Abstract
Mansonella ozzardi (Nematoda: Onchocercidae) is an understudied filarial nematode, originally described by Patrick Manson in 1897, that can be transmitted by two families of dipteran vectors, biting midges (most of them members of the genus Culicoides) and black flies (genus Simulium). With a patchy geographic distribution from southern Mexico to northwestern Argentina, human infection with M. ozzardi is highly prevalent in some of the Caribbean islands, along riverine communities in the Amazon Basin, and on both sides of the border between Bolivia and Argentina. There is no clinical entity unequivocally associated with M. ozzardi infection, although fever, arthralgia, headache, cold lower extremities, and itchy cutaneous rashes are occasionally mentioned in case report series. More recently, ocular manifestations (especially keratitis) have been associated with mansonelliasis, opening an important area of investigation. Here, we briefly review the biology, epidemiology, pathogenesis, and clinical aspects of M. ozzardi infection and point to some existing knowledge gaps, aiming to stimulate a research agenda to help filling them.
Keywords: Mansonella ozzardi, Nematode, Microfilariae, Amazon, Pathogenesis, Diagnosis
Introduction
Mansonella ozzardi (Nematoda: Onchocercidae) is one of the several filarial nematodes that infect humans. This relatively unknown parasite has a patchy geographic distribution across Latin America and the Caribbean, from southern Mexico to northwestern Argentina. Most infected people, regardless of the parasite density, are asymptomatic or have few symptoms. As a consequence, infections with M. ozzardi usually remain undiagnosed and untreated. Ill-defined and unspecific symptoms such as fever, arthralgia, headache, cold lower extremities, and itchy cutaneous rashes are occasionally reported by patients, but whether they are caused by M. ozzardi infection remains to be determined. Nevertheless, ocular manifestations potentially associated with mansonelliasis, especially keratitis, have attracted substantial interest from ophthalmologists in recent years. Here, we summarize key biological, epidemiological, and clinical aspects of M. ozzardi infection. We explore recent developments in pathogenesis, laboratory diagnosis, and chemotherapy and discuss the potential public health impact of this highly prevalent but largely neglected New World parasite.
Biological features
Three filarial nematodes of the genus Mansonella are known to cause human mansonelliasis: Mansonella streptocerca, which is endemic to Africa; Mansonella perstans, which is commonly found in Africa but also occurs in South America; and M. ozzardi, which is found exclusively in the Americas and the Caribbean islands.1 Only humans appear to be naturally infected with M. ozzardi; African patas monkeys (Erythrocebus patas), but not chimpanzees, rhesus, capuchin, or squirrel monkeys, are susceptible to experimental infection with this nematode.2
Sir Patrick Manson (1844–1922) first described the microfilariae of M. ozzardi in the late 1890s, while examining the peripheral blood of Amerindians living in the interior of the former British Guyana.3 The parasite, originally named Filaria ozzardi by Manson, was placed by Faust in the new genus Mansonella in the late 1920s.4 Several decades later, Orihel and Eberhard described the elusive adult male and female worms recovered from experimentally infected patas monkeys.2
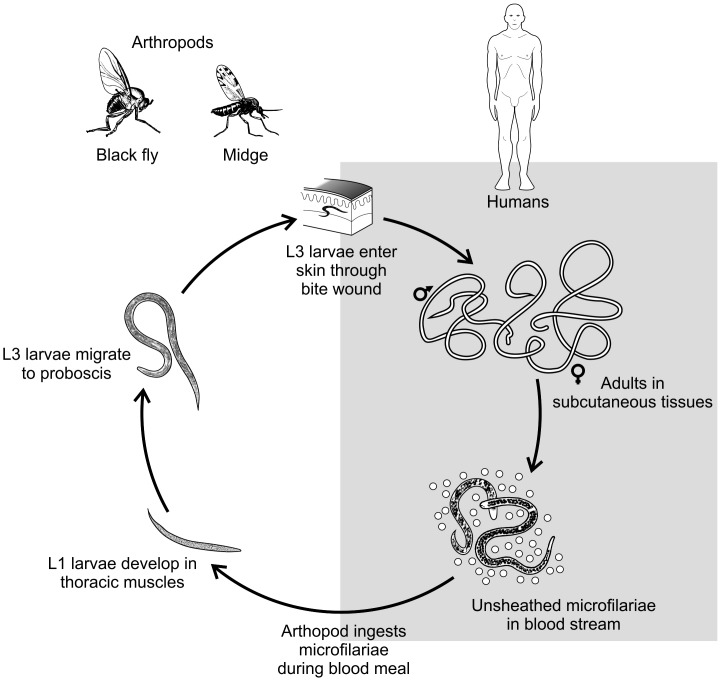
Natural infection with M. ozzardi begins with the bite of infected vectors, either biting midges (most of them of the genus Culicoides) or blackflies (genus Simulium), which deposits third-stage (L3) larvae onto the skin of the human host (Fig. 1). These L3 larvae undergo two further molts and develop into adult worms Adults are cylindrical in shape; the females measure 32–61 mm in length and 0.13–0.16 mm in diameter and the males 24–28 × 0.07–0.08 mm.5 Their presumed habitat in humans remains uncertain; in experimentally infected patas monkeys, small numbers of adult M. ozzardi worms have been recovered from subcutaneous tissues, but not from the abdominal cavity or mesenteries.2
Figure 1.
Schematic representation of the life cycle of Mansonella ozzardi. Modified from: Centers for Disease Control and Prevention. DPDx – Laboratory Identification of Parasitic Diseases of Public Health Concern. Mansonellosis. 2013. Available at: http://www.cdc.gov/dpdx/mansonellosis/index.html.
Unsheathed microfilariae with sharp tails are released by the viviparous female worms and reach the bloodstream. Circulating microfilariae are detected all day long, but moderate periodic fluctuation has been described in microfilarial density in the capillary blood of infected subjects.6,7 This fluctuation is not necessarily synchronous, with different peak hours among hosts originating a pattern of crypto-periodicity that may be difficult to discern.8 Microfilariae may also be occasionally found in the skin of infected subjects.9–11M. ozzardi microfilariae harbor maternally transmitted bacterial endosymbionts of the genus Wolbachia,12 with potential immunologic and therapeutic implications that have been recently reviewed elsewhere.13 The prepatent period in human infections is unknown, but in patas monkeys the first microfilariae are detected in the bloodstream 149–186 (mean, 168) days after subcutaneous inoculation of L3 larvae.2
Microfilariae measure 207–232 (mean, 220) μm in length and 3–4 μm in maximum diameter, when formalin-fixed and stained with hematoxylin, and 185–214 (mean, 200) μm × 4–5 μm, when methanol-fixed and stained with hematoxylin or Giemsa.5 The tip of the microfilaria’s sharp tail is devoid of nuclei, while its anterior extremity has a cephalic space (7–9 μm) that ends at the point where the nuclear column begins. The 2–3 anterior-most nuclei are typically found in a single line just caudal to the cephalic space,5 but occasionally two paired nuclei followed by a single nucleus are found in atypical M. ozzardi microfilariae described in Brazil14 and Peru.15
The microfilariae of M. ozzardi are usually smaller than those of Onchocerca volvulus (that causes human onchocerciasis), which measure 186–286 (mean, 253) μm in length.16 However, sizes may overlap, posing a major diagnostic challenge when unsheathed microfilariae are found in skin biopsies from communities in South America, where both species co-exist.16,17 In Brazil, M. ozzardi occurs sympatrically with O. volvulus in some areas within the Amazonian onchocerciasis focus.17–19 Further morphological16 and molecular19,20 analyses of skin-dwelling microfilariae are required to prevent misidentifying M. ozzardi as O. volvulus in these areas.
Similarly, M. perstans is also found in sympatry with M. ozzardi across the Amazon, in Southern Colombia,21 Western Guyana,22 and Venezuela.23
The microfilariae are ingested by the vectors (either biting midges of the genus Culicoides or black flies of the genus Simulium) during blood meals. Their subsequent development in the black flies, which have been described in detail by Tidwell and colleagues,24 is briefly summarized here. The ingested microfilariae migrate within 2 h of the blood meal from the stomach wall, through the hemocoel, to the thoracic musculature. The larvae shorten, reaching the minimum length (123 μm) within 48 h. The first molt is usually completed 4.5 days after infection, leading to early second-stage larvae measuring 291 μm in length and 23 μm in width. Following the second molt, third-stage infective larvae are first observed between days 5 and 6, mostly in the head (in the proboscis), but also in the thorax and intestine of the vectors, and measure approximately 630 × 18 μm. These larvae can be transmitted to the definitive host during the blood meal.
One parasite, two families of vectors
The arthropod species that are currently known to transmit M. ozzardi are listed in Table 1, but data from several endemic settings remain incomplete or absent. Vectors belonging to two dipteral families are involved in transmission. Biting midges, members of the genus Culicoides and, less frequently, Leptoconops (Diptera: Ceratopogonidae), were first identified as vectors on several Caribbean islands and Mexico, while black flies of Simulium genus (Diptera: Simuliidae) were shown to transmit this parasite in Central and South America. The biting midge Culicoides furens was first shown to transmit this parasite on St. Vincent Island in the early 1930s.25,26 Biting midges were later found to be M. ozzardi vectors on other Caribbean islands27–30 and the Yucatan Peninsula of Mexico.31 Subsequently, Culicoides phlebotomus was reported in endemic sites of Haiti27 and Trinidad.32 Several black flies, such as Simulium sanguineum, Simulium amazonicum, and Simulium argentiscutum, and the biting midge Culicoides insiniatus were reported in endemic sites of Colombia.24,33 S. amazonicum, S. argentiscutum, and Simulium oyapockense have been implicated as the main vectors in the Amazon Basin of Brazil,34–37Simulium sanchezi in Venezuela,38,39 S. sanguineum in Panama,40, and Simulium minusculum in Guyana.41 Romaña and Wigodzinsky described Culicoides paraensis as a vector of M. ozzardi in Tucumán province of northern Argentina.42 Shelley and Coscarón reported that, in the Jujuy province of Argentina, Culicoides lahillei was the main vector, while C. paraensis and Simulium exiguum were secondary vectors.43 C. lahillei, Culicoides debilipalpis, and C. paraensis were recently described as the main vectors of M. ozzardi in northwestern Argentina and southwest Bolivia.44 Interestingly, M. ozzardi microfilariae were also found to develop in the musculature of other experimentally infected dipterans, such as Anopheles aquasalis, Anopheles albitarsis, and Aedes aegypti.45
Table 1.
Mansonella ozzardi vectors across Latin America
| Region | Country | Biting midges | Black flies | Key references |
|---|---|---|---|---|
| North America | Mexico | Culicoides furens | Biagi et al.31 | |
| Central America | Panama | Simulium sanguineum | Petersen et al.40 | |
| Caribbean | Haiti | C. furens, C. barbosai | Lowrie et al.27–29 | |
| Leptoconops bequaerti | ||||
| St. Vincent | C. furens, C. paraensis? | Buckley25,26 | ||
| Trinidad | C. phlebotomus | Nathan30,32 | ||
| South America | Argentina | C. paraensis | Nathan and Wygodzinsky42 | |
| C. lahillei, C. paraensis | S. exiguum | Shelley and Coscarón43 | ||
| C. debilipalpis, C. lahillei, C. paraensis | Veggiani-Aybar et al.44 | |||
| Brazil | S. amazonicum, S. argentiscutum | Cerqueira34 | ||
| S. oyapockense s.l. or S. roraimense | Shelley et al.35–37 | |||
| Colombia | C. insinuatus, C. caprilesi? | S. amazonicum, S. argentiscutum | Tidwell et al.24,33 | |
| S. sanguineum | ||||
| Guyana | S. oyapockense s.l. | Nathan et al.41 | ||
| Suriname | C. guttatus? | Bruijning96 | ||
| Venezuela | S. oyapockense s.l., S. guyanensis | Yarzábal et al. and González38,39 |
Modified from Shelley and Coscarón.43
The finding that vectors from different insect families are able to transmit M. ozzardi led to the hypothesis that highly divergent parasite populations with contrasting vector preference circulated in the Caribbean and the Amazon.46 However, there is compelling evidence that M. ozzardi isolates from equatorial Colombia and subtropical Argentina can infect both black flies and biting midges.33,43,47 Moreover, M. ozzardi strains from the Amazon and the Caribbean are morphologically identical at the ultrastructural level.21
A patchy geographic distribution
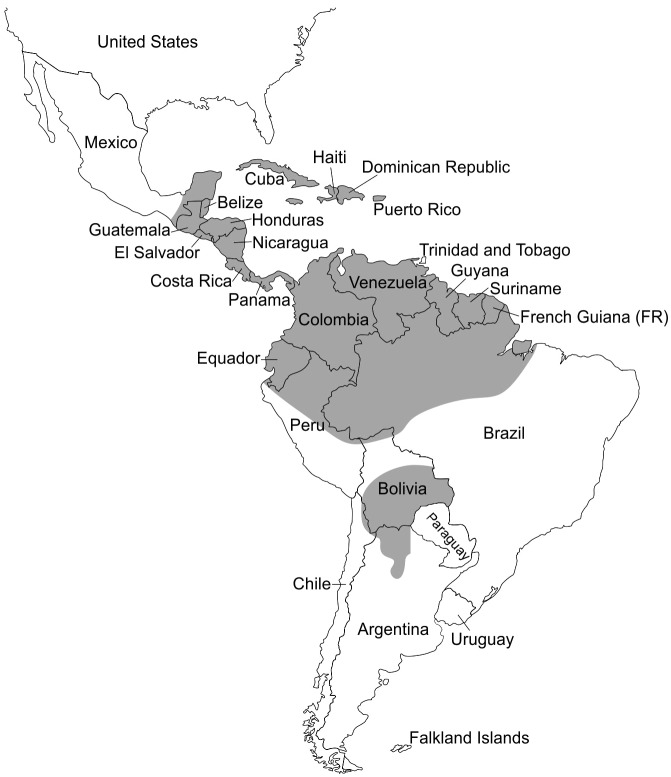
Human infections with M. ozzardi have been diagnosed exclusively in the Americas, from southern Mexico to northwestern Argentina (Fig. 2), with prevalence rates detected by conventional thick-smear microscopy ranging between zero and 46% in the general population (Table 2). Available data are fragmentary and should be interpreted with caution since studies differ according to the laboratory techniques used for diagnosis (which in turn differ in sensitivity), the age composition of surveyed populations, and several other factors that may affect prevalence estimates. Moreover, studies were carried out over several decades, and transmission levels may have varied with time in many endemic settings.
Figure 2.
Presumed geographic distribution of Mansonella ozzardi across the Americas. Regions where this parasite has been reported (either in population-based surveys or case reports) and those that are contiguous with known endemic areas are shaded, but within each shaded region the transmission tends to be focal, with high-prevalence pockets surrounded with areas with no transmission. See main text and Table 2 for details.
Table 2.
Prevalence of Mansonella ozzardi infection in selected populations, as determined by microscopic and molecular methods on blood samples
| Country | Sites | No. samples examined | Age range (years) | Prevalence (%) | Diagnostic technique | References |
|---|---|---|---|---|---|---|
| Argentina | Tucumán Province (El Cercado, Orán, El Churqui, El Molino, La Aguadita and El Timbó) | 7141 | 31–80 | 31.3 (El Molino) | Thick-smear microscopy | Mühlens et al.62 |
| Salta Province (Tartagal and Peña Morada) | 39.1 (Arroyo Colorado) | |||||
| Jujuy Province (Arroyo Colorado, Santa Clara, Santa Bárbara and San Pedro de Jujuy) | ||||||
| Argentina | Salta Province (El Oculto) | 29 | Mean, 45 | 20.7 | Thick-smear microscopy | Taranto and Castelli97 |
| Argentina | Jujuy Province (Río Colorado, Quebarchal, Barroso, Candelaria, Normenta, Arrayanal, Marta, Sauces, Loma del Medio, San Borja and Trementinal) | 107 | ≥5 | 41.1 | Thick-smear microscopy | Remondegui et al.63 |
| Argentina | Salta Province (Balderrama and Metán) | 32 | >40 | 50.0 | Two methods combineda | Zaidenberg64 |
| Argentina | Tucumán Province (Santa Ana) | 92 | Unspecified | 57.6 | Venous blood PCR | Degese et al.98 |
| 50.0 | Knott | |||||
| 27.2 | Membrane filtration | |||||
| Argentina | Salta Province (Acambuco, Aguas Blancas, El Oculto, San Ramón de la Nueva Orán, Algarrobal, Pichanal, Embarcación, General Ballivian, General Mosconi, Tartagal, Aguaray, Campo Durán and Salvador Mazza) | 417 | Adults | 92.3 (Salta Province) | Thick-smear microscopy | Veggiani-Aybar et al.44 |
| 46.9 (Tartagal) | ||||||
| 30.1 (San Ramón de la Nueva Orán) | ||||||
| Jujuy Province (Palma Sola, Isla Chica, San Borja, San Pedro de Jujuy and Libertador General San Martín) | 85.9 (Jujuy Province) | |||||
| 56.4 (Libertador General San Martín) | ||||||
| 20.0 (San Pedro de Jujuy) | ||||||
| Bolivia | Parapeti and Yuti rivers, Camiri, Cordillera Province (Guarani Indians and mestizos) | 296 | <1–81 | 26.0 | Thick-smear microscopy | Bartoloni et al.68 |
| Bolivia | Pilcomayo river, Villa Montes, Gran Chaco Province (Guarani Indians and mestizos) | 298 | <1–85 | 0.7 | Thick-smear microscopy | Bartoloni et al.68 |
| Brazil | Solimões river, Tabatinga (Ticuna Indians) | 800 | All ages | 45.7 | Thick-smear microscopy | Moraes et al.10 |
| Brazil | Içana river (Baniwa Indians) | 24 | >10 | 62.5 | Thick-smear microscopy | Lawrence et al.49 |
| Brazil | Marari river (Yanomama Indians) | 11 | >10 | 0 | Thick-smear microscopy | Lawrence et al.49 |
| Brazil | Solimões river (Ticuna Indians) | 56 | >10 | 30.3 | Thick-smear microscopy | Lawrence et al.49 |
| Brazil | Juruá river tributaries (Kanamari Indians) | 82 | >10 | 1.2 | Thick-smear microscopy | Lawrence et al.49 |
| Brazil | Purus river (Jaminawa Indians) | 15 | >10 | 0 | Thick-smear microscopy | Lawrence et al.49 |
| Brazil | Javari river (Marubo Indians) | 20 | >10 | 5.0 | Thick-smear microscopy | Lawrence et al.49 |
| Brazil | Juruá river tributaries (Kashinawa Indians) | 37 | >10 | 0 | Thick-smear microscopy | Lawrence et al.49 |
| Brazil | Envira river (Katukina Indians) | 42 | >10 | 0 | Thick-smear microscopy | Lawrence et al.49 |
| Brazil | Tacutu, Maú, Surumu and Cotingo rivers, western Roraima State (Makuxi and Wapixana Indians, 15 indigenous communities) | 652 | All ages | 3.2 | Thick-smear microscopy | Moraes et al.37 |
| Brazil | Purus river, Pauini (6 indigenous communities) | 169 | 2–71 | 28.4 | Thick-smear microscopy | Medeiros et al.48 |
| Brazil | Ituxi river, Lábrea (12 riverine communities) | 129 | ≥2 | 30.2 | Thick-smear microscopy | Medeiros et al.66 |
| Brazil | Purus river, Boca do Acre (9 riverine communities) | 282 | ≥2 | 27.3 | Thick-smear microscopy | Medeiros et al.52 |
| Brazil | Pauini river, Pauini (5 riverine communities) | 177 | 2 | 24.9 | Thick-smear microscopy | Medeiros et al.18 |
| Brazil | Purus rivers, Pauini (30 riverine communities) | 744 | ≥2 | 24.6 | Thick-smear microscopy | Medeiros et al.18 |
| Brazil | Purus river, Lábrea (23 riverine communities) | 694 | ≥2 | 20.7 | Thick-smear microscopy | Medeiros et al.57 |
| Brazil | Solimões river, urban area of Coari | 1069 | All ages | 10.2 | Thick-smear microscopy | Martins et al.61 |
| Brazil | Solimões river, rural area of Coari (10 riverine communities) | 664 | All ages | 18.4 | Thick-smear microscopy | Martins et al.61 |
| Brazil | Mamoré, Madeira, Guaporé, Machado and Preto rivers (urban populations and riverine communities) | 4452 | >5 | 0 | Thick-smear microscopy | Basano et al.54 |
| Brazil | Tefé river, Tefé (11 riverine communities) | 300 | 2–82 | 6.3 | Thick-smear microscopy | Medeiros et al.99 |
| Brazil | Acre river, Porto Acre and Vila Antimary | 217 | >18 | 12.9 | Knott | Adami et al.53 |
| Brazil | Antimary river (two riverine communities) | 78 | >18 | 1.3 | Knott | Adami et al.53 |
| Brazil | Purus river, Boca do Acre (2 riverine communities) | 60 | >18 | 60.0 | Knott | Adami et al.53 |
| Brazil | Solimões river, Codajás (riverine communities) | 109 | All ages | 44.9 | Venous blood PCR | Medeiros et al.87 |
| 25.7 | Thick-smear microscopy | |||||
| Brazil | Solimões river, Coari (riverine communities) | 105 | All ages | 44.8 | Venous blood PCR | Medeiros et al.87 |
| 29.5 | Capillary blood (FTA) PCR | |||||
| 22.9 | Thick-smear microscopy | |||||
| Brazil | Solimões river, Codajás (7 riverine communities) | 245 | All ages | 10.2 | Thick-smear microscopy | Medeiros et al.100 |
| Brazil | Solimões river, Coari 5 (riverine communities) | 127 | All ages | 22.0 | Thick-smear microscopy | Medeiros et al.100 |
| Colombia | Amazonas river, Comisaría del Amaznonas, Letícia (several indigenous peoples and mestizos) | 535 | >10 | 47.1 | Knott | Kozek et al.101 |
| Colombia | Amazonas river, Comisaría del Guainía, Puerto Inírida (several indigenous peoples and mestizos) | 604 | All ages | 20.0 | Knott | Kozek et al.102 |
| Guyana | Six districts country wide (indigenous communities) | 9506 | All ages | 1.5 | Thick-smear microscopy | Orihel22 |
| Haiti | Bayeux, north coast | 1165 | All ages | 16.1 | Thick-smear microscopy | Raccurt et al.103 |
| Haiti | Corail, Grande Anse region, north coast | 462 | All ages | 16.5 | Thick-smear microscopy | Raccurt et al.56 |
| Mexico | Northern Yucatan Peninsula | 296 | All ages | 61.1 | Thick-smear microscopy | Biagi et al.31 |
| Panama | Chucunaque river, Darien (villages of Morti, Uala and Membrillo) | 312 | All ages | 67.5 (Morti) | Thick-smear microscopy | Petersen et al.40 |
| 18.3 (Uala) | ||||||
| 10.9 (Membrillo) | ||||||
| Peru | Amazonas river, Iquitos (periurban villages) | 433 | All ages | 1.4 | Thick-smear microscopy | Arróspide et al.74 |
| Peru | Santa Maria de Nanay, Alto Nanay district, Loreto | 134 | All ages | 47.8 | Thick-smear microscopy | Vargas et al.67 |
| Trinidad | Blanchisseuse, Northern Range montains (coastal community) | 602 (1980) | All ages | 23.3 (1980) | Two methods combinedb | Chadee et al.84 |
| 348 (1992) | 21.5 (1992) | Two methods combinedb | ||||
| 348 (1992) | 19.2 (1992) | Thick-smear microscopy | ||||
| 384 (1992) | 11.8 (1992) | Membrane filtration | ||||
| Venezuela | Southwestern Bolívar State (8 indigenous communities) | 139 | All ages | 57.6 | Thick-smear microscopy | Godoy et al.104 |
| Venezuela | Orinoco river, Southeastern Orinoquia (13 indigenous communities) | 806 | >10 | 22.2 | Thick-smear microscopy | Medrano et al.51 |
| Venezuela | Orinoco and Negro basins, Amazonas and Bolívar State (17 riverine communities) | 1057 | Unspecified | 9.9 | Knott | Gómez and Guerrero105 |
Thick-smear microscopy and Knott combined.
Thick-smear microscopy and membrane filtration combined.
Despite the parasite’s versatility regarding vector preference, M. ozzardi transmission is clearly focal. Many transmission hotspots in the Amazon Basin map to indigenous communities, such as the Ticuna on Solimões river,10 the Apurinã on Purus river,48 and other Amerindian villages in Brazil49 and Venezuela.50,51 Endemicity levels vary widely among rural villages situated a few kilometers apart along the same rivers in the western Amazon Basin of Brazil.52,53 Nevertheless, some riverside villages remain free of infection despite the presence of competent simuliid vectors.54 The environmental factors that limit M. ozzardi spread beyond well-characterized hotspots along major rivers (mostly Solimões, Purus, Negro, and Orinoco) and their tributaries in the Amazon Basin remain largely undetermined, but a similarly patchy geographic distribution has been described for ceratopogonid-transmitted M. perstans in rural Africa.55 In Haiti, where C. furens larvae breed in both brackish and freshwater while C. barbosai breeds exclusively in mangrove salty marshes, all major foci are located in coastal areas.56 Transmission levels appear to have increased in recent years in known endemic areas of Brazil. For example, substantially higher positivity rates were detected by microscopy in riverine communities along the Purus river revisited in the 2000s52,53,57 compared to the prevalence rates found at the time of the first survey, in the 1970s.58 Relatively few cases of mansonelliasis have been documented in major cities of Amazonian Brazil,59,60 but urban M. ozzardi transmission appears to occur in towns and small cities along the Solimões61 and Acre53 rivers.
The highest prevalence rates in northwestern Argentina are currently found in sites covered by subtropical mountainous rainforest (Yungas), such as Tartagal and San Ramón de la Nueva Orán, both in Salta province, and Libertador General San Martín and San Pedro de Jujuy, both in Jujuy province.44 This is consistent with previous studies in the provinces of Jujuy 62,63 and Salta.64 The number of M. ozzardi infections diagnosed by microscopy has declined sharply in northwestern Argentina since 1986, when malaria transmission started to decrease and active case detection of malaria parasite carriers became less intensive.44
The prevalence of M. ozzardi microfilaraemia in most endemic communities in the Amazon and the Caribbean typically increases with age, being highest in middle-age adults.10,44,48,49,53,56,60,65–67 There is also evidence that average microfilaria counts, among microscopy-positive subjects, increase with age,48,68 consistent with increased exposure in adults leading to frequent superinfection in the absence of effective acquired immunity. Adult males are more often affected than females,18,44,48,52 with greater average microfilaremias in males,48 suggesting that the risk of infection may be associated with occupation-related exposure (e.g. subsistence farming and fishing). Gender-related differences in the prevalence of infection, which are more pronounced in the adult population, are also seen in perstans mansonelliasis, lymphatic filariasis, and onchocerciasis in Africa.55 In the Chaco region of Bolivia, however, similar prevalences of infection with M. ozzardi are found in males and females.68 The close proximity between individuals’ dwellings and main rivers is another well-recognized risk factor for infection.58,61
Is M. ozzardi entirely harmless?
Unspecific symptoms and clinical signs classically associated with ozzardi mansonelliasis include fever, articular pain, headache, cold lower extremities, cutaneous rashes and lymphadenopathy.53,69,70 However, available descriptions of clinical symptoms are mostly derived from relatively small case series with no control group in which other infectious and noninfectious conditions have been explicitly ruled out. Eosinophilia is commonly observed, being reversed after treatment.70,71 Deposition of circulating immune complexes, which have been detected in M. ozzardi infection,72 might trigger articular inflammation and pain. However, not all community-based surveys revealed significant associations between clinical manifestations and the presence of infection.68,73,74 As noted by Bartoloni and colleagues,68 age may be a strong confounder of this association. In unadjusted analysis, they found a significant association between infection and unspecific clinical manifestations such as arthralgia, headache, and pruritus. However, they argue that this association may have been confounded by age since the prevalence of infection and that of unspecific symptoms in the general population increase with age. No association between clinical manifestations and M. ozzardi infection in the Chaco region of Bolivia was observed after Bartoloni and colleagues adjusted their analysis for age and gender.68
Since the early 2000s, ocular lesions have been described in M. ozzardi-infected subjects in indigenous and non-indigenous communities along the Negro and Solimões rivers in the Amazon Basin of Brazil.75–77 No O. volvulus transmission has been documented in these areas17 and in two of these studies all subjects tested negative for O. volvulus microfilariae in skin biopsies.75,76 Garrido and Campos first described multiple nummular infiltrates in the cornea, with ≤2 mm in diameter, in 55% of 140 infected subjects (mostly indigenous) from the upper Negro river, with 2–8 lesions per eye.75 None of the 358 uninfected subjects examined had similar corneal lesions. Most lesions were peripheral and did not affect the visual acuity of affected subjects.75 A subsequent cross-sectional survey was carried out in riverine communities in Coari, along the Solimões river, where the overall prevalence rate of ozzardi mansonelliasis is estimated at 19%.76 Corneal lesions were diagnosed in 15% of 95 infected subjects; punctate keratitis accounted for most (12 of 14) corneal lesions diagnosed, with sclerosing and nummular keratitis being found in only one subject each.76 No microfilariae were found by microscopy or polymerase chain reaction (PCR) in conjuntival and limbal biopsies of five keratitis patients and corneal biopsies of three patients.76 Finally, another cross-sectional survey in Coari found corneal lesions in 14 of 56 (25%) microfilaremic subjects and 8 of 156 (5%) uninfected controls.77 These authors also provided the first report of M. ozzardi microfilariae identified in the cornea. They found, by confocal microscopy, linear lesions consistent with M. ozzardi microfilariae in the cornea of seven subjects; two of them had microfilariae detected on Giemsa-stained smears prepared with blood from the limbal conjuntiva.77
Although there is no definitive evidence that migrating M. ozzardi microfilariae can directly cause corneal lesions, a careful eye examination is recommended to be part of the routine clinical care of infected subjects, regardless of any symptoms. In areas of Central and South America where M. ozzardi and O. volvulus co-occur, skin biopsies are mandatory to rule out onchocerciasis as a cause of corneal lesions. Moreover, further studies are required to determine the extent to which the corneal lesions so far described are associated with visual symptoms and reduced visual acuity and whether lesions are responsive to therapeutic interventions to suppress microfilaremia and reduce ocular inflammation.
Chronic infections with tissue-invasive helminths typically affect immune responses to co-infecting pathogens by creating an immunoregulatory environment dominated by interleukin (IL)-10 and transforming growth factor (TGF)-β. The impact of filarial infections on antimalarial immunity has been characterized in detail in Africa, with an IL-10-dependent decrease in IL-12p70, interferon (IFN)-γ, and IFN-γ-induced protein 10 (IP-10) responses upon T-cell stimulation with malarial antigens,78–80 but no data are available for chronic, mostly undiagnosed and untreated M. ozzardi infections in the Amazon.
Microscopic and molecular diagnosis
Most infections with M. ozzardi have been diagnosed by microscopic examination of Giemsa-stained thick blood smears. Because malaria and ozzardi mansonelliasis co-occur in several endemic settings, microfilariae are often found on thick smears originally prepared for malaria diagnosis67, and sometimes co-infection with Plasmodium parasites were reported.62,67,81
As microfilariae circulate in the peripheral blood all day long, diagnostic capillary or venous blood samples can be obtained at any time. Concentration methods are required to diagnose low-density microfilaremias. A widely used concentration technique was originally described by Knott in 1939.82,83 It involves mixing 5 ml of anticoagulated venous blood with 50 ml of a 2% formalin solution in a polystyrene tube and recovering microfilariae in the sediment after a brief centrifugation at 400g. A carefully collected aliquot of the sediment is used to prepare a thick smear, which is fixed with methanol, stained with Giemsa and examined under a microscope.
Alternatively, polycarbonate membrane filtration may be used, allowing for the examination of relatively large blood volumes. Anticoagulated venous blood (up to 10 ml) is diluted in 0.85% saline solution or phosphate-buffered saline and filtered through a 13 or 25 mm polycarbonate membrane (pore size, 3 μm) adapted to a sterile syringe. After this filtering procedure, followed by several washes with saline solution, the wet membrane is placed on a glass slide, fixed with methanol, stained with Giemsa or hematoxylin, and examined for retained microfilariae under 10–40× magnification. A survey in Trinidad showed an 1.6 × higher diagnostic sensitivity of thick-smear microscopy (using 20-μl finger-prick blood samples) compared to membrane filtration (5 ml of venous blood filtered through 25-mm Nuclepore filters with 3-μm porosity).84
Although M. ozzardi microfilariae can be found in the skin of infected subjects, skin biopsies should be obtained and examined for diagnostic purposes only in areas where ozzardi mansonelliasis and onchocerciasis are known or suspected to co-occur.9–11 M. ozzardi microfilariae have also been found in the ascitic fluid of a patient.85
Molecular diagnosis may be used to detect M. ozzardi microfilariae in the peripheral blood, skin biopsies and other tissues. PCR-based amplification of species-specific target sequences allows for increased diagnostic sensitivity, compared with microscopic methods, and reliable differentiation between M. ozzardi and co-endemic filarial species such as O. volvulus and M. perstans.19,20,86,87
Key factors that determine the diagnostic sensitivity of PCR include sample storage conditions prior to DNA extraction, sample volume, and methods used to isolate DNA.87 The PCR method described by Tang and colleagues19 was 1.5 × more sensitive when DNA templates were prepared from 2.5-μl of venous blood, compared with those isolated from 0.6 μl of dried blood from FTA membranes.87 Moreover, venous blood-based PCR was 1.8 × more sensitive than conventional thick smear microscopy (one smear examined per subject).87
Therapeutic approaches
Unlike most other filarial species, diethylcarbamazine (DEC) has little or no effect on microfilariae of M. ozzardi.88 Accordingly, DEC-based mass chemotherapy (montly doses of 6 mg/kg of DEC citrate over 12 months administered between 1980 and 1981) has eliminated W. bancrofti from a rural community of Trinidad, but had virtually no impact on the local prevalence of M. ozzardi infection.84 In contrast, the daily administration of levamisole (150 mg/day, adult dose) for 2–3 months has been found to suppress M. ozzardi microfilaremia in a limited number of patients.70
Ivermectin (one single dose of 0.14–0.2 mg/kg) is currently the treatment of choice,89,90 although it remains uncertain whether it has any effect against adult worms. A single dose of 0.15 mg/kg of ivermectin has recently been reported to suppress M. ozzardi microfilaremia for at least 12 months in 53 subjects from Brazil, suggesting some effect on female worm survival or fertility.71 Adverse effects that are reminiscent of Mazzotti reaction,91 such as fever, chills, malaise, headache, arthralgia, dizziness, and dyspnea, have been often observed after the administration of ivermectin, but nearly all patients recover rapidly without specific therapy.71,89,92 The severity of post-treatment reaction appears to correlate positively with pretreatment microfilarial density in O. volvulus91 and W. bancrofti93 infections, but whether this holds for M. ozzardi remains to be determined.
Because M. ozzardi harbors the endosymbiotic bacteria Wolbachia,12 doxycycline may be an effective therapy to eliminate adult worms,94 as recently shown for M. perstans.95 However, no trials with doxycycline (either alone or in combination with ivermectin) have been conducted for M. ozzardi infection. Similarly, there are no data regarding the therapeutic efficacy of mebendazole or albendazole against M. ozzardi microfilariae or adult worms, but these benzimidazoles appear to be of poorly effective against M. perstans.55
Conclusions
Although infections with M. ozzardi are highly prevalent in areas of the Caribbean, the Amazon, and on both sides of the border between Bolivia and Argentina, several knowledge gaps persist. For example, there is no clinical entity unequivocally associated with human infection with M. ozzardi, but corneal lesions putatively caused by migrating microfilariae are a major reason for concern. It remains to be examined whether chronic mansonelliasis may exert a strong immunomodulatory effect, as many other helminthic infections. The habitat of adult worms in human hosts is uncertain and more affordable experimental models are surely needed to scrutinize the parasite’s biology. Potential vector species have been carefully characterized in some Caribbean islands, parts of the Amazon and northern Argentina, but little is known for most other endemic settings. Despite recent evidence for ivermectin efficacy against microfilariae, it remains unclear whether this antihelmintic, either alone or combined with other drugs, is able to kill adult worms. These unknowns render M. ozzardi a typical neglected parasite that affects mostly poor rural populations across Latin America and the Caribbean.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (2013/12723-7). NFL receives a PhD scholarship from FAPESP (2013/26928-0) and MUF receives a senior researcher scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Ethics approval
Not applicable.
All named authors contributed equally to this article.
References
- 1.Downes BL, Jacobsen KH. A systematic review of the epidemiology of mansonelliasis. Afr J Infect Dis. 2010;4:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orihel TC, Lowrie RC Jr, Eberhard ML, Raccurt C, Kozek WJ, Tidwell MA, et al. Susceptibility of laboratory primates to infection with Mansonella ozzardi from man. Am J Trop Med Hyg. 1981;30:790–4. [DOI] [PubMed] [Google Scholar]
- 3.Manson P. On certain new species of nematode hematozoa occurring in America. Br Med J. 1897;2:1837–8. [Google Scholar]
- 4.Bain O, Mutafchiev Y, Junker K, Guerrero R, Martin C, Lefoulon E, Uni S. Review of the genus Mansonella Faust, 1929 sensu lato (Nematoda: Onchocercidae), with descriptions of a new subgenus and a new subspecies. Zootaxa. 2015;3918:151–93. 10.11646/zootaxa.3918.2 [DOI] [PubMed] [Google Scholar]
- 5.Orihel TC, Eberhard ML. Mansonella ozzardi: a redescription with comments on its taxonomic relationships. Am J Trop Med Hyg. 1982;31:1142–7. [DOI] [PubMed] [Google Scholar]
- 6.Rachou RC, Lacerda NB. Da variação horária da microfilaremia de Mansonella ozzardi [On hourly variation of Mansonella ozzardi microfilaremia]. Rev Bras Malariol Doenças Trop. 1954;6:343–8. [PubMed] [Google Scholar]
- 7.Moraes MAP. Estudo sobre a variação nictimeral de microfilaremia de “Mansonella ozzardi” [A study on nictemeral variation of Mansonella ozzardi microfilaremia]. Hospital (Rio J). 1959;56:869–74. [PubMed] [Google Scholar]
- 8.Pichon G. Crypto-periodicity in Mansonella ozzardi. Trans R Soc Trop Med Hyg. 1983;77:331–3. 10.1016/0035-9203(83)90155-4 [DOI] [PubMed] [Google Scholar]
- 9.Moraes MAP. Mansonella ozzardi microfilariae in skin snips. Trans R Soc Trop Med Hyg. 1976;70:16. [DOI] [PubMed] [Google Scholar]
- 10.Moraes MA, Almeida MM, Lovelace JK, Chaves GM. Mansonella ozzardi among Ticuna Indians of the State of Amazonas, Brazil. Bol Oficina Sanit Panam. 1978;85:16–25. [PubMed] [Google Scholar]
- 11.Ewert A, Smith JH, Corredor A. Microfilariae of Mansonella ozzardi in human skin biopsies. 1981;30:988–91. [DOI] [PubMed] [Google Scholar]
- 12.Casiraghi M, Favia G, Cancrini G, Bartoloni A, Bandi C. Molecular identification of Wolbachia from the filarial nematode Mansonella ozzardi. Parasitol Res. 2001;87:417–20. 10.1007/s004360000368 [DOI] [PubMed] [Google Scholar]
- 13.Slatko BE, Luck AN, Dobson SL, Foster JM. Wolbachia endosymbionts and human disease control. Mol Biochem Parasitol. 2014;195:88–95. 10.1016/j.molbiopara.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Adami YL, Moraes MAP, Lanfredi RM, Maia-Herzog M. An atypical microfilaria in blood samples from inhabitants of Brazilian Amazon. Parasitol Res. 2008;104:95–9. 10.1007/s00436-008-1164-4 [DOI] [PubMed] [Google Scholar]
- 15.Marcos LA, Arrospide N, Recuenco S, Cabezas C, Weil GJ, Fischer PU. Genetic characterization of atypical Mansonella (Mansonella) ozzardi microfilariae in human blood samples from northeastern Peru. Am J Trop Med Hyg. 2012;87:491–4. 10.4269/ajtmh.2012.11-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post RJ, Adams Z, Shelley AJ, Maia-Herzog M, Luna Dias AP, Coscarón S. The morphological discrimination of microfilariae of Onchocerca volvulus from Mansonella ozzardi. Parasitology. 2003;127:21–7. 10.1017/S003118200300324X [DOI] [PubMed] [Google Scholar]
- 17.Shelley AJ. Human onchocerciasis in Brazil: an overview. Cad Saude Publica. 2002;18:1167–77. 10.1590/S0102-311X2002000500009 [DOI] [PubMed] [Google Scholar]
- 18.Medeiros JF, Py-Daniel V, Barbosa UC, Izzo TJ. Mansonella ozzardi in Brazil: prevalence of infection in riverine communities in the Purus region, in the state of Amazonas. Mem Inst Oswaldo Cruz. 2009;104:74–80. 10.1590/S0074-02762009000100012 [DOI] [PubMed] [Google Scholar]
- 19.Tang TH, López-Vélez R, Lanza M, Shelley AJ, Rubio JM, Luz SL. Nested PCR to detect and distinguish the sympatric filarial species Onchocerca volvulus, Mansonella ozzardi and Mansonella perstans in the Amazon Region. Mem Inst Oswaldo Cruz. 2010;105:823–8. 10.1590/S0074-02762010000600016 [DOI] [PubMed] [Google Scholar]
- 20.Morales-Hojas R, Post RJ, Shelley AJ, Maia-Herzog M, Coscarón S, Cheke RA. Characterization of nuclear ribosomal DNA sequences from Onchocerca volvulus and Mansonella ozzardi (Nematoda: Filarioidea) and development of a PCR-based method for their detection in skin biopsies. Int J Parasitol. 2001;31:169–77. 10.1016/S0020-7519(00)00156-9 [DOI] [PubMed] [Google Scholar]
- 21.Kozek WJ, Raccurt C. Ultrastructure of Mansonella ozzardi microfilaria, with a comparison of the South American (simuliid-transmitted) and the Caribbean (culicoid-transmitted) forms. Tropenmed Parasitol. 1983;34:38–53. [PubMed] [Google Scholar]
- 22.Orihel TC. Infections with Dipetalonema perstans and Mansonella ozzardi in the aboriginal Indians of Guyana. Am J Trop Med Hyg. 1967;16:628–35. [DOI] [PubMed] [Google Scholar]
- 23.Formica S, Botto C. Filariasis focus due to Mansonella ozzardi and Mansonella perstans in the Amazon Federal Territory of Venezuela. J Trop Med Hyg. 1990;93:160–5. [PubMed] [Google Scholar]
- 24.Tidwell MA, Tidwell MA, Hoyos PM. Development of Mansonella ozzardi in a black fly species of the Simulium sanguineum group from eastern Vaupés, Colombia. Am J Trop Med Hyg. 1980;29:1209–14. [DOI] [PubMed] [Google Scholar]
- 25.Buckley JJC. On the development, in Culicoides furens Poey, of Filaria (=Mansonella) ozzardi Manson, 1897. J Helminthol. 1934;12:99–118. 10.1017/S0022149X00003229 [DOI] [Google Scholar]
- 26.Buckley JJC. A note on the development of Filaria ozzardi in Culicoides furens Poey. J Helminthol. 1933;11:257–8. 10.1017/S0022149X00001899 [DOI] [Google Scholar]
- 27.Lowrie RC Jr, Raccurt C. Mansonella ozzardi in Haiti II. Arthropod vector studies. Am J Trop Med Hyg. 1981;30:598–603. [DOI] [PubMed] [Google Scholar]
- 28.Lowrie RC Jr, Raccurt CP. Assessment of Culicoides barbosai as a vector of Mansonella ozzardi in Haiti. Am J Trop Med Hyg. 1984;33:1275–7. [DOI] [PubMed] [Google Scholar]
- 29.Lowrie RC Jr, Raccurt CP, Eberhard ML, Katz SP. Assessment of Leptoconops bequaerti as a potential vector of Mansonella ozzardi in Haiti. Am J Trop Med Hyg. 1983;32:1013–15. [DOI] [PubMed] [Google Scholar]
- 30.Nathan MB. Transmission of the human filarial parasite Mansonella ozzardi by Culicoides phlebotomus (Williston) (Diptera: Ceratopogonidae) in coastal north Trinidad. Bull Entomol Res. 1981;71:97–105. 10.1017/S0007485300051063 [DOI] [Google Scholar]
- 31.Biagi F, Tay J, de Biagi A. Observaciones sobre mansonelosis en La península de Yucatán. Culicoides furens como transmisor [Observations on mansonelliasis in Yucatán Peninsula. Culicoides furens as a vector]. Rev Med México. 1958;38:377–9. [PubMed] [Google Scholar]
- 32.Nathan NB. Culicoides phlebotomus, a vector of M. ozzardi in coastal North Trinidad West Indies. Trans R Soc Trop Med Hyg. 1978;72:436–437. [Google Scholar]
- 33.Tidwell MA, Tidwell MA. Development of Mansonella ozzardi in Simulium amazonicum, S. argentiscutum, and Culicoides insinuatus from Amazonas, Colombia. Am J Trop Med Hyg. 1982;31:1137–41. [DOI] [PubMed] [Google Scholar]
- 34.Cerqueira NL. Sobre a transmissão de Mansonella ozzardi [On Mansonella ozzardi transmission]. J Bras Med. 1959;1:885–914. [Google Scholar]
- 35.Shelley AJ, Shelley A. Further evidence for the transmission of Mansonella ozzardi by Simulium amazonicum in Brazil. Ann Trop Med Parasitol. 1976;70:213–7. 10.1080/00034983.1976.11687114 [DOI] [PubMed] [Google Scholar]
- 36.Shelley AJ, Luna Dias AP, Moraes MA. Simulium species of the amazonicum group as vectors of Mansonella ozzardi in the Brazilian Amazon. Trans R Soc Trop Med Hyg. 1980;74:784–8. 10.1016/0035-9203(80)90200-X [DOI] [PubMed] [Google Scholar]
- 37.Moraes MAP, Shelley AJ, Luna-Dias APA. Mansonella ozzardi no Território Federal de Roraima: Distribuição e achado de um novo vetor na área do Rio Surumu [Mansonella ozzardi in the Federal Territory of Roraima: distribution and finding of a new vector in Surumu river area]. Mem Inst Oswaldo Cruz. 1985;80:395–400. 10.1590/S0074-02761985000400003 [DOI] [PubMed] [Google Scholar]
- 38.Yarzábal L, Basáñez MG, Ramírez-Pérez J, Ramírez A, Botto C, Yarzábal A. Experimental and natural infection of Simulium sanchezi by Mansonella ozzardi in the Middle Orinoco region of Venezuela. Trans R Soc Trop Med Hyg. 1985;79:29–33. 10.1016/0035-9203(85)90226-3 [DOI] [PubMed] [Google Scholar]
- 39.González R. Estudo de la participación de Simulium pintoi (D’Andretta & D’Andretta, 1946) en la transmissón de Mansonella ozzardi (Manson, 1897) en San José de Cayama, Estado Bolívar. Infección natural y experimental [A study on the role of Simulium pintoi (D’Andretta & D’Andretta, 1946) in Mansonella ozzardi (Manson, 1897) transmission in San José de Cayama, Bolívar State. Natural and experimental infection. Thesis. Ciudad Bolívar, thesis]. Universidad de Oriente, Ciudad Bolívar (Venezuela); 1987. [Google Scholar]
- 40.Petersen JL, Bawden MP, Wignall FS, Latorre CR, Johnson CM, Miranda CR. Mansonella ozzardi in Darién (Panama). Rev Med Panama. 1984;9:236–46. [PubMed] [Google Scholar]
- 41.Nathan MB, Tikasingh ES, Munroe P. Filariasis in Amerindians of Western Guyana with observations on transmission of Mansonella ozzardi by a Simulium species of the amazonicum group. Tropenmed Parasitol. 1982;33:219–22. [PubMed] [Google Scholar]
- 42.Romaña M, Wygodzinsky P. Acerca de la transmisión de Mansonella ozzardi (Manson) (Filaria tucumana Biglieri y Araoz) [On transmission of Mansonella ozzardi (Manson) (Filaria tucumana Biglieri and Araoz)]. An Inst Med Reg. 1950;3:29–34. [Google Scholar]
- 43.Shelley AJ, Coscarón S. Simuliid blackflies (Diptera: Simuliidae) and ceratopogonid midges (Diptera: Ceratopogonidae) as vectors of Mansonella ozzardi (Nematoda: Onchocercidae) in northern Argentina. Mem Inst Oswaldo Cruz. 2001;96:451–8. [DOI] [PubMed] [Google Scholar]
- 44.Veggiani-Aybar CA, Dantur-Juri MJ, Zaidenberg MO. Mansonella ozzardi in Neotropical region of Argentina: Prevalence through time (1986–2010). Acta Trop. 2016;153:1–6. 10.1016/j.actatropica.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 45.Davis N. A study on the transmission of filaria in Northern Argentina. Am J Trop Med Hyg. 1928;8:457–466. [Google Scholar]
- 46.Nelson GS, Pester FR. The identification of infective filarial larvae in Simuliidae. Bull World Health Organ. 1962;27:473–81. [PMC free article] [PubMed] [Google Scholar]
- 47.Lowrie RC Jr, Orihel TC, Eberhard ML. Culicoides variipennis, a laboratory vector for the Amazon form of Mansonella ozzardi. Am J Trop Med Hyg. 1982;31:166–7. [DOI] [PubMed] [Google Scholar]
- 48.Medeiros JF, Py-Daniel V, Barbosa UC, Farias ES. Epidemiological studies of Mansonella ozzardi (Nematoda: Onchocercidae) in indigenous communities of Pauini municipality, Amazonas, Brazil. Acta Amazon. 2007;37:241–6. [Google Scholar]
- 49.Lawrence DN, Erdtmann B, Peet JW, Nunes de Mello JA, Healy GR, Neel JV, et al. Epidemiologic studies among Amerindian populations of Amazonia. II. Prevalence of Mansonella ozzardi. Am J Trop Med Hyg. 1979;28:991–6. [DOI] [PubMed] [Google Scholar]
- 50.Godoy GA, Volcan G, Medrano C, Teixeira A, Matheus L. Mansonella ozzardi infections in Indians of the Southwestern part of the state of Bolivar, Venezuela. Am J Trop Med Hyg. 1980;29:373–6. [DOI] [PubMed] [Google Scholar]
- 51.Medrano CE, Volcán GS, Godoy GA. Mansonelliasis in the southeast Venezuelan Orinoquia region. Rev Inst Med Trop S Paulo. 1992;34:61–70. [PubMed] [Google Scholar]
- 52.Medeiros JF, Py-Daniel V, Barbosa UC, Ogawa GM. Occurrence of Mansonella ozzardi (Nematoda, Onchocercidae) in riverine communities of the Purus river, Boca do Acre municipality, Amazonas State, Brazil. Cad Saude Publica. 2009;25:1421–26. [DOI] [PubMed] [Google Scholar]
- 53.Adami YL, Rodrigues G, Alves MC, Moraes MAP, Banic DM, Maia-Herzog M. New records of Mansonella ozzardi: a parasite that is spreading from the state of Amazonas to previously uninfected areas of the state of Acre in the Purus River region. Mem Inst Oswaldo Cruz. 2014;109:87–92. 10.1590/0074-0276130243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basano SA, Aranha-Camargo JS, Vera LJ, Velasques SN, Ogawa GM, Medeiros JF, et al. Investigation of the occurrence of Mansonella ozzardi in the State of Rondônia, Western Amazonia, Brazil. Rev Soc Bras Med Trop. 2011;44:600–3. 10.1590/S0037-86822011005000055 [DOI] [PubMed] [Google Scholar]
- 55.Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop. 2011;120(Suppl 1):S109–20. 10.1016/j.actatropica.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 56.Raccurt CP, Brasseur P, Boncy J. Mansonelliasis, a neglected parasitic disease in Haiti. Mem Inst Oswaldo Cruz. 2014;109:709–11. 10.1590/0074-0276140107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medeiros JF, Py-Daniel V, Barbosa UC. Prevalence of Mansonella ozzardi among riverine communities in the municipality of Lábrea, State of Amazonas, Brazil. Rev Soc Bras Med Trop. 2011;44:186–90. 10.1590/S0037-86822011005000006 [DOI] [PubMed] [Google Scholar]
- 58.Shelley AJ. A preliminary survey of the prevalence of Mansonella ozzardi in some rural communities on the river Purus, state of Amazonas, Brazil. Ann Trop Med Parasitol. 1975;69:407–12. 10.1080/00034983.1975.11687027 [DOI] [PubMed] [Google Scholar]
- 59.Deane MP. Sobre a incidência de filárias humanas em Manaus, Estado do Amazonas [On the incidence of human filariae in Manaus, Amazonas State]. Rev Serv Esp Saude Publ. 1949;2:849–58. [Google Scholar]
- 60.Rachou RG. Distribuição geográfica das filarioses humanas no Brasil [Geographic distribution of human filariases in Brazil]. Rev Bras Malariol Doenças Trop. 1957;9:79–100. [PubMed] [Google Scholar]
- 61.Martins M, Pessoa FA, de Medeiros MB, de Andrade EV, Medeiros JF. Mansonella ozzardi in Amazonas, Brazil: prevalence and distribution in the municipality of Coari, in the middle Solimões River. Mem Inst Oswaldo Cruz. 2010;105:246–53. 10.1590/S0074-02762010000300002 [DOI] [PubMed] [Google Scholar]
- 62.Mühlens P, Dios R, Petrocchi S, Zuccarini J. Las filariosis argentinas. La microfilaria humana. Estudio sobre el paludismo y hematologías en el norte argentino [Argentinean filariases. The human microfilaremia. Study on malaria and haematologic conditions in northern Argentine]. Rev Inst Bact. 1925;4:336–42. [Google Scholar]
- 63.Remondegui C, Zaforoff G, Ripio C, Arce M, Neder de Roman L, Esquivel O. Mansonella ozzardi: Estudio clínico epidemiológico de un foco endémico en la Provincia de Jujuy [Mansonella ozzardi: Clinical-epidemiologic study of an endemic focus in Jujuy Province]. Acta Infectol. 1988;4:3–13. [Google Scholar]
- 64.Zaidenberg M. Filariasis en Balderrama, Provincia de Salta. Aspectos epidemiológicos, año 1997 [Filariases in Balderrama, Salta Province. Epidemiologic aspects, 1997]. Medicina (Bs As). 1997;55:23. [Google Scholar]
- 65.Marinkelle CJ, German E. Mansonelliasis in the Comisaría del Vaupes of Colombia [Mansonelliasis in the Comisaría del Vaupes of Colombia]. Trop Geogr Med. 1970;22:101–11. [PubMed] [Google Scholar]
- 66.Medeiros JF, Py-Daniel V, Barbosa UC, Ogawa GM. Current profile of Mansonella ozzardi (Nematoda: Onchocercidae) in communities along the Ituxi river, Lábrea municipality, Amazonas, Brazil. Mem Inst Oswaldo Cruz. 2008;103:409–11. [DOI] [PubMed] [Google Scholar]
- 67.Vargas J, Arróspide N, Gutiérrez S, Obregón C, Valencia P, Mormontoy H. Mansonellosis by Mansonella ozzardi in volunteers undergoing screening for malaria in the Peruvian Amazon. Rev Peru Med Exp Salud Publica. 2015;32:265–71. 10.17843/rpmesp.2015.322.1617 [DOI] [PubMed] [Google Scholar]
- 68.Bartoloni A, Cancrini G, Bartalesi F, Marcolin D, Roselli M, Arce CC, et al. Mansonella ozzardi infection in Bolivia: prevalence and clinical associations in the Chaco region. Am J Trop Med Hyg. 1999;61:830–3. [DOI] [PubMed] [Google Scholar]
- 69.Batista B, Oliveira WR, Rabello VD. Estudo da patogenicidade de Mansonella ozzardi e da sintomatologia da mansonelose [A study on Mansonella ozzardi pathogenicity and mansonellosis symptomatology]. Rev Inst Med Trop Sao Paulo. 1960;2:281–9. [Google Scholar]
- 70.Restrepo M, Ochoa N. Treatment with levamisole of infection by Mansonella ozzardi. Rev Inst Med Trop Sao Paulo. 1986;28:104–10. 10.1590/S0036-46651986000200007 [DOI] [PubMed] [Google Scholar]
- 71.Basano SA, Fontes G, Medeiros JF, Aranha-Camargo JS, Souza-Vera LJ, Parente-Araújo MP, et al. Sustained clearance of Mansonella ozzardi infection after treatment with ivermectin in the Brazilian Amazon. Am J Trop Med Hyg. 2014;90:1170–5. 10.4269/ajtmh.13-0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Godoy GA. Circulating immune complexes in Mansonella ozzardi infection. Ann Trop Med Parasitol. 1998;92:895–6. 10.1080/00034989858952 [DOI] [PubMed] [Google Scholar]
- 73.McNeeley DF, Raccurt CP, Boncy J, Lowrie RC Jr. Clinical evaluation of Mansonella ozzardi in Haiti. Trop Med Parasitol. 1989;40:107–10. [PubMed] [Google Scholar]
- 74.Arróspide N, Reyna O, Montenegro-Idrogo JJ, Palomino M, Lucero J, Villaseca P, et al. Prevalencia y factores associados com la filariosis por Mansonella ozzardi em 2 comunidades periurbanas de Iquitos, 2009 [Prevalence and risk factors associated with Mansonella ozzardi filariasis in two periurban communities of Iquitos, 2009]. Infectio. 2015;19:124–130. 10.1016/j.infect.2015.03.003 [DOI] [Google Scholar]
- 75.Garrido C, Campos M. First report of presumed parasitic keratitis in Indians from the Brazilian Amazon. Cornea. 2000;19:817–9. 10.1097/00003226-200011000-00011 [DOI] [PubMed] [Google Scholar]
- 76.Cohen JM, Ribeiro JA, Martins M. Ocular manifestations in mansonelliasis. Arq Bras Oftalmol. 2008;71:167–71. [DOI] [PubMed] [Google Scholar]
- 77.Vianna LM, Martins M, Cohen MJ, Cohen JM, Belfort R Jr. Mansonella ozzardi corneal lesions in the Amazon: a cross-sectional study. BMJ Open. 2012;2:e001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metenou S, Dembélé B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, et al. Patent filarial infection modulates malaria-specific type 1cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population. J Immunol. 2009;183:916–24. 10.4049/jimmunol.0900257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Metenou S, Babu S, Nutman TB. Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS. 2012;7:231–8. 10.1097/COH.0b013e3283522c3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Layland LE, Specht S. Helpful or a hindrance: co-infections with helminths during malaria. Adv Exp Med Biol. 2014;828:99–129. 10.1007/978-1-4939-1489-0 [DOI] [PubMed] [Google Scholar]
- 81.Dantur Juri MJ, Veggiani Aybar CA, Ortega ES, Galante GB, Zaidenberg MO. Plasmodium vivax and Mansonella ozzardi co-infection in north-western Argentina. Malar J. 2013;12:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knott J. A method for making microfilarial surveys on day blood. Trans R Soc Trop Med Hyg. 1939;33:191–6. 10.1016/S0035-9203(39)90101-X [DOI] [Google Scholar]
- 83.Raccurt C, Lowrie RC Jr, Boncy J, Katz SP. Mansonella ozzardi in Haiti. III. A comparison of the sensitivity of four sampling methods in detecting infections. Am J Trop Med Hyg. 1982;31:275–9. [PubMed] [Google Scholar]
- 84.Chadee DD, Tilluckdharry CC, Rawlins SC, Doon R, Nathan MB. Mass chemotherapy with diethylcarbamazine for the control of Bancroftian filariasis: a twelve-year follow-up in northern Trinidad, including observations on Mansonella ozzardi. Am J Trop Med Hyg. 1995;52:174–6. [DOI] [PubMed] [Google Scholar]
- 85.Figueroa JM. Presence of microfilaria of Mansonella ozzardi in ascitic fluid. Acta Cytol. 1973;17:73–5. [PubMed] [Google Scholar]
- 86.Vera LJ, Basano SA, Aranha-Camargo JS, França AK, Ferreira RG, Casseb AA, et al. Improvement of a PCR test to diagnose infection by Mansonella ozzardi. Rev Soc Bras Med Trop. 2011;44:380–2. [DOI] [PubMed] [Google Scholar]
- 87.Medeiros JF, Almeida TA, Silva LB, Rubio JM, Crainey JL, Pessoa FA, et al. A field trial of a PCR-based Mansonella ozzardi diagnosis assay detects high-levels of submicroscopic M. ozzardi infections in both venous blood samples and FTA card dried blood spots. Parasit Vectors. 2015;8:280. 10.1186/s13071-015-0889-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bartholomew CF, Nathan MB, Tikasingh ES. The failure of diethylcarbamazine in the treatment of Mansonella ozzardi infections. Trans R Soc Trop Med Hyg. 1978;72:423–4. 10.1016/0035-9203(78)90141-4 [DOI] [PubMed] [Google Scholar]
- 89.Nutman TB, Nash TE, Ottesen EA. Ivermectin in the successful treatment of a patient with Mansonella ozzardi infection. J Infect Dis. 1987;156:662–5. 10.1093/infdis/156.4.662 [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez AA, Chadee DD, Rawlins SC. Ivermectin treatment of mansonellosis in Trinidad. West Indian Med J. 1999;48:231–4. [PubMed] [Google Scholar]
- 91.Francis H, Awadzi K, Ottesen EA. The Mazzotti reaction following treatment of onchocerciasis with diethylcarbamazine: clinical severity as a function of infection intensity. Am J Trop Med Hyg. 1985;34:529–36. [DOI] [PubMed] [Google Scholar]
- 92.Krolewiecki AJ, Cajal SP, Villalpando C, Gil JF. Ivermectin-related adverse clinical events in patients treated for Mansonella ozzardi infections. Rev Argent Microbiol. 2011;43:48–50. [DOI] [PubMed] [Google Scholar]
- 93.Keiser PB, Coulibaly YI, Keita F, Traore D, Diallo A, Diallo DA, et al. Clinical characteristics of post-treatment reactions to ivermectin/albendazole for Wuchereria bancrofti in a region co-endemic for Mansonella perstans. Am J Trop Med Hyg. 2003;69:331–5. [PubMed] [Google Scholar]
- 94.Hoerauf A. Mansonella perstans – the importance of an endosymbiont. N Engl J Med. 2009;361:1502–4. 10.1056/NEJMe0905193 [DOI] [PubMed] [Google Scholar]
- 95.Coulibaly YI, Dembele B, Diallo AA, Lipner EM, Doumbia SS, Coulibaly SY, et al. A randomized trial of doxycycline for Mansonella perstans infection. N Engl J Med. 2009;361:1448–58. 10.1056/NEJMoa0900863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bruijning CF. Notes on the common species of Culicoides (Diptera: Ceratopogonidae) from Surinam in relation to ozzardi filariasis. Doc Med Geogr Trop. 1957;9:169–72. [PubMed] [Google Scholar]
- 97.Taranto N, Castelli E. Detección de un foco de microfilariasis en el noroeste argentino [Detection of a microfilariasis focus in northwestern Argentina]. Rev Arg Microbiol. 1988;20:49–51. [PubMed] [Google Scholar]
- 98.Degese MF, Cabrera MG, Krivokapich SJ, Irazu LE, Rodriguez MA, Guarnera RA. Aporte de la técnica de PCR en el diagnóstico de Mansonella ozzardi en zonas endémicas de la Argentina [Contribution of the PCR technique in the diagnosis of Mansonella ozzardi in endemic areas of Argentina]. Rev Arg Microbiol. 2012;44:97–100. [PubMed] [Google Scholar]
- 99.Medeiros JF, Costa CA, Lima AM, Pessoa FA. Mansonella ozzardi (Nematoda: Onchocercidae) in the riverine population of the Tefé River, State of Amazonia, Brazil. Rev Soc Bras Med Trop. 2014;47:113–5. 10.1590/0037-8682-0031-2012 [DOI] [PubMed] [Google Scholar]
- 100.Medeiros JF, Pessoa FAC, Rodrigues MS, Martins M. Epidemiological snapshot of the mansonelliasis infection in the Amazonian riverine communities in two contiguous municipalities of Somiões river, Amazonas State, Brazil. Rev Pan Amaz Saude. 2015;6:83–7. 10.5123/S2176-62232015000200011 [DOI] [Google Scholar]
- 101.Kozek WJ, D’Alessandro A, Silva J, Navarette SN. Filariasis in Colombia: prevalence of mansonellosis in the teenage and adult population of the Colombian bank of the Amazon, Comisaria del Amazonas. Am J Trop Med Hyg. 1982;31:1131–6. [DOI] [PubMed] [Google Scholar]
- 102.Kozek WJ, Palma G, Henao A, García H, Hoyos M. Filariasis in Colombia: prevalence and distribution of Mansonella ozzardi and Mansonella (=Dipetalonema) perstans infections in the Comisaría del Guainía. Am J Trop Med Hyg. 1983;32:379–84. [DOI] [PubMed] [Google Scholar]
- 103.Raccurt C, Lowrie RC Jr, McNeeley DF. Mansonella ozzardi in Haiti. 1. Epidemiological survey. Am J Trop Med Hyg. 1980;29:803–8. [DOI] [PubMed] [Google Scholar]
- 104.Godoy GA, Volcan G, Medrano C, Teixeira A, Matheus L. Mansonella ozzardi infections in Indians of the Southwestern part of the state of Bolivar, Venezuela. Am J Trop Med Hyg. 1980;29:373–6. [DOI] [PubMed] [Google Scholar]
- 105.Gómez J, Guerrero R. Environmental factors and the distribution of mansonelliases in Southern Venezuela. Parasite. 2000;7:71–6. 10.1051/parasite/2000072071 [DOI] [PubMed] [Google Scholar]