Abstract
Although abdominal pain is a symptom of several structural gastrointestinal disorders (eg, peptic ulcer disease), this comprehensive review will focus on the 4 most common nonstructural, or functional, disorders associated with abdominal pain: functional dyspepsia, constipation-predominant and diarrhea-predominant irritable bowel syndrome, and functional abdominal pain syndrome. Together, these conditions affect approximately 1 in 4 people in the United States. They are associated with comorbid conditions (eg, fibromyalgia, depression), impaired quality of life, and increased health care utilization. Symptoms are explained by disordered gastrointestinal motility and sensation, which are implicated in a variety of peripheral (eg, postinfectious inflammation, luminal irritants) and/or central (eg, stress and anxiety) factors. These disorders are defined and can generally be diagnosed by symptoms alone. Often prompted by alarm features, selected testing is useful to exclude structural disease. Identifying the specific diagnosis (eg, differentiating between functional abdominal pain and irritable bowel syndrome) and establishing an effective patient-physician relationship are the cornerstones of therapy. Many patients with mild symptoms can be effectively managed with limited tests, sensible dietary modifications, and over-the-counter medications tailored to symptoms. If these measures are not sufficient, pharmacotherapy should be considered for bowel symptoms (constipation or diarrhea) and/or abdominal pain; opioids should not be used. Behavioral and psychological approaches (eg, cognitive behavioral therapy) can be very helpful, particularly in patients with chronic abdominal pain who require a multidisciplinary pain management program without opioids.
Keywords: Abdominal Pain, Chronic Pain, Constipation, Diarrhea, Gastroenterology
Introduction
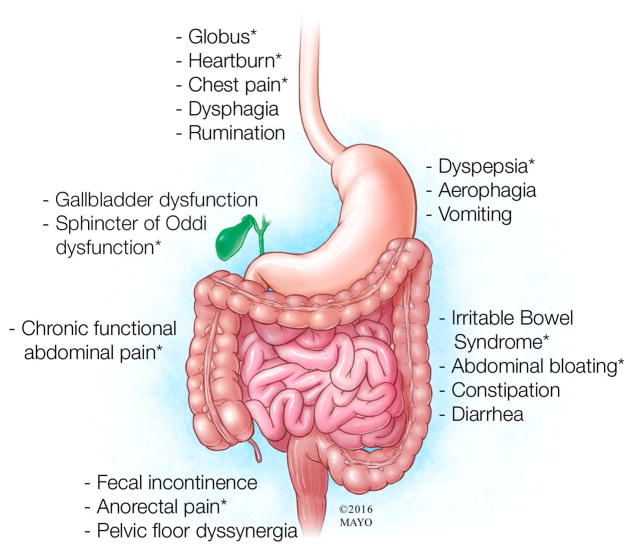
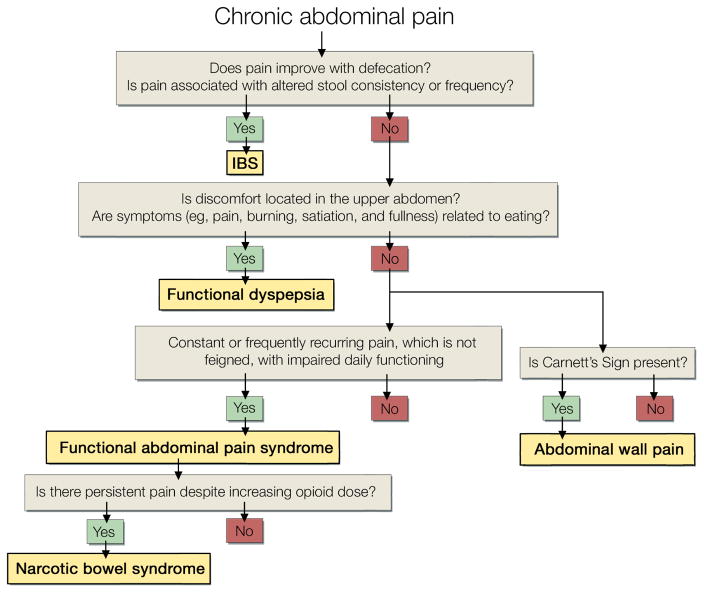
This review will focus on nonstructural, or functional, rather than structural (eg, peptic ulcer disease, ulcerative colitis) gastrointestinal (GI) disorders associated with abdominal pain.1 Symptoms provide the basis for classifying the functional disorders, which affect the entire GI tract (Figure 1). Many physicians refer to these conditions with the umbrella term irritable bowel syndrome (IBS). It is critical to recognize that while these conditions share several features, they can and should be distinguished from each other primarily on the basis of the nature of symptoms (Figure 2). These specific diagnoses are not only more precise but also facilitate management, which is tailored to the specific symptoms. This review will focus on the 4 most common functional disorders associated with abdominal pain: functional dyspepsia, constipation-predominant and diarrhea-predominant IBS, and functional abdominal pain syndrome.
Figure 1.
Common Functional Gastrointestinal Disorders. These disorders are defined by specific criteria but often coexist. Conditions associated with pain are marked with an asterisk (*).
Figure 2.
Diagnostic Criteria and Algorithm for Common Functional Gastrointestinal Disorders Associated With Abdominal Pain. This algorithm applies the Rome criteria, which are widely used in research studies. Irritable bowel syndrome is defined by abdominal pain accompanied by 2 of these 3 criteria: relief with defecation, altered stool consistency (loose or hard stools), or altered frequency (less or more frequent). The other syndromes are defined by the listed criteria. The criteria also specify the duration and frequency of symptoms, in general, a duration of 6 months and frequency of 2 days every week or more often.
Methods
We searched MEDLINE on the PubMed and Ovid platforms, as well as the Cochrane Database of Systematic Reviews, using the keywords abdominal pain, chronic abdominal pain, abdominal wall pain, visceral pain, narcotic bowel, functional abdominal pain for English-language articles with no date restrictions. Search terms were cross-referenced with review articles, and additional articles were identified by manually searching reference lists.
Epidemiology and Natural Course
In North America, approximately 20% of adults have symptoms of dyspepsia and 10% to 15% have symptoms of IBS.2,3 Among the latter, approximately 5% each have diarrhea- and constipation-predominant IBS, which are more common in men and women, respectively.2 By comparison, the prevalence of functional abdominal pain is much lower (0.5%–1.7%).4 Even this figure is probably an overestimate, since the definition of functional abdominal pain in these studies did not incorporate all the criteria for functional abdominal pain syndrome, such as the loss of daily function associated with the pain. Most cases of IBS are diagnosed by primary care specialists.5
It is not uncommon for patients to simultaneously have symptoms of 2 or more disorders (eg, dyspepsia and constipation).6 The severity and nature of symptoms vary with time. Over the long term, symptoms were unchanged in 50%, worse in approximately 20%, and improved in 30% of patients with IBS seen in clinics.7 In the general population, approximately 20% of patients with IBS had the same symptoms, 40% had no symptoms, and 40% had different symptoms at follow-up 12 years later.8 The nature of symptoms may change over time, most frequently from constipation- or diarrhea-predominant IBS to mixed type or vice versa.9 In postinfectious IBS, the prognosis is better; symptoms resolve in approximately 50% of patients after 6 to 8 years.10
Relevant Anatomy, Physiology, and Pathophysiology
Clinically Oriented Introduction to GI Motor and Sensory Functions
Motility is regulated by coordinated neurohormonal mechanisms that influence smooth muscle contractility.11 Gut motor activity is primarily controlled by the intrinsic or enteric nervous system. The central nervous system modulates gut motor activity via the extrinsic sympathetic and parasympathetic pathways, while descending pathways in the spinal cord modulate transmission of sensory input from the dorsal horn to supraspinal centers. Visceral sensation is conveyed via afferents that travel to the spinal cord and ultimately to the cerebral cortex, and through the vagus to the brainstem. There is a 10:1 ratio of afferent to efferent fibers in the vagus at the level of the diaphragm. The vagus primarily conveys subnoxious messages, while the spinal afferents convey nonnoxious and noxious input12 to the dorsal horn of the spinal cord. Thereafter, information is conveyed via the spinothalamic tract to the medial and posterior thalamus and subsequently to the primary somatosensory cortex, which localizes and discriminates somatic and visceral sensations.13 The spinoreticular and spinomesencephalic tracts project to the brainstem reticular formation and medulla, from which information is conveyed to the medial thalamus and the anterior cingulate cortex and insula. These areas process the affective-motivational aspects of pain.
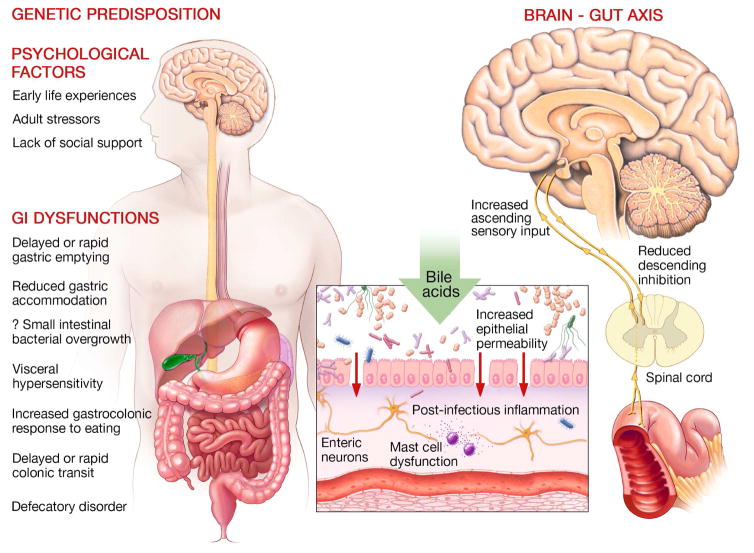
Descending inhibitory fibers from the anterior cingulate cortex to the dorsal horn of the spinal cord utilize endogenous opioids, serotonin, and noradrenaline to modulate or gate visceral sensation, including pain, such that only some messages from the viscera are conveyed to higher centers (Figure 3).13
Figure 3.
Pathogenesis of Common Functional Gastrointestinal Disorders. Genetic predisposition and psychological factors contribute to a variety of gastrointestinal sensory and motor dysfunctions that contribute to symptoms (left panel). Center panel shows some factors (eg, bile acids, postinfectious inflammation) that alter mucosal permeability, stimulate enterochromaffin cells, and activate immune mechanisms to stimulate afferent nerves. Central sensitization may result from this peripheral sensitization and/or reduced descending inhibition, which normally gates visceral sensation in the spinal cord.
Pathogenesis of Symptoms
Current concepts suggest that the functional GI disorders result from the combined effect of biological, psychological, and social factors (ie, the biopsychosocial model). Exemplifying this model, postinfectious IBS, which occurs after the infection resolves in 4% to 40% of patients with infectious gastroenteritis,10 is more likely to occur after severe or prolonged gastroenteritis in women older than 60 years, smokers, and patients with anxiety, depression, hypochondriasis, or an adverse life event in the preceding 3 months.10 The following factors have been implicated in the pathogenesis of functional GI disorders.
Luminal Irritants
Luminal irritants primarily include bile acids, nonabsorbed complex carbohydrates, and the products of their bacterial metabolism (ie, short-chain fatty acids).14 Bile acids15 and short-chain fatty acids activate secretion of enteroendocrine cell products (eg, 5-hydroxytryptamine [5-HT]) and stimulate colonic motility and defecation. Enteric microbiota, including both commensal and pathogenic organisms, also communicate with the host.16
Increased Visceral Sensitivity
Increased visceral sensitivity is documented with increased perception of GI (eg, rectal) balloon distention. Healthy people have little awareness of GI physiologic processes except for the sensation of postprandial fullness after a satiating meal or the desire to defecate. Increased sensitivity refers to an exaggerated awareness of normal events and differs from hyperalgesia, wherein painful stimuli evoke more pain than usual, or allodynia, which refers to perception of nonnoxious stimuli as being painful. Increased sensitivity may be due to sensitization of peripheral afferent receptors or spinal dorsal horn neurons, alterations in descending modulation, or central amplification.13 Sensitization of peripheral receptors may be secondary to low-grade inflammation, which activates silent nociceptors and increases the sensitivity and field of peripheral receptors. Peripheral sensitization may increase afferent input to the dorsal horn, causing central sensitization. Exemplifying this phenomenon, acid infusion in the distal esophagus causes hyperalgesia not only in the acid-exposed distal esophagus but also in the unexposed proximal esophagus.13 Repeated distention of the sigmoid colon causes rectal hyperalgesia.13 Central amplification or sensitization is the hallmark of fibromyalgia.17 The central mechanisms (eg, reduced descending modulation or central amplification) (Figure 3) that may explain increased visceral sensitivity have been evaluated in fibromyalgia and IBS.13,17
Normal and Disordered GI Motility
Disordered GI motility may contribute to symptoms. Normally, the stomach relaxes or accommodates after a meal, providing room for food to be digested into smaller particles. Impaired gastric accommodation may explain postprandial symptoms (eg, early fullness or satiety) in functional dyspepsia.11 The colonic response to feeding, or gastrocolonic response, refers to reflex contraction of the distal small intestine and colon in response to nutrients in the stomach and small intestine. This reflex, which is mediated by afferent vagal pathways and efferent sacral parasympathetic pathways, explains why healthy people experience the desire to defecate after eating. An exaggerated response may explain postprandial discomfort and diarrhea in patients with diarrhea-predominant IBS. The contractile response to colonic distention may also evoke abdominal discomfort. Indeed, the perception of distention may be due to the contractile response to distention rather than distention per se.
Decreased motility may affect the stomach, small intestine, and/or colon; delay GI transit; and explain symptoms.18 For example, opioids markedly delay colonic transit and may predispose to colonic retention of stool, colonic distention, and abdominal distention. Pelvic floor dysfunction (ie, a disturbance in the normal rectoanal coordination during defecation) may impair rectal evacuation of stool, which also predisposes to rectal and colonic distention with stool and similar symptoms.
Hormonal and Other Chemical Mediators
Serotonin, which is released from enterochromaffin cells, initiates the peristaltic reflex and activation of extrinsic vagal and spinal afferents that activate extrinsic reflexes and sensation.11 This reflex involves excitatory (eg, acetylcholine, tachykinins) and inhibitory (primarily vasoactive intestinal pepide and nitric oxide) neurotransmitters. IBS is associated with serotoninergic disturbances.19 Ingestion of calories stimulates the release of hormones such as cholecystokinin and GLP-1, which stimulate vagal afferents and mediate postprandial symptoms such as nausea and satiety.11
Abdominal Wall Accommodation
Normally, meal ingestion is accompanied by relaxation of the diaphragm (ie, diaphragmatic ascent), which provides extra space in the upper abdominal cavity, and compensatory contraction of the upper anterior abdominal wall, which prevents abdominal distention in the upright position.20 In contrast, patients with dyspepsia have aberrant abdominal accommodation characterized by paradoxical contraction of the diaphragm (ie, diaphragmatic descent) and relaxation of the upper anterior abdominal wall, which increases abdominal girth.21 Similar disturbances (ie, diaphragmatic contraction and relaxation of the lower rectus and internal oblique) have been observed during intestinal gas infusion in IBS and functional bloating.22
Psychosocial Factors
Early life experiences (eg, verbal, sexual, or physical abuse), adult stressors (eg, divorce, bereavement), lack of social support, and other social learning experiences can affect an individual’s physiologic and psychological responses. The changes in these responses can lead to maladaptive earned-illness behaviors23 and predispose to functional GI and psychiatric disorders.24 Women with IBS are more likely to have experienced abuse.25 These experiences may affect the brain-gut axis and lead to increased visceral sensitivity. Earlier studies suggested that psychosocial factors influence illness behavior (ie, the decision to seek care, excessive expressions of suffering) rather than symptoms per se. More recent studies suggest that psychological disturbances are common even among people with IBS in the community, many of whom have not sought medical attention.24 Whether psychological and somatic symptoms (eg, chronic fatigue, fibromyalgia) reflect the shared expression of a common substrate or whether they cause each other is unknown. Somatization, hypervigilance, and catastrophizing amplify GI and non-GI symptoms in patients with IBS.26
Clinical Features
Characteristics of Pain, Physical Examination, and Associated Conditions
The location of pain may suggest its origin; for example, postprandial epigastric pain likely originates from the stomach. The pattern of pain, particularly its relationship to meals, and associated symptoms are also useful. Patients with dyspepsia, which is derived from the Greek word for indigestion, have pain that occurs shortly (eg, within 60 minutes) after meals, often associated with upper GI symptoms such as nausea, bloating, early satiety, or heartburn (Figure 2). However, dyspepsia is not associated with disordered bowel habits. In contrast, patients with IBS have intermittent abdominal pain, often related to meals. Moreover, the abdominal pain is associated with 2 of these 3 features: improvement with defecation, harder and/or less frequent stools in constipation-predominant IBS, and the opposite in diarrhea-predominant IBS. Some patients with functional constipation and diarrhea also have abdominal pain; however, the pain is not temporally associated with bowel symptoms.
On physical examination, patients may have tenderness to abdominal palpation, but not guarding or rigidity. The absence of features of autonomic arousal (eg, tachycardia) when patients report severe pain suggests a functional rather than a structural disorder. When the abdomen is palpated, patients with functional pain might wince with their eyes closed (closed eyes sign), while patients with an acute abdominal pain episode usually keep their eyes open in anxious anticipation. When the abdomen is palpated with a stethoscope, the behavioral response to pain decreases in functional but increases in acute causes of abdominal pain (stethoscope sign).27 In constipated patients, stool may be palpable in the colon.
Somatic symptoms (eg, chronic fatigue, fibromyalgia, cardiopulmonary symptoms) that are not due to an organic disorder and psychiatric disorders (major depression, anxiety, and somatization) are also common among patients with IBS.24,28,29 Among patients with chronic abdominal pain, the prevalence of depression and impaired overall functioning is comparable to that in patients with chronic back pain.30 Hence, all patients with moderate or severe symptoms should be screened for psychiatric symptoms and illness-related disability either during the interview or, perhaps more efficiently, with a screening questionnaire (eg, GAD-7 or PHQ-9, Health Anxiety Inventory, Sheehan Disability Scale). Physicians should also inquire about the relationship of stressors to symptoms; a history of sexual and/or physical abuse, which often is not volunteered by patients; and the presence of social support networks, which are often limited, resulting in maladaptive strategies (eg, catastrophizing thoughts, frustration) to cope with symptoms. The physical and mental quality of life in patients with severe IBS is comparable to or worse than in patients with severe chronic obstructive lung disease or congestive heart failure.31–33 The mental distress is less severe than in patients with severe depression but worse than in patients with cancer.34,35
Other Conditions
Musculoskeletal Abdominal Pain
In contrast to visceral abdominal pain, musculoskeletal abdominal pain is sharp, localized to an area of the abdominal wall usually smaller than 2 cm, and associated with Carnett sign.36 Patients with abdominal wall pain are likely to be women, often are obese, and have painful comorbid conditions (eg, IBS, functional dyspepsia). Carnett sign is elicited by palpating the abdomen before and during contraction of the abdominal muscles (eg, by raising the head from the bed without using the arms); abdominal wall pain and visceral abdominal pain are characterized by more and less pain, respectively, during than before contraction. A positive Carnett sign has a diagnostic accuracy of 97% for abdominal wall pain.37 Conversely, less than 10% of patients with visceral pain had a positive Carnett sign.38
Opioid Bowel Dysfunction, Opioid-Induced Constipation, and Narcotic Bowel Syndrome
These conditions can occur with opioid use for any intra-abdominal or extra-abdominal disease.39 Opioid bowel dysfunction (OBD) comprises the full spectrum of peripheral, μ-opioid-receptor–mediated GI adverse effects, including anorexia, nausea, vomiting, gastroparesis, biliary pain, gastroesophageal reflux, constipation (ie, opioid-induced constipation [OIC]), ileus, pseudo-obstruction, abdominal bloating, and straining. In contrast, narcotic bowel syndrome (NBS) is characterized by incompletely controlled abdominal pain that cannot be explained by another known or previous diagnosis in patients receiving steady or increasing doses of opioids. In contrast to OBD, NBS is mediated by centrally mediated visceral hyperalgesia rather than GI dysmotility. Approximately 50% to 60% of patients on opioid therapy have OBD, 40% have OIC, and 5% have NBS. NBS may coexist with OBD and OIC.
Structural Diseases
Functional and structural diseases may coexist. For example, persistent abdominal pain in patients with quiescent inflammatory bowel disease likely reflects a coexistent functional GI disorder. Alarm features (ie, symptom onset after age 50 years; severe or progressive symptoms; unexplained weight loss; nocturnal pain or diarrhea; family history of organic GI diseases, including colon cancer, celiac disease, or inflammatory bowel disease; rectal bleeding or anemia; unexplained iron deficiency anemia) should increase the index of suspicion for structural diseases. However, many patients with structural disease do not have alarm symptoms.
Diagnostic Testing
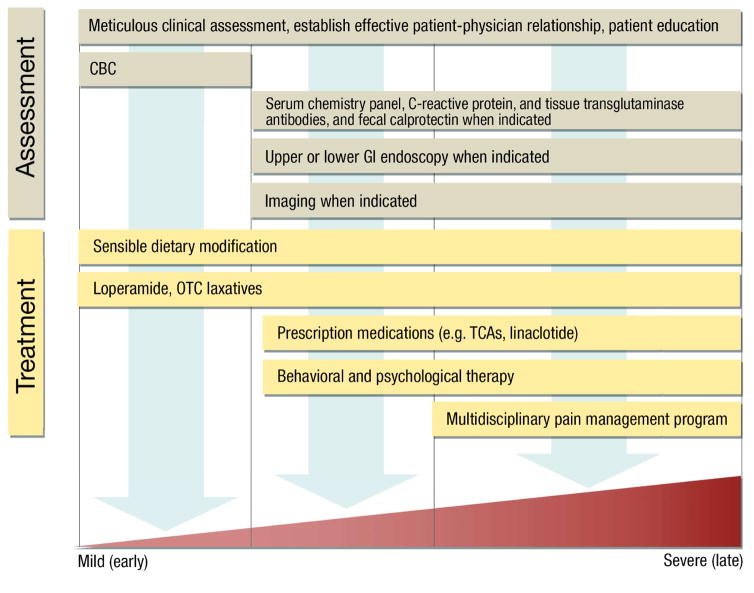
Diagnostic testing should be limited and tailored to the clinical features, alarm symptoms, symptom severity, and response to prior therapy (Figure 4). Absent alarm features, symptoms generally suffice to diagnose functional GI disorders.28 While alarm features may prompt additional testing, most patients with alarm features will have negative test results. After a negative evaluation for structural lesions, the risk is less than 5% that a patient with diagnosed IBS will have a structural disease diagnosed in future.7 A complete blood cell count should be checked in all patients. Some guidelines recommend consideration of a blood test (eg, tissue transglutaminase antibodies) or duodenal mucosal biopsies to diagnose celiac disease, given the potential long-term consequences of missing this diagnosis.28 Contradicting this recommendation, the prevalence of celiac disease was lower in patients with than without IBS in a US population.40 While abdominal pain and diarrhea occur in inflammatory bowel disease as well as in IBS, rectal bleeding and systemic symptoms are much more common in inflammatory bowel disease. In a prospective study, inflammatory bowel disease was later diagnosed in less than 1% of patients with IBS symptoms and in no controls.41 Noninvasive biomarker testing is a cost-effective approach to screen for inflammatory bowel disease. When the C-reactive protein level is less than 0.5 mg/dL or the fecal calprotectin level is less than 40 μg/g, the risk of inflammatory bowel disease is less than 1% in patients with typical IBS symptoms.28 A minority of patients with diarrhea-predominant IBS, especially patients older than 50 years or those who have nocturnal stools, weight loss, shorter duration of diarrhea, recent introduction of new drugs, or comorbid autoimmune diseases.42
Figure 4.
Multidisciplinary Management of Functional Gastrointestinal Disorders. After a meticulous clinical assessment, many patients with mild symptoms can be effectively managed with limited tests, sensible dietary modifications, and over-the-counter medications tailored to symptoms. Gastrointestinal endoscopy and imaging should be performed only when indicated. Behavioral and psychological approaches can be very helpful, particularly in patients with chronic abdominal pain who require a multidisciplinary pain management program without opioids.
In constipated patients, colorectal cancer and, more importantly, pelvic floor dysfunction (ie, defecatory disorders) should be considered. Absent concerning features, less than 1% of patients with typical constipated IBS have colorectal cancer, which is not greater than in asymptomatic controls. Hence, only age-appropriate colorectal cancer screening is required.43,44 Defecatory disorders (DD), which are attributed to the dyscoordination among the abdominal wall, anal sphincter, and pelvic floor muscles during defecation, are a common but underrecognized cause of constipation and abdominal symptoms such as pain, discomfort, and bloating.45,46 While selected symptoms (eg, the need for digital maneuvers to facilitate defecation) and a meticulous rectal examination may suggest DD, anorectal testing is necessary diagnose DD, which are appropriately managed with pelvic floor retraining through biofeedback therapy rather than with laxatives. Hence, patients with constipation unresponsive to laxatives should be referred for evaluation of rectoanal dysfunction with a digital rectal examination, anorectal manometry, balloon expulsion testing, or anorectal imaging.45,46
Mechanical small bowel obstruction generally presents with acute or recurrent acute symptoms rather than constant abdominal pain. Although adhesions and chronic abdominal pain may occur concurrently after surgery, whether adhesions cause pain is debatable.47
Rare causes of intermittent or chronic abdominal pain include hereditary angioedema, acute intermittent porphyria, and endometriosis.48,49 Endometriosis is identified in up to 80% of women presenting with chronic pelvic pain (defined as noncyclic lower abdominal pain lasting for at least 6 months). It typically presents with perimenstrual lower abdominal pain and dyspareunia; dysuria, urgency, and hematuria are other symptoms. The diagnosis is confirmed by laparoscopy-guided biopsy.50
Management
Establishing an Effective Patient-Physician Relationship
Many patients with functional GI disorders feel abandoned and undertreated and seek care from multiple doctors with limited success. Physicians caring for these patients may feel frustrated because of the lack of specific diagnostic tests and/or patient dissatisfaction. Therefore, it is essential to establish an effective patient-physician relationship by approaching patients’ symptoms with empathy, reassuring patients that life-threatening medical conditions have been excluded after reasonable testing, educating them about the disease, setting reasonable expectations for treatment, and involving them in the management.
Empathy
Empathy involves an acknowledgment by the physician that the patient’s symptoms and the disability are real (Table 1). Empathy increases patient satisfaction and adherence to recommendations.51
Table 1.
Suggested Approaches When Caring for Patients With Chronic Abdominal Pain and Other Functional Gastrointestinal Disorders
| Goal | Prefer | Avoid |
|---|---|---|
| Express empathy | Acknowledge patient suffering (eg, “I am sorry you feel this way…I can see that the pain has really affected your life. I will do my best to help you.”) | Dismissing symptoms (eg, “There is nothing wrong with you.”) |
| Assess the patient’s insight into the functional nature of the pain | Ask open-ended questions (eg, “Can you tell me what you think is causing your symptoms?” or “Tell me about what concerns you the most about your symptoms.”) | Closed-ended questions (eg, “Do you think your pain is caused by eating?”) |
| Understand the patient’s expectation from the physician | Ask open-ended questions (eg, “Tell me a little about what you were expecting from this consultation” or “I see that you have been suffering from pain for many years. Could you tell me a little bit about what made you come to see me today?”) | Judgmental statements (eg, “I am not sure I can help you. You have been to so many doctors already.”) |
| Understand the patient’s expectations from treatment(s) | Ask probing, open-ended questions (eg, “If I asked you what would be a reasonably tolerable pain level that we can try to achieve, what would you say?”) | Imposing a treatment plan (eg, “My plan is to refer you to the psychiatrist and the pain specialist.”) |
| Assess the patient’s understanding of education provided by the physician | “I provided you with quite a bit of information today and want to make sure you understood what I said. Can you tell me what you have understood so far?” | Unilateral flow of information (eg, “I hope you understood all the things we discussed today and implement the suggestions I gave you.”) |
| Help the patient take responsibility for the illness | Suggest that the patient keep a diary of symptoms for 3–4 wk | Prescribing treatments in which the patient is a passive recipient |
Education
Education entails assessing and increasing patients’ understanding of the cause of their symptoms, explaining the benign nature of the disease, and addressing unrealistic expectations (Table 1). Providing analogies to other diseases (eg, arthritis, back pain) is useful to convey that chronic abdominal pain is unlikely to be “cured” but can often be improved. A brief explanation of the role of central sensitization may be helpful. This broader framework (ie, the biopsychosocial model) is more readily accepted by patients than the psychosomatic model, wherein physical symptoms are attributed to mental or emotional problems rather than a physical illness. Education provided by a trained nurse in an individual or group setting is as effective as longer education programs or those involving specialists.52 Interactive patient education was superior to self-education obtained from an IBS guidebook.53
Helping the Patient Take Responsibility
Engaging patients in their treatment increases the likelihood of success and improves patient and physician satisfaction.51 A 2-week symptom diary may identify precipitating and alleviating factors and point to appropriate behavioral treatment strategies. This task should be done with the explicit understanding that it is intended for implementing change and not for uncovering some, as yet, undiscovered pathway of disease.
Designing a Treatment Strategy Based on Severity of Symptoms
The approaches include dietary modification, pharmacotherapy, and behavioral or psychological therapy. These should be tailored to the symptoms and individual patient preferences and integrated whenever necessary.51 For instance, lifestyle changes (eg, dietary advice) and psychological therapy may suffice for patients with mild symptoms related to dietary triggers or other stressors. Patients with severe, disabling symptoms typically will also require prescription medications and behavioral and/or psychological therapy (Figure 4).
Dietary Measures
Food triggers symptoms in most patients with IBS,54 underscoring the importance of dietary modification. While reducing fat intake seems sensible, fat has not been selectively eliminated in controlled clinical trials. Other food intolerances have been implicated, for example, to sugar alcohols (eg, mannitol, sorbitol, xylitol, maltitol, erythritol), to caffeine, and nonceliac gluten sensitivity. Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) diets contain reduced content of these and other ingredients may improve symptoms, and have adverse effects.55 A recent controlled trial observed that a low-FODMAP diet was as effective as a sensible, frequently recommended diet for IBS; the latter is characterized by regular meals, avoidance of large meals, and reduced intake of fat, insoluble fiber, caffeine, and gas-producing foods, such as beans, cabbage, and onions.56
Dietary fiber supplementation with a soluble (eg, psyllium) but not an insoluble (eg, bran) fiber preparation is effective, and often the initial approach, for managing constipation (Table 2).45 The dose should be increased gradually, and patients should be reminded that the effect may not be evident for up to 12 weeks. Probiotics (eg, VSL#3, Bifidobacterium infantis) improve bloating and flatulence in IBS;65,66,67 B. infantis also improved abdominal pain and the ease of defecation, but not stool frequency or consistency.67
Table 2.
Over-the-Counter and Prescription Treatments for Functional Bowel Disorders
| Treatment | NNT (95% CI)a | Benefits and Suggestions for Use |
|---|---|---|
| Over-the-counter agents | ||
| Fiber: psyllium | 4.5, NA 45 b | Start with low dose and increase gradually. Effects are not as pronounced as for laxatives and manifest over time |
| Laxatives: polyethylene glycol | NA, 3 (2–4) 45 b | More evidence in CC than IBS-C. Improved bowel symptoms but not abdominal pain in IBS-C57 |
| Antidiarrheals: loperamide | NA | Beneficial for diarrhea but not abdominal pain in IBS-D |
| Probiotics | 7 (4–12.5) 58 | Studies used different species, strains, preparations, and doses in various patient populations; therefore, results are challenging to interpret. Possibly beneficial for bloating and flatulence |
| Antispasmodics: peppermint oil | 3 (2–4) 58 | Improves abdominal pain and global symptom relief |
| Prescription medications | ||
| Antidepressants: TCAs, SSRIs, SNRIs | 4 (3–6) 58 | Use TCAs for IBS-D and SSRIs for IBS-C |
| Linaclotide | 7 (5–8), 6 (5–8) 59–61 | Improves abdominal pain, bloating, and global IBS symptoms in IBS-C |
| Lubiprostone | 13, 4 (3–7) 45 | Improves abdominal bloating, discomfort, constipation severity in opioid-induced constipation62 |
| Prucalopride | NA, 6 (5–9) 45 | Not approved for use in the United States |
| Antibiotics: rifaximin | 9 (6–12.5) 58 | Improves global symptoms, bloating, and abdominal pain in IBS |
| Clonidine | NA 63 | Improves bowel symptoms in IBS-D |
| Other therapies | ||
| Psychological and behavioral therapy | 4 (35) 64 | Cognitive behavioral therapy, hypnotherapy, dynamic psychotherapy, relaxation training, and multi-component psychological therapy all improved global symptoms in IBS |
Abbreviations: CC, chronic constipation; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome of constipation type; IBS-D, irritable bowel syndrome of diarrhea type; NA, not available; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
NNT indicates number needed to treat for IBS unless specified otherwise.
NNT for IBS-C, chronic constipation.
Pharmacotherapy for Symptoms
Visceral Pain
Except for very select indications (eg, managing intractable diarrhea when all other options have failed), opioids should not be used to manage functional GI symptoms because of the likelihood of abuse, dependency, and addiction and the adverse effects of opioids, particularly NBS.
The pharmacotherapy of pain is often guided by associated symptoms (Table 2). Antidepressants enhance the sense of general well-being; may remedy the psychological comorbidity (eg, anxiety), which can amplify the pain experience; and facilitate central pain modulation, perhaps by increasing descending inhibition.68 In addition, tricyclic antidepressants (TCAs) prolong and many selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs) accelerate intestinal transit, which may be beneficial. A Cochrane meta-analysis observed that 59% of patients treated with antidepressants improved, compared to 39% of patients receiving placebo; the number needed to treat was 5.69 TCAs are the first choice for patients with chronic abdominal pain. The initial dose is 10 to 50 mg daily, titrated to effectiveness and adverse effects up to a range of 25 to 150 mg daily. The dose should be increased only if necessary and as tolerated. In the largest controlled trial, desipramine (150 mg/d) was not significantly different than placebo for patients with moderate or severe functional bowel disorders.70 However, after the 25% of patients who had dropped out were excluded, desipramine was superior to placebo. Patients should be reassured that, as in other painful medical conditions (eg, migraine, diabetic neuropathy), antidepressants are not being used for a psychiatric condition, but as central analgesic agents. While adverse effects occur early, beneficial effects may take 4 to 6 weeks to appear. In addition, many symptoms attributed to adverse effects of desipramine often predated treatment, which suggests that they were not related to drug per se.71
SSRIs can reduce anxiety and depression and improve the sense of overall well-being. However, there are limited data to suggest that these drugs reduce abdominal pain, perhaps because, in contrast to TCAs and SNRIs, they do not have much noradrenergic effect. Adverse effects (eg, diarrhea, sexual dysfunction, nightmares) are less common with SSRIs than TCAs, which can be beneficial for patients who have a low threshold for adverse effects.
SNRIs block norepinephrine and serotonin reuptake, which should reduce pain sensation.13 They also have other beneficial effects; for example, venlafaxine reduced the colonic contractile response to a meal and also colonic perception of balloon distention.72 Duloxetine is effective for treating peripheral diabetic neuropathic pain and fibromyalgia.73 Despite a lack of evidence, SNRIs may be considered for patients with severe refractory abdominal pain. Because they lack anticholinergic and antihistaminic adverse effects, SNRIs are mostly better tolerated than TCAs. When a single antidepressant is not helpful, augmentation therapy, such as multiple antidepressants, or combining an antidepressant with psychological treatment may be tried.27 Excess sedation can be reduced by lowering the dose of the tricyclic agent and/or by supplementing with a SSRI. A benzodiazepine agent or a low dose TCA at bedtime can reduce “jitteriness" from an SSRI. For patients with dyspepsia and anxiety, the 5-HT1 agonist buspirone, an anxiolytic agent that improved gastric accommodation and reduced postprandial fullness and bloating, should be considered.72 Pregabalin may be considered in patients with a general anxiety disorder or fibromyalgia and abdominal wall pain.27
Constipation
In patients who do not have a DD, a stepwise approach beginning with dietary fiber supplementation and escalating as necessary to simple osmotic laxatives (eg, polyethylene glycol, milk of magnesia) and stimulant laxatives (eg, bisacodyl, senna) and then to a secretory agent (linaclotide or lubiprostone) is recommended (Table 2).45 In each class, the alternative agent should be considered if one fails. Both lubiprostone and linaclotide increase intestinal secretion of fluids and electrolytes; linaclotide may also have antihyperalgesic effects.74 Among constipated patients with severe abdominal symptoms (eg, bloating, fullness, discomfort, pain), approximately 60% treated with linaclotide vs 30% treated with placebo reported adequate relief of IBS symptoms at week 12 of therapy.74
Diarrhea
The initial options are loperamide, taken regularly or on demand (eg, 2 to 4 mg 30 minutes before meals, up to a maximum of 8 tablets daily), and anticholinergic agents such as diphenoxylate and amitriptyline; amitriptyline may also reduce rectal urgency.75 Up to 30% of patients with idiopathic diarrhea have idiopathic bile salt malabsorption, suggesting a role for bile acid–binding resins.75,76 However, these drugs have not been evaluated in controlled clinical trials. Cholestyramine is inexpensive but often associated with bloating, which is not a common adverse effect with colesevelam. Eluxadoline, rifaximin, and alosetron are approved by the US Food and Drug Administration for treating diarrhea-predominant IBS.77 Alosetron is available under a restricted-use program.77 Clonidine, an α2-adrenergic agonist, improves fluid and electrolyte absorption and improved symptoms in a phase II study in diarrhea-predominant IBS63; clonidine also reduces the perception of colonic distention.78 At a dose of 0.1 mg tablet twice daily, it is well tolerated, and adverse effects (eg, dry mouth, sedation, hypotension) are uncommon.
Abdominal Wall Pain
Injection of a local anesthetic (eg, lidocaine, bupivacaine) with or without a corticosteroid (eg, methylprednisolone acetate) at the site of maximal tenderness is both diagnostic and therapeutic.79 Other options, supported by limited evidence, include lidocaine patches,80 local application of heat, low-dose TCAs, and gabapentin.36 In one series, a variety of approaches eased the pain in 47% of patients.36
Narcotic Bowel Syndrome
Treatment requires withdrawal of narcotics, often in combination with medications to control withdrawal symptoms (eg, clonidine and lorazepam), and use of antidepressants (eg, paroxetine, desipramine, duloxetine, cyclobenzaprine) to control visceral pain and decrease anxiety.81,82
Nausea and Vomiting
Phenothiazines (eg, prochlorperazine) and antihistamine agents (including promethazine), which can be administered as a suspension or suppositories, should be tried initially,14 followed when necessary by serotonin 5-HT3 receptor antagonists (eg, ondansetron), which are more expensive. The neurokinin-1 receptor antagonist aprepitant is approved for treating emesis due to chemotherapy.
Psychological and Behavioral Interventions
These interventions are tailored to the symptoms, functional impairment, psychological distress, and symptom expression. Patients should be reassured that the goals are to manage pain, improve daily function, and relieve psychological distress.
Cognitive behavioral therapy (CBT), which is the most effective psychological therapy for functional abdominal pain, is generally imparted by therapists, but can be self-administered by patients, with similar efficacy.83 CBT helps patients identify maladaptive thoughts, perceptions, and behaviors. Thereafter, they are taught to develop new approaches to increase control over their reactions and functioning, including an emphasis on stress management, decreasing illness behaviors, increasing social support, and more effective problem solving. Any intervention that enhances the ability to relax will give the patient a sense of control over symptoms and induce more parasympathetic activity. A recent meta-analysis of 41 trials involving 2,290 patients observed that psychological therapy is moderately effective for improving IBS symptoms for up to 1 year after therapy.84 CBT was effective whether it was administered in person or online, individually or in a group. The number, duration, and frequency of sessions did not affect its efficacy.
Behavioral interventions targeted to specific symptoms can be very useful. By activating the diaphragm, diaphragmatic breathing is a form of habit reversal training that serves as a competing response and thereby prevents belching, regurgitation, and vomiting.85,86 Patients should begin diaphragmatic breathing at the onset of cues of belching and continue to engage the technique until the cues subside. Measures to prevent aerophagia (eg, cease drinking through a straw) should be emphasized in patients with belching.
Multidisciplinary Pain Treatment Centers
Comprehensive pain rehabilitation involves physical therapy, occupational therapy, and CBT in an intensive, interdisciplinary outpatient setting. Most pain rehabilitation centers offer daily treatment for 2 to 4 weeks. The emphasis is on physical reconditioning and elimination of medications for pain and other symptoms (eg, benzodiazepines), along with activity management and behavior therapy.87 Patients who benefit from this approach do so because of a change in their behavior, beliefs, and physical status. These programs are very successful. In a study of 356 patients with chronic pain (abdominal pain in 8%), 132 of 135 patients receiving opioids discontinued them after a pain rehabilitation program.88
Conclusion
Identifying the precise phenotype, which is predominantly based on clinical features, is essential for appropriate and cost-effective diagnosis and treatment of nonstructural, or functional, GI disorders associated with abdominal pain. The need for and choice of diagnostic tests are guided by the clinical features and alarm symptoms. Testing should evaluate for conditions that are not widely recognized and are amenable to therapy (eg, pelvic floor dysfunction). Multidisciplinary treatment approaches that integrate dietary modification, pharmacotherapy, and behavioral or psychological therapy and are tailored to the symptoms should be considered. The focus is on improving symptoms and restoring quality of life.
Acknowledgments
This study was supported in part by USPHS NIH Grant R01 DK78924 from the National Institutes of Health.
Abbreviations
- 5-HT
5-hydroxytryptamine
- CBT
cognitive behavioral therapy
- DD
defecatory disorders
- FODMAP
Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- NBS
narcotic bowel syndrome
- OBD
opioid bowel dysfunction
- OIC
opioid-induced constipation
- SNRI
serotonin and norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressant
Footnotes
Conflicts of Interest: Dr. Bharucha reports personal fees from Allergan Inc, personal fees from Johnson and Johnson Inc, personal fees and other from Medspira, personal fees from Ironwood Pharma, personal fees from GI Care Pharma, personal fees from National Center for Pelvic Pain Research, personal fees from Salix, personal fees from Macmillan Medical Communications, personal fees from Forum Pharmaceuticals, outside the submitted work; In addition, Dr. Bharucha has a patent Anorectal manometry device with royalties paid to Medspira Inc, and a pending patent Anorectal manometry probe fixation device licensed to Medtronic Inc. Drs. Chakraborty and Sletten have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130(5):1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97(8):1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Ford AC. Functional Dyspepsia. N Engl J Med. 2015;373(19):1853–1863. doi: 10.1056/NEJMra1501505. [DOI] [PubMed] [Google Scholar]
- 4.Clouse RE, Mayer EA, Aziz Q, et al. Functional abdominal pain syndrome. Gastroenterology. 2006;130(5):1492–1497. doi: 10.1053/j.gastro.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 5.Locke GR, 3rd, Yawn BP, Wollan PC, Melton LJ, 3rd, Lydick E, Talley NJ. Incidence of a clinical diagnosis of the irritable bowel syndrome in a United States population. Aliment Pharmacol Ther. 2004;19(9):1025–1031. doi: 10.1111/j.1365-2036.2004.01938.x. [DOI] [PubMed] [Google Scholar]
- 6.Locke GR, 3rd, Zinsmeister AR, Fett SL, Melton LJ, 3rd, Talley NJ. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17(1):29–34. doi: 10.1111/j.1365-2982.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Pilgrim P, Schoenfeld P. Systemic review: Natural history of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19(8):861–870. doi: 10.1111/j.1365-2036.2004.01929.x. [DOI] [PubMed] [Google Scholar]
- 8.Halder SLS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133(3):799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Garrigues V, Mearin F, Badia X, et al. Change over time of bowel habit in irritable bowel syndrome: a prospective, observational, 1-year follow-up study (RITMO study) Aliment Pharmacol Ther. 2007;25(3):323–332. doi: 10.1111/j.1365-2036.2006.03197.x. [DOI] [PubMed] [Google Scholar]
- 10.Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J Neurogastroenterol Motil. 2012;18(3):258–268. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131(2):640–658. doi: 10.1053/j.gastro.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61(5):1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- 13.Farmer AD, Aziz Q. Mechanisms and management of functional abdominal pain. J R Soc Med. 2014;107(9):347–354. doi: 10.1177/0141076814540880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2013;368(6):578–579. doi: 10.1056/NEJMc1214185. [DOI] [PubMed] [Google Scholar]
- 15.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282(3):G443–449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 16.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clauw DJ. Fibromyalgia and Related Conditions. Mayo Clin Proc. 2015;90(5):680–692. doi: 10.1016/j.mayocp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha AE, Pemberton JH, Locke GR., 3rd American gastroenterological association technical review on constipation. Gastroenterology. 2013;144(1):218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burri E, Cisternas D, Villoria A, et al. Abdominal accommodation induced by meal ingestion: differential responses to gastric and colonic volume loads. Neurogastroenterol Motil. 2013;25(4):339–e253. doi: 10.1111/nmo.12068. [DOI] [PubMed] [Google Scholar]
- 21.Accarino A, Perez F, Azpiroz F, Quiroga S, Malagelada JR. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology. 2009;136(5):1544–1551. doi: 10.1053/j.gastro.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 22.Barba E, Burri E, Accarino A, et al. Abdominothoracic mechanisms of functional abdominal distension and correction by biofeedback. Gastroenterology. 2015;148(4):732–739. doi: 10.1053/j.gastro.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Chitkara DK, van Tilburg MAL, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103(3):765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choung RS, Locke GR, 3rd, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol. 2009;104(7):1772–1779. doi: 10.1038/ajg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drossman DA. Abuse, trauma, and GI illness: is there a link? Am J Gastroenterol. 2011;106(1):14–25. doi: 10.1038/ajg.2010.453. [DOI] [PubMed] [Google Scholar]
- 26.Keefer L, Mandal S. The potential role of behavioral therapies in the management of centrally mediated abdominal pain. Neurogastroenterol Motil. 2015;27(3):313–323. doi: 10.1111/nmo.12474. [DOI] [PubMed] [Google Scholar]
- 27.Sperber AD, Drossman DA. Review article: the functional abdominal pain syndrome. Aliment Pharmacol Ther. 2011;33(5):514–524. doi: 10.1111/j.1365-2036.2010.04561.x. [DOI] [PubMed] [Google Scholar]
- 28.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313(9):949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 29.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123(6):2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 30.Townsend CO, Sletten CD, Bruce BK, Rome JD, Luedtke CA, Hodgson JE. Physical and emotional functioning of adult patients with chronic abdominal pain: comparison with patients with chronic back pain. J Pain. 2005;6(2):75–83. doi: 10.1016/j.jpain.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Creed F, Ratcliffe J, Fernandez L, et al. Health-related quality of life and health care costs in severe, refractory irritable bowel syndrome. Ann Intern Med. 2001;134(9 Pt 2):860–868. doi: 10.7326/0003-4819-134-9_part_2-200105011-00010. [DOI] [PubMed] [Google Scholar]
- 32.Smith B, Forkner E, Zaslow B, et al. Disease management produces limited quality-of-life improvements in patients with congestive heart failure: evidence from a randomized trial in community-dwelling patients. Am J Manag Care. 2005;11(11):701–713. [PubMed] [Google Scholar]
- 33.Stahl E, Lindberg A, Jansson SA, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes. 2005;3:56. doi: 10.1186/1477-7525-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coulehan JL, Schulberg HC, Block MR, Madonia MJ, Rodriguez E. Treating depressed primary care patients improves their physical, mental, and social functioning. Arch Intern Med. 1997;157(10):1113–1120. [PubMed] [Google Scholar]
- 35.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–1068. doi: 10.1016/s0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 36.Costanza CD, Longstreth GF, Liu AL. Chronic abdominal wall pain: clinical features, health care costs, and long-term outcome. Clin Gastroenterol Hepatol. 2004;2(5):395–399. doi: 10.1016/s1542-3565(04)00124-7. [DOI] [PubMed] [Google Scholar]
- 37.Greenbaum DS, Greenbaum RB, Joseph JG, Natale JE. Chronic abdominal wall pain. Diagnostic validity and costs. Dig Dis Sci. 1994;39(9):1935–1941. doi: 10.1007/BF02088128. [DOI] [PubMed] [Google Scholar]
- 38.Gray DW, Dixon JM, Seabrook G, Collin J. Is abdominal wall tenderness a useful sign in the diagnosis of non-specific abdominal pain? Ann R Coll Surg Engl. 1988;70(4):233–234. [PMC free article] [PubMed] [Google Scholar]
- 39.Kurlander JE, Drossman DA. Diagnosis and treatment of narcotic bowel syndrome. Nat Rev Gastroenterol Hepatol. 2014;11(7):410–418. doi: 10.1038/nrgastro.2014.53. [DOI] [PubMed] [Google Scholar]
- 40.Choung RS, Rubio-Tapia A, Lahr BD, et al. Evidence Against Routine Testing of Patients With Functional Gastrointestinal Disorders for Celiac Disease: A Population-based Study. Clin Gastroenterol Hepatol. 2015;13(11):1937–1943. doi: 10.1016/j.cgh.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chey WD, Nojkov B, Rubenstein JH, Dobhan RR, Greenson JK, Cash BD. The yield of colonoscopy in patients with non-constipated irritable bowel syndrome: results from a prospective, controlled US trial. Am J Gastroenterol. 2010;105(4):859–865. doi: 10.1038/ajg.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macaigne G, Lahmek P, Locher C, et al. Microscopic colitis or functional bowel disease with diarrhea: a French prospective multicenter study. Am J Gastroenterol. 2014;109(9):1461–1470. doi: 10.1038/ajg.2014.182. [DOI] [PubMed] [Google Scholar]
- 43.Norgaard M, Farkas DK, Pedersen L, et al. Irritable bowel syndrome and risk of colorectal cancer: a Danish nationwide cohort study. Br J Cancer. 2011;104(7):1202–1206. doi: 10.1038/bjc.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsiao CW, Huang WY, Ke TW, et al. Association between irritable bowel syndrome and colorectal cancer: a nationwide population-based study. Eur J Intern Med. 2014;25(1):82–86. doi: 10.1016/j.ejim.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 45.American Gastroenterological A. Bharucha AE, Dorn SD, Lembo A, Pressman A. American gastroenterological association medical position statement on constipation. Gastroenterology. 2013;144(1):211–217. doi: 10.1053/j.gastro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109(8):1141–1157. doi: 10.1038/ajg.2014.190. (Quiz) 1058. [DOI] [PubMed] [Google Scholar]
- 47.Ten Broek RPG, Bakkum EA, Laarhoven CJHM, van Goor H. Epidemiology and Prevention of Postsurgical Adhesions Revisited. Ann Surg. 2016;263(1):12–19. doi: 10.1097/SLA.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 48.Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008;359(10):1027–1036. doi: 10.1056/NEJMcp0803977. [DOI] [PubMed] [Google Scholar]
- 49.Besur S, Hou W, Schmeltzer P, Bonkovsky HL. Clinically important features of porphyrin and heme metabolism and the porphyrias. Metabolites. 2014;4(4):977–1006. doi: 10.3390/metabo4040977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butrick CW. Patients with chronic pelvic pain: endometriosis or interstitial cystitis/painful bladder syndrome? JSLS. 2007;11(2):182–189. [PMC free article] [PubMed] [Google Scholar]
- 51.Drossman DA. Functional abdominal pain syndrome. Clin Gastroenterol Hepatol. 2004;2(5):353–365. doi: 10.1016/s1542-3565(04)00118-1. [DOI] [PubMed] [Google Scholar]
- 52.Ringstrom G, Storsrud S, Simren M. A comparison of a short nurse-based and a long multidisciplinary version of structured patient education in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2012;24(8):950–957. doi: 10.1097/MEG.0b013e328354f41f. [DOI] [PubMed] [Google Scholar]
- 53.Ringstrom G, Storsrud S, Posserud I, Lundqvist S, Westman B, Simren M. Structured patient education is superior to written information in the management of patients with irritable bowel syndrome: a randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22(4):420–428. doi: 10.1097/MEG.0b013e3283333b61. [DOI] [PubMed] [Google Scholar]
- 54.Lacy BE, Chey WD, Lembo AJ. New and Emerging Treatment Options for Irritable Bowel Syndrome. Gastroenterol Hepatol (N Y) 2015;11(4 Suppl 2):1–19. [PMC free article] [PubMed] [Google Scholar]
- 55.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 56.Bohn L, Storsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–1407. e1392. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 57.Chapman RW, Stanghellini V, Geraint M, Halphen M. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol. 2013;108(9):1508–1515. doi: 10.1038/ajg.2013.197. [DOI] [PubMed] [Google Scholar]
- 58.Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2–26. doi: 10.1038/ajg.2014.187. quiz S27. [DOI] [PubMed] [Google Scholar]
- 59.Bharucha AE, Locke GR, Pemberton JH. AGA Practice Guideline on Constipation:Technical Review. Gastroenterology. 2013;144(1):218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Videlock EJ, Cheng V, Cremonini F. Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(9):1084–1092. e1083. doi: 10.1016/j.cgh.2013.04.032. quiz e1068. [DOI] [PubMed] [Google Scholar]
- 61.Atluri DK, Chandar AK, Bharucha AE, Falck-Ytter Y. Effect of linaclotide in irritable bowel syndrome with constipation (IBS-C): a systematic review and meta-analysis. Neurogastroenterol Motil. 2014;26(4):499–509. doi: 10.1111/nmo.12292. [DOI] [PubMed] [Google Scholar]
- 62.Spierings EL, Rauck R, Brewer R, Marcuard S, Vallejo R. Long-Term Safety and Efficacy of Lubiprostone in Opioid-induced Constipation in Patients with Chronic Noncancer Pain. Pain Pract. 2015 doi: 10.1111/papr.12347. [DOI] [PubMed] [Google Scholar]
- 63.Camilleri M, Kim D-Y, McKinzie S, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2003;1(2):111–121. doi: 10.1053/cgh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 64.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58(3):367–378. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 65.Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 66.Kim HJ, Vazquez Roque MI, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17(5):687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 67.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 68.Tornblom H, Drossman DA. Centrally targeted pharmacotherapy for chronic abdominal pain. Neurogastroenterol Motil. 2015;27(4):455–467. doi: 10.1111/nmo.12509. [DOI] [PubMed] [Google Scholar]
- 69.Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460. doi: 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125(1):19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 71.Thiwan S, Drossman DA, Morris CB, et al. Not all side effects associated with tricyclic antidepressant therapy are true side effects. Clin Gastroenterol Hepatol. 2009;7(4):446–451. doi: 10.1016/j.cgh.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G130–137. doi: 10.1152/ajpgi.00266.2002. [DOI] [PubMed] [Google Scholar]
- 73.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao SSC, Quigley EMM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol. 2014;12(4):616–623. doi: 10.1016/j.cgh.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 75.Nee J, Zakari M, Lembo AJ. Current and emerging drug options in the treatment of diarrhea predominant irritable bowel syndrome. Expert Opin Pharmacother. 2015;16(18):2781–2792. doi: 10.1517/14656566.2015.1101449. [DOI] [PubMed] [Google Scholar]
- 76.Bajor A, Tornblom H, Rudling M, Ung K-A, Simren M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64(1):84–92. doi: 10.1136/gutjnl-2013-305965. [DOI] [PubMed] [Google Scholar]
- 77.Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for Irritable Bowel Syndrome with Diarrhea. N Engl J Med. 2016;374(3):242–253. doi: 10.1056/NEJMoa1505180. [DOI] [PubMed] [Google Scholar]
- 78.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol. 1997;273(5 Pt 1):G997–1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- 79.Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Randomized clinical trial of trigger point infiltration with lidocaine to diagnose anterior cutaneous nerve entrapment syndrome. Br J Surg. 2013;100(2):217–221. doi: 10.1002/bjs.8958. [DOI] [PubMed] [Google Scholar]
- 80.Saber AA, Elgamal MH, Rao AJ, Itawi EA, Martinez RL. Early experience with lidocaine patch for postoperative pain control after laparoscopic ventral hernia repair. Int J Surg. 2009;7(1):36–38. doi: 10.1016/j.ijsu.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Grunkemeier DMS, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007;5(10):1126–1139. doi: 10.1016/j.cgh.2007.06.013. quiz 1121–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berna C, Kulich RJ, Rathmell JP. Tapering Long-term Opioid Therapy in Chronic Noncancer Pain: Evidence and Recommendations for Everyday Practice. Mayo Clin Proc. 2015;90(6):828–842. doi: 10.1016/j.mayocp.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Holroyd K. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. 2008;6(8):899–906. doi: 10.1016/j.cgh.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laird KT, T-SE, Russell AC, Hollon SD, Walker LS. Short-term and Long-term Efficacy of Psychological Therapies for Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 85.Halland M, Parthasarathy G, Bharucha AE, Katzka DA. Diaphragmatic breathing for rumination syndrome: efficacy and mechanisms of action. Neurogastroenterol Motil. 2016;28(3):384–391. doi: 10.1111/nmo.12737. [DOI] [PubMed] [Google Scholar]
- 86.Chitkara DK, Van Tilburg M, Whitehead WE, Talley NJ. Teaching diaphragmatic breathing for rumination syndrome. Am J Gastroenterol. 2006;101(11):2449–2452. doi: 10.1111/j.1572-0241.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 87.Rome JD, Sletten CD, Bruce BK. A rehabilitation approach to chronic pain in rheumatologic practice. Curr Opin Rheumatol. 1996;8(2):163–168. doi: 10.1097/00002281-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 88.Rome JD, Townsend CO, Bruce BK, Sletten CD, Luedtke CA, Hodgson JE. Chronic noncancer pain rehabilitation with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Mayo Clin Proc. 2004;79(6):759–768. doi: 10.4065/79.6.759. [DOI] [PubMed] [Google Scholar]