Abstract
Background
Early detection of structural changes in left atrium (LA) prior to atrial fibrillation (AF) development could be helpful in identification of those at higher risk for AF. Using CMR imaging, we examined the association of LA volume and function and incident AF in a multi-ethnic population free of clinical cardiovascular diseases.
Methods and Results
In a case-cohort study embedded in the Multi-Ethnic Study of Atherosclerosis (MESA), baseline LA size and function assessed by CMR feature-tracking were compared between 197 participants with incident AF and 322 participants randomly selected from the whole MESA cohort. Participants were followed for 8 years. Incident AF cases had a larger LA volume and decreased passive, active and total LA emptying fractions (LAEF) and peak global LA longitudinal strain (peak LA strain) at baseline. In multivariable analysis, elevated LA maximum volume index (HR: 1.38 per SD, 95% CI: 1.01–1.89) and decreased peak LA strain (HR: 0.68 per SD, 95%CI: 0.48–0.96), passive and total LA emptying fractions (HR for passive LAEF: 0.55 per SD, 95%CI: 0.40–0.75, and HR for active LAEF: 0.70 per SD, 95%CI: 0.52–0.95) but not active LA emptying fraction were associated with incident AF.
Conclusions
Elevated LA volumes as well as decreased passive and total LAEF were independently associated with incident AF in an asymptomatic multi-ethnic population. Including LA functional variables along with other risk factors of AF may help to better risk stratify individuals at risk of AF development.
Keywords: left atrial function, atrial fibrillation, feature tracking CMR
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with both cardiovascular and cerebrovascular morbidity and mortality. According to a population-based study, it is estimated that over 2 million US adults have been diagnosed with AF and by 2050 this number is expected to increase to 5.6 million 1. Given the high prevalence of AF, finding methods to identify individuals at risk for AF development is of great value in both clinical and population-based studies.
Cross-sectional studies using echocardiography or CMR have shown that left trial (LA) functional and structural variables are impaired in patients with AF. 2–4 These markers have been used to risk stratify patients with AF to predict risks of stroke5, 6, LA reverse remodeling 7, outcomes of catheter ablation 8, 9 or cardioversion 10. More importantly, it is suggested that structural changes in LA may precede development of AF 11. Therefore, early detection of these changes in asymptomatic individuals at risk of AF will be helpful for risk stratification and early intervention purposes. Few prospective studies investigating the role of LA in prediction of AF have been limited to measuring LA size12–14, or only one aspect of LA function15, 16 and have been conducted on an elderly referral population 16. In addition, these studies have used Doppler imaging or more recently speckle tracking echocardiography for analyzing LA function. However, given the anatomical location of the LA, the thin atrial wall and the presence of pulmonary veins and LA appendage, assessment of LA function can be challenging 3. On the other hand, cardiac magnetic resonance (CMR) imaging is a well-established method for the assessment of cardiac deformation given its excellent ability to define endocardial and epicardial borders. Therefore, CMR has been proposed as the reference method to measure atrial and ventricular volumes 17.
In this study, we sought to examine the association of LA size and function measured with feature-tracking CMR and incident AF in a large asymptomatic multi-ethnic population. We hypothesized that reduced left atrial function, assessed as decreased LA emptying fractions and longitudinal LA strain, is associated with incidence of AF.
Methods
Study design
The present study was designed as a case-cohort study within the Multi-Ethnic Study of Atherosclerosis (MESA). The MESA study protocol has previously been described in detail 18. Briefly, between July 2000 and August 2002, 6814 individuals were recruited from 6 U.S. communities in North Carolina, New York, Maryland, Illinois, Minnesota and California. The participants were aged between 45 and 84 years from 4 different self-reported racial/ethnic backgrounds (white, African-American, Hispanic and Chinese). All participants were free of any clinically apparent cardiovascular disease. The study protocol was approved by the institutional review board of each study site.
Follow up
Approximately every 9 months, each participant was contacted by a telephone interviewer to inquire about hospital admissions, cardiovascular outpatient diagnoses, and deaths. Medical records and information were successfully obtained on 98% of reported hospitalized cardiovascular events and 95% of reported outpatient cardiovascular diagnostic encounters.
Identification of atrial fibrillation cases
Incident cases of AF during follow-up were identified through MESA event surveillance and, for participants enrolled in fee-for-service Medicare, from inpatient Medicare claims data. As part of standard event surveillance procedures in the MESA cohort, all hospitalizations are identified during follow-up calls to study participants or a proxy. Discharge diagnostic and procedure codes from those hospitalizations are abstracted. AF was considered to be present if an International Classification of Diseases; Ninth Revision (ICD9) diagnosis code 427.31 or 427.32 was present. AF hospitalizations associated with open cardiac surgery were excluded. If the first AF claim occurred before the baseline MESA exam, the participant was considered to have prevalent AF and thus was excluded from the analysis.
Covariates
Standardized questionnaires were used at baseline to collect information about age, sex, racial/ethnic background, cigarette smoking; medication inventory was used to collect information on prescription and non-prescription medications. Cigarette smoking was categorized as current, former, or never. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2) with weight measured to the nearest 0.5 kg and height to the nearest 0.1 cm. Blood pressure was measured 3 times using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon; Tampa, FL) while the participants were resting in a seated position. The average of the last 2 measurements was used in the analysis. Fasting blood glucose, total and high-density lipoprotein (HDL) cholesterol levels were measured after fasting for 12 hours. Fasting glucose was obtained by a thin-film adaptation of the glucose oxidase method (Johnson & Johnson Clinical Diagnostics, Inc; Rochester, NY). Diabetes was defined as a fasting glucose of ≥126 mg/dl or use of hypoglycemic medication. N-terminal pro-brain-natriuretic peptide (NT-proBNP) was analyzed centrally at the core laboratory (University of Vermont, Burlington).
Image analysis
Baseline MR images were acquired using 1.5T MR scanners: Signa LX or CVi (GE Medical Systems, Waukesha, WI), or Symphony or Sonata (Siemens Medical Systems, Erlangen, Germany). Long-axis cine images were obtained from 2-chamber and 4-chamber views, using ECG-gated fast gradient-echo pulse sequence. All cine images were acquired with a temporal resolution of ~50 msec. A stack of short-axis images recorded at end diastole was obtained for the LV mass assessment. The detailed MR protocol has previously been described. 19
Multimodality Tissue Tracking software (MTT; version 5.0, Toshiba, Japan) was used to quantify LA strain and volume from baseline 4- and 2-chamber cine CMR images. 20 This method has been previously validated with great reproducibility (intraclass correlation coefficients between 0.90 to 0.97 for LA volumes and strain).20,21A single experienced operator blinded to the case status of the participant defined endocardial and epicardial borders of the LA at end-systole. Using the marked points, the software creates endocardial and epicardial borders and then tracks LA tissue in subsequent frames (video). The endocardial and epicardial contours generated by the software are then followed by the operator during the cardiac cycle for quality control. Maximum, minimum and pre-atrial contraction LA volumes were extracted from volume curves which were created using the area–length method from apical 4- and 2-chamber views, volume = (0.848 · area4ch X area2ch)/([length2ch + length4ch]/2) 22. An example of LA volume cure in a cardiac cycle has been shown in figure 1. All measured LA volumes were subsequently indexed by body surface area. The variables of LA volumes included in our study were:
Figure 1.
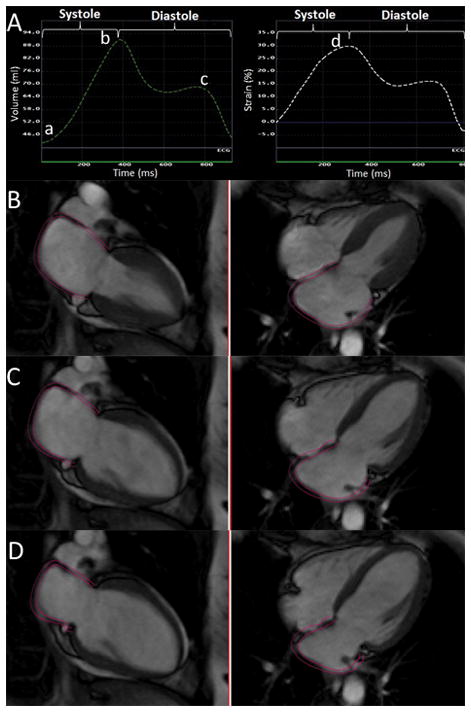
A: Phasic LA volume (left) and global longitudinal strain (right) curves during ventricular systole and diastole. Points “a”, “b” and ‘c” are representing minimum, and maximum LA volume and LA volume before atrial contraction respectively. On the longitudinal strain curve (left), point “d” represents peak LA longitudinal strain. Feature tracking MRI in different phases of cardiac cycle,: end systole (B), before atrial contraction (C), and end diastole (D).
Maximum LA volume (LAVmax): LA volume at end-systole before mitral valve opening.
Minimum LA volume (LAVmin): LA volume at end-diastole right after mitral valve closure.
Pre-atrial contraction LA volume (LAVPreA): LA volume before atrial contraction.
Using the measured LA volumes at different points of cardiac cycle, LA function was calculated as follow:
Passive LA emptying fraction (passive LAEF): (LAVmax - LAVPreA)/LAVmax
Active LA emptying fraction (active LAEF): (LAVPreA – LAVmin)/LAVPreA
Total LA emptying fraction (total LAEF): (LAVmax-LAVmin)/LAVmax
Strain measurement
The software calculates global longitudinal atrial strain by averaging longitudinal strain of all LA segments in 2- and 4-chamber views during each cardiac cycle. Global peak longitudinal left atrial strain (peak LA strain) was measured from the global longitudinal strain curve (figure 1).
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are presented as frequencies and percentages. Differences between group means were evaluated with t tests for continuous variables or chi-square analysis for categorical variables. Logarithmic transformation was applied to NT-proBNP before entry into the models because of its skewed distribution. We used modified Cox proportional hazard models weighted for the stratified case-cohort sampling design to estimate the hazard ratios of developing AF as a function of measured LA variables in three models 23. Cox models were adjusted for significant predictors of AF from a backward selection among the following variables: age, sex, race/ethnicity, body mass index, cigarette smoking, systolic and diastolic blood pressure, hypertensive medication use, diabetes, and left ventricular ejection fraction in Model 1. We additionally adjusted for left ventricular mass and ln NT-proBNP in Model 2. To avoid co-linearity, correlations between continuous variables were tested using the Spearman Correlation Coefficient and variables with r>0.50 were not included in the same multivariable model. All statistical analyses were performed using Stata software (Version 11.2, Stata Corp, Texas, USA).
Results
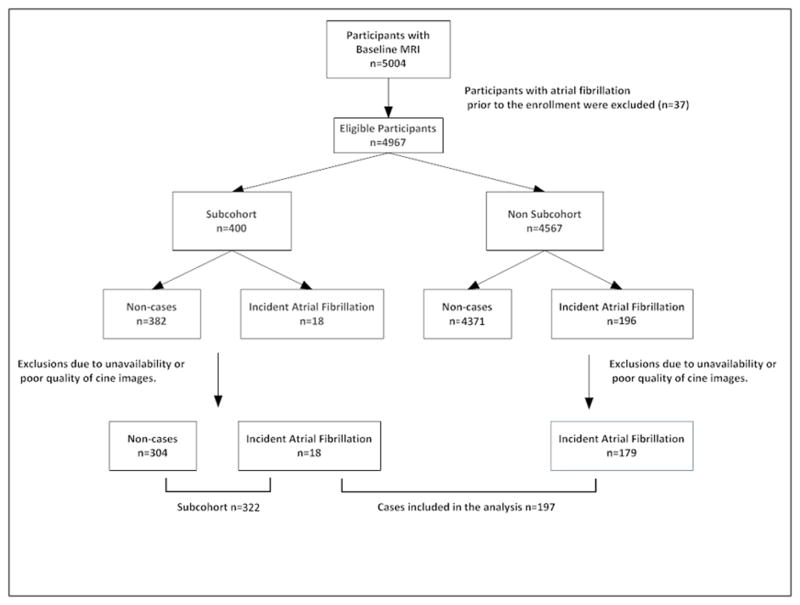
The flowchart of the study is illustrated in Figure 2. Out of 5004 participants with baseline MRI, 37 were excluded due to the diagnosis of AF before enrolling into the MESA. The subcohort consisted of 400 participants who were randomly selected from the MESA cohort irrespective of their case status. The cases were 214 participants who developed AF over a median follow up of 7.6 years. It was feasible to measure LA variables in 197 cases of AF and 304 non-cases.
Figure 2.
Case-cohort design of the study.
The analysis included 322 participants in the subcohort and 197 participants who developed AF; 18 participants were included in this analysis as both AF cases and members of the subcohort. The subcohort and MESA cohort were similar in demographics, baseline characteristics and incidence of cardiovascular events (supplemental table). The baseline characteristics of participants who developed AF and the subcohort are summarized in Table 1. Participants with incident AF were older (70 versus 61 years), and were more likely to be male (62% versus 48%) compared to the subcohort. Higher systolic blood pressure, hypertension diagnosis and being on hypertension medications were all more common in AF cases compared to the subcohort. Although the LV ejection fraction was similar in the two groups, AF cases had a higher LV mass and NT-proBNP.
Table 1.
Baseline characteristics of AF cases and subcohort.
| Subcohort (n=322) | AF Cases (n=197) | |
|---|---|---|
| Age, years | 61.2 ± 10.3 | 70.0 ± 7.6 |
| Male, % | 48.4 | 62.6 |
| Ethnicity, % | ||
| White | 36.3 | 53.5 |
| Chinese | 9.6 | 7.1 |
| Black | 27.6 | 20.7 |
| Hispanic | 26.4 | 18.7 |
| Systolic blood pressure, mmHg | 126 ± 21 | 135 ± 22 |
| Diastolic Blood pressure, mmHg | 71.6 ± 10.0 | 72.2 ± 10.7 |
| Hypertension, % | 45.3 | 65.7 |
| Medications for hypertension, % | 39.4 | 57. 4 |
| Pulse rate, beats/min | 63 ± 9 | 62 ±10 |
| Body mass index, kg/m2 | 27.7 ± 4.9 | 28.2 ± 5.0 |
| Smoking , % | ||
| Never | 49.7 | 44.4 |
| Former | 35.1 | 45.9 |
| Current | 15.2 | 10.7 |
| Diabetes, % | 12.1 | 12.1 |
| LV ejection fraction, % | 69 ± 7 | 69 ± 9 |
| LV mass , g | 144 ± 37 | 165 ± 47 |
| NT-pro BNP (pg/mL) | 105 ± 206 | 267 ± 398 |
LA structure and function
The LA volume was 17% larger at baseline in patients with incident AF compared with subcohort participants (LAVImax : 41 ± 15ml/m2 versus 35 ± 10ml/m2, p<0.001 and LAVImin: 26 ± 11ml/m2 versus 20 ± 7ml/m2, p<0.001, Table 2). In parallel, peak LA strain and all LA emptying fractions (passive, active and total) were lower in AF cases (p<0.001 for all).
Table 2.
Baseline LA variables in AF cases and subcohort.
| LA Variables | Subcohort (n=322) | AF Cases (n=197) |
|---|---|---|
| LAVmax(ml) | 65 ± 20 | 78 ± 28 |
| LAVImax(ml/m2) | 35 ± 10 | 41 ± 15 |
| LAVmin | 36 ± 13 | 49 ± 21 |
| LAVImin (ml/m2) | 20 ± 7 | 26 ± 11 |
| Passive LAEF, (%) | 19 ± 8 | 15 ± 7 |
| Active LAEF, (%) | 31 ± 9 | 28 ± 10 |
| Total LAEF, (%) | 44± 9 | 39 ± 10 |
| Peak LA Strain, (%) | 31 ± 13 | 25 ± 12 |
LAVImax: maximum left atrial volume index. LAVImin minimum left atrial volume index, LAEF: left atrial emptying fraction
Association of LA function and incident AF
Multivariable Cox regression models to examine the association of incident AF with LA structural and functional variables have been summarized in Table 3. Because of high correlation between LAVImax and LAVImin (r=0.92) only LAVImax was included the multivariable models. In Model 1 after adjustment for age, sex, ethnicity, hypertensive medications, and systolic blood pressure, all LA variables were significantly associated with incident AF. In Model 2 after additionally adjusting for ln NT-proBNP and LV mass, the association remained significant for all variables except for active LAEF. Larger LA was positively associated with incident AF (HR for LAVImax: 1.38 per SD, P-value: 0.042). Decreased LA function assessed as decreased passive and total emptying fractions and peak LA strain were also associated with incident AF (HRs per SD for passive LAEF: 0.55 P-value <0.001, for total LAEF: 0.70 P-value 0.020 and for peak LA strain: 0.68 P-value: 0.031).
Table 3.
Association of LA function and incident AF.
| LA Variables | Model 1 * | Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
|
|
|
|
||
| LAVImax | 1.82 (1.40–2.36) | <0.001 | 1.38 (1.01–1.89) | 0.042 |
| Passive LAEF | 0.68 (0.54–0.88) | 0.003 | 0.55 (0.40–0.75) | <0.001 |
| Active LAEF | 0.74 (0.58–0.94) | 0.014 | 0.95 (0.70–1.27) | 0.717 |
| Total LAEF | 0.61 (0.48–0.79) | <0.01 | 0.70 (0.52–0.95) | 0.020 |
| Peak LA Strain | 0.68 (0.50–0.93) | 0.014 | 0.68 (0.48–0.96) | 0.031 |
Model 1 was adjusted for age, sex, ethnicity, hypertensive medications, and systolic blood pressure.
Model 2 was additionally adjusted for LV mass and Ln NT-ProBNP.
LAVImax: maximum left atrial volume index. LAEF: left atrial emptying fraction
Discussion
In this case-cohort study, reduced LA function measured by CMR feature tracking was associated with incident AF. Parallel to enlarged LA size, passive and total LAEF and also peak LA strain were lower at baseline in participants who developed AF. This association was independent of traditional cardiovascular risk factors, LV mass, and NT-ProBNP. To the best of our knowledge, this is the first study using feature-tracking CMR to assess this association in a large multi-ethnic cohort.
Structural and functional changes in LA are present before AF development. Therefore early detection of these changes in individuals at higher risk for AF has a great clinical impact. As both animal and human studies have shown, LA remodeling, especially in the earlier stages, could be reversible after using certain medications 24–26. Although multiple imaging modalities have been proposed to measure LA volume and assess LA function, given the far field location of LA and the thin asymmetric wall, the assessment of its function is quite challenging. In this study we used CMR feature tracking to measure phasic changes in the volume and assess phasic function of the LA.
LA size and incident AF
In the absence of valvular disease or AF, LA volume has been shown to reflect the burden of cardiovascular disease 27, 28. Based on multiple studies performed with echocardiography, enlarged LA is a well-known risk factor for AF development 12–14, 29. According to a study on 1924 subjects in the Framingham Heart Study, every 5mm increase in LA diameter measured with M-mode echocardiography was associated with 42% increase in the risk of AF 13. Later studies using 2D echocardiography were able to examine this association with LA volume. Based on a study performed by Tsang et al. on 317 participants, LAVI was a stronger marker of cardiovascular events including AF compared to LA diameter and area 29. In another study of 574 adults, both LAVmin and LAVmax measured with 2D echocardiography were independently associated with AF/flutter development 14.
The findings of our study, which has been performed using feature-tracking MRI, are in agreement with prior studies using echocardiography. In our study, increased LA volume was significantly associated with incident AF.
LA function and incident AF
Left atrium (LA) modulates left ventricular filling and function in three distinct phases. It functions as a reservoir for pulmonary venous return during ventricular systole, as a conduit for pulmonary venous return during early ventricular diastole, and as a booster pump that augments ventricular filling at the end of ventricular diastole. In patients with atrial fibrillation, not only the booster pump function is absent, but also reservoir and conduit functions are decreased. Studies suggest these functional changes precede development of AF, therefore, assessment of LA function in a population at risk of AF is of great interest. Traditionally Doppler imaging has been used to assess the LA wall motion. In a study of 942 participants in the Framingham study, decreased transmitral Doppler was associated with new onset AF independent of LA size, suggesting impaired LA booster pump function preceding AF development 15. Phasic LA function can be also assessed using volumetric and strain analysis methods. In volumetric method, total, passive and active LA emptying fractions (LAEF) are surrogates of reservoir, conduit, and booster pump functions respectively. Peak LA longitudinal strain is also used as a representative of LA reservoir function.30 In a study performed by Abhayaratna et al., total LAEF measured with 2D Echocardiography was associated with new onset AF/flutter in 574 adults older than 65 years of age who were referred for clinically indicated echocardiography 16. In a recent study with speckle tracking echocardiography on 580 adults with 32 new onset AF patients, out of phasic LA function, strain and strain rate measurements, only active LAEF was independently associated with incident AF 31. In this study a cut-off of active LAEF ≤20% had a sensitivity and specificity of 88% and 81% respectively for new onset AF. In our study, similar to the study of Hirose et al, active LAEF was significantly associated with incident AF after adjusting for significant predictors of AF (model 1). However, after adjusting for NT-ProBNP and LV mass the association became non-significant. We further explored the association of NT-ProBNP and emptying fractions, and found that active LAEF but not passive LAEF is inversely associated with NT-ProBNP (r=−0.19, p=0.027). To our knowledge there are no other studies comparing the association of passive and active LAEF with incident AF in general population. Although on 1120 subjects with AF in ENGAGE AF_TIMI 48 cohort, increased AF burden was associated with lower passive and total LAEF but not active LAEF. 2 Previously we also found that in patients with AF undergoing catheter ablation, CMR measured passive LAEF but not active LAEF is associated with increased LA late gadolinium enhancement. 4 These findings may suggest that passive LAEF is a more sensitive and independent marker of LA remodeling in patients with AF or at risk of having AF. In our study peak LA strain, total and passive LAEF, as measures of LA reservoir, global and conduit phase functions respectively, were independently associated with new onset AF.
LA remodeling, especially in early stages, can be reversible. Many studies have shown that certain medications such as angiotensin converting enzyme inhibitors 26 or angiotensin II receptor antagonists 32 are associated with improvement of LA function as well as reduction of new onset AF in individuals at risk of AF. 33 In addition, based on animal studies, aldosterone antagonists such as spironolactone have been shown to reduce LA dilation and fibrosis in rats with heart failure after myocardial infarction.34 LA remodeling is present and detectable using non-invasive methods such as feature tracking CMR before development of AF. Inclusion of LA functional variables together with other known risk factors of AF may help to create stronger predictive models to identify population at risk of AF and therefore to initiate earlier targeted intervention. This approach could be tested in future trials based on LA variables used in this study.
Strengths and Limitations
To our knowledge, our study is among the first studies to investigate the association of LA function and new onset AF using CMR imaging. Other strengths are the wide age range of the study population, in contrast to most other studies on LA function, and also the availability of comprehensive clinical and imaging data as well as biomarkers. We also acknowledge a few study limitations. Considering the fact that images were acquired using Gradient Echo Sequences (GRE), the CMR images were not interpretable in 15% of the study population. However, this rate is about the same or lower compared to the other studies using speckle tracking echocardiography in measurements of LA function 35, 36. We used area-length method to measure LA volumes. Therefore, the calculated volumes might be an underestimation of the true volumes by 11.5–20% depending on the alignment of the acquired slice with true orientation of LA. 22, 37 However, this method is widely used in clinical and research studies.38 Incident AF was identified based on hospital discharge codes, which may not be accurate and also may underestimate AF diagnosis as many of AF cases are asymptomatic and do not require hospitalization. However, a validation substudy on 45 MESA participants with the diagnosis of AF based on hospital discharge codes confirms the diagnosis of AF in 93% of hospitalizations 39. Additionally, based on a systematic review using information from 16 unique studies, a large proportion of prevalent AF cases identified by ICD-9 code were valid (positive predictive value 70%–96%, median 89%).40
Conclusions
In this multi-ethnic study population free of clinical cardiovascular disease, elevated LA volume and decreased LA reservoir and conduit functions measured with feature-tracking CMR imaging, were associated with incident AF. This association is suggesting of alterations of LA function prior the AF development. Moreover, CMR feature-tracking was feasible to analyze phasic LA function. Assessment of LA function in addition to LA volume may add further information in detecting individuals at higher risk of AF development.
Supplementary Material
Clinical Perspective.
Atrial fibrillation (AF) is associated with functional, structural, and electrical changes in the left atrium (LA). These changes precede development of AF and worsen with AF maintenance. Functional LA measures include total, passive and active emptying fractions, and strain analysis, which is traditionally assessed using speckle tracking echocardiography. However, echocardiographic assessment of LA function is challenging due to the posterior location and thin wall of the LA. In this study we examined the association of phasic LA function with incident AF in a multi-ethnic asymptomatic population using the novel method of feature tracking MRI. We found an inverse association between LA functional parameters (total and passive emptying fraction and peak longitudinal strain) with incident AF independent of clinical risk factors, and biomarkers. Measurement of LA function using CMR in addition to clinical risk factors might be useful to detect individuals at risk of AF in order to start early preventive interventions.
Acknowledgments
We thank all the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding: This research was supported by contracts N01-HC-95159 through N01-HC-95166, and N01-HC-95168 from the National Heart, Lung, and Blood Institute; and UL1-RR-024156 and UL1-RR-025005 from National Center for Research Resources, National Institutes of Health
Footnotes
Disclosures: None.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, Deenadayalu N, Hoffman E, Patel I, Shi M, Mercuri M, Mitrovic V, Braunwald E, Solomon SD. Effective aNticoaGulation with factor x AnGiAFTIMIESI. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. European heart journal. 2014;35:1457–65. doi: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circulation Cardiovascular imaging. 2010;3:231–9. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 4.Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K, Spragg D, Ashikaga H, Rickard J, Marine JE, Calkins H, Nazarian S. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circulation Cardiovascular imaging. 2015;8:e002769. doi: 10.1161/CIRCIMAGING.114.002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, Macleod RS, Marrouche NF. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. Journal of the American College of Cardiology. 2011;57:831–8. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih JY, Tsai WC, Huang YY, Liu YW, Lin CC, Huang YS, Tsai LM, Lin LJ. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. Journal of the American Society of Echocardiography. 2011;24:513–9. doi: 10.1016/j.echo.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, Zeppenfeld K, Holman E, Schalij MJ, Bax JJ. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. Journal of the American College of Cardiology. 2011;57:324–31. doi: 10.1016/j.jacc.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 8.Dodson JA, Neilan TG, Shah RV, Farhad H, Blankstein R, Steigner M, Michaud GF, John R, Abbasi SA, Jerosch-Herold M, Kwong RY. Left atrial passive emptying function determined by cardiac magnetic resonance predicts atrial fibrillation recurrence after pulmonary vein isolation. Circulation Cardiovascular imaging. 2014;7:586–92. doi: 10.1161/CIRCIMAGING.113.001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 10.Di Salvo G, Caso P, Lo Piccolo R, Fusco A, Martiniello AR, Russo MG, D’Onofrio A, Severino S, Calabro P, Pacileo G, Mininni N, Calabro R. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005;112:387–95. doi: 10.1161/CIRCULATIONAHA.104.463125. [DOI] [PubMed] [Google Scholar]
- 11.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–63. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 12.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clinic proceedings. 2001;76:467–75. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 13.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–30. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 14.Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, Tsang TS. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. European journal of echocardiography. 2009;10:282–6. doi: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 15.Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, Benjamin EJ National Heart L, Blood Institute NIoH. Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study) The American journal of cardiology. 2003;91:1079–83. doi: 10.1016/s0002-9149(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 16.Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TS. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. The American journal of cardiology. 2008;101:1626–9. doi: 10.1016/j.amjcard.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 17.Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance. 2010;12:65. doi: 10.1186/1532-429X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 20.Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G, Bluemke DA, Lima JA. Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovascular imaging. 2014;7:570–9. doi: 10.1016/j.jcmg.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zareian M, Ciuffo L, Habibi M, Opdahl A, Chamera EH, Wu CO, Bluemke DA, Lima JA, Venkatesh BA. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. Journal of cardiovascular magnetic resonance. 2015;17:52. doi: 10.1186/s12968-015-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith HJ, Ihlen H. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. International journal of cardiac imaging. 1999;15:397–410. doi: 10.1023/a:1006276513186. [DOI] [PubMed] [Google Scholar]
- 23.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. Journal of clinical epidemiology. 1999;52:1165–72. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovascular research. 2002;54:456–61. doi: 10.1016/s0008-6363(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Li W, Yang B, Han W, Dong D, Xue J, Li B, Yang S, Sheng L. Effects of Cilazapril on atrial electrical, structural and functional remodeling in atrial fibrillation dogs. Journal of electrocardiology. 2007;40:100e1–6. doi: 10.1016/j.jelectrocard.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Tsang TS, Barnes ME, Abhayaratna WP, Cha SS, Gersh BJ, Langins AP, Green TD, Bailey KR, Miyasaka Y, Seward JB. Effects of quinapril on left atrial structural remodeling and arterial stiffness. The American journal of cardiology. 2006;97:916–20. doi: 10.1016/j.amjcard.2005.09.143. [DOI] [PubMed] [Google Scholar]
- 27.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. The American journal of cardiology. 2002;90:1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 28.Miller JT, O’Rourke RA, Crawford MH. Left atrial enlargement: an early sign of hypertensive heart disease. American heart journal. 1988;116:1048–51. doi: 10.1016/0002-8703(88)90158-5. [DOI] [PubMed] [Google Scholar]
- 29.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? Journal of the American College of Cardiology. 2006;47:1018–23. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 30.Hoit BD. Left atrial size and function: role in prognosis. Journal of the American College of Cardiology. 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 31.Hirose T, Kawasaki M, Tanaka R, Ono K, Watanabe T, Iwama M, Noda T, Watanabe S, Takemura G, Minatoguchi S. Left atrial function assessed by speckle tracking echocardiography as a predictor of new-onset non-valvular atrial fibrillation: results from a prospective study in 580 adults. European heart journal cardiovascular Imaging. 2012;13:243–50. doi: 10.1093/ejechocard/jer251. [DOI] [PubMed] [Google Scholar]
- 32.Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. Journal of the American College of Cardiology. 2003;41:2197–204. doi: 10.1016/s0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 33.Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. Journal of the American College of Cardiology. 2005;45:712–9. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 34.Milliez P, Deangelis N, Rucker-Martin C, Leenhardt A, Vicaut E, Robidel E, Beaufils P, Delcayre C, Hatem SN. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. European heart journal. 2005;26:2193–9. doi: 10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 35.Saraiva RM, Demirkol S, Buakhamsri A, Greenberg N, Popovic ZB, Thomas JD, Klein AL. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010;23:172–80. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–5. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 37.Vardoulis O, Monney P, Bermano A, Vaxman A, Gotsman C, Schwitter J, Stuber M, Stergiopulos N, Schwitter J. Single breath-hold 3D measurement of left atrial volume using compressed sensing cardiovascular magnetic resonance and a non-model-based reconstruction approach. Journal of cardiovascular magnetic. 2015;17:47. doi: 10.1186/s12968-015-0147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–6. doi: 10.1136/heartjnl-2013-304724. [DOI] [PubMed] [Google Scholar]
- 40.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):141–7. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.