Abstract
Objective:
Sentinel lymph node biopsy (SLNB) with a superparamagnetic iron oxide (SPIO) tracer was shown to be non-inferior to the standard combined technique in the SentiMAG Multicentre Trial. The MRI subprotocol of this trial aimed to develop a magnetic alternative for pre-operative lymphoscintigraphy (LS). We evaluated the feasibility of using MRI following the administration of magnetic tracer for pre-operative localization of sentinel lymph nodes (SLNs) and its potential for non-invasive identification of lymph node (LN) metastases.
Methods:
Patients with breast cancer scheduled to undergo SLNB were recruited for pre-operative LS, single photon emission CT (SPECT)-CT and SPIO MRI. T1 weighted turbo spin echo and T2 weighted gradient echo sequences were used before and after interstitial injection of magnetic tracer into the breast. SLNs on MRI were defined as LNs with signal drop and direct lymphatic drainage from the injection site. LNs showing inhomogeneous SPIO uptake were classified as metastatic. During surgery, a handheld magnetometer was used for SLNB. Blue or radioactive nodes were also excised. The number of SLNs and MR assessment of metastatic involvement were compared with surgical and histological outcomes.
Results:
11 patients were recruited. SPIO MRI successfully identified SLNs in 10 of 11 patients vs 11 of 11 patients with LS/SPECT-CT. One patient had metastatic involvement of four LNs, and this was identified in one node on pre-operative MRI.
Conclusion:
SPIO MRI is a feasible technique for pre-operative localization of SLNs and, in combination with intraoperative use of a handheld magnetometer, provides an entirely radioisotope-free technique for SLNB. Further research is needed for the evaluation of MRI characterization of LN involvement using subcutaneous injection of magnetic tracer.
Advances in knowledge:
This study is the first to demonstrate that an interstitially administered magnetic tracer can be used both for pre-operative imaging and intraoperative SLNB, with equal performance to imaging and localization with radioisotopes.
INTRODUCTION
Sentinel lymph node biopsy (SLNB) is the standard care for surgical staging of the axilla in clinically and radiologically node-negative patients with breast cancer.1–3 The gold standard for SLNB is the combined technique, using a radioisotope and blue dye, to identify the sentinel lymph nodes (SLNs) intraoperatively using a gamma probe and/or by visual identification of blue dye. Planar lymphoscintigraphy (LS), or optionally single photon emission CT (SPECT)-CT, provides the surgeon with pre-operative information on the location and number of SLNs. Although the value of pre-operative LS has been questioned in breast cancer,4 it is required for SLNB in melanoma in order to decide which lymphatic basin(s) require surgical exploration. Furthermore, LS is still the standard of care in breast cancer in many countries, and the European Association of Nuclear Medicine recommends the procedure because of the potential added value over the use of the gamma probe alone.5 However, the use of radioisotopes has important drawbacks: it exposes patients and medical staff to radiation and is governed by stringent legislation. Furthermore, LS provides low spatial resolution, unlike MRI.
Three separate trials have evaluated a radioisotope-free surgical technique for SLNB, using an interstitially administered superparamagnetic iron oxide (SPIO) tracer in combination with a handheld magnetometer, and demonstrated an identification rate non-inferior to the combined technique.6–8 To be able to perform an entirely radioisotope-free SLNB procedure, an alternative for LS is needed. SPIO contrast agents are known to provide “negative contrast” on T2* weighted MRI because the uptake of the tracer results in a drop of signal intensity. Intravenously injected SPIOs were first used as MRI contrast agents for the identification of metastases in the liver and spleen.9,10 Several animal and patient studies have demonstrated that if SPIOs are injected interstitially, they are taken up by SLNs, and therefore, SPIO MRI can be used as an alternative to SPECT-CT for pre-operative localization of SLNs. However, all these studies still rely on the use of radioisotopes for intraoperative detection of the sentinel nodes.11–15 To date, only one study16 has involved both pre-operative SPIO MRI and intraoperative SLN detection with a handheld magnetometer in nine patients. However, this study did not compare the MRI technique with the gold standard of the combined technique.
The application of magnetic nanoparticles is not necessarily limited to localization of SLNs as it has the potential to identify metastatic involvement of lymph nodes (LNs). Intravenously administered ultrasmall superparamagnetic iron oxides (USPIOs) are taken up in the healthy LN tissue and not in metastatic areas, and consequently, areas affected by tumour have relatively high signal intensity on T2 weighted images compared with normal tissue.17–19 If localization of SLNs could be coupled with accurate detection of metastasis, it could potentially provide an alternative to current routine surgical axillary staging, i.e. SLNB.20 Although SLNB is a minimally invasive procedure, it is associated with morbidity, e.g. lymphoedema, loss of arm mobility, numbness and pain.21,22 USPIO-MRI has been successfully used as a non-invasive method for evaluating metastatic LN involvement in various types of cancer including breast cancer.17–19 A non-invasive method that accurately diagnoses SLN metastases would prevent patients undergoing unnecessary SLNB, thereby decreasing physical and psychological morbidity and treatment costs.21–23
In this imaging subprotocol of the SentiMAG Multicentre trial (NTR3238, http://www.trialregister.nl), both SPIO MRI and intraoperative SLN detection using subcutaneous injection of magnetic tracer and a handheld magnetometer were performed and quantitatively compared with the current gold standard of LS/SPECT-CT and SLNB with the combined technique. This is believed to be the first time such a comparison has been made and that the magnetic tracer has been used for imaging. The purpose of this study was to demonstrate the feasibility of SPIO MRI for pre-operative localization of SLNs in patients with breast cancer as an alternative to LS and/or SPECT-CT and to evaluate its potential to identify LN metastasis.
METHODS AND MATERIALS
Patients
Between July 2012 and March 2013, patients with histologically confirmed breast cancer who were clinically and radiologically node-negative and scheduled to undergo SLNB were eligible for inclusion. Patients were recruited after Ethics Committee approval had been obtained (reference NL39018.044.11) and after informed consent was obtained from each patient. Ethics Committee approval was obtained from Medisch Ethische Toetsingscommissie Twente (Enschede, Netherlands). Exclusion criteria were known intolerance to iron or dextran compounds, iron overload disorder and the standard MRI exclusion criteria. Furthermore, patients scheduled for a 1-day protocol were excluded for logistical reasons.
Planar lymphoscintigraphy and single photon emission CT-CT
The day before surgery, patients received two peritumoral, 0.5 ml injections of 99mTc-albumin-colloid (Nanocoll®; GE Healthcare, Eindhoven, Netherlands) with a total activity of approximately 140 MBq, as per standard practice. LS was performed 2 h after injection, using an E-cam dual-head gamma camera (Siemens Medical Solutions, Erlangen, Germany). Anterior and anterior-oblique images were acquired. In addition to LS, which is the standard of care, SPECT-CT (Symbia™ T6, Siemens Medical Solutions) was performed. Imaging was performed in the same position as MRI: patients were placed in the prone position on an MR breast imaging mattress, with the arms adducted and parallel to the body. This positioning facilitates comparison of SPECT-CT images and MR images post-acquisition.
Superparamagnetic iron oxide MRI
The day before surgery, MRI was performed with a 1.5-T system (Intera, Philips Medical Systems, Best, Netherlands). Patient positioning was similar to SPECT-CT (see Planar lymphoscintigraphy and single photon emission CT). The SENSE Breast-7 coil was used for eight patients and the SENSE Body coil for three patients, the latter being used for larger patients.
The magnetic tracer Sienna+® (Endomagnetics Ltd, Cambridge, UK) is a CE-marked injectable medical device, consisting of a sterile aqueous suspension of SPIO particles in injectable water, containing circa 27 mg iron ml−1. The particles have a carboxydextran coating and a mean hydrodynamic diameter of 59 nm (Z-averaged diameter).24
Before tracer injection, transverse images were obtained using T1 weighted turbo spin echo (TSE) sequence (repetition time (TR)/echo time (TE) 734/16 ms; flip angle (FA) 90°; slice thickness 3.0 mm; field of view 30 cm; matrix 320 × 320 pixels; imaging time approximately 4 min). In addition, a T1 weighted TSE sequence in the coronal plane (TR/TE 727/16 ms; FA 90°; slice thickness 3.0 mm; field of view 30 cm; matrix 320×320 pixels imaging time 3 min) was obtained to distinguish LNs located closely to each other.
While the patient was still positioned in the scanner, a periareolar subcutaneous injection of 2 ml magnetic tracer (diluted with 3 ml normal saline) was administered followed by 5-min massage. In 9 of 11 patients, the magnetic tracer was administered after injection of the radioisotope, and in 2 of 11 patients, the magnetic tracer was injected first. In the first two patients, the injection site was not massaged, and in the remaining nine patients, the injection site was massaged for 3–5 min, to promote lymphatic drainage. Post-contrast imaging of the breast and axillary region was started approximately 5 min after injection.
Pre- and post-contrast T2* weighted gradient echo (GRE) images (TR/TE 500/4.6 ms; FA 18°; field of view 30 cm; matrix 400 × 400 pixels; slice thickness 3.0 mm; imaging time 5 min) were acquired in the transverse plane to localize the LNs with SPIO uptake and assess the SPIO distribution within the LNs. This sequence was repeated post-injection until SPIO uptake in LNs was observed.
The total imaging time (including time required for tracer injection) of approximately 30 min for the SPIO MRI was comparable with the imaging time (approximately 25 min without time for injection) for SPECT-CT. LS was less time consuming with an imaging time of approximately 10–15 min.
Image analysis
LS and SPECT-CT images were evaluated on a syngo® Multimodality Workplace (Siemens Medical Solutions) by a nuclear medicine physician (WIdB). LNs demonstrating uptake of the radioactive tracer and presenting with an afferent lymphatic vessel leading from the injection site to the LN are defined to be SLNs. If no afferent vessel can be identified, the first LN appearing from the injection site is defined to be SLN. The number of SLNs and their locations were registered both for LS and SPECT-CT.
The MRI data were analysed independently by two radiologists experienced in breast imaging (RB and CAHK), using a DynaCAD® workstation (Philips Healthcare). Any disagreements were resolved by consensus. All axillary LNs were identified on the T1 weighted TSE images, and their number and anatomical level (in relation to pectoralis minor muscle) were noted. The pre- and post-contrast T2* weighted GRE images were compared to identify LNs with decreased signal intensity owing to SPIO uptake. The number and anatomical level of LNs demonstrating SPIO uptake were recorded. LNs showing SPIO uptake from direct lymphatic drainage of the injection site were considered to be SLNs on SPIO MRI, according to the definition of radioactive SLNs. The number of SLNs and their locations were registered.
MR criteria for detection of metastases
SLNs showing heterogeneous SPIO uptake were classified as metastatic; nodes with homogeneous uptake were classified as non-metastatic. The classification was compared with the histopathology results of the resected SLNs.
Surgery
SLNB was performed as previously described.6 Patent Blue V® (Laboratoire Guerbet, France) was administered intraoperatively after induction of anaesthesia. The blue dye was administered with a periareolar injection. The surgeon initially used a handheld magnetometer (SentiMAG®) for SLNB. A gamma probe (Europrobe 3, Euromedical Instruments, Le Chesnay, France) was used for subsequent confirmation of the magnetometer results. The magnetometer consists of a base unit and a handheld probe with a diameter of 24 mm. The device generates an alternating magnetic field, which magnetizes the SPIO particles in vicinity of the probe. The resulting change in magnetization can be used as a measure for the amount of tracer present. The obtained signal is shown on the numerical display and produces an audible signal. Any iron-containing and/or radioactive and/or blue SLNs were removed. A correlation was made between the number of SLNs identified on LS/SPECT-CT and SPIO MR, and the number of radioactive SLNs and magnetic SLNs removed during surgery. Routine histological assessment was performed on all resected SLNs to determine the presence of metastasis.
RESULTS
The study recruited 11 patients, 8 with invasive ductal carcinoma and 3 with ductal carcinoma in situ. The mean age of the patients was 58.5 years (range 45–71 years). In 10 of 11 patients (91%), SLNs were successfully identified by SPIO MRI. The injection site, lymphatic tracts and SLNs could be identified on post-contrast T2* weighted images (Figures 1 and 2). An artefact at the injection site was seen in all patients; however, this did not affect axillary imaging in any patient. The same LNs could be identified both before and after massage of the injection site in all patients. A total of 21 SLNs (mean 1.9, range 0–4) were identified on MRI. LS/SPECT-CT identified a total of 13 SLNs (mean 1.2, range 1–2). In all patients, the number of SLNs on LS and SPECT-CT was concordant.
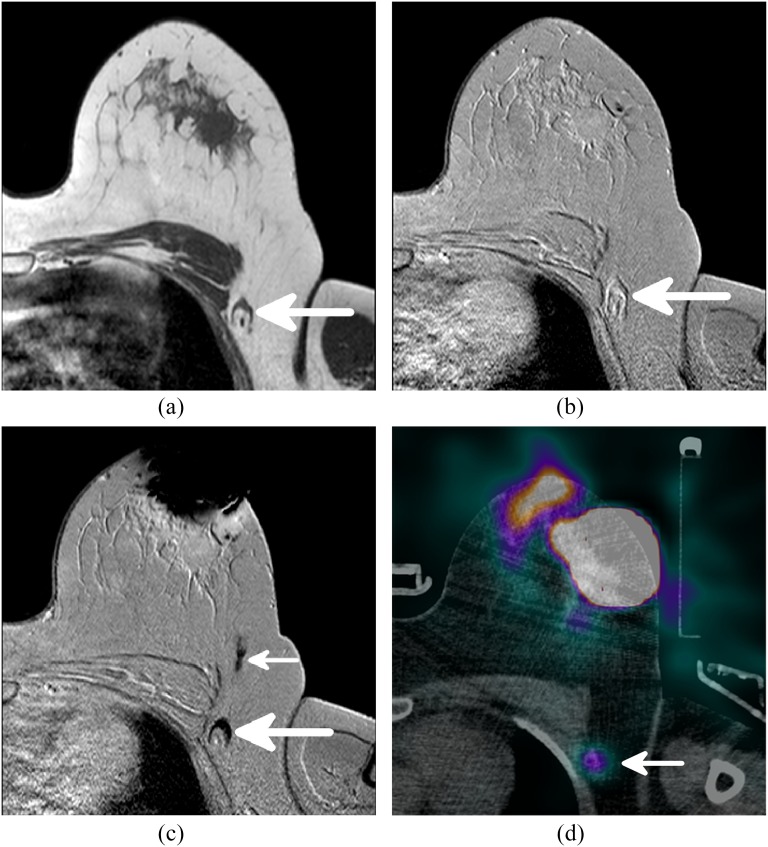
Figure 1.
The different MR sequences and single photon emission CT (SPECT)-CT of a single patient. (a) A T1 weighted image showing the anatomy and morphology, one axillary lymph node (LN) is visible (arrow). (b) The same axillary LN, visible (arrow), on a pre-contrast T2 weighted scan. (c) A large decrease in signal is observed at the periareolar injection site. The axillary LN also shows a signal decrease owing to uptake of superparamagnetic iron oxide (SPIO) and is therefore considered sentinel lymph node (SLN) (large arrow). A SPIO-filled lymphatic vessel draining from the injection site to the SLN is indicated with the small arrow. (d) The radioactive SLN (large arrow) identified by SPECT-CT corresponds to the SLN identified by MRI.
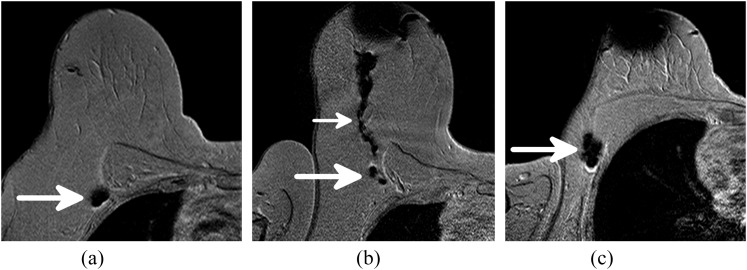
Figure 2.
Post-contrast T2 weighted gradient echo images of three patients. (a) One sentinel lymph node (SLN) with magnetic tracer uptake (arrow). (b) Two SLNs with magnetic tracer uptake (large arrow) with a lymphatic vessel (small arrow) draining from the periareolar injection site. (c) Two closely located SLNs (arrow) result in one large area with decreased signal.
SLNs were successfully identified during surgery using the handheld magnetometer and the combined technique in 11 of 11 patients. A total of 22 SLNs were excised in 11 patients (mean 2.0, range 1–4). Of these, 11 nodes were magnetic, radioactive and blue, 8 were magnetic and radioactive and 3 were solely magnetic. A correlation between the number of SLNs identified by pre-operative imaging and the number of SLNs removed during surgery is shown in Table 1.
Table 1.
Correlation between the number of sentinel lymph nodes (SLNs) identified by pre-operative MRI and lymphoscintigraphy (LS)/single photon emission CT (SPECT)-CT and the number of magnetic and radioactive SLNs removed during surgery
| Patient | SLNs on SPIO MRI (n) | SLNs on LS/SPECT-CT (n) | Magnetic SLNs resected (n) | Radioactive SLNs resected (n) |
|---|---|---|---|---|
| 1 | 4 | 1 | 4 | 1 |
| 2 | 0 | 1 | 3 | 3 |
| 3 | 1 | 1 | 1 | 1 |
| 4 | 3 | 1 | 2 | 2 |
| 5 | 2 | 1 | 2 | 2 |
| 6 | 3 | 1 | 2 | 2 |
| 7 | 2a | 1b | 1 | 1b |
| 8 | 1 | 1 | 2 | 2 |
| 9 | 2 | 2 | 2 | 2 |
| 10 | 1a | 1 | 1 | 1 |
| 11 | 2 | 2 | 2 | 2 |
LN, lymph node; SPIO, superparamagnetic iron oxide.
In these patients, one additional LN with iron uptake was identified, but it was not clear whether these LNs received lymphatic drainage directly from the injection site or from one of the SLNs. These LNs were therefore not classified as SLNs.
One additional parasternal SLN was identified by LS/SPECT-CT. The intercostal space was not explored during surgery, and the parasternal node was therefore not removed and not shown in this table.
The pre-operative MRI showed the same number of magnetic SLNs as found during surgery in 6 of 11 patients (55%). Of the remaining five patients, three patients showed one additional SLN on MRI which was not identified during surgery and one patient showed one additional SLN removed during surgery which was not identified on MRI. In one patient (Patient 2, Table 1), no SLNs were identified on MRI; however, three magnetic SLNs were removed during surgery.
LS/SPECT-CT accurately predicted the number of radioactive SLNs identified during surgery in 6 of 11 patients (55%). Four patients had one additional SLN removed during surgery which was not identified on nuclear imaging. In one patient (Patient 2, Table 1), two additional radioactive SLNs were removed during surgery.
Histopathological analysis of the resected SLNs revealed nodal involvement in two patients (Patients 1 and 2, Table 1). Patient 2 (Table 1) was excluded in the analysis of nodal metastases because no SPIO uptake in the LNs was observed during MRI examination. All four magnetic SLNs of Patient 1 harboured macrometastasis; one of these four SLNs showed heterogeneous SPIO uptake (Figure 3) and was correctly classified as metastatic. Two histopathologically node-negative patients were falsely classified as metastatic.
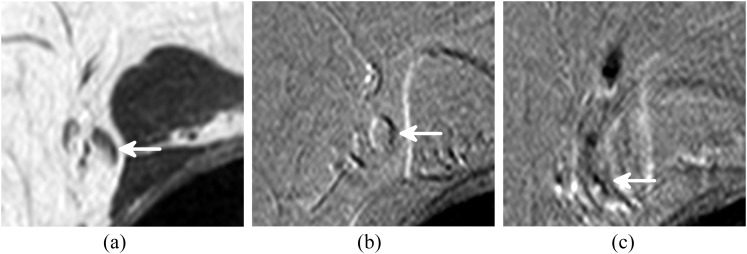
Figure 3.
A sentinel lymph node (SLN) showing inhomogeneous superparamagnetic iron oxide uptake. The arrow indicates the SLN on (a) T1 weighted turbo spin echo image, (b) pre-contrast T2 weighted gradient echo (GRE) image and (c) post-contrast T2 weighted GRE image. The position of the lymph node is different in the post-contrast scan owing to the movement of the patient during the procedure.
DISCUSSION
This study demonstrates the feasibility of SPIO MRI for pre-operative localization of SLNs. SPIO MRI successfully identified SLNs in 10 of 11 patients compared with 11 of 11 patients with the standard combined technique. The concordance between the number of nodes identified by imaging and during surgery was 55% for both the magnetic and the combined technique.
Poor spatial resolution of LS is a recognized drawback of SLNB with radioisotopes. Several animal and patient studies with SPIO MRI have been performed to improve pre-operative localization of SLNs to provide a surgical roadmap.11–15 However, these studies still rely on the use of radioisotopes for intraoperative detection of the sentinel nodes. Shiozawa et al16 was the first to present an entirely radioisotope-free procedure, using SPIO MRI for pre-operative localization and a handheld magnetometer for intraoperative detection of SLNs. They successfully identified SLNs with MRI in nine of nine patients; however, MRI only predicted the number of SLNs accurately in three of nine (33%) patients. We achieved a concordance between the number of nodes identified by imaging and during surgery of 55%, equal to the combined technique. Shiozawa et al16 used a different, possibly less sensitive, magnetometer which could explain this difference.
In the first two patients in our study, highly time-variable distribution of SPIO was observed. In the first patient, SPIO uptake was seen immediately after injection. However, in the second patient, no SPIO uptake was observed in LNs on MRI, 35 min after injection. Nevertheless, a low-signal lymphatic tract extending towards the axilla was seen 22 min after injection of SPIO. Three magnetic SLNs were identified during surgery the next day, so it is apparent that lymphatic drainage was slow, and it is most likely that SLNs would have been detected by MRI if scanning had continued for a longer period. Other studies25–27 have shown that many factors (e.g. body mass index, age, tumour size) affect the identification rate of SLNs, and it is hypothesized that these factors also alter lymphatic drainage. To improve drainage of the tracer, i.e. to prevent unsuccessful SLN uptake of SPIO, a massage of the injection site was performed in the remaining nine patients. This is in line with the practice guideline of the European Association of Nuclear Medicine/Society of Nuclear Medicine and Molecular Imaging (EANM/SNMMI).5 Immediate tracer uptake in the SLNs was observed in the remaining nine patients, indicating that massaging improves lymphatic drainage and should therefore be performed for this application.
Concordance rates between the number of nodes identified by imaging and during surgery of the combined technique reported in the literature range from 39% to 73%,28–31 which is in line with our findings. In all the (five of five) discordant procedures, LS/SPECT-CT underestimated the number of SLNs while SPIO MRI overestimated the number of SLNs in three of five discordant procedures. A possible explanation for this observation is the limited resolution of LS; closely located SLNs may appear as one hotspot. The high resolution of MRI allows to distinguish these closely located SLNs. The fact that MRI is more sensitive to very small concentrations of SPIO than the handheld magnetometer may result in a higher number of SLNs on imaging compared with surgery. The differences in the number of SLNs identified by LS/SPECT-CT and MRI could also be explained by difference in lymphatic drainage from the peritumoral and periareolar injection sites. There remains considerable debate about the different drainage patterns. Large differences between the drainage patterns from the different sites were recently reported in a small study,32 while Caruso et al33 concluded that the same SLNs were reached from different injection sites. In our study, the SLNs identified during surgery were both magnetic and hot in all but one patient, indicating similar drainage patterns, and thus supporting the conclusion drawn by Caruso.
The clinical significance of discordance between the number of SLNs found on imaging and during surgery remains unclear. In a recent retrospective study,31 no higher axillary recurrence rate was found when fewer SLNs were identified during surgery than were seen on LS.
The magnetic tracer generated an artefact at the injection site in the breasts of all patients. This was not noted by Shiozawa et al16 and Motomura et al,34 using a similar magnetic tracer (Resovist®; Fujifilm RI Farma Co., Ltd, Kyobashi, Tokyo). Motomura administered approximately 150× less magnetic tracer compared with our study, which could explain this finding. However, Shiozawa used a similar dosage to that in our study.
Although the artefact at the injection site did not affect axillary imaging, future work should evaluate whether this artefact persist and focus on limiting the artefact by optimizing dosage, injection site and scan parameters. The technique should not be used in patients who will subsequently require breast MRI surveillance.
The secondary goal of this study was to evaluate the potential of SPIO MRI to identify LN metastasis pre-operatively. An accurate method could alter the current treatment pathway of patients with early breast cancer by sparing patients without nodal involvement, an unnecessary SLNB procedure, while patients with nodal involvement can directly undergo axillary LN dissection without having an SLNB procedure. In this study, only 1 of 10 patients presented with (macro) metastases. One of the four involved SLNs of this patient was accurately classified as metastatic on MRI, but there were false negative results in the other three nodes. Moreover, two patients were falsely classified as node-positive. Since the primary goal of this study was the localization of SLNs rather than their characterization, choices in the protocol were made which differed from previously used methods for the characterization of SLNs. The trials involving intravenous injected USPIOs17–19 all performed post-contrast scans after a minimum waiting time of 24 h to ensure that the magnetic nanoparticles could transport to and distribute through the LNs. Motomura et al34 used a similar interval of 18–24 h before post-contrast imaging was performed in their study with interstitial administered SPIO. To be able to perform our procedure in the current clinical schedule for SLNB, we performed post-contrast scanning immediately after injection. As described earlier, this was suitable for the localization of SLNs but may not be ideal for LN characterization. Johnson et al35 observed a significant correlation between the time of SPIO injection and LN resection and the SPIO distribution in the LN. A longer time interval between injection and resection resulted in distribution throughout the whole LN. A possible explanation for the false positive results in this study is that distribution through the whole LN was not yet complete at the time of scanning.
Since the handheld magnetometer is not as sensitive for low SPIO concentrations as MRI, a high dose of SPIO was used to facilitate intraoperative detection with the handheld magnetometer. High SPIO concentrations do not only shorten T2 relaxation in their vicinity but also influence relaxation in the surrounding voxels. As a result of this “blooming effect”, LNs with inhomogeneous SPIO uptake can appear to show complete homogeneous uptake, resulting in false negative findings. Shiozawa et al16 used a similar dosage and waiting time (45 mg iron, 20 min) as we did and obtained 100% sensitivity, specificity and accuracy. They included nine patients of whom only one had metastasis in two SLNs. Further evaluation is essential to find the optimal dosage and timing for characterization of involved LNs.
The use of SPIO MRI for SLNB has several advantages. It provides the surgeon with detailed anatomical information on the location of the SLNs, which serves as a detailed surgical roadmap. When combined with the intraoperative use of a handheld magnetometer, the need for radioisotopes can be completely eliminated. The magnetometer does not achieve equal transcutaneous penetration depth as the gamma probe; however, the pre-operative SPIO MRI can be used to determine the optimal incision site. Accurate localization may also facilitate the introduction of targeted removal of SLNs under local anaesthetic, further reducing the morbidity associated with a surgical intervention.
In addition, the technique has the potential to non-invasively characterize the involvement of the sentinel nodes, although further optimization is needed. In the future, the ability to non-invasively characterize the SLNs could eliminate the need for axillary surgery in part of the patients.
Since this study is a feasible study, the number of included patients is small and insufficient to perform meaningful statistical analysis. A limited number of patients were recruited because patients could participate in the surgical trial without participating in this imaging subprotocol. Furthermore, a lack of access to MRI scanning slots limited the number of recruited patients. Larger prospective studies to validate and optimize both pre-operative SPIO MRI and the surgical magnetic SLNB are needed. Secondly, the number of patients with nodal metastases is too small to draw valid conclusions about the efficacy of SPIO MRI to characterize SLN involvement.
CONCLUSION
This study demonstrated that SPIO MRI for pre-operative localization of SLNs is a feasible alternative to LS/SPECT-CT and, in combination with the intraoperative use of a handheld magnetometer, provides an entirely radioisotope-free technique for SLNB in patients with breast cancer. Further research is needed for the evaluation of MRI characterization of LN involvement using subcutaneous injection of magnetic tracer.
FUNDING
This research was supported by the Dutch Technology Foundation STW, which is part of the Netherlands Organisation for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs. This research was supported by an unrestricted Educational Grant from Endomagnetics Ltd, UK. This research is part of an UK National Institute for Health Research (NIHR) adopted trial.
Acknowledgments
ACKNOWLEDGMENTS
This research was performed in collaboration with Laboratorium Pathologie Oost Nederland, Hengelo, Netherlands. This work was undertaken in collaboration with both Kings College London/Guy's and St Thomas' Hospitals and University College London Hospital/University College London, which receive funding from the department of Health's National Institute for Health Research Biomedical Research Centre funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health.
Contributor Information
Joost J Pouw, Email: j.j.pouw@utwente.nl.
Maarten R Grootendorst, Email: maarten.grootendorst@kcl.ac.uk.
Roland Bezooijen, Email: r.bezooijen@mst.nl.
Caroline A H Klazen, Email: c.klazen@mst.nl.
Wieger I De Bruin, Email: w.debruin@mst.nl.
Joost M Klaase, Email: j.klaase@mst.nl.
Margaret A Hall-Craggs, Email: margaret.hall-craggs@uclh.nhs.uk.
Michael Douek, Email: michael.douek@kcl.ac.uk.
Bennie ten Haken, Email: b.tenhaken@utwente.nl.
REFERENCES
- 1.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003; 349: 546–53. doi: 10.1056/NEJMoa012782 [DOI] [PubMed] [Google Scholar]
- 2.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006; 98: 599–609. doi: 10.1093/jnci/djj158 [DOI] [PubMed] [Google Scholar]
- 3.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. ; National Surgical Adjuvant Breast and Bowel Project. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 2007; 8: 881–8. doi: 10.1016/S1470-2045(07)70278-4 [DOI] [PubMed] [Google Scholar]
- 4.Goyal A, Mansel RE. Does imaging in sentinel node scintigraphic localization add value to the procedure in patients with breast cancer? Nucl Med Commun 2005; 26: 845–7. doi: 10.1097/00006231-200510000-00001 [DOI] [PubMed] [Google Scholar]
- 5.Giammarile F, Alazraki N, Aarsvold JN, Audisio RA, Glass E, Grant SF, et al. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur J Nucl Med Mol Imaging 2013; 40: 1932–47. doi: 10.1007/s00259-013-2544-2 [DOI] [PubMed] [Google Scholar]
- 6.Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D, et al. ; SentiMAG Trialists Group. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol 2014; 21: 1237–45. doi: 10.1245/s10434-013-3379-6 [DOI] [PubMed] [Google Scholar]
- 7.Thill M, Kurylcio A, Welter R, van Haasteren V, Grosse B, Berclaz G, et al. The Central-European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast 2014; 23: 175–9. doi: 10.1016/j.breast.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 8.Rubio IT, Diaz-Botero S, Esgueva A, Rodriguez R, Cortadellas T, Cordoba O, et al. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur J Surg Oncol 2015; 41: 46–51. doi: 10.1016/j.ejso.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, et al. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology 1988; 168: 297–301. doi: 10.1148/radiology.168.2.3393649 [DOI] [PubMed] [Google Scholar]
- 10.Weissleder R, Hahn PF, Stark DD, Elizondo G, Saini S, Todd LE, et al. Superparamagnetic iron oxide: enhanced detection of focal splenic tumors with MR imaging. Radiology 1988; 169: 399–403. doi: 10.1148/radiology.169.2.3174987 [DOI] [PubMed] [Google Scholar]
- 11.Kitamura N, Kosuda S, Araki K, Tomifuji M, Mizokami D, Shiotani A, et al. Comparison of animal studies between interstitial magnetic resonance lymphography and radiocolloid SPECT/CT lymphoscintigraphy in the head and neck region. Ann Nucl Med 2012; 26: 281–5. doi: 10.1007/s12149-011-0565-0 [DOI] [PubMed] [Google Scholar]
- 12.Madru R, Kjellman P, Olsson F, Wingardh K, Ingvar C, Ståhlberg F, et al. 99mTc-labeled superparamagnetic iron oxide nanoparticles for multimodality SPECT/MRI of sentinel lymph nodes. J Nucl Med 2012; 53: 459–63. doi: 10.2967/jnumed.111.092437 [DOI] [PubMed] [Google Scholar]
- 13.Mizokami D, Kosuda S, Tomifuji M, Araki K, Yamashita T, Shinmoto H, et al. Superparamagnetic iron oxide-enhanced interstitial magnetic resonance lymphography to detect a sentinel lymph node in tongue cancer patients. Acta Oto-laryngol 2013; 133: 418–23. doi: 10.3109/00016489.2012.744143 [DOI] [PubMed] [Google Scholar]
- 14.McCauley TR, Rifkin MD, Ledet CA. Pelvic lymph node visualization with MR imaging using local administration of ultra-small superparamagnetic iron oxide contrast. J Magn Reson Imaging 2002; 15: 492–7. doi: 10.1002/jmri.10089 [DOI] [PubMed] [Google Scholar]
- 15.Iida S, Imai K, Matsuda S, Itano O, Hatakeyama M, Sakamoto S, et al. In vivo identification of sentinel lymph nodes using MRI and size-controlled and monodispersed magnetite nanoparticles. J Magn Reson Imaging 2013; 38: 1346–55. doi: 10.1002/jmri.24108 [DOI] [PubMed] [Google Scholar]
- 16.Shiozawa M, Kobayashi S, Sato Y, Maeshima H, Hozumi Y, Lefor AT, et al. Magnetic resonance lymphography of sentinel lymph nodes in patients with breast cancer using superparamagnetic iron oxide: a feasibility study. Breast cancer 2014; 21: 394–401. doi: 10.1007/s12282-012-0401-y [DOI] [PubMed] [Google Scholar]
- 17.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003; 348: 2491–9. doi: 10.1056/NEJMoa022749 [DOI] [PubMed] [Google Scholar]
- 18.Memarsadeghi M, Riedl CC, Kaneider A, Galid A, Rudas M, Matzek W, et al. Axillary lymph node metastases in patients with breast carcinomas: assessment with nonenhanced versus uspio-enhanced MR imaging. Radiology 2006; 241: 367–77. doi: 10.1148/radiol.2412050693 [DOI] [PubMed] [Google Scholar]
- 19.Michel SC, Keller TM, Fröhlich JM, Fink D, Caduff R, Seifert B, et al. Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology 2002; 225: 527–36. doi: 10.1148/radiol.2252011605 [DOI] [PubMed] [Google Scholar]
- 20.Ahmed M, Usiskin SI, Hall-Craggs MA, Douek M. Is imaging the future of axillary staging in breast cancer? Eur Radiol 2014; 24: 288–93. doi: 10.1007/s00330-013-3009-5 [DOI] [PubMed] [Google Scholar]
- 21.Verbelen H, Gebruers N, Eeckhout FM, Verlinden K, Tjalma W. Shoulder and arm morbidity in sentinel node-negative breast cancer patients: a systematic review. Breast Cancer Res Treat 2014; 144: 21–31. doi: 10.1007/s10549-014-2846-5 [DOI] [PubMed] [Google Scholar]
- 22.Teshome KV, McCall LM, Cormier JN, Giuliano AE, Hunt K, eds Long-term Incidence of lymphedema after Sentinel lymph node dissection for early Stage breast Cancer: ACOSOG Z0010 (Alliance). Society of Surgical Oncology 67th Annual Cancer Symposium. Phoenix, Arizona: Springer; 2014. [Google Scholar]
- 23.Meng Y, Ward S, Cooper K, Harnan S, Wyld L. Cost-effectiveness of MRI and PET imaging for the evaluation of axillary lymph node metastases in early stage breast cancer. Eur J Surg Oncol 2011; 37: 40–6. doi: 10.1016/j.ejso.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 24.Pouw JJ, Ahmed M, Anninga B, Schuurman K, Pinder SE, Van Hemelrijck M, et al. Comparison of three magnetic nanoparticle tracers for sentinel lymph node biopsy in an in vivo porcine model. Int J Nanomedicine 2015; 10: 1235–43. doi: 10.2147/IJN.S76962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chagpar AB, Martin RC, Scoggins CR, Carlson DJ, Laidley AL, El-Eid SE, et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery 2005; 138: 56–63. doi: 10.1016/j.surg.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Gschwantler-Kaulich D, Riegler-Keil M, Ruecklinger E, Singer CF, Seifert M, Kubista E. Factors influencing the identification rate of the sentinel node in breast cancer. Eur J Cancer Care (Engl) 2011; 20: 627–31. doi: 10.1111/j.1365-2354.2011.01241.x [DOI] [PubMed] [Google Scholar]
- 27.Cox CE, Dupont E, Whitehead GF, Ebert MD, Nguyen K, Peltz ES, et al. Age and body mass index may increase the chance of failure in sentinel lymph node biopsy for women with breast cancer. Breast J 2002; 8: 88–91. doi: 10.1046/j.1524-4741.2002.08203.x [DOI] [PubMed] [Google Scholar]
- 28.Carmon M, Hain D, Shapira J, Golomb E. Preoperative lymphatic mapping does not predict the number of axillary sentinel lymph nodes identified during surgery in breast cancer patients. Breast J 2006; 12: 424–7. doi: 10.1111/j.1075-122X.2006.00296.x [DOI] [PubMed] [Google Scholar]
- 29.Mathew MA, Saha AK, Saleem T, Saddozai N, Hutchinson IF, Nejim A. Pre-operative lymphoscintigraphy before sentinel lymph node biopsy for breast cancer. Breast 2010; 19: 28–32. doi: 10.1016/j.breast.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 30.Teal CB, Slocum JP, Akin EA, Kelly TA. Correlation of lymphoscintigraphy with the number of sentinel lymph nodes identified intraoperatively in patients with breast cancer. Am J Surg 2005; 190: 567–9. doi: 10.1016/j.amjsurg.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 31.Volders JH, van la Parra RF, Bavelaar-Croon CD, Barneveld PC, Ernst MF, Bosscha K, et al. Discordance between number of scintigraphic and perioperatively identified sentinel lymph nodes and axillary tumour recurrence. Breast 2014; 23: 159–64. doi: 10.1016/j.breast.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 32.Noushi F, Spillane AJ, Uren RF, Cooper R, Allwright S, Snook KL, et al. High discordance rates between sub-areolar and peri-tumoural breast lymphoscintigraphy. Eur J Surg Oncol 2013; 39: 1053–60. doi: 10.1016/j.ejso.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 33.Caruso G, Cipolla C, Costa R, Morabito A, Latteri S, Fricano S, et al. Lymphoscintigraphy with peritumoral injection versus lymphoscintigraphy with subdermal periareolar injection of technetium-labeled human albumin to identify sentinel lymph nodes in breast cancer patients. Acta Radiol 2014; 55: 39–44. doi: 10.1177/0284185113493775 [DOI] [PubMed] [Google Scholar]
- 34.Motomura K, Ishitobi M, Komoike Y, Koyama H, Noguchi A, Sumino H, et al. SPIO-enhanced magnetic resonance imaging for the detection of metastases in sentinel nodes localized by computed tomography lymphography in patients with breast cancer. Ann Surg Oncol 2011; 18: 3422–9. doi: 10.1245/s10434-011-1710-7 [DOI] [PubMed] [Google Scholar]
- 35.Johnson L, Pinder SE, Douek M. Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology 2013; 62: 481–6. doi: 10.1111/his.12019 [DOI] [PubMed] [Google Scholar]