Abstract
Objective:
Compared with standard, whole-gland (WG) therapies for prostate cancer, focal approaches may provide equivalent oncologic outcomes with fewer adverse effects. The purpose of this study was to compare organ-at-risk (OAR) dosimetry between hemigland (HG) and WG stereotactic body radiotherapy (SBRT) plans.
Methods:
Volumetric-modulated arc radiotherapy-based SBRT plans were designed to treat the left HG, right HG and WG in eight patients, using five fractions of 8 Gy. OARs of interest included the contralateral HG, rectum, urinary bladder, urethra, penile bulb and contralateral neurovascular bundle.
Results:
Rectal V80% (the percentage of a normal structure receiving a dose of 80%) and V90% were significantly lower with HG plans than with WG plans (median values of 4.4 vs 2.5 cm3 and 2.1 vs 1.1 cm3, respectively, p < 0.05 by Student's t-test). Bladder V50% was also reduced significantly in HG plans (32.3 vs 17.4 cm3, p < 0.05), with a trend towards reduction of V100% (3.4 vs 1.3 cm3, p = 0.09). Urethral maximum dose and mean doses to the penile bulb and contralateral neurovascular bundle were also reduced significantly (42.0 vs 39.7 Gy, p < 0.00001; 13.3 vs 9.2 Gy, p < 0.05; and 40.2 vs 19.3 Gy, p < 0.00001, respectively).
Conclusion:
Targeting an HG volume rather than a WG volume when delivering SBRT can offer statistically significant reductions for all OARs. Given the large magnitude of the reduction in dose to these OARs, it is anticipated that HG SBRT could offer a superior toxicity profile when compared with WG SBRT. This is likely to be most relevant in the context of salvaging a local failure after radiation therapy.
Advances in knowledge:
The dosimetric feasibility of HG SBRT is demonstrated. When compared with WG SBRT plans, the HG plans demonstrate statistically significant and large magnitude reduction in doses to the rectum, bladder, urethra, penile bulb and contralateral neurovascular bundle, suggesting the possibility of improved toxicity outcomes with HG SBRT. This is likely to be most relevant in the context of salvaging a local failure after radiation therapy.
INTRODUCTION
While pathological studies1,2 demonstrate that prostate cancer (CaP) is a multifocal disease, recent data suggest that the largest and highest grade lesion, sometimes called the “index lesion”, is the true driver of the disease's natural history. This, in combination with multiparametric MRI (mpMRI) techniques that allow visualization of any such index lesions, raises the possibility of performing focal therapy for CaP.3,4 The theoretical advantage of such focal approaches is achieving a reduction in acute- and late-term toxicities by treating a smaller volume of the prostate while still maintaining oncologic efficacy. This may be particularly important in the context of salvage therapy for a local failure after definitive external beam radiotherapy (EBRT). In this setting, studies2,4,5 suggest that the most common site of recurrence is at the site of the “index lesion” and whole-gland (WG) retreatment series report rates of incontinence and fistula ranging from 6.2% to 49.7% and from 1.6% to 3.6%, respectively.
The optimal modality for focal therapy of CaP remains unknown. Investigated techniques include cryosurgery,6,7 high-intensity focused ultrasound,8 laser ablation,9 photodynamic therapy,10 and low-dose rate (LDR) brachytherapy.11,12 A recent consensus statement regarding LDR-based focal therapy outlined three broad strategies: a form of focused therapy, in which the WG is treated but the area of the index lesion is given a higher dose; hemigland (HG) treatment, in which the lobe of the prostate that contains the index lesion is treated; and ultrafocal treatment, in which only the index lesion is treated.13 It is not known which of these strategies is the most ideal. An HG approach may be the most practical as it does not necessitate an MRI–ultrasound fusion device or a perfect understanding of radiological–pathological correlation between the location of the index lesion on MRI and the location of the index lesion on pathology. Most prior planning studies of focal radiotherapy techniques have focused on either high-dose rate (HDR)14,15 and LDR brachytherapy.16 Indeed, only one prior report17 has evaluated the dosimetric advantages of focal EBRT techniques.
On the basis of multiple prospective studies, 18–22 the National Comprehensive Cancer Centre guidelines now consider stereotactic body radiotherapy (SBRT), a standard option for the definitive treatment of low- or intermediate-risk CaP. SBRT, when delivered via volumetric-modulated arc radiotherapy, allows for highly conformal dose delivery. As it requires only five treatments, it is considerably more cost-effective than conventionally fractionated EBRT and also takes radiobiological advantage of the low alpha/beta ratio of CaP.23–26 While it is obvious that an HG treatment will reduce doses to organs at risk (OARs) relative to a WG plan, the magnitude of these dose reductions and, more importantly, their clinical relevance remain unknown. In the upfront setting, the risk of serious (i.e. ≥grade 3) late Common Terminology Criteria for Adverse Events (CTCAE) genitourinary (GU) toxicity following SBRT ranges from 1.0% to 3.6%.27–29 Owing to the well-established low risk of serious late adverse events after SBRT in the upfront setting, the absolute marginal benefit of pursuing HG SBRT is expected to be modest. In the reirradiation setting, however, rates of long-term adverse are likely to be higher, and clinical data are still emerging. Several recent series30–32 exploring the outcomes of salvage SBRT in this setting have reported late CTCAE grade ≥3 GU toxicity rates after salvage SBRT approach of 7%. The purpose of this study was to assess the magnitude of the dosimetric advantages offered by performing HG prostate SBRT, with the hypothesis that the dosimetric benefits would be large enough to suggest a clinical benefit, particularly in the salvage setting.
METHODS AND MATERIALS
Patient population and hemigland definition
Eight patients with clinically localized CaP received HDR monotherapy for CaP at University of California, Los Angeles. We used the simulation CT scans for CaP who received HDR because all had Foley catheters placed, allowing accurate delineation of the urethra; patients treated with SBRT do not have catheters placed during simulation CT. All patients had a bowel preparation prior to their HDR brachytherapy catheter insertion, and at the time of simulation, the bladder was instilled with saline and contrast to improve visualization on CT imaging. The prostate and proximal seminal vesicles (SVs) were contoured to generate a WG clinical target volume (CTV). The HG contour was created from the WG contour by dividing the CTV into a left and right HG based on the Foley catheter as a surrogate for the urethra.
Contouring and planning
Because mpMRI imaging was not available for the patients in this study—who were all initially treated with HDR—all contours were based on simulation CT scans only. For WG treatments, the CTV was expanded by 5 mm in all directions except posteriorly to form a planning target volume (PTV); the posterior expansion was 4 mm. For HG treatments, the PTV was pulled back 2 mm from the urethra. SBRT plans were designed to deliver 8 Gy in five fractions and were generated using the Eclipse™ treatment planning system (Varian® Medical Systems, Inc., Palo Alto, CA). Contoured OARs included the rectum, urinary bladder, urethra, penile bulb and neurovascular bundles. Neurovascular bundles were contoured as presented in a published atlas.33 Dosimetric goals were as follows (note that, in all instances, an abbreviation Vx indicates the percentage of a normal structure receiving a dose “x”): PTV: V50%isodose/PTV < 4.0 (where V50%isodose refers to the volume of the 50% isodose cloud) and PTVV100% ≥ 95%; rectum: V50% ≤ 50% (i.e. volume of rectum receiving ≤50% of the prescription dose), V80% ≤ 20%, V90% ≤ 10% and V100% ≤ 5%; bladder: V50% ≤ 40%, V100% ≤ 10%; femoral head: V40Gy ≤ 5%; urethra, maximum dose (Dmax) < 42 Gy. Plans were designed to be delivered via volumetric-modulated arc radiotherapy with collimator angles of 30° and 300°, with two 360° arcs for WG treatments and two 180° arcs for HG treatments. No special planning adjustments were made to account for the presence of a Foley catheter or the presence of HDR brachytherapy catheters; other groups have reported that treating patients with SBRT with Foley catheters in place34 and the dosimetric impact of HDR catheters themselves are expected to be minimal.
Statistical analysis
A Student's t-test was used to analyse potential differences between WG and HG plans. Significance was set at a p-value <0.05.
RESULTS
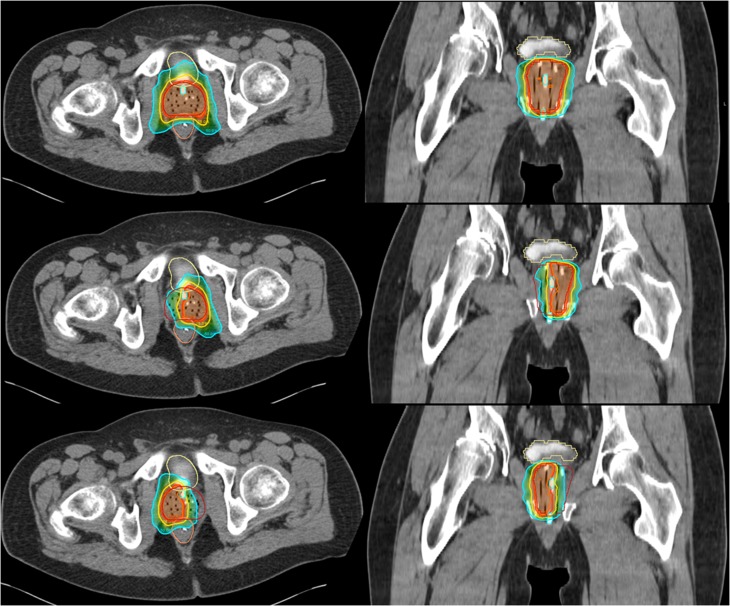
Figure 1 shows WG and corresponding HG SBRT plans for a sample patient. WG and HG comparative dosimetric data are summarized in Table 1. The HG values represent the average of the right and left HG plans. The median WG PTV size was 105.3 cm3 (range, 88.1–158.5 cm3), and the median HG PTV size was 47.8 cm3 (range, 39.5–78.9 cm3). As a result of diminished PTV size, PTVV50%/PTV was significantly larger for HG treatments (p < 0.05, Student's t-test). Rectal V80% and V90% were significantly reduced with HG treatments, with relative reductions with respect to V80% and V90% in the respective WG plans that ranged from 16.9% to 67.7% (median, 37.3%) for rectal V80% and from 11.8% to 79.4% (median, 38.5%) for rectal V90%. The reduction in rectal V50% showed a trend towards statistical significance (p = 0.055). Bladder V50% was significantly reduced in HG plans, while bladder V100% showed a trend towards reduction (p < 0.00001 and p = 0.09, respectively). Relative reductions afforded by the HG plans when compared with that of the WG plans ranged from 12.2% to 61.2% (median, 41.2%) for bladder V50% and from 33.3% to 94.1% (median, 50.0%) for bladder V100%. Although not optimized upon, urethral Dmax was significantly lower with HG treatments (39.7 vs 43.0 Gy, p < 0.001). With respect to the WG plan, urethral Dmax values were reduced by 4.9%–10.6% with HG plans (median, 7.9%). Mean penile bulb doses and contralateral neurovascular bundle doses were also significantly lower with HG plans (9.2 vs 13.3 Gy, p < 0.05 and 19.3 vs 40.2 Gy, p < 0.00001, respectively). Femoral head V40% averaged 0.2% in WG treatments and was not significantly reduced with HG treatment. The median contralateral “spill” volume, reported as V50% to the contralateral HG as a percentage, was 69.3% (range, 54.2–84.4%).
Figure 1.
Sample stereotactic body radiotherapy: whole-gland,35 left hemigland (HG) (middle) and right HG (bottom) plans. The colour code for the isodose colour wash is as follows: orange, 100%; red, 95%; yellow, 75%; teal, 50%. For colour image see online.
Table 1.
Whole-gland (WG) and hemigland (HG) dosimetric and volumetric organ-at-risk parameters for stereotactic body radiotherapy (SBRT)
| Dose–volume histogram parameter | WG (SD) | HG (SD) | HG/WG × 100% (SD) |
|---|---|---|---|
| CTV (cm3) | 56.2 (16.0) | 24.1 (6.9) | 42.8 (1.7) |
| V50%isodose/VCTV | 6.9 (0.7) | 10.5 (1.0) | 151.5 (15.5) |
| PTV (cm3) | 105.3 (28.5) | 51.5 (13.0)a | 44.8 (1.3) |
| V50%isodose/VPTV | 3.4 (0.1) | 4.6 (0.12)a | 144.9 (14.8) |
| PTV V100% (%) | 95.0 (0) | 95.0 (0) | – |
| Bladder V50% (cm3) | 32.3 (15.0) | 17.4 (9.3)a | 58.8 (12.8) |
| Bladder V100% (cm3) | 3.4 (2.2) | 1.3 (1.2) | 53.8 (9.6) |
| Rectum V50% (cm3) | 16.6 (4.9) | 12.0 (4.8) | 74.4 (14.3) |
| Rectum V80% (cm3) | 4.4 (1.1) | 2.5 (1.0)a | 58.4 (15.3) |
| Rectum V90% (cm3) | 2.1 (0.7) | 1.1 (0.64)a | 54.3 (17.2) |
| Rectum V100% (cm3) | 0.3 (0.3) | 0.15 (0.19) | 155.7 (15.4) |
| Urethra Dmax (Gy) | 43.0 (0.80) | 39.7 (0.54)a | 92.2 (1.5) |
| Mean penile bulb dose (Gy) | 13.3 (6.1) | 9.15 (4.2) | 82.6 (27.8) |
| Mean neurovascular bundle dose (Gy) | 40.2 (1.1) | 19.3 (2.8) | 48.1 (7.7) |
| Contralateral V50% “spill” volume (%) | – | 69.3 (9.0) | – |
CTV, clinical target volume; Dmax, maximum dose; PTV, planning target volume; SD, standard deviation.
All data are presented as median values, with SD in parentheses. In all instances, the abbreviation Vx indicates the percentage of a normal structure receiving a dose “x”, and the abbreviation V50%isodose refers to the volume of the 50% isodose cloud.
Statistically significant difference (p < 0.05) as compared with WG SBRT; HG, presented as average of right and left HG plans.
DISCUSSION
Focal therapy approaches for CaP are conceptually attractive because of their potential to offer more favourable toxicity profiles than WG treatments while still providing long-term tumour control. The ideal focal therapy modality remains unknown, and even when considering only radiation-based approaches, there are many options to choose from: LDR, HDR, conventional fractionated EBRT, hypofractionated EBRT and SBRT. In this study, we chose to focus on SBRT as a focal therapy modality of interest because it is non-invasive, cost-effective, allows treatment to be completed in five appointments and takes advantage of the low alpha/beta ratio of CaP.23–26 Clinical outcome data following SBRT demonstrate high prostate-specific antigen control rates and low significant acute and late toxicities.18–22 These excellent results and SBRT's favourable toxicity profile contrast significantly with non-radiation ablative techniques such as cryotherapy, for which WG treatments produce much greater toxicity, and focal approaches are necessary in order to achieve the “trifecta” of prostate-specific antigen control, urinary continence and potency.36,37 Because standard SBRT (i.e. WG SBRT) already offers a favourable toxicity profile, it is unclear as to what clinical benefit may be gleaned from a focal SBRT approach. This is compounded by the fact that initial focal therapy approaches have not actually yielded significantly lower toxicity rates.38 Thus, it is ultimately not merely a reduction in dose but rather the magnitude of reduction in dose and its relation to baseline toxicity rates, which is the most important consideration when estimating whether a focal approach will provide a meaningful translation into improved patient quality-of-life (QOL) outcomes.
Our results demonstrate that HG SBRT plans can offer statistically significant reductions in rectal, bladder and urethral doses when compared with WG SBRT plans. The magnitude of these reductions ranged from 12% to 79% for rectal V80–90% and from 12% to 61% for bladder V50%. Urethral Dmax was reduced by a median of 3.3 Gy, mean penile bulb dose was reduced by a median of 21 Gy and mean contralateral neurovascular bundle dose was reduced by a median of 20.7 Gy. In relative terms, reductions ranged from 41.6% to 45.7% for rectal V80–90%, 41.2% for bladder V50%, 7.8% for urethral Dmax, 17.4% for mean penile bulb dose and 51.9% for mean contralateral neurovascular bundle dose. Trying to understand whether this may translate into a clinically meaningful reduction requires a review of the SBRT literature regarding acute- and late-term morbidity (Table 2).
Table 2.
Literature review of stereotactic body radiotherapy late (>6 months) toxicity outcomes
| Study | n | Median follow-up (months) | Gastrointestinal | Genitourinary | Sexual |
|---|---|---|---|---|---|
| Rivin del Campo et al39 | 477 | 72 | RTOG Grade 3: 1.7% |
||
| King et al18 | 304 | 60 | RTOG Grade 2: 4.51% |
RTOG Grade 2: 8.18% Grade 3: 1.64% |
Percentage developed ED: 25% |
| Sher et al25 | 269 | 36 | CTCAE v. 3 Grade ≥2: 41.4% Grade 3: 1.5% |
||
| Katz and Kang40 | 100 | 36 | RTOG Grade 1: 2% Grade 2: 1% |
RTOG Grade 1: 4% Grade 2: 3% Grade 3: 1% |
|
| Mirallbell et al24 | 100 | 27.6 | CTCAE v. 3 Grade 1: 11% |
CTCAE v. 3 Grade 1: 26% Grade 2: 17% Grade 3: 1% |
Percentage developed ED: 21% |
| Obayomi-Davies et al41 | 84 | 50.8 | RTOG Grade 2: 7.14% Grade 4: 1.19% |
RTOG Grade 2: 5.95% |
|
| Resnick et al42 | 67 | 32.4 | RTOG Grade 1: 14% Grade 2: 2% Grade 3 0% |
RTOG Grade 1: 23% Grade 2: 5% Grade 3: 3.5% |
|
| Kupelian et al26 | 56 | 35.5 | CTCAE v. 4 Grade 1: 19.6% Grade 2: 19.6% Grade 3: 3.6% |
||
| Gomez et al43 | 32 | 35.5 | Percentage developed ED: 33% | ||
| Wiegner and King44 | 97 | 32.4 | Percentage developed ED: 45.6% | ||
| Marien et al36 | 864 | 36 | EPIC-26 bowel −0.85 points at 3 years |
EPIC-26 urinary +0.4 points at 3 years |
EPIC-26 sexual −7.3 points at 3 years |
| Kim et al45 | 228 | 45.6 | EPIC-26 bowel −0.8 points at 2 years |
EPIC-26 urinary −1.9 points at 2 years |
EPIC-26 sexual −7.5 points at 2 years |
| Jones et al37 | 174 | 46.8 | EPIC-26 bowel −2.4 points at 3 years |
EPIC-26 urinary −2.5 points at 3 years |
EPIC-26 sexual −7.5 points at 2 years |
CTCAE, common terminology criteria for adverse events; ED, erectile dysfunction; EPIC-26, Expanded Prostate Cancer Index Composite-26; RTOG, Radiation Therapy and Oncology Group.
With regard to gastrointestinal (GI) toxicity, King et al35 recently published patient-reported QOL outcomes for 864 patients treated with SBRT. They found that Expanded Prostate Cancer Index Composite-26 (EPIC-26) bowel domain scores decreased by 12 points from baseline to 3 months post SBRT but improved to 3.5 points below baseline at 6 months and returned to baseline at 3 years. Another group reported that the percentage of patients reporting a decrease in the EPIC-26 bowel domain summary score of >5 points was 46.8% at 1 month, 29.6% at 6 months, 29% at 12 months and 22.4% at 36 months.46 Reported rates of physician-scored toxicities, such as CTCAE and Radiation Therapy Oncology Group grade ≥3 toxicities, have ranged from 1% to 3%.27,47 Thus, while acute bowel toxicity is fairly common, it is typically low grade and largely resolved by 6 months post treatment. With regards to dosimetric correlations with GI toxicity, King et al reported that bowel QOL was decreased significantly among patients in the top 25th percentile of rectal V90% and V100%.43 Kim et al45 found that late CTCAE grade ≥3 rectal toxicity was correlated with rectal wall dosimetry (V50Gy >3 cm3 or V39Gy > 35%). In this context, the relative reductions in rectal V50–90% with HG plans are large enough to suggest improved toxicity profiles. However, it is not clear whether the resources necessary to investigate this hypothesis would be worthwhile, given that the baseline rates of serious GI toxicities in the upfront setting are low.
HG plans also reduced doses significantly to GU OARs. In general, post-SBRT GU toxicity is more frequent than GI toxicity both at acute and late time points, although most patients have resolution of symptoms at long-term follow-up. King et al35 reported an 8.7 point decrease in EPIC-26 urinary domain scores at 3 months and a 0.95 decrease at 6 months, with normalization of scores at 3 years. Similarly, the Georgetown University group reported that the percentage of patients reporting a decrease in the EPIC-26 urinary domain summary score of >2 points was 68.4% at 1 month, 43.2% at 6 months, 51.6% at 12 months and 41.8% at 36 months.46 They also found that <10% of patients felt that obstructive voiding symptoms were a moderate to big problem at 2 years post treatment.28 The same group reported 2-year incidences of 39.5% and 41.1% for acute and late CTCAE grade ≥2 toxicities, respectively.28 However, the authors identified a 1.5% incidence of late grade ≥3 toxicity and noted that the majority of grade 2 toxicities were based on the prescription of medications rather than clinical presentation. Indeed, Katz and Kang47 reported the incidence of late grade 2 urinary toxicity to be 9.1%. Correlations between GU toxicity and certain dosimetric parameters have also been reported. King et al43 recently reported that a significantly greater reduction in urinary EPIC-26 QOL was observed among patients with a high PTV V100% and high bladder V100%. The significant reduction in urinary QOL associated with high PTV V100% was thought to be related to urethral Dmax. Another study29 found that the overall risk of any CTCAE grade ≥2 urinary toxicity was associated with larger prostate volume, urethral V44Gy and bladder V19Gy. As with GI toxicity and rectal dosimetry, it is expected that the large reductions in bladder and urethral doses seen in this study might translate into clinically meaningful benefits in toxicity. However, given that most post-SBRT GU toxicities seem to be self-limited, the added benefit of pursuing an HG SBRT approach in the upfront setting is unclear.
Thus, one must consider whether a benefit may present itself with respect to post-SBRT erectile dysfunction (ED). The analysis of sexual side effects following any form of therapy for CaP is complicated by the confounding effects of normal ageing and medical comorbidities; in addition, the relevant OARs for ED remain unknown.39 In terms of the development of ED following SBRT, EPIC-26 sexual domain scores appear to decrease by 5.1 points at 3 months, but rather than normalizing, they continue to decrease, with an average decrease of 7.3 points at 3 years and 13.7 points at 6 years.35 Another study44 found that the baseline ED rate increased from 38% to 71% after treatment but noted that more than half of the males younger than 70 years at the time of SBRT remained without ED at the last follow-up, compared with only 15% of those males aged ≥70 years. Other studies40,41,48 have confirmed these findings, noting a stable, long-term decline in EPIC sexual scores, and ED incidence rates that depend on baseline potency and age. Mean doses to both the penile bulb and the contralateral neurovascular bundles were significantly reduced with HG plans. With the aforementioned caveats that post-treatment ED is difficult to attribute to any one particular cause, the steady decline in sexual function, as opposed to bowel or bladder function, following a typical treatment with SBRT suggests that this may be a domain in which focal therapy may be of particular value. This may be most reasonable in the context of males who do not have ED at baseline. However, these males constitute a small proportion of incident CaP cases.42 Furthermore, a large proportion of patients who do not have ED at baseline may be younger, and the desire for potency preservation in this population must be weighed against selecting an oncologically appropriate therapy.
Alternatively, and perhaps most practically, HG SBRT may prove to have a greater clinical benefit in the salvage setting. In general, the frequency of serious adverse events following post-radiotherapy salvage treatments is significantly greater than in the upfront setting.5 No OAR dosimetric constraints have been established in the salvage reirradiation setting, and therefore, following the principle of “as low as reasonably achievable” with respect to OAR sparing is reasonable. This, in turn, would support using a focal approach, as long as it is oncologically isoeffective to a WG approach. Fuller et al32 recently published the results of a prospective study of 29 patients treated with salvage SBRT with 34 Gy in five fractions. Late CTCAE grade ≥3 toxicity was seen in two patients, one of whom had received prior LDR. There does appear to be a dose–response for GU toxicity, as an earlier study using 30 Gy in five fractions reported a 7% incidence of late grade 3 GU toxicity, and no late grade 3 toxicities were identified in a small cohort of patients treated with 25–30 Gy in five fractions.30,31 Serious GI toxicity was not reported in any of these studies. Given that the grade ≥3 toxicities appear to be at least twice as common with salvage SBRT compared with upfront SBRT, the dosimetric improvements seen with HG plans may be more clinically meaningful in the salvage setting.
To our knowledge, only one other report17 has investigated the dosimetry of an HG EBRT approach. Amini et al17 compared two hypofractionated plans for 10 consecutive patients, one a standard WG plan in which the entire prostate received 70 Gy in 28 fractions, and the other a dose-painted plan in which the involved lobe received 70 Gy in 28 fractions while the contralateral lobe received 50.4 Gy in 28 fractions. Compared with the WG plans, the dose-painted plans demonstrated significantly lower doses to the contralateral neurovascular bundles. However, the mean rectal dose, rectal V70Gy, bladder V70Gy and mean penile bulb doses were not significantly reduced. Our study, on the other hand, showed significant dosimetric advantages with respect to rectal, bladder, urethral, penile bulb and neurovascular bundle doses, and this difference is likely explained by the sparing of the contralateral HG in our study.
This work has several limitations. First, despite the significant dosimetric benefits offered by HG SBRT vs WG SBRT, this study does not provide accompanying clinical data to support the hypothesis that the toxicity profile of HG SBRT is superior to that of WG SBRT. Second, the CTVs included the proximal SVs. It is possible that the dosimetric benefits seen in this study would have been different had the SVs not been included in the CTV. Third, it is unclear whether the penile bulb and/or neurovascular bundle are the OARs responsible for sexual adverse effects after SBRT; therefore, the fact that HG SBRT spares them more than WG SBRT may not translate into a clinical benefit. Clearly, judicious treatment of patients on clinical protocols would be required to demonstrate any clinical consequences of the dosimetric differences reported here. Another limitation is that our proposed HG salvage SBRT approach would only be indicated in cases in which a lateralized local recurrence is suspected. In addition, our choice of 8 × 5 Gy as a prescription dose was largely based on our institutional experience at UCLA utilizing this dose-fractionation regimen. Arguably, the prescription dose should be lowered given concerns in the setting of focal salvage reirradiation of increased toxicity. We have certainly already learned this in HDR prostate brachytherapy in which data with salvage HDR are in the range of 6 × 6 Gy compared with closer to 7.25 × 6 Gy in the definitive monotherapy setting.49,50 Alternatively, if an HG SBRT approach allows improved OAR dosimetry, the prescription dose could be maintained at a higher level in the focal salvage reirradiation setting sans an increased risk of adverse effects. In addition, because of the retrospective nature of this planning study, in which patients who had already been treated with HDR were identified for the development of HG SBRT plans as a proof of principle, mpMRI images—which, in the salvage setting particularly, would help delineate the highest risk areas—were not available. Finally, the margins chosen for this planning study are adapted from those used in a previously reported prospective SBRT trial;51 our institution typically treats patients with SBRT using a linear accelerator. It is possible that utilization of a CyberKnife platform could allow further reduction of margins, potentially further reducing OAR doses.
CONCLUSION
Taken together, our results suggest that meaningful improvements in QOL might be possible with HG plans but that most frequent acute- and/or late-term toxicity following SBRT warranting an attempt at an improved toxicity profile is ED. More practically, HG SBRT may be useful in the context of salvage of a local failure after prior EBRT in which serious GU toxicities are more frequent. Whether the dosimetric advantages of an HG approach translate into a clinical benefit will require prospective analysis on a clinical trial.
Contributor Information
Amar U Kishan, Email: aukishan@gmail.com.
Sang J Park, Email: SPark@mednet.ucla.edu.
Christopher R King, Email: crking@mednet.ucla.edu.
Kristofer Roberts, Email: Kwroberts@mednet.ucla.edu.
Patrick A Kupelian, Email: pkupelian@mednet.ucla.edu.
Michael L Steinberg, Email: MSteinberg@mednet.ucla.edu.
Mitchell Kamrava, Email: mkamrava@mednet.ucla.edu.
REFERENCES
- 1.Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 2009; 15: 559–65. doi: 10.1038/nm.1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopra S, Toi A, Taback N, Evans A, Haider MA, Milosevic M, et al. Pathological predictors for site of local recurrence after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82: e441–8. doi: 10.1016/j.ijrobp.2011.05.035 [DOI] [PubMed] [Google Scholar]
- 3.Ahmed HU, Pendse D, Illing R, Allen C, van der Meulen JH, Emberton M. Will focal therapy become a standard of care for men with localized prostate cancer? Nat Clin Pract Oncol 2007; 4: 632–42. doi: 10.1038/ncponc0959 [DOI] [PubMed] [Google Scholar]
- 4.Valerio M, Ahmed HU, Emberton M, Lawrentschuk N, Lazzeri M, Montironi R, et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol 2014; 66: 732–51. doi: 10.1016/j.eururo.2013.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parekh A, Graham PL, Nguyen PL. Cancer control and complications of salvage local therapy after failure of radiotherapy for prostate cancer: a systematic review. Semin Radiat Oncol 2013; 23: 222–34. doi: 10.1016/j.semradonc.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 6.Bahn D, de Castro Abreu AL, Gill IS, Hung AJ, Silverman P, Gross ME, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol 2012; 62: 55–63. doi: 10.1016/j.eururo.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 7.Onik G, Vaughan D, Lotenfoe R, Dineen M, Brady J. The “male lumpectomy”: focal therapy for prostate cancer using cryoablation results in 48 patients with at least 2-year follow-up. Urol Oncol 2008; 26: 500–5. doi: 10.1016/j.urolonc.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Muto S, Yoshii T, Saito K, Kamiyama Y, Ide H, Horie S. Focal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancer. Jpn J Clin Oncol 2008; 38: 192–9. doi: 10.1093/jjco/hym173 [DOI] [PubMed] [Google Scholar]
- 9.Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, Atri M, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol 2009; 182: 1371–7. doi: 10.1016/j.juro.2009.06.035 [DOI] [PubMed] [Google Scholar]
- 10.Arumainayagam N, Moore CM, Ahmed HU, Emberton M. Photodynamic therapy for focal ablation of the prostate. World J Urol 2010; 28: 571–6. doi: 10.1007/s00345-010-0554-2 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen PL, Chen MH, Zhang Y, Tempany CM, Cormack RA, Beard CJ, et al. Updated results of magnetic resonance imaging guided partial prostate brachytherapy for favorable risk prostate cancer: implications for focal therapy. J Urol 2012; 188: 1151–6. doi: 10.1016/j.juro.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosset JM, Cathelineau X, Wakil G, Pierrat N, Quenzer O, Prapotnich D, et al. Focal brachytherapy for selected low-risk prostate cancers: a pilot study. Brachytherapy 2013; 12: 331–7. doi: 10.1016/j.brachy.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Langley S, Ahmed HU, Al-Qaisieh B, Bostwick D, Dickinson L, Veiga FG, et al. Report of a consensus meeting on focal low dose rate brachytherapy for prostate cancer. BJU Int 2012; 109: 7–16. doi: 10.1111/j.1464-410X.2011.10825.x [DOI] [PubMed] [Google Scholar]
- 14.Banerjee R, Park SJ, Anderson E, Demanes DJ, Wang J, Kamrava M. From whole gland to hemigland to ultra-focal high-dose-rate prostate brachytherapy: a dosimetric analysis. Brachytherapy 2015; 14: 366–72. doi: 10.1016/j.brachy.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 15.Mason J, Al-Qaisieh B, Bownes P, Thwaites D, Henry A. Dosimetry modeling for focal high-dose-rate prostate brachytherapy. Brachytherapy 2014; 13: 611–7. doi: 10.1016/j.brachy.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Al-Qaisieh B, Mason J, Bownes P, Henry A, Dickinson L, Ahmed HU, et al. Dosimetry modeling for focal low-dose-rate prostate brachytherapy. Int J Radiat Oncol Biol Phys 2015; 92: 787–93. doi: 10.1016/j.ijrobp.2015.02.043 [DOI] [PubMed] [Google Scholar]
- 17.Amini A, Westerly DC, Waxweiler TV, Ryan N, Raben D. Dose painting to treat single-lobe prostate cancer with hypofractionated high-dose radiation using targeted external beam radiation: is it feasible? Med Dosim 2015; 40: 256–61. doi: 10.1016/j.meddos.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 18.King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013; 109: 217–21. doi: 10.1016/j.radonc.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 19.Katz AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol 2013; 8: 118. doi: 10.1186/1748-717X-8-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network I. NCCN Clinical Practice Guidelines in Oncology (NCCN). 2015. [Google Scholar]
- 21.Henderson DR, Tree AC, van As NJ. Stereotactic body radiotherapy for prostate cancer. Clin Oncol (R Coll Radiol) 2015; 27: 270–9. doi: 10.1016/j.clon.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 22.Meier R. Dose-escalated robotic SBRT for Stage I–II prostate cancer. Front Oncol 2015; 5: 48. doi: 10.3389/fonc.2015.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002; 52: 6–13. doi: 10.1016/S0360-3016(01)02664-5 [DOI] [PubMed] [Google Scholar]
- 24.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys 2012; 82: e17–24. doi: 10.1016/j.ijrobp.2010.10.075 [DOI] [PubMed] [Google Scholar]
- 25.Sher DJ, Parikh RB, Mays-Jackson S, Punglia RS. Cost-effectiveness analysis of SBRT versus IMRT for low-risk prostate cancer. Am J Clin Oncol 2014; 37: 215–21. doi: 10.1097/COC.0b013e31827a7d2a [DOI] [PubMed] [Google Scholar]
- 26.Kupelian P, Mehta NH, King C, Steinberg M, Finkelstein SE, Fernandez E. Stereotactic body radiation therapy for prostate cancer: rational and reasonable. Pract Radiat Oncol 2015; 5: 188–92. doi: 10.1016/j.prro.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 27.Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol 2013; 8: 58. doi: 10.1186/1748-717X-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arscott WT, Chen LN, Wilson N, Bhagat A, Kim JS, Moures RA, et al. Obstructive voiding symptoms following stereotactic body radiation therapy for prostate cancer. Radiat Oncol 2014; 9: 163. doi: 10.1186/1748-717X-9-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seymour ZA, Chang AJ, Zhang L, Kirby N, Descovich M, Roach M, 3rd, et al. Dose-volume analysis and the temporal nature of toxicity with stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol 2015; 5: e465–72. doi: 10.1016/j.prro.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 30.Jereczek-Fossa BA, Beltramo G, Fariselli L, Fodor C, Santoro L, Vavassori A, et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82: 889–97. doi: 10.1016/j.ijrobp.2010.11.031 [DOI] [PubMed] [Google Scholar]
- 31.Zerini D, Jereczek-Fossa BA, Fodor C, Bazzani F, Maucieri A, Ronchi S, et al. Salvage image-guided intensity modulated or stereotactic body reirradiation of local recurrence of prostate cancer. Br J Radiol 2015; 88: 20150197. doi: 10.1259/bjr.20150197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller DB, Wurzer J, Shirazi R, Bridge SS, Law J, Mardirossian G. High-dose-rate stereotactic body radiation therapy for postradiation therapy locally recurrent prostatic carcinoma: preliminary prostate-specific antigen response, disease-free survival, and toxicity assessment. Pract Radiat Oncol Jun 2015. Epub ahead of print. doi: 10.1016/j.prro.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 33.Wright JL, Newhouse JH, Laguna JL, Vecchio D, Ennis RD. Localization of neurovascular bundles on pelvic CT and evaluation of radiation dose to structures putatively involved in erectile dysfunction after prostate brachytherapy. Int J Radiat Oncol Biol Phys 2004; 59: 426–35. doi: 10.1016/j.ijrobp.2003.11.022 [DOI] [PubMed] [Google Scholar]
- 34.Fuller DB, Naitoh J, Mardirossian G. Virtual HDR CyberKnife SBRT for localized prostatic carcinoma: 5-year disease-free survival and toxicity observations. Front Oncol 2014; 4: 321. doi: 10.3389/fonc.2014.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King CR, Collins S, Fuller D, Wang PC, Kupelian P, Steinberg M, et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 2013; 87: 939–45. doi: 10.1016/j.ijrobp.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 36.Marien A, Gill I, Ukimura O, Betrouni N, Villers A. Target ablation–image-guided therapy in prostate cancer. Urol Oncol 2014; 32: 912–23. doi: 10.1016/j.urolonc.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 37.Jones JS, Rewcastle JC, Donnelly BJ, Lugnani FM, Pisters LL, Katz AE. Whole gland primary prostate cryoablation: initial results from the cryo on-line data registry. J Urol 2008; 180: 554–8. doi: 10.1016/j.juro.2008.04.027 [DOI] [PubMed] [Google Scholar]
- 38.Giannarini G, Gandaglia G, Montorsi F, Briganti A. Will focal therapy remain only an attractive illusion for the primary treatment of prostate cancer? J Clin Oncol 2014; 32: 1299–301. doi: 10.1200/JCO.2013.54.8214 [DOI] [PubMed] [Google Scholar]
- 39.Rivin del Campo E, Thomas K, Weinberg V, Roach M, 3rd. Erectile dysfunction after radiotherapy for prostate cancer: a model assessing the conflicting literature on dose-volume effects. Int J Impot Res 2013; 25: 161–5. doi: 10.1038/ijir.2013.28 [DOI] [PubMed] [Google Scholar]
- 40.Katz AJ, Kang J. Stereotactic body radiotherapy as treatment for organ confined low- and intermediate-risk prostate carcinoma, a 7-year study. Front Oncol 2014; 4: 240. doi: 10.3389/fonc.2014.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obayomi-Davies O, Chen LN, Bhagat A, Wright HC, Uhm S, Kim JS, et al. Potency preservation following stereotactic body radiation therapy for prostate cancer. Radiat Oncol 2013; 8: 256. doi: 10.1186/1748-717X-8-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resnick MJ, Barocas DA, Morgans AK, Phillips SE, Chen VW, Cooperberg MR, et al. Contemporary prevalence of pretreatment urinary, sexual, hormonal, and bowel dysfunction: defining the population at risk for harms of prostate cancer treatment. Cancer 2014; 120: 1263–71. doi: 10.1002/cncr.28563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez CL, Xu X, Qi XS, Wang PC, Kupelian P, Steinberg M, et al. Dosimetric parameters predict short-term quality-of-life outcomes for patients receiving stereotactic body radiation therapy for prostate cancer. Pract Radiat Oncol 2015; 5: 257–62. doi: 10.1016/j.prro.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 44.Wiegner EA, King CR. Sexual function after stereotactic body radiotherapy for prostate cancer: results of a prospective clinical trial. Int J Radiat Oncol Biol Phys 2010; 78: 442–8. doi: 10.1016/j.ijrobp.2009.07.1748 [DOI] [PubMed] [Google Scholar]
- 45.Kim DW, Cho LC, Straka C, Christie A, Lotan Y, Pistenmaa D, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014; 89: 509–17. doi: 10.1016/j.ijrobp.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 46.Woo JA, Chen LN, Wang H, Cyr RA, Bhattasali O, Kim JS, et al. Stereotactic body radiation therapy for prostate cancer: what is the appropriate patient-reported outcome for clinical trial design? Front Oncol 2015; 5: 77. doi: 10.3389/fonc.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katz AJ, Kang J. Quality of life and toxicity after SBRT for organ-confined prostate cancer, a 7-year study. Front Oncol 2014; 4: 301. doi: 10.3389/fonc.2014.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattasali O, Chen LN, Woo J, Park JW, Kim JS, Moures R, et al. Patient-reported outcomes following stereotactic body radiation therapy for clinically localized prostate cancer. Radiat Oncol 2014; 9: 52. doi: 10.1186/1748-717X-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CP, Weinberg V, Shinohara K, Roach M, 3rd, Nash M, Gottschalk A, et al. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-year outcomes. Int J Radiat Oncol Biol Phys 2013; 86: 324–9. doi: 10.1016/j.ijrobp.2013.01.027 [DOI] [PubMed] [Google Scholar]
- 50.Demanes DJ, Martinez AA, Ghilezan M, Hill DR, Schour L, Brandt D, et al. High-dose-rate monotherapy: safe and effective brachytherapy for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 2011; 81: 1286–92. doi: 10.1016/j.ijrobp.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 51.King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC, Jr. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 2009; 73: 1043–8. doi: 10.1016/j.ijrobp.2008.05.059 [DOI] [PubMed] [Google Scholar]