Abstract
Purpose
Obesity, as measured by body mass index (BMI), is a risk factor for distant recurrence and decreased survival in breast cancer. We sought to determine whether BMI correlated with local recurrence and reduced survival in a cohort of predominantly obese women treated with breast conservation therapy.
Methods and Materials
From 1998–2010, 154 women with early stage invasive breast cancer and 39 patients with ductal carcinoma in situ (DCIS) underwent prone whole breast irradiation. Cox proportional hazards regression, Kaplan-Meier methods with log-rank test, and multivariate analysis were used to explore the association of outcomes with BMI.
Results
The median patient age was 60 years, and the median follow-up was 73 months. The median BMI was 33.2 kg/m2; 91% of patients were overweight (BMI≥25 kg/m2) and 69% of patients were clinically obese (BMI≥30 kg/m2). BMI was significantly associated with local-regional recurrence-free interval for invasive and DCIS patients (hazard ratio 1.09, p=0.047), and there was a trend for increased local-regional recurrence with higher BMI (p=0.09) for patients with invasive disease, which was significant when examining BMI above and below the median value of 33.2 (p=0.008). BMI was also significantly associated with decreased distant recurrence-free interval (DRFI; HR 1.09, p=0.011) and overall survival (OS; HR 1.09, p=0.004); this association remained on multivariate analysis (DRFI, p=0.034; overall survival, p=0.0007).
Conclusions
These data suggest that BMI may impact the rate of local-regional recurrence in breast cancer patients. Higher BMI predicted worse distant recurrence-free interval and overall survival. This investigation adds to growing evidence that BMI is an important prognostic factor in early stage breast cancer treated with breast conservation therapy.
Keywords: Body Mass Index, local control, breast cancer, overall survival, distant metastasis, breast conservation therapy, radiation
Introduction
The prevalence of obesity has risen dramatically in the United States over the past 25 years, with over one-third of adult women in the United States estimated to be obese (1). Obesity is a risk factor for the development of several malignancies, including breast cancer. The association of obesity with breast cancer is best-established in post-menopausal women, while the contribution of obesity to the development of pre-menopausal breast cancer is less clear (2, 3). In addition, obesity, as measured by body mass index (BMI), has been associated with worse breast cancer-specific survival, distant metastasis-free survival, and overall survival in breast cancer patients (4–6). However, the role of BMI or obesity in increasing the risk of local or regional breast cancer recurrence has not been well established.
Several potential mechanisms have been proposed to underlie the association of obesity with less favorable breast cancer outcomes. We hypothesized that the factors in obese patients that promote breast cancer growth systemically could also affect local control. Systemic therapies lead to improved local control of breast cancer (7), and thus less effective systemic therapy in obese individuals could increase the risk of a local recurrence. For invasive breast cancer, a deleterious effect on local control by additional obesity related factors could contribute to the worse breast cancer-specific survival and/or distant metastasis-free survival seen in this population, given that improved local control from radiotherapy has been shown to increase breast cancer specific survival (7).
Here, we used a unique patient population to examine the association of BMI with local control; all patients in our cohort underwent prone whole breast irradiation as part of breast conservation therapy. The primary indication for prone breast irradiation at our institution is large/pendulous breasts or larger body habitus (8, 9), and thus the majority of the patients in our cohort were overweight and more than two-thirds were obese. We examined the association of obesity with local-regional control and additional survival and breast cancer-specific outcomes in a predominantly obese cohort of women that underwent breast conservation therapy.
Methods and Materials
This study was performed under the auspices of an institutional review board protocol approved by the Medical College of Wisconsin. Investigators completed training in human research and patient privacy. A retrospective chart review was performed of women with early stage breast cancer who underwent post-lumpectomy whole breast irradiation in the prone position between 1998 and 2010, using three-dimensional conformal radiation therapy (3DCRT) under the guidance of Medical College of Wisconsin radiation oncologists, as previously described (8). The primary indication for breast radiotherapy in the prone position was large body habitus and/or large or pendulous breasts. Patients with invasive breast cancer received breast conservation surgery and uniformly underwent axillary surgery in the form of sentinel lymph node biopsy and/or axillary dissection. The details of 3DCRT for the patients in the prone position were reported previously (8). Systemic therapy was recommended when appropriate.
Clinicopathologic characteristics and outcomes were obtained from patient records. BMI at diagnosis was obtained by calculating the patient weight in kilograms (kg) divided by the height in meters squared (m2). The cohort consisted of patients with non-invasive ductal carcinoma in situ (DCIS; N=39) and early-stage invasive breast cancer (N=154). Outcomes for patients with invasive cancer were used in analyses of survival, as described below. Patients with DCIS were analyzed with all other patients only to examine factors associated with local-regional control.
The primary study endpoint was local-regional control probability among all patients; this probability was also calculated separately for the invasive cohort. The secondary outcomes were distant recurrence-free interval and overall survival. Prognostic factors that were examined for association with local control and survival outcomes included hormone positivity (estrogen receptor and progesterone receptor), BMI, Bloom-Richardson grade, tumor size, nodal stage (N0 versus N1), chemotherapy, age, and diabetes at diagnosis. Breast cancer-specific outcomes were defined as described by Hudis et al. (10). Local-regional failure was defined as any recurrence in the ipsilateral breast or ipsilateral regional lymph nodes. Survival was calculated starting from the time of definitive surgery.
Univariate Cox proportional hazards models were performed for each outcome, including local-regional recurrence-free interval, distant recurrence-free survival, and overall survival. For multivariate analyses, adjusted Cox proportional hazards models were performed for local-regional recurrence-free interval, distant recurrence-free survival, and overall survival. No adjusted Cox proportional hazards models were run for local-regional recurrence due to low event rates. All multivariate models were run on data from invasive cancer patients only. Survival rates were compared via Kaplan-Meier methods and the log-rank test with BMI as a non-continuous variable; the cohorts were broken into subgroups with BMI above and below the median value. Statistical analyses were performed using Graphpad Prism Version 6.0d, MedCalc Version 15 software, and SAS version 9.3. P<0.05 was considered significant.
Results
Characteristics of the patient population are summarized in Table 1. Patients with invasive breast cancer (N=154) had a median age of 60 years, and a median invasive tumor size of 1.2 cm. Seventy-nine percent of patients with invasive cancer had estrogen receptor/progesterone receptor-positive disease, and 17% had Her2/NEU-positive or -amplified tumors. Seventy-five percent of patients initiated anti-endocrine treatment, and 41% of patients received chemotherapy after surgery. Patients with DCIS (N=39) had a median age of 57 years and a median DCIS size of 1.5 cm. The median follow-up for all patients was 73 months. The median BMI for the entire population was 33.0 kg/m2 (range 19–61 kg/m2), with a median BMI of 33.2 kg/m2 for patients with invasive cancer and 32.0 kg/m2 for patients with DCIS. Ninety percent of all patients were overweight (BMI≥25 kg/m2), more than two-thirds were obese (BMI≥30 kg/m2), and more than one-third fulfilled the criteria for World Health Organization class II obesity (BMI≥35 kg/m2, Table 1).
TABLE 1.
Patient and tumor characteristics.
| Characteristic | Invasive Tumors (N=154) | All Patients (N=193) | ||||
|---|---|---|---|---|---|---|
| % | Mean | Median (Range) | % | Mean | Median (Range) | |
| Age at diagnosis, years | 60.3 | 60.0 (27–91) | 56.7 | 59.0 (27–91) | ||
| Follow-up, months | 81.1 | 73.2 (10–191) | 80.7 | 73.2 (10–191) | ||
| BMI, kg/m2 | 34.3 | 33.2 (20–62) | 34.1 | 33.0 (19–62) | ||
| Overweight (BMI≥25) | 91% | 90% | ||||
| Obese (BMI≥30) | 69% | 68% | ||||
| WHO II Obese (BMI≥35) | 37% | 37% | ||||
| Diabetes at diagnosis | 8% | 7% | ||||
| Tumor size, cm* | 1.0 | 1.2 | 1.0 | 1.2 | ||
| T stage | ||||||
| T0 | 0% | 20% | ||||
| T1 | 76% | 60% | ||||
| T2 | 21% | 17% | ||||
| T3 | 3% | 2% | ||||
| N stage | ||||||
| N0 | 82% | 86% | ||||
| N1 | 18% | 14% | ||||
| ER/PR positive | 79% | 77% | ||||
| Her-2/neu positive/amplified* | 17% | 13% | ||||
| Chemotherapy | 41% | 33% | ||||
| Anti-endocrine therapy | 75% | 70% | ||||
| Post-menopausal | 69% | 68% | ||||
Invasive cancers only.
Abbreviations: BMI, body mass index; ER/PR, estrogen receptor/progesterone receptor; WHO, World Health Organization.
The 5-year local-regional control probability was 97.7% for all patients and 97.1% for patients with invasive disease. Seven of 154 patients with invasive tumors had local-regional recurrences (4.5%), five of the seven local-regional recurrences were local: four of these were isolated local failures and one was identified at the same time as a distant recurrence. The remaining two patients had a regional recurrence, one as an isolated site of failure and one identified with a distant recurrence. In patients with DCIS, one patient experienced an isolated local recurrence.
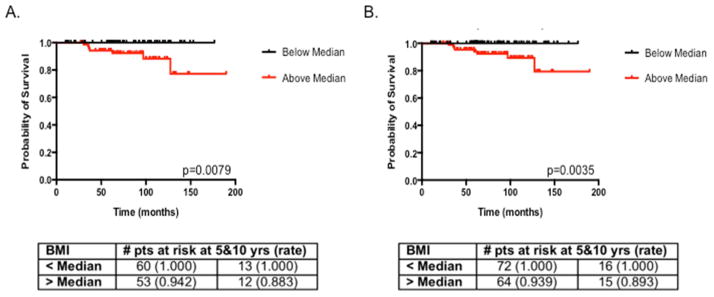
We examined predictors of local-regional recurrence in patients with invasive disease (N=154) only and then for the entire patient population (N=193). Predictors of local-regional recurrence were examined only using univariate analysis due to the low number of events (seven and eight events for patients with invasive cancer only and the total population, respectively). Patients with invasive breast cancer displayed a trend toward a higher risk of local-regional recurrence when BMI increased as a continuous variable (p=0.0886, Table 2). When patients were divided into those with BMI above and below the median of 33.2 kg/m2, higher BMI was significantly associated with local-regional recurrence-free interval (p=0.008, Fig. 1A). When the entire cohort was examined for factors predicting local-regional recurrence-free survival, BMI was significant when examined as a continuous variable (p=0.047, Table 2) and when the population was divided into subgroups with BMI above and below the median value of 33 kg/m2 (p=0.004, Fig. 1B).
TABLE 2.
Univariate analysis of local-regional recurrence-free interval.
| Invasive Cancers | All Patients* | |||
|---|---|---|---|---|
|
| ||||
| Parameter | Hazard Ratio (Range) | P-value | Hazard Ratio (Range) | P-value |
| ER-PR- vs. ER+/PR+ (invasive tumors) | 0.81 (0.04–4.87) | 0.8500 | 0.81 (0.04–4.79) | 0.8482 |
| Tumor subtype (DCIS vs. invasive) | - | - | 0.53 (0.03–3.10) | 0.5555 |
| Body Mass Index | 1.09 (0.98–1.19) | 0.0886 | 1.09 (1.00–1.19) | 0.0474 |
| Bloom-Richardson grade (invasive tumors) | 0.94 (0.35–2.58) | 0.9094 | 1.13 (0.57–2.34) | 0.7275 |
| Tumor size | 0.913 (0.33–1.79) | 0.8323 | 1.06 (0.50–1.82) | 0.8694 |
| N stage | 2.28 (0.33–10.59) | 0.3250 | 2.47 (0.36–10.73) | 0.2692 |
| Chemotherapy (yes/no) | 1.85 (0.41–9.38) | 0.4227 | 2.01 (0.48–8.52) | 0.3230 |
| Diabetes at diagnosis | 1.85 (0.10–10.85) | 0.5712 | 1.67 (0.90–9.46) | 0.6314 |
| Age | 0.976 (0.919–1.039) | 0.4345 | 0.98 (0.92–1.04) | 0.4877 |
Both invasive cancers and DCIS cases
Abbreviations: DCIS, ductal carcinoma in situ; ER/PR, estrogen receptor/progesterone receptor.
Figure 1.
Kaplan-Meier curves for local-regional recurrence-free interval with BMI stratified above (red) and below (black) the median value of 33 kg/m2 for invasive cancer patients only (A) and all patients (B).
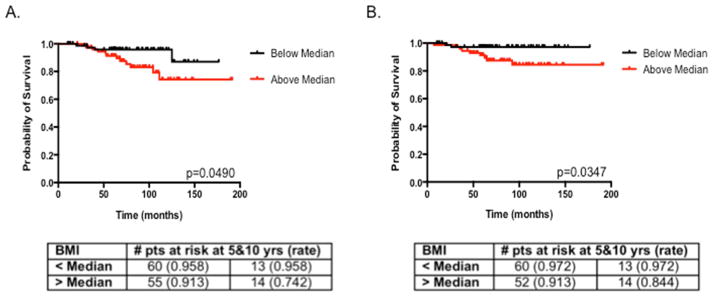
Overall survival and distant recurrence-free interval were examined only in patients with invasive breast cancers; the 5-year overall survival rate was 93.6% and the 5-year distant recurrence-free interval was 94.3%. Univariate analysis of outcomes and potential risk factors revealed that BMI, tumor size, and age were significantly associated with overall survival in invasive breast cancer patients (Table 2), while BMI (hazard ratio 1.12, p=0.0007) and age (hazard ratio 1.09, p=0.0015) remained significant on multivariate analysis (Table 3). On univariate analysis, BMI, tumor grade, and tumor size were significantly associated with distant recurrence-free interval (Table 4); these factors remained significant on multivariate analysis (Table 4). When patients were stratified by BMI, BMI greater than the median value was significantly associated with lower overall survival (p=0.049, Fig. 2A) and with shorter distant recurrence-free intervals (p=0.035, Fig. 2B).
TABLE 3.
Univariate and multivariate analysis of overall survival of patients with invasive breast cancer.
| Univariate
| ||
|---|---|---|
| Parameter | Hazard Ratio (Range) | P-value |
| ER-PR- vs. ER+/PR+ | 2.75 (0.93–7.44) | 0.0511 |
| Body Mass Index | 1.09 (1.03–1.16) | 0.0039 |
| Bloom-Richardson grade | 1.78 (0.92–3.60) | 0.0971 |
| Tumor size | 1.52 (1.03–2.10) | 0.0193 |
| Nodal stage | 2.24 (0.7–6.18) | 0.1350 |
| Chemotherapy (yes/no) | 1.11 (0.40–2.97) | 0.8431 |
| Diabetes at diagnosis | 1.53 (0.24–5.48) | 0.5751 |
| Age | 1.06 (1.01–1.10) | 0.0101 |
|
| ||
| Multivariate | ||
|
| ||
| Parameter | Hazard Ratio (Range) | P-value |
|
| ||
| Body Mass Index | 1.12 (1.05–1.20) | 0.0007 |
| Tumor size | 1.36 (0.89–1.93) | 0.1150 |
| Age | 1.09 (1.03–1.15) | 0.0015 |
Abbreviations: ER/PR, estrogen receptor/progesterone receptor.
TABLE 4.
Univariate and multivariate analysis of distant recurrence-free interval of patients with invasive breast cancer.
| Univariate
| ||
|---|---|---|
| Parameter | Hazard Ratio (Range) | P-value |
| ER-PR- vs. ER+/PR+ | 2.53 (0.66–8.37) | 0.1394 |
| Body Mass Index | 1.09 (1.02–1.17) | 0.0113 |
| Bloom-Richardson grade | 3.08 (1.30–8.48) | 0.0167 |
| Tumor size | 1.94 (1.35–2.67) | 0.0001 |
| N stage | 3.04 (0.795–10.07) | 0.0766 |
| Chemotherapy (yes/no) | 2.54 (0.77–9.71) | 0.1363 |
| Diabetes at diagnosis | 0.00 (0.00–2.10) | 0.9938 |
| Age | 1.01 (0.96–1.06) | 0.7820 |
|
| ||
| Multivariate | ||
|
| ||
| Parameter | Hazard Ratio (Range) | P-value |
|
| ||
| Body Mass Index | 1.09 (1.00–1.19) | 0.0341 |
| Bloom-Richardson grade | 2.63 (1.14–2.52) | 0.0338 |
| Tumor size | 1.77 (1.18–2.52) | 0.0024 |
Abbreviations: ER/PR, estrogen receptor/progesterone receptor.
Figure 2.
Kaplan-Meier curves in patient with invasive breast cancer for overall survival (A) and distant recurrence-free interval (B) with BMI stratified above (red) and below (black) the median value of 33.0 kg/m2.
Discussion
Obesity has been associated with worse breast cancer-specific survival, distant metastasis-free survival, and overall survival in breast cancer patients (4, 5, 11). However, there is a relative paucity of data linking obesity with local control. As improved local control can optimize breast cancer survival (7), the role of BMI in local recurrence may have important implications. In our cohort of predominantly overweight and obese patients with early-stage breast cancer receiving breast conservation therapy, BMI >33.2 kg/m2 was significantly associated with local-regional recurrence (Fig. 1). In addition, we detected a trend toward a significant association between BMI and local-regional recurrence when BMI was examined as a continuous variable in patients with invasive disease, and this trend was significant when all patients were examined (including those with DCIS) (Table 2). BMI also predicted worse distant recurrence-free interval and overall survival (Tables 3 and 4, Fig. 2).
Interestingly, typical predictors of local-regional recurrence, such as stage and age, were not related to breast cancer outcomes in univariate analysis (Table 2) in this study, likely reflecting that most patients were relatively lower risk being predominantly early stage, node negative, hormone sensitive, and with predominantly negative surgical margins and receipt of appropriate systemic therapy. Thus, in this population of women with a very high median BMI, BMI may be a relatively more important factor contributing to in-breast local-regional recurrences. It should be noted that this is not meant to suggest that these patients are not well-suited for breast conservation, as the overall local control and survival outcomes were excellent, with a 5-year local-regional control probability was 97.7%, overall survival rate of 93.6% and a distant recurrence-free interval of 94.3% for patients with invasive cancer.
Several mechanisms have been proposed to explain the association of obesity with less favorable breast cancer outcomes. We hypothesized that obesity-related factors that affect the risk of distant breast cancer recurrences would also affect the local growth of breast cancer cells. Increased adipose tissue may increase the levels of circulating estrogens, insulin and insulin-like growth factors, and adipocytokines, which can promote cancer cell growth (12–15). Obesity may also lead to increased oxidative stress and chronic low levels of inflammation, which can promote tumor growth and lead to increased estrogen levels (16–20), and some obese patients with invasive breast cancer may receive less systemic therapy due to dose-capping practices or toxicities (21, 22). Obesity has been associated with the development of breast cancer, especially the development of ER+ tumors in post-menopausal women (23), which may be related to the relative increase in estrogen production in the presence of increase adiposity (20). The patients in our study were predominantly post-menopausal with ER+ tumors, and thus the effect of BMI on recurrences, both local and distant, may reflect the effect of the relatively higher endogenous estrogen levels in obese post-menopausal patients.
While the association of BMI with overall and breast cancer-specific survival has been examined in numerous studies, the extent to which BMI affects local and regional control is not well-established. Few investigations have examined the association of obesity with local and regional control in the context of modern technologies and modern systemic therapy. Older studies of patients treated from 1940–1965 (24) and in the 1980s (25) reported that higher body weight/BMI was associated with any breast cancer recurrence, as well as overall survival (24, 25). It is not clear from these publications what proportion of recurrences consisted of local-regional recurrences, and optimal systemic treatments would not have been administered during these periods. A more recent study from Saudi Arabia that examined local recurrence in 112 women with non-metastatic breast cancer in relation to BMI does not offer any further corroboration. It found that patients with a BMI of 26–30 kg/m2 had an increased likelihood of local-regional recurrence, but other ranges of BMI—including higher BMI—had no effect on the likelihood of local-regional recurrence (26). These patients underwent mastectomy with or without radiation (71%) or breast conservation therapy as local treatment. In contrast, our patients received breast conservation therapy as the only local treatment. The trends toward worse local-regional control with increased BMI detected here (Table 2 and Fig. 1) suggest that additional studies in larger cohorts are warranted.
Our patient population enabled us to examine the association between BMI and local-regional recurrences, but it is not necessarily representative of all patients with early-stage breast cancer receiving breast conservation therapy, as the median BMI and proportion of overweight and obese patients was high. However, given that the prevalence of obesity in the United States continues to rise, it is likely that over time the proportion of overweight and obese patients receiving breast conservation therapy will also continue to increase. This study is limited by being retrospective in nature, and thus there could be another selection bias present that is influencing outcomes. However, the majority of patients were recommended to have prone positioning because of their body habitus/ BMI and there were very few patients over this time period unable to tolerate prone positioning, but other unknown sources of selection bias could be present. It is anticipated that these hypothesis-generating findings could in the future be examined in more detail using a propensity-matched analysis among larger cohorts of patients with a wider range of BMIs, and ultimately in prospective clinical trials. Additional insight on the effect of BMI on local cancer recurrence can be potentially gained from the Alliance A011401 “Randomized Phase III Trial Evaluating the Role of Weight Loss In Adjuvant Treatment of Overweight and Obese Women with Early Breast Cancer” that will examine the effects of weight loss on survival outcomes and enrollment on this study for this population is recommended. Further determining the mechanisms for potential worsened local control in obese individuals may eventually lead to therapies to counteract the growth-promoting factors that worsen patient outcomes.
Conclusion
Overall, this study examined the association of BMI with local-regional control in a retrospective cohort of early-stage breast cancer patients receiving breast conservation therapy. This study population was unique in that the median BMI was >33 kg/m2, and two-thirds of patients were obese. BMI was associated with increased local-regional recurrence, worse distant recurrence-free survival, and worse overall survival. However, obese patients still had very favorable outcomes. These findings are hypothesis-generating and warrant study in larger cohorts.
Summary.
While obesity is associated with breast cancer outcomes such as breast cancer-specific survival and overall survival, the association of obesity and local-regional control is not well established. Data from this cohort of predominantly overweight, early stage breast cancer patients receiving breast conservation surgery and whole breast irradiation suggest that higher body mass index may increase the rate of local-regional recurrence.
Acknowledgments
FUNDING SUPPORT
This research was supported in part by the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health (NIH), through Grant #8KL2TR000056 and grant #8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
ABBREVIATIONS
- BMI
Body mass index
- DCIS
ductal carcinoma in situ
- ER
estrogen receptor
- PR
progesterone receptor
- 3DCRT
three-dimensional conformal radiation therapy
Footnotes
A preliminary version of this study was presented at the 2014 American Society of Radiation Oncology 56th Annual Meeting, September 15, 2014, in San Francisco, California.
CONFLICT OF INTEREST DISCLOSURES
A. Szabo and C. Bergom report grants from NIH NCATS during the conduct of the study. The authors have no other financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Cheraghi Z, Poorolajal J, Hashem T, et al. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PloS One. 2012;7:e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 6.Ewertz M, Jensen M-B, Gunnarsdóttir KÁ, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 7.Buchholz TA, Tucker SL, Erwin J, et al. Impact of systemic treatment on local control for patients with lymph node-negative breast cancer treated with breast-conservation therapy. J Clin Oncol. 2001;19:2240–2246. doi: 10.1200/JCO.2001.19.8.2240. [DOI] [PubMed] [Google Scholar]
- 8.Bergom C, Kelly T, Morrow N, et al. Prone whole-breast irradiation using three-dimensional conformal radiotherapy in women undergoing breast conservation for early disease yields high rates of excellent to good cosmetic outcomes in patients with large and/or pendulous breasts. Int J Radiat Oncol Biol Phys. 2012;83:821–828. doi: 10.1016/j.ijrobp.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stegman LD, Beal KP, Hunt MA, et al. Long-term clinical outcomes of whole-breast irradiation delivered in the prone position. Int J Radiat Oncol Biol Phys. 2007;68:73–81. doi: 10.1016/j.ijrobp.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 10.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 11.Ewertz M, Jensen M-B, Gunnarsdóttir KÁ, et al. Effect of Obesity on Prognosis After Early-Stage Breast Cancer. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.29.7614. JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin PJ, Ennis M, Bahl M, et al. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. 2009;114:517–525. doi: 10.1007/s10549-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 13.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28:4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin PJ. Obesity, insulin resistance and breast cancer outcomes. Breast. 2015;24(Suppl 2):S56–59. doi: 10.1016/j.breast.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 16.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunter MJ, Wang T, Cushman M, et al. Circulating Adipokines and Inflammatory Markers and Postmenopausal Breast Cancer Risk. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda M, Shimomura I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–e341. doi: 10.1016/j.orcp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Dai Q, Gao Y-T, Shu X-O, et al. Oxidative Stress, Obesity, and Breast Cancer Risk: Results From the Shanghai Women’s Health Study. J Clin Oncol. 2009;27:2482–2488. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and Inflammation: New Insights into Breast Cancer Development and Progression. Am Soc Clin Oncol Educ Book. 2013;33:46–51. doi: 10.1200/EdBook_AM.2013.33.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griggs JJ, Sorbero MES, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 22.Shayne M, Crawford J, Dale DC, et al. Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2006;100:255–262. doi: 10.1007/s10549-006-9254-4. [DOI] [PubMed] [Google Scholar]
- 23.Ahn J, Schatzkin A, Lacey JV, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167:2091–2102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- 24.Donegan WL, Hartz AJ, Rimm AA. The association of body weight with recurrent cancer of the breast. Cancer. 1978;41:1590–1594. doi: 10.1002/1097-0142(197804)41:4<1590::aid-cncr2820410449>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Hebert JR, Augustine A, Barone J, et al. Weight, height and body mass index in the prognosis of breast cancer: early results of a prospective study. Int J Cancer. 1988;42:315–318. doi: 10.1002/ijc.2910420302. [DOI] [PubMed] [Google Scholar]
- 26.Al Saeed EF, Ghabbban AJ, Tunio MA. Impact of BMI on Locoregional Control among Saudi Patients with Breast Cancer after Breast Conserving Surgery and Modified Radical Mastectomy. Gulf J Oncolog. 2015;1:7–14. [PubMed] [Google Scholar]