volume and OAR dose constraints when you use IMRT for non-trial patients with breast cancer? | i) Yes |
| ii) No | |
| How is breast boost radiotherapy delivered for non trial patients with breast cancer at your centre? | a) Single electron field |
| b) 3DCRT/IMRT photon treatment | |
| If answer is 3DCRT/IMRT, did you implement this after joining IMPORT HIGH? | |
| i) Yes | |
| ii) No | |
| Treatment verification | |
| How do you perform treatment verification for non trial patients with breast cancer at your centre? | a) Single MV/KV planar image at the tangential beam angles |
| b) 2D MV/KV image pairs | |
| c) 3D MV/KV volumetric CT | |
| d) Others. Please specify | |
| What information do you use for breast radiotherapy verification for non trial patients with breast cancer at your centre? Please choose all that apply. | a) Lung and heart depth measurement |
| b) Breast tissue/bony anatomy matching | |
| c) Surgical clips/seeds matching | |
| d) 3D volumetric tumour bed matching | |
| Have you ever used implanted markers information to guide breast radiotherapy verification before joining IMPORT HIGH? | i) Yes |
| ii) No | |
| Do you use implanted markers information to guide breast radiotherapy verification for non trial patients with breast cancer after joining IMPORT HIGH? | i) Yes |
| ii) No | |
3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; OAR, organs at risk.
The Royal College of Radiologists audit data
In 2011, a national audit of adjuvant breast RT technique and tumour bed boost practice in early breast cancer after breast-conserving surgery was carried out by the Royal College of Radiologists (RCR). 50 of 58 RT centres submitted data for the RCR audit including 1 private provider. In order to compare the clinical practice of breast RT between IMPORT HIGH and non–IMPORT HIGH centres, parts of the audit results were used in this study. The questions quoted from the audit were:
(1) Was there a tumour bed radiotherapy delivered? If yes, were clips or other fiducials inserted by the surgeons to mark the surgical cavity?
(2) How was the whole breast radiotherapy planned?
(3) What treatment modality was used for the tumour bed boost?
RESULTS
Study questionnaire
All centres returned completed questionnaires by January 2014.
Volumes delineation
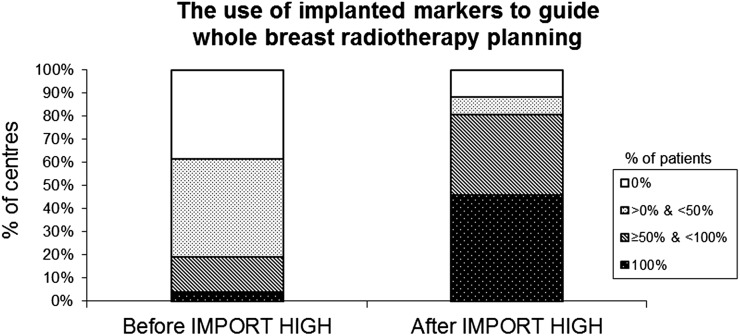
After joining IMPORT HIGH, the number of participating centres using tumour bed clips to guide placement of whole-breast treatment fields in ≥50% patients rose from 5 (19%) to 21 (81%) centres (Figure 2).
Figure 2.
Change in percentages of IMPORT centres using implanted markers to guide whole-breast radiotherapy planning for non-trial patients with breast cancer pre- and post-joining trial (n = 26).
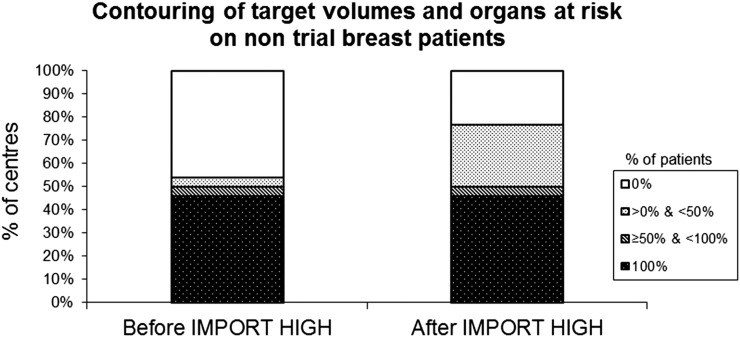
The number of centres outlining target volumes and organs at risk (OARs) in at least a proportion of non-trial patients increased from 14 to 20 centres (Figure 3). Following participation in IMPORT HIGH, fewer centres rely on traditional skin mark-up for tumour bed delineation in breast boost RT (Table 2). The most common tumour bed delineation method among the IMPORT HIGH centres is now based on localization of surgical clips marking tumour bed on the planning CT.
Figure 3.
Change in percentages of IMPORT centres performing target volumes and organs at risk delineations on non-trial patients with breast cancer pre- and post-joining trial (n = 26).
Table 2.
A summary of tumour bed delineation methods used in breast boost radiotherapy by IMPORT HIGH centres pre- and post-joining trial
| Delineation methods | Clinical markup | CT only | Ultrasound | Clips with CT |
|---|---|---|---|---|
| Before IMPORT HIGH | 10 | 4 | 0 | 10 |
| After IMPORT HIGH | 4 | 3 | 1 | 16 |
Data provided in the table represent number of centres.
Radiotherapy planning and treatment
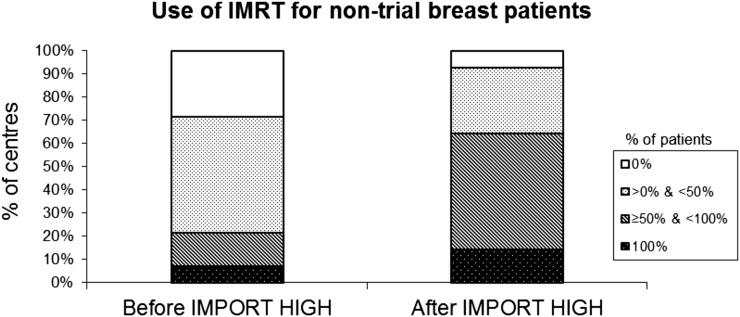
14/26 (54%) IMPORT HIGH centres use inverse-planned IMRT, and the other 12 centres use forward-planned IMRT in the trial. Of those 14 inverse-planned IMRT centres, the number of centres treating ≥50% of their non-trial patients with breast cancer with IMRT (forward-planned or inverse-planned) after taking part in IMPORT HIGH has increased from 3 (21%) to 9 (64%) (Figure 4). The stated reasons for using IMRT were to improve dose homogeneity and to treat challenging anatomy such as pectus excavatum. 10/14 (71%) centres using IMRT in off-trial patients with breast cancer use the IMPORT HIGH trial protocol for target volume and OARs dose constraints.
Figure 4.
Change in percentages of IMPORT centres using intensity-modulated radiotherapy (IMRT) for non-trial patients with breast cancer pre- and post-joining trial (n = 14).
18/26 (69%) centres use a single electron boost field routinely for non-trial patients with breast cancer' boost treatment. As influenced by the IMPORT HIGH trial, 8 (31%) centres have implemented three-dimensional conformal RT (3DCRT) or IMRT for planning boost treatments of at least a proportion of non-trial patients.
Treatment verification
The current treatment verification techniques used for non-trial patients with breast cancer include: a single megavoltage/kilovoltage (MV/KV) planar image at the tangential beam angles (20/26, 77%), a two-dimensional MV/KV image pair (4/26, 15%) and three-dimensional (3D) MV/KV volumetric CT (2/26, 8%).
In terms of information used for assessing verification images, most centres use breast tissue and bony anatomy matching (23/26 centres) with lung and heart measurements (21/26 centres). In addition to these assessment measures, seven centres use surgical clips/seeds matching and two centres use 3D volumetric tumour beds matching as their routine practice.
IMPORT HIGH 24/26 (92%) centres never used clips information for treatment verification of patients with breast cancer before. After taking part in the trial, seven (27%) centres started using clips for image matching during the whole-breast and boost treatments for non-trial patients with breast cancer.
The Royal College of Radiologists audit data
The RCR audit provided data on 1386 patients, representing 1402 breasts with inclusion of bilateral cases. Only 31/1386 patients received breast RT within a clinical trial, and they were most commonly in the intraoperative breast RT trial. At the time of the audit, there were 17 UK centres recruiting patients into the IMPORT HIGH trial. 13 IMPORT HIGH centres submitted data to the audit (n = 408 breasts), whereas 37 centres not participating in the trial submitted data (n = 994 breasts).
As suggested by the RCR audit, there was 499/1402 with a tumour bed RT delivered. Of those 499 cases, the audit had either positive or negative replies to the question: “were clips or other fiducials inserted by the surgeons to mark the surgical cavity?” in 471 cases. The remainder (28/499 cases) were either blank or “don't know” answers. 124/150 (83%) cases from IMPORT HIGH centres had surgical clips implanted and compared with 214/321 (67%) cases from non-IMPORT HIGH centres.
Regarding whole-breast RT planning, all 408 cases from IMPORT HIGH centres were treated using CT-guided planning with full 3D dose compensation (including forward-/inverse-planned IMRT). This only applied to 749/994 (75%) of cases from non-IMPORT HIGH centres; the remaining cases were treated using conventional two-dimensional treatment planning with optimization of dose homogeneity on central axis plane only.
The RCR audit showed that a boost was delivered in 499/1402 cases in which 158 cases were from IMPORT HIGH centres and 341 cases were from non–IMPORT HIGH centres. 424/499 (85%) cases were treated with electron boost RT. 48/158 (30%) cases from IMPORT HIGH centres were treated with photon boost RT compared with 26/341 (8%) cases from non-IMPORT HIGH centres.
DISCUSSION
Acceptance as a participating site for the IMPORT HIGH trial required some RT centres to further develop multiple aspects of their breast RT process and implement completely new techniques, in order to comply with the IMPORT HIGH RT QA programme.
This survey shows that a significant proportion of IMPORT HIGH centres have gone one step further by incorporating advanced RT technologies, including IMRT and IGRT, as a standard of care in appropriate subgroups of patients. These changes were facilitated by the multidisciplinary group comprising the Trial Management Group (TMG), who developed a trial protocol incorporating a comprehensive RT QA regimen. By implementing changes within this secure framework, it is anticipated that staff at participating centres felt supported as they gained confidence and expertise in advanced breast RT techniques. The fact that the IMPORT HIGH centres seem to have adopted these new techniques into their routine clinical practice more quickly than the non-IMPORT HIGH centres (100 vs 75%) is supported by results from the RCR audit. Only 2% (31/1389) of the patients captured by the audit were treated within clinical trials. This suggests that the RCR audit data not only represent centres' routine clinical breast RT practice regardless of whether they were recruiting patients into trials or not but also indicates that the centres are not reserving advanced RT for trial patients only.
Surgical clips implanted following national guidelines in combination with the RT planning CT have been shown to be important for whole-breast as well as boost RT.3–6 Surgical clips with CT is the most common method used for target volume delineation among the IMPORT HIGH centres for non-trial patients with breast cancer as shown in Table 2. The RCR audit suggested that it is more common for patients to have surgical clips implanted if their RT centre was participating in IMPORT HIGH (83 vs 67%). Discussions between the IMPORT TMG and the British Association of Surgical Oncology led to a recommendation that all patients undergoing breast-conserving surgery should have surgical clips on the wall of the tumour bed as standard care in the UK.6 It is likely that the adoption of this practice may have been less widespread and rapid without the associations formed within the TMG. Another improvement in routine practice relates to planning and delivery of the RT boost dose to the tumour bed. Most IMPORT HIGH centres previously used a simple electron tumour bed boost field in the majority of non-trial patients with breast cancer, without being able to quantify the risk of under dosage at depth in heavy-breasted females.7 Since taking part in the IMPORT HIGH trial, centres increasingly use titanium clips and CT information for boost treatments, and also for whole-breast RT, as indicated in Figure 2 and Table 2.
With the development of IMRT and volumetric modulated RT, studies have demonstrated their feasibility and potential benefits in patients with breast cancer.8–11 Dose–volume histograms (DVHs) of target volumes and OARs are increasingly used in breast RT planning and serve as predictors of normal tissue toxicity, especially cardiac toxicity.12 As implied by Figures 3 and 4, the comprehensive IMPORT HIGH trial protocol and planning pack has provided centres with guidance to use IMRT to localize and treat breast cancer in routine clinical settings, especially for patients with challenging anatomy such as pectus excavatum. This has been illustrated by the RCR audit which indicated that IMPORT HIGH centres used 3DCRT photon boost RT more often than the non-IMPORT HIGH centres (30 vs 8%). Although 30% may seem low, it should be borne in mind that there are national resource implications associated with planning and delivering a 3DCRT photon boost such that the use of the 3DCRT is likely to be limited to those with the greatest clinical need. The OAR constraints needed to implement this were developed as part of the planning pack, and although this may have been adopted in a small number of centres without the trial, this implementation may have been less widespread and more variable without the guidance provided by the IMPORT HIGH planning pack.
Using the simple and effective tangential pair whole-breast technique, a single-planar image verification method provides adequate information to verify patients' treatment position based on breast tissue and bony anatomy matching with lung and heart measurements. However, the soft-tissue-based tumour bed cannot be directly visualized using only MV/KV image. Surgical clips are a good surrogate of the lumpectomy cavity (tumour bed) provided they have been implanted according to national protocol.13,14 As the treatment delivery techniques adopt smaller planning target volume margins, IGRT using surgical clips is needed to ensure that the radiation is delivered to the desired treatment area accurately and toxicity is minimized.15 With the experience gained through IMPORT HIGH, nearly 30% of centres have implemented treatment verification process involving the surgical clips information in whole-breast and tumour bed boost RT.
It has been suggested before that RT QA programmes for clinical trials can lead to improved standards of RT delivery to all patients treated at participating centres.16 The UK RTTQA group has become a national resource for facilitating discussion between centres with similar equipment.2 The 32-page IMPORT HIGH planning pack prepared jointly by the RT QA group and trial TMG, detailed the trial requirements on volume delineation, RT planning, treatment delivery and verification and on how centres could adapt their local techniques to tackle these practical challenges. Chow et al17 reported that clinical outcomes were improved in patients treated within clinical trials as compared with non-trial patients such that the percentage of patients included in a trial could be used as a quality indicator for a RT centre. Clearly, the simultaneous boost RT as under investigation within IMPORT HIGH has not been adopted as routine practice outside the trial because outcome measures are not yet mature. However, results from this survey indicate that >50% of IMPORT HIGH centres used some aspects of the planning pack as guidance when considering requirements for their routine clinical patients.
A limitation of this study is that the questionnaire was not designed to investigate why some IMPORT HIGH centres did not adopt advanced RT techniques into routine clinical practice. As the complexity of the treatment planning and delivery increases, additional resources including staffing, planning and treatment machine verification time are required. Some centres may have been limited by staffing issues and/or the number of treatment machines capable of delivering IMRT and IGRT.
The great difficulty here is attributing the change of practice solely to the influence of the IMPORT HIGH trial as a number of other levers for change could have been in place. The authors acknowledge that there was no proper baseline measurement and the questionnaire results were dependent on interpretations of the people who filled in the questionnaire. In addition, centres were not asked the question: “Did your practice change for non-trial patients as a direct result of participating in IMPORT HIGH?” but rather “Has your practice changed since participating in IMPORT HIGH?” Therefore, the exact cause–effect relationship can only be inferred. In support of this possible cause–effect relationship, there was no other significant national guideline pertaining to technical breast RT during this time period, which may have substantially influenced a practice change.
CONCLUSION
The study suggests that participation in the IMPORT HIGH trial has played an important part in providing the guidance and support networks needed for the safe integration of advanced RT techniques, where appropriate, as a standard of care for patients with breast cancer treated at participating cancer centres.
FUNDING
The IMPORT HIGH trial is supported by Cancer Research UK (grant number C1491/A16831). We thank all the patients who participated in this study, and the doctors, nurses, radiographers, physicists and data managers at the participating centres. We acknowledge the support of The Royal College of Radiologists (UK). We acknowledge NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London.
Y Tsang, L Ciurlionis and K Venables are supported by a grant to the National Radiotherapy Quality Assurance team from the National Institute of Health Research.
Dr Charlotte Coles is supported by the Cambridge National Institute of Health Research Biomedical Research Centre.
Contributor Information
Yat Tsang, Email: yatmantsang@nhs.net.
Laura Ciurlionis, Email: laura.ciurlionis@yahoo.co.uk.
Anna M Kirby, Email: Anna.Kirby@rmh.nhs.uk.
Imogen Locke, Email: Imogen.Locke@rmh.nhs.uk.
Karen Venables, Email: karen.venables@nhs.net.
John R Yarnold, Email: John.Yarnold@icr.ac.uk.
Jenny Titley, Email: Jenny.Titley@icr.ac.uk.
Judith Bliss, Email: Judith.Bliss@icr.ac.uk.
Charlotte E Coles, Email: charlotte.coles@addenbrookes.nhs.uk.
REFERENCES
- 1.Miles E, Venables K. Radiotherapy quality assurance: facilitation of radiotherapy research and implementation of technology. Clin Oncol (R Coll Radiol) 2012; 24: 710–12. doi: 10.1016/j.clon.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 2.Venables K, Tsang Y, Ciurlionis L, Coles CE, Yarnold JR. Does participation in clinical trials influence the implementation of new techniques? A look at changing techniques in breast radiotherapy in the UK. Clin Oncol (R Coll Radiol) 2012; 24: e100–5. doi: 10.1016/j.clon.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Coles C, Yarnold J; IMPORT Trials Management Group. The IMPORT trials are launched (September 2006). Clin Oncol (R Coll Radiol) 2006; 18: 587–90. doi: 10.1016/j.clon.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 4.Coles C, Yarnold J. Localising the tumour bed in breast radiotherapy. Clin Oncol (R Coll Radiol) 2010; 22: 36–8. doi: 10.1016/j.clon.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 5.Coles CE, Wilson CB, Cumming J, Benson JR, Forouhi P, Wilkinson JS, et al. Titanium clip placement to allow accurate tumour bed localisation following breast conserving surgery: audit on behalf of the IMPORT Trial Management Group. Eur J Surg Oncol 2009; 35: 578–82. doi: 10.1016/j.ejso.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Association of Breast Surgery at BASO 2009. Surgical guidelines for the management of breast cancer. Eur J Surg Oncol 2009; 35 (Suppl. 1): 1–22. doi: 10.1016/j.ejso.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 7.Donovan EM, Ciurlionis L, Fairfoul J, James H, Mayles H, Manktelow S, et al. Planning with intensity-modulated radiotherapy and tomotherapy to modulate dose across the breast to reflect recurrence risk (the IMPORT HIGH trial). Int J Rad Oncol Biol Phys 2011; 79: 1064–72. doi: 10.1016/j.ijrobp.2009.12.052 [DOI] [PubMed] [Google Scholar]
- 8.Coon AB, Dickler A, Kirk MC, Liao Y, Shah AP, Strauss JB, et al. Tomotherapy and multifield intensity-modulated radiotherapy planning reduce cardiac doses in left-sided breast cancer patients with unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys 2010; 78: 104–10. doi: 10.1016/j.ijrobp.2009.07.1705 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez VJ, Buchholz DJ, Langen KM, Olivera GH, Chauhan B, Meeks SL, et al. Evaluation of two tomotherapy-based techniques for the delivery of whole-breast intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2006; 65: 284–90. doi: 10.1016/j.ijrobp.2005.12.044 [DOI] [PubMed] [Google Scholar]
- 10.Lohr F, El-Haddad M, Dobler B, Grau R, Wertz HJ, Kraus-Tiefenbacher U, et al. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys 2009; 74: 73–80. doi: 10.1016/j.ijrobp.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 11.Reynders T, Tournel K, De Coninck P, Heymann S, Vinh-Hung V, Van Parijs H. et al. Dosimetric assessment of static and helical TomoTherapy in the clinical implementation of breast cancer treatments. Radiother Oncol 2009; 93: 71–9. doi: 10.1016/j.radonc.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368: 987–98. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 13.Coles CE, Harris EJ, Donovan EM, Bliss P, Evans PM, Fairfoul J, et al. Evaluation of implanted gold seeds for breast radiotherapy planning and on treatment verification: a feasibility study on behalf of the IMPORT trialists. Radiother Oncol 2011; 100: 276–81. doi: 10.1016/j.radonc.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Leonard CE, Tallhamer M, Johnson T, Hunter K, Howell K, Kercher J, et al. Clinical experience with image-guided radiotherapy in an accelerated partial breast intensity-modulated radiotherapy protocol. Int J Radiat Oncol Biol Phys 2010; 76: 528–34. doi: 10.1016/j.ijrobp.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Harris EJ, Mukesh M, Jena R, Baker A, Bartelink H, Brooks C, et al. A multicentre observational study evaluating image-guided radiotherapy for more accurate partial-breast intensity-modulated radiotherapy: comparison with standard imaging technique. Southampton, UK: Efficacy and Mechanism Evaluation; 2014. [PubMed] [Google Scholar]
- 16.Weber DC, Tomsej M, Melidis C, Hurkmans CW. QA makes a clinical trial stronger: evidence-based medicine in radiation therapy. Radiother Oncol 2012; 105: 4–8. doi: 10.1016/j.radonc.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 17.Chow CJ, Habermann EB, Abraham A, Zhu Y, Vickers SM, Rothenberger DA. et al. Does enrollment in cancer trials improve survival? J Am Coll Surg 2013; 216: 774–80. doi: 10.1016/j.jamcollsurg.2012.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]