Abstract
Moving in synchrony leads to cooperative behaviour and feelings of social closeness, and dance (involving synchronisation to others and music) may cause social bonding, possibly as a consequence of released endorphins. This study uses an experimental paradigm to determine which aspects of synchrony in dance are associated with changes in pain threshold (a proxy for endorphin release) and social bonding between strangers. Those who danced in synchrony experienced elevated pain thresholds, whereas those in the partial and asynchrony conditions experienced no analgesic effects. Similarly, those in the synchrony condition reported being more socially bonded, although they did not perform more cooperatively in an economic game. This experiment suggests that dance encourages social bonding amongst co-actors by stimulating the production of endorphins, but may not make people more altruistic. We conclude that dance may have been an important human behaviour evolved to encourage social closeness between strangers.
Keywords: Dance, Social bonding, Synchrony, Endorphins, Weak-link coordination game
1. Introduction
Around the world people sing, make music and dance - activities which are often conducted in a group setting, accompanied by strong emotions, and can be broadly defined as “musicking” (Small, 1998). The evolutionary origins of dance, which involves synchrony of movement to others and to music, remains unclear. One prominent theory is that this behaviour might have played an important role in increasing interpersonal cooperation and feelings of social closeness, thereby helping to establish and maintain group cohesion (Freeman, 2000; Kirschner & Tomasello, 2010; Reddish, Fischer, & Bulbulia, 2013; Tarr, Launay, & Dunbar, 2014).
Like most anthropoid primates, humans live in bonded social groups (Dunbar & Shultz, 2010). Bonded social groups allow their members to mount a coordinated (passive and active) defense against predators or conspecific raiders (Lehmann, Lee, & Dunbar, 2014), and provide direct fitness benefits by buffering individuals against the stresses of social life (Wittig et al., 2008) and enhancing infant survival (monkeys: Silk, Alberts, & Altmann, 2003; Silk, 2007; humans: Spence, 1954; Oesch & Dunbar, 2015). Allogrooming is a conventional mechanism for social bonding in primates, including humans, but is very expensive in terms of time, and therefore imposes a limit on the size of networks or groups that can be effectively bonded (Dunbar, Korstjens, & Lehmann, 2009). It would have been advantageous for humans to develop additional behaviours that allow bonding between multiple individuals simultaneously so as to allow us to increase the size of our social networks and communities (Dunbar, 2012a). Musicking may facilitate efficient large-scale bonding: when moving together to music, individuals can establish social closeness with the whole of the group involved (Dunbar, 2012b; Kirschner & Tomasello, 2010; Wiltermuth & Heath, 2009).
To date, empirical evidence that dance can lead to social bonding has focused on the role of our innate capacity to perceive and synchronise to a rhythmic pattern (Patel, Iversen, Chen, & Repp, 2005), particularly beats embedded in music (Demos, Chaffin, Begosh, Daniels, & Marsh, 2012) or those produced by another human (Kirschner & Tomasello, 2009).
Synchronisation is a pervasive behaviour in many animals, playing a part in female identification of conspecific males (e.g. fireflies: Moiseff & Copeland, 2010), pair formation displays (e.g. western grebes: Nuechterlein & Storer, 1982), and courtship (e.g. fiddler crabs: Backwell, Jennions, & Passmore, 1998). The capacity to synchronise specifically to a musical beat is not uniquely human, and we share this aspect of music cognition with certain other species (Patel et al., 2008; Patel, Iversen, Bregman, & Schulz, 2009). Although there is some evidence that chimpanzees are capable of learning to spontaneously synchronise to an auditory beat (Hattori, Tomonaga, & Matsuzawa, 2013), our proclivity to produce organized rhythmic sound (music) and our mutual entrainment as occurs when we dance, remains characteristically human (Fitch, 2012).
Like mimicry (e.g. Chartrand & Lakin, 2013), synchrony has received much attention in accounts of human social-cognitive functioning (Macrae, Duffy, Miles, & Lawrence, 2008). When people perform the same movements at the same time (i.e. synchronise), there is a co-activation of action and perception networks which is believed to blur a sense of ‘other’ and ‘self’ (Overy & Molnar-Szakacs, 2009), leading to a social bond between co-performers (e.g. Decety & Sommerville, 2003). This mechanism is argued to explain why small movement synchrony (e.g. finger tapping) increases participants’ feelings of affiliation towards a tapping partner, as measured by self-reported similarity in personality (Valdesolo & Desteno, 2011) and how much participants like their co-actor (Hove & Risen, 2009; Valdesolo & Desteno, 2011). This effect is evident with real and virtual partners (Launay, Dean, & Bailes, 2014), and also manifests in prosocial behaviours such as willingness to help a partner with whom someone has synchronised (Kirschner & Tomasello, 2010; Kokal, Engel, Kirschner, & Keysers, 2011; Valdesolo & Desteno, 2011), and positive behaviour in economic games (Launay, Dean, & Bailes, 2013; Wiltermuth & Heath, 2009).
Synchronisation has been shown to facilitate entitativity – the feeling of being ‘on the same team’ (e.g. Lakens, 2010) – which can then lead to enhanced cooperation and prosociality, possibly due to a sense of collective fate (Wiltermuth & Heath, 2009). Synchronised action has also been described as increasing action understanding of others via “motor resonance” (Macrae et al., 2008), whereby self-other attentional coupling facilitates social cognition (Blakemore & Decety, 2001) by facilitating observational learning (Wilson & Knoblich, 2005) and enhancing person-related processing (Knoblich & Sebanz, 2006). This seemingly primes co-actors to establish trust and so coordinate better, as demonstrated by the fact that synchronised movement can predict success in a later joint activity that demands collaboration (Valdesolo, Ouyang, & DeSteno, 2010). Furthermore, people preferentially direct compassion and altruism toward similar others (e.g. Strürmer, Snyder, Kropp, & Siem, 2006), and synchrony (which enhances perception of similarity between co-actors) may be a means of creating a unified in-group. As a result of these various socio-cognitive effects, it is hypothesised that the prosocial effects encouraged during synchrony would be evolutionarily advantageous in other domains which require smooth coordination such as hunting, gathering, building shelters together and mutual defence against predators or conspecific raiders.
Although action-perception matching is often cited as the main mechanism underpinning the social bonding effects of synchronisation, it has also been suggested that social activities such as musicking may trigger the Endogenous Opioid System (EOS; Dunbar, Kaskatis, MacDonald, & Barra, 2012; Tarr et al., 2014), which is known to be involved in social bonding in non-human primates (e.g. Ragen, Maninger, Mendoza, Jarcho, & Bales, 2013). The EOS consists of opioid-producing nuclei in the hypothalamus and opioid receptors that are distributed throughout the central nervous system and is generally studied in humans for its analgesic and reward-inducing effects (Bodnar, 2008). The Brain Opioid Theory of Social Attachment (BOTSA) highlights the fact that social attachment involves elevated levels of opioids in the brain (Machin & Dunbar, 2011; Nummenmaa et al., 2015), and that the positive effects of social interaction are similar to those induced by opiates (Machin & Dunbar, 2011). Activation of the EOS is associated with feelings of euphoria (Bodnar, 2008), interpersonal warmth, well-being, and bliss (Depue & Morrone-Strupinsky, 2005), reward (Olmstead & Franklin, 1997), social motivation (Chelnokova et al., 2014), and pleasure and pain perception (Leknes & Tracey, 2008). Given the role of the EOS in social bonding in mammals generally (Broad, Curley, & Keverne, 2006), it is argued that human behaviours which activate the EOS lead to perception of closer social bonds between co-actors (e.g. Dunbar, 2004, 2012b). According to BOTSA, the EOS may have been ‘co-opted’ from its more general role in pain relief and positivity to reinforce social behaviours (Eisenberger, 2015; Macdonald & Leary, 2005; Panksepp, 1999).
Group activities which increase pain threshold (a recognised proxy measure of endorphin levels; Mueller et al., 2010) include laughter (Dezecache & Dunbar, 2012; Dunbar, Baron, et al., 2012), group exercise (Sullivan, Rickers, Gagnon, Gammage, & Peters, 2011) and synchronised sport (Cohen, Ejsmond-Frey, Knight, & Dunbar, 2010; Sullivan, Rickers, & Gammage, 2014; Sullivan & Rickers, 2013). Rowing in synchrony elevates pain threshold compared to rowing alone (Cohen et al., 2010) or when unsynchronised (Sullivan et al., 2014), irrespective of whether the rowers are strangers or acquaintances (Sullivan & Rickers, 2013). Furthermore, active participation in group music-based activities is similarly associated with increased pain threshold (Dunbar, Kaskatis, et al., 2012). Although these studies did not measure social closeness directly, they postulate that EOS activation (specifically elevated endorphin levels) as indexed by pain threshold may play a role in the bonding that is associated with these various social activities.
The current experiment investigates changes in social bonding and pain thresholds associated with synchronised dance in groups of strangers. Existing research on the link between synchrony and social bonding has predominantly focused on synchronisation of small movements such as rocking in a chair (Demos et al., 2012), walking in step (Wiltermuth & Heath, 2009), finger tapping (Launay et al., 2013), or the performance of simple arm and leg movements in time with others or a metronome (Reddish et al., 2013). These studies demonstrate that synchronisation of simple movements by pairs of people or small groups leads to increased social bonding, as measured by both self-report and behavioural measures. Nevertheless, dance is arguably more than scaled up finger tapping. Few studies have investigated the effect in groups larger than two with music, or with movement conditions representative of dance (e.g. instead using conditions of walking, singing, waving cups: Kirschner & Tomasello, 2010; Wiltermuth & Heath, 2009).
In the present study, groups of four individuals performed dance movements to popular music. We used a ‘silent disco’ paradigm in which participants dancing in a group heard music through individual headphones; thus, any social bonding that occurs can be attributed to behavioural synchrony of dance actions. The silent disco technology allowed us to compare the synchronous condition to two non-synchronous conditions: partial synchrony (counterbalanced movements, same music) and asynchrony (unique movements and different music). Previous studies report a group synchrony effect in comparison to no-movement conditions (e.g. Wiltermuth & Heath, 2009) or sequential (cannon) movements (e.g. Reddish, Bulbulia, & Fischer, 2014; Reddish et al., 2013) and it is unclear whether the positive effects associated with synchrony are due to synchronisation itself, or negative effects that arise in certain non-synchronous conditions. In addition to self-report questions and a behavioural measure of social closeness (the weak link coordination game adapted from Wiltermuth & Heath (2009)), the present study includes pain threshold as a proxy measure of EOS activation.
2. Materials and Methods
2.1. Participants
After exclusions, a final sample of 94 participants (74 females; x̄ age = 24.29, SD = 5.29 years) was recruited in Oxford. To avoid biases in pain threshold measurements, the sample excluded pregnant, lactating or diabetic individuals (McKinney, Tims, Kumar, & Kumar, 1997), and participants who had smoked or drunk alcohol within the two hours prior to the experiment.
2.2. General study design
Test groups consisting of four strangers were randomly assigned to a movement condition (synchrony, partial synchrony or asynchrony; see section 2.3 for details). An accelerometer Actiwatch was attached to each participant’s right wrist to provide an ‘activity count’ per unit of time, which was interpreted as a measure of the intensity of movement (CambridgeNeurotechnology, 2008). Participants’ pain thresholds were measured at the start of the experiment and immediately after the silent disco using a standard method (see section 2.4). A pre-activity questionnaire included demographic information, a personality scale (Cooper, Smillie, & Corr, 2010), and a Positive and Negative Affect Scale (PANAS: Mackinnon et al., 1999 see ESM1 for more details).
In a private cubicle, participants first learned four basic dance moves from a video (ESM2). Each movement was named and at the end of the video participants rated their recall confidence. Following this, participants stood in a square facing inwards, each on a marked space separated from one another by 1 meter, and engaged in a silent disco. During the silent disco (which lasted 13 minutes), headphones relayed music with a pre-recorded voice-over providing a sequence of dance movements. Music was chosen from current popular hits (ESM1). A post-activity questionnaire included a series of questions relating to social closeness (see section 2.4), the PANAS, and questions relating to participants’ experience of the experiment (ESM1). Due to the fact that social bonding following synchrony may be influenced by perceived success (Launay et al., 2013), participants rated how well they had followed the audio instructions, and those in the synchrony condition additionally rated their success at synchronising. The first 58 participants also played a weak-link coordination game (Wiltermuth & Heath, 2009), but this game was omitted for later participants due to doubts about the relevance of the test (Burton-Chellew & West, 2013) and time constraints in running the experiment. Nonetheless, we report the results here for completeness.
2.3. Manipulation of synchrony
In the synchrony and partial synchrony conditions, all participants learned the same dance moves (e.g. ESM2.1 Dance training video A), whereas in the asynchronous condition, each participant was taught a unique repertoire of movements (ESM2.1 Dance training video A; ESM2.2 Dance training video B; ESM2.3 Dance training video C; ESM2.4 Dance training video D). In the synchrony condition, all four participants heard the same music during the silent disco, with the same auditory instructions delivered at the same time. In the partial synchrony condition, all participants heard the same music, but with a unique sequence of instructions, meaning that no two participants performed the same movement at any one time. In the asynchrony condition, the order of the music tracks differed for each participant (resulting in no rhythmic or tempo congruity between participant’s stimuli), and the auditory instructions were tailored for each participant’s repertoire.
2.4. Dependent measures
2.4.1. Pain threshold
Pain threshold was measured by inducing ischemic pain through the inflation of a blood pressure cuff on the participant’s upper arm and noting the pressure sustained, a standard procedure used in previous studies (Cohen et al., 2010; Sullivan et al., 2014; Tarr, Launay, Cohen, & Dunbar, 2015). Participants indicated when the pressure became uncomfortable, and to avoid ceiling effects data from those who reached the maximum pressure measurable by the cuff (300mmHg; a total of 9 individuals) were excluded. Within every testing group, each participant was assigned a research assistant who recorded their pain threshold in a private cubicle. Participants thus had no cues as to how other participants were performing. Research assistants were blind to the condition and hypothesis.
2.4.2. Self-report social closeness
Social closeness was measured using 7-point Likert scale questions (ESM1) including an adapted version of the Inclusion of Other in Self (IOS) scale (Aron, Aron, & Smollan, 1992), and questions of connectedness (Wiltermuth & Heath, 2009), likeability (Hove & Risen, 2009), and ratings of similarity in personality (Valdesolo & Desteno, 2011). A combined ‘social closeness index’ was created by averaging these scores (on the basis of sufficiently high reliability: Cronbach’s α = 0.76).
2.4.3. Weak-link coordination game
The weak-link coordination game (adapted from: Wiltermuth & Heath, 2009) was programmed in z-Tree (Fischbacher, 2007). This game was played using electronic tokens to represent money. Each participant was given an endowment of 20 tokens at the start of each of 5 rounds. They could keep as many tokens as they wanted, and could decide to donate any number of the tokens to a public pot. The lowest contribution to the public pot was doubled and then paid back out to each participant. This was repeated each round. The assumption is that people will donate more if they expect others in the group to also donate generously: in effect, the game provides a measure of trust in a coordinated strategy (van Huyck, Battalio, & Beil, 1990). At the end of each round, the participants received information about the minimum contribution made so they could adjust their next decision accordingly. Over the 5 rounds, a well-coordinated group will demonstrate a consistent average donation, indicating that the group has established a joint strategy (Camerer, 2003).
2.5. Statistical methods
The change in pain threshold and social closeness indices were normally distributed in each movement condition (Kolmogorov-Smirnov tests, p > 0.05; ESM1, Table S1). Social closeness data had homogenous variance, but the pain threshold data had non-homogenous variance (Levene’s statistic = 4.297, df1 = 2, df2 = 91, p = 0.016; ESM1, Table S1). Given that exertion may have a positive and independent effect on pain threshold and social bonding (Tarr et al., 2015), each participant’s average Actiwatch ‘Activity Count’ during the silent disco was included as a covariate in all analyses.
Due to the hierarchical nature of the data, multilevel liner modeling was used to account for individual variation, repeated measures, and membership of testing group. The repeated measure dependent variables (i.e. within-subject measures of pain threshold and PANAS) were modeled using the fixed factors of time point (i.e. at the start and end), and movement condition (synchrony, partial synchrony and asynchrony), including interactions between these effects.
3. Results
3.1. Baseline differences
The movement conditions differed with respect to three baseline measures: conscientiousness (F(2) = 3.232, n = 94, p = 0.044), extraversion (F(2) = 3.640, n = 94, p = 0.041) and self-reported confidence in ability to remember the dance moves (F(2) = 4.658, n = 94, p = 0.012; ESM1, Table S2). These three variables were included as covariates in all subsequent analyses, although omitting them did not change the overall results. There were no significant differences between conditions in participants’ rating of fun, difficulty, embarrassment, or enjoyment of the silent disco, nor their perception of success (ESM1, Table S3). There was a significant overall increase in positive affect (F(1) = 86.564, p <0.001) and a decrease in negative affect (F(1) = 33.845, p < 0.001), but these changes did not differ between movement conditions (ESM1, Table S4).
3.2. Pain threshold
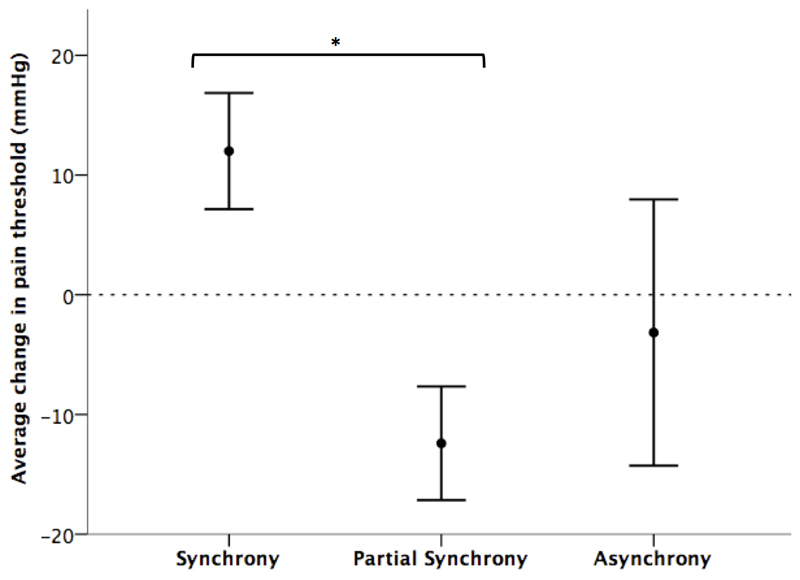
There was a significant main effect of movement condition on change in pain threshold (F(2) = 3.654, n = 94, p = 0.030), with the synchrony and partial synchrony conditions differing significantly (t = -2.562, df = 86, p = 0.012; see Figure 1). Those in the synchrony condition experienced a positive significant increase in pain threshold (x̄ = 12.000, SD = 35.303, n =53; t = 2.475, df = 52, p = 0.017), whereas those in the partial synchrony condition had a significantly lower pain threshold after compared to before (x̄ = -12.409, SD = 22.260, n = 22; t = -2.615, df = 21, p = 0.016). In the asynchrony condition, the change in pain threshold after dancing compared with before was in the same direction as in the partial synchrony condition and the difference between these conditions was not statistically significant (x̄ = -3.158, SD = 48.467, n = 19; t = 0.813, p = 0.418).
Figure 1.
Average (±ISE) change in pain threshold (end - start) for each movement condition, n = 94, * p ≤ 0.05.
3.3. Social closeness index and weak-link coordination game
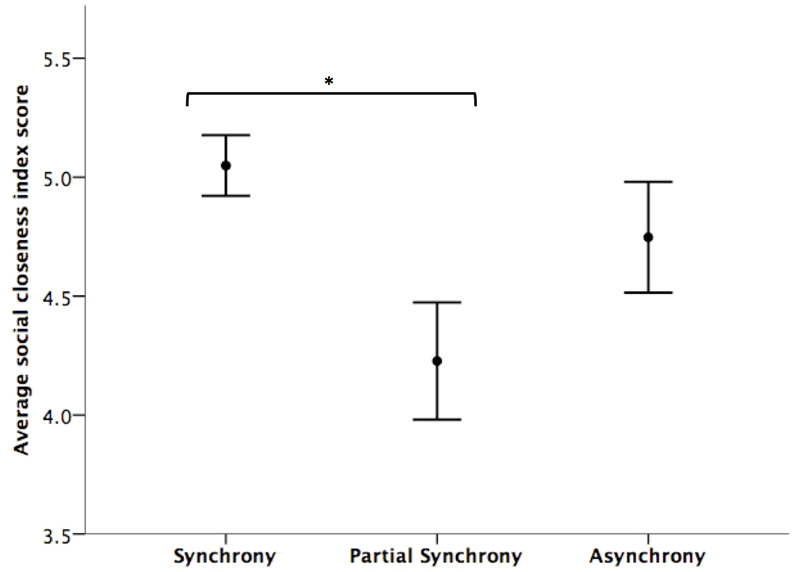
As predicted, there was a significant main effect of movement condition on the social closeness index (F(2) = 5.450, n = 96, p = 0.011), and pairwise comparisons indicated that those who danced in synchrony felt more socially close (x̄ = 5.049, SD = 0.928, n =53) than those in the partial synchrony condition (x̄ = 4.227, SD = 1.155, n =22; t = -3.300, df = 25, p = 0.003; see Figure 2). There was no significant difference between the closeness of those who had danced in asynchrony and either of the other movement conditions.
Figure 2.
Average (±ISE) social closeness index for each movement condition, n = 94, * p ≤ 0.05.
There was no significant effect of movement condition on donations to the public good in the weak-link coordination game (ESM1, Table S5). Analysis of donations at each round showed no significant difference between conditions except for the second round (F(2) = 3.325, n = 58, p = 0.044), where those in the synchrony condition donated significantly fewer tokens (x̄ = 8.41, SD = 3.589, n = 17) than those in the asynchrony condition (x̄ = 10.63, SD = 4.728, n = 19; t = 3.800, p = 0.037).
4. Discussion
This study investigated whether synchrony influences social bonding and pain threshold during a group dancing activity; synchronising full-body dance movements increased strangers’ self-reported feelings of social closeness to one another and elevated pain thresholds. These effects arose when participants synchronised with each other and the music, rather than merely with the music. Moving in asynchrony or a partially synchronised manner caused no change or a decrease in pain threshold respectively, and social closeness was highest for those in the synchrony condition. This is reflected in the fact that the change in pain threshold in the synchronized condition was significantly different from that in the combined non-synchronised conditions (i.e. partial synchrony and asynchrony conditions combined).
These data demonstrate that the established relationship between synchrony and feelings of social closeness holds in a more ecologically valid experimental paradigm in which participants are doing more than tapping fingers, rocking, or walking in time with one-another. Furthermore, given the relationship between pain threshold and activation of the EOS (particularly endorphin release), these data support the hypothesis that synchronised musicking elevates endorphin uptake (Dunbar, Kaskatis, et al., 2012; Tarr et al., 2015). Based on the role that opioids play in social bonding in other mammals (Machin & Dunbar, 2011), it is plausible that opioid and other neurohormonal mechanisms are important in the social closeness that arises during group movement activities such as dance. However, how these mechanisms relate to ‘self-other merging’ during synchrony is yet to be determined. It is possible that synchrony has an (as yet unknown) inherent relationship with the EOS, meaning that activities involving synchronisation also have social bonding effects.
During the silent disco, the positive effects associated with synchronising arose even though dancers wore individual headphones and were not aware of what the other participants could hear. The lack of effect in the case of the non-synchrony conditions may in part at least arise because participants have to ‘switch off’ from attending to the others in order to be able to concentrate on maintaining rhythm. Individuals are less likely to look at another participant if they are moving out of time (Hadley, Tidhar, & Woolhouse, 2012; Woolhouse & Tidhar, 2010) and attending to one another less can negatively affect social perception and likeability (Kleinke, 1986).
It is notable that the partial synchrony condition led to the lowest ratings of social bonding and an even greater decrease in pain thresholds than in the asynchrony condition. One possible explanation for this may lie in differences in how participants experienced being out of synchrony in the partial and asynchrony conditions. In the asynchrony condition, the fact that participants performed entirely different movements (unfamiliar to the other members of the group) may have masked the temporal dissonance that is evident when people are making the same movements at different times (as in e.g. Wiltermuth & Heath, 2009). As a result, participants in the asynchrony condition might have blocked out what others were doing and concentrated on their own moves, whereas those in the partial synchrony condition may have found the partial synchrony of the other participants particularly distracting. This may have resulted in the partial synchrony condition having a negative effect on bonding and pain threshold because it was more distracting than the asynchrony condition, in which the performance of a unique repertoire allowed participants to ‘switch off’ from each other.
It is also likely that the partial synchrony and asynchrony sessions provided differential signals to those participating. Hagen and Bryant (2003) have suggested that synchrony signals group cohesion and coalition strength, and it is possible that the partial synchrony condition was perceived by the participants as a failed attempt at synchrony, thereby signaling low levels of coalitional and bonding quality. This would conceivably have a negative effect on the bonding and pain threshold measures for these participants, in comparison to both the synchrony condition and those doing completely independent repertoires in the asynchrony condition. This apparent contrast between the two non-synchronous conditions requires further investigation, especially given that control conditions in the majority of previous studies are comparable to the partial synchrony condition used here.
Social bonds are developed on the basis of initial prosocial feelings, for example liking, feelings of similarity, and perception of inclusion as part of a group, which are considered meaningful measures of closeness (Gächter, Starmer, & Tufano, 2015). Nonetheless, although strangers dancing in synchrony reported feeling socially close, they did not make higher donations in the weak-link coordination game than those in the partial synchrony and asynchrony conditions. This contrasts with Wiltermuth & Heath (2009), who reported higher cooperation in the weak-link coordination game following synchrony. It is possible that Wiltermuth & Heath’s results were anomalous: Schachner & Garvin (2010) replicated their methods, and found no effect of synchrony condition on economic game behavior, which they attributed to the fact that the experimenters running the replication study were blind to the hypothesis and synchrony condition. However, an alternative explanation is that there are different forms of social bonding and although people feel closer after synchronising this need not manifest in all types of prosocial behavior. The use of different headphones (and therefore different sources of attention) in the current experiment may have changed the kind of social bonding experienced by group members and led to less of a development of economic trust. Indeed, the suitability of economic games for accurately assessing how socially close strangers feel in laboratory based experiments has been questioned (Burton-Chellew, El Mouden, & West, 2016; Burton-Chellew & West, 2013). Economic games assume a trading relationship between participants which may reflect societal norms (Henrich et al., 2005) rather than social bonding between newly acquainted people. If these games really are less suitable for assessing bonding, then within-subject testing may be more appropriate for capturing any differences in cooperation that result from manipulation of synchrony (as in Launay et al., 2013).
Anthropologists formulating early hypotheses about why synchrony in dance leads to social closeness emphasized the role of positive emotional states and joint arousal (e.g. collective effervescence; Durkheim, 1915). In the present study, positive affect increased following the silent disco, but did not differ according to movement condition, suggesting a dissociation between positive affect and social closeness, which is also evident in previous studies. For example, synchronised walkers who felt and acted more prosocial were not happier than non-synchronised walkers (Wiltermuth & Heath, 2009), positive interpersonal evaluations were unrelated to cooperative behavior in an economic game after conversations of varying convergence (Manson, Bryant, Gervais, & Kline, 2013), and hedonistic mood was not related to willingness to help following either a synchronised or unsynchronised group activity in another study (Reddish et al., 2014). Similarly, while laughter was associated with changes in pain threshold, affect was not (Dunbar, Baron, et al., 2012). Further investigation may be required to determine if this dissociation is due to an uncoupling of positive affect and bonding, or an artifact of the self-report measures used to assess mood.
Conclusions
The ability to synchronise to rhythmic beats and/or with conspecifics exists in a variety of species, and its occurrence and role in human musicking is of ongoing scientific interest. Although the use of individual headphones was necessary to create the non-synchronised conditions, resulting in a fairly contrived dance experience, this study extends previous research on the link between synchrony and social bonding by having participants perform full-bodied movements to popular music in a group setting.
Given that dancing does not necessarily involve matching movements with others and in time with the music, future studies are needed to determine how temporally synchronised, and which body parts need to be involved, for this effect to arise. Dance and musicking are atavistic behaviours evident in ritual, identity, and human expression around the world. Whilst various evolutionary benefits of group dancing are likely due to a range of different elements (e.g. complex sensorimotor coordination, improvisation, creative expression), this study provides prima facie evidence that dancing in synchrony has positive effects on social bonding between strangers and leads to greater increases in pain threshold compared with movements made out of time. In so far as tightly bonded and well coordinated groups face better survival odds than those which are less so, bonding activities which foster social cohesion and trust can be considered collectively advantageous and adaptive (Dunbar & Shultz, 2010). Once a close-knit group is formed, synchronised movement can have a number of additional, related functions, including signaling coalition strength to others (Hagen & Bryant, 2003), and enhancing perception (by synchronised individuals) of their relative formidability compared to an adversary (Fessler & Holbrook, 2014).
This study provides support for the theory that musicking may be a bio-cultural adaptation which is well suited for fostering social closeness. The capacity of group synchronised dancing to bond multiple people simultaneously suggests that social bonding is a likely evolutionary function of dance.
Supplementary Material
Acknowledgments
This study was funded by an ERC Advanced Investigator grant to the senior author (295663). We thank the many research assistants who helped throughout data collection, including colleagues at our Research Lab, and Matei Cristea for modeling the dance movements in the training videos.
References
- Aron A, Aron EN, Smollan D. Inclusion of Other in the Self Scale and the Structure of Interpersonal Closeness. Journal of Personality and Social Psychology. 1992;63(4):596–612. [Google Scholar]
- Backwell P, Jennions M, Passmore N. Synchronized courtship in fiddler crabs. Nature. 1998;391:31–2. [Google Scholar]
- Blakemore S-J, Decety J. From the perception of action to the understanding of intention. Nature Reviews Neuroscience. 2001 Aug;2:561–7. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2007. Peptides. 2008;29(12):2292–375. doi: 10.1016/j.peptides.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB. Mother-infant bonding and the evolution of mammalian social relationships. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2006;361(1476):2199–214. doi: 10.1098/rstb.2006.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton-Chellew MN, El Mouden C, West SA. Conditional cooperation and confusion in public-goods experiments. PNAS. 2016:1–6. doi: 10.1073/pnas.1509740113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton-Chellew MN, West SA. Prosocial preferences do not explain human cooperation in public-goods games. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(1):216–21. doi: 10.1073/pnas.1210960110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CambridgeNeurotechnology. The Actiwatch User Manual V7.2. CamNtech Ltd; Cambridge, UK: 2008. Retrieved from www.camntech.com/files/The_Actiwatch_User_Manual_V7.2.pdf. [Google Scholar]
- Camerer CF. Behavioural Game Theory. Princeton University Press; 2003. [Google Scholar]
- Chartrand TL, Lakin JL. The antecedents and consequences of human behavioral mimicry. Annual Review of Psychology. 2013;64:285–308. doi: 10.1146/annurev-psych-113011-143754. [DOI] [PubMed] [Google Scholar]
- Chelnokova O, Laeng B, Eikemo M, Riegels J, Løseth G, Maurud H, Leknes S, et al. Rewards of beauty: the opioid system mediates social motivation in humans. Molecular Psychiatry. 2014:1–2. doi: 10.1038/mp.2014.1. [DOI] [PubMed] [Google Scholar]
- Cohen E, Ejsmond-Frey R, Knight N, Dunbar RIM. Rowers’ high: behavioural synchrony is correlated with elevated pain thresholds. Biology Letters. 2010;6(1):106–8. doi: 10.1098/rsbl.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ, Smillie LD, Corr PJ. A confirmatory factor analysis of the Mini-IPIP five-factor model personality scale. Personality and Individual Differences. 2010;48(5):688–91. doi: 10.1016/j.paid.2010.01.004. [DOI] [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and other: a social cognitive neuroscience view. Trends in Cognitive Sciences. 2003;7(12):527–33. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Demos AP, Chaffin R, Begosh KT, Daniels JR, Marsh KL. Rocking to the beat: Effects of music and partner’s movements on spontaneous interpersonal coordination. Journal of Experimental Psychology General. 2012;141(1):49–53. doi: 10.1037/a0023843. [DOI] [PubMed] [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. The Behavioral and Brain Sciences. 2005;28(3):313–95. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Dezecache G, Dunbar RIM. Sharing the joke: the size of natural laughter groups. Evolution and Human Behavior. 2012;33(6):775–9. doi: 10.1016/j.evolhumbehav.2012.07.002. [DOI] [Google Scholar]
- Dunbar RIM. Language, music, and laughter in evolutionary perspective. In: Oller DK, Griebel U, editors. Evolution of Communication Systems: A Comparative Approach. Vol. 54. MIT Press; Cambridge, Massachusetts; London, England: 2004. pp. 257–74. [DOI] [Google Scholar]
- Dunbar RIM. Bridging the bonding gap: the transition from primates to humans. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2012a;367(1597):1837–46. doi: 10.1098/rstb.2011.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM. On the Evolutionary Function of Song and Dance. In: Bannan N, Mithen S, editors. Music, Language and Human Evolution. Oxford University Press; 2012b. pp. 201–214. [Google Scholar]
- Dunbar RIM, Baron R, Frangou A, Pearce E, van Leeuwen EJC, Stow J, van Vugt M. Social laughter is correlated with an elevated pain threshold. Proceedings of the Royal Society B: BiologicalSciences. 2012;279(1731):1161–7. doi: 10.1098/rspb.2011.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM, Kaskatis K, MacDonald I, Barra V. Performance of music elevates pain threshold and positive affect: implications for the evolutionary function of music. Evolutionary Psychology. 2012;10(4):688–702. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23089077. [PubMed] [Google Scholar]
- Dunbar RIM, Korstjens aH, Lehmann J. Time as an ecological constraint. Biological Reviews of the Cambridge Philosophical Society. 2009;84(3):413–29. doi: 10.1111/j.1469-185X.2009.00080.x. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S. Bondedness and sociality. Behaviour. 2010;147(7):775–803. doi: 10.1163/000579510X501151. [DOI] [Google Scholar]
- Durkheim E. The Elementary Forms of the Religious Life (J W Swain Trans.) Free Press; New York: 1915. Retrieved from http://www.archive.org/stream/elementaryformso00durk#page/n1/mode/2up. [Google Scholar]
- Eisenberger NI. Social Pain and the Brain: Controversies, Questions, and Where to Go from Here. Annual Review of Psychology. 2015;66:601–29. doi: 10.1146/annurev-psych-010213-115146. [DOI] [PubMed] [Google Scholar]
- Fessler DMT, Holbrook C. Marching into battle: synchronized walking diminishes the conceptualized formidability of an antagonist in men. Biology Letters. 2014;10(20140592) doi: 10.1098/rsbl.2014.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbacher U. Z-Tree: Zurich Toolbox for Ready-made Economic Experiments. Experimental Economics. 2007;10(2):171–8. [Google Scholar]
- Fitch WT. The biology and evolution of rhythm: unravelling a paradox. In: Rebuschat P, Rohrmeier M, Hawkins J, Cross I, editors. Language and Music as Cognitive Systems. Oxford University Press; Oxford, UK: 2012. pp. 73–96. [Google Scholar]
- Freeman WJ. A neurobiological role of music in social bonding. In: Wallin N, Merkur B, Brown S, editors. The Origins of Music. MIT Press; Cambridge, MA: 2000. pp. 411–24. [Google Scholar]
- Gächter S, Starmer C, Tufano F. Measuring the Closeness of Relationships: A Comprehensive Evaluation of the “Inclusion of the Other in the Self” Scale. PloS ONE. 2015;10(6):1–19. doi: 10.1371/journal.pone.0129478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley L, Tidhar D, Woolhouse M. Effects of Observed Music-Gesture Synchronicity on Gaze and Memory. In: Cambouropoulos E, Tsougras C, Mavromatis P, Pastiadis K, editors. Proceedings of the 12th International Conference on Music Perception and Cognition and the 8th Triennial Conference of the European Society for the Cognitive Sciences of Music; Thessaloniki, Greece. 2012. pp. 384–388. [Google Scholar]
- Hagen EH, Bryant GA. Music and dance as a coalition signalling system. Human Nature. 2003;14(1):21–51. doi: 10.1007/s12110-003-1015-z. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Tomonaga M, Matsuzawa T. Spontaneous synchronized tapping to an auditory rhythm in a chimpanzee. Nature: Scientific Reports. 2013;3(1566):1–6. doi: 10.1038/srep01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, Boyd R, Bowles S, Camerer CF, Fehr E, Gintis H, Tracer D, et al. “Economic man” in cross-cultural perspective: behavioral experiments in 15 small-scale societies. The Behavioral and Brain Sciences. 2005;28(6):795–815. doi: 10.1017/S0140525X05000142. discussion 815–55. [DOI] [PubMed] [Google Scholar]
- Hove MJ, Risen JL. It’s all in the timing: Interpersonal synchrony increases affiliation. Social Cognition. 2009;27(6):949–61. [Google Scholar]
- Kirschner S, Tomasello M. Joint drumming: social context facilitates synchronization in preschool children. Journal of Experimental Child Psychology. 2009;102(3):299–314. doi: 10.1016/j.jecp.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Kirschner S, Tomasello M. Joint music making promotes prosocial behavior in 4-year-old children. Evolution and Human Behavior. 2010;31(5):354–64. doi: 10.1016/j.evolhumbehav.2010.04.004. [DOI] [Google Scholar]
- Kleinke CL. Gaze and eye contact: a research review. Psychological Bulletin. 1986;100(1):78–100. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3526377. [PubMed] [Google Scholar]
- Knoblich G, Sebanz N. The social nature of perception and action. Current Directions in Psychological Science. 2006;15(3):99–104. [Google Scholar]
- Kokal I, Engel A, Kirschner S, Keysers C. Synchronized drumming enhances activity in the caudate and facilitates prosocial commitment-if the rhythm comes easily. PloS ONE. 2011;6(11):e27272. doi: 10.1371/journal.pone.0027272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Movement synchrony and perceived entitativity. Journal of Experimental Social Psychology. 2010;46(5):701–708. doi: 10.1016/j.jesp.2010.03.015. [DOI] [Google Scholar]
- Launay J, Dean RT, Bailes F. Synchronization can influence trust following virtual interaction. Experimental Psychology. 2013;60(1):53–63. doi: 10.1027/1618-3169/a000173. [DOI] [PubMed] [Google Scholar]
- Launay J, Dean RT, Bailes F. Synchronising movements with the sounds of a virtual partner enhances partner likeability. Cognitive Processing. 2014 doi: 10.1007/s10339-014-0618-0. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Lee PC, Dunbar RIM. Unravelling the evolutionary function of communities. In: Dunbar RIM, Gamble C, Gowlett JAJ, editors. Lucy to Language: the Benchmark Papers. Oxford University Press; Oxford: 2014. pp. 245–76. [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nature Reviews Neuroscience. 2008;9(4):314–20. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin. 2005;131(2):202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- Machin A, Dunbar RIM. The brain opioid theory of social attachment: a review of the evidence. Behaviour. 2011;148(9):985–1025. doi: 10.1163/000579511X596624. [DOI] [Google Scholar]
- Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the Positive and Negative Affect Schedule: evaluation of factorial validity and invariance across demographic variables in a community sample. Personality and Individual Differences. 1999;27:405–416. [Google Scholar]
- Macrae CN, Duffy OK, Miles LK, Lawrence J. A case of hand waving: Action synchrony and person perception. Cognition. 2008;109(1):152–6. doi: 10.1016/j.cognition.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Manson JH, Bryant GA, Gervais MM, Kline MA. Convergence of speech rate in conversation predicts cooperation. Evolution and Human Behavior. 2013;34(6):419–26. doi: 10.1016/j.evolhumbehav.2013.08.001. [DOI] [Google Scholar]
- McKinney CH, Tims FC, Kumar A, Kumar M. The effect of selected classical music and spontaneous imagery on plasma beta-endorphin. Journal of Behavioral Medicine. 1997;20(1):85–99. doi: 10.1023/a:1025543330939. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9058181. [DOI] [PubMed] [Google Scholar]
- Moiseff A, Copeland J. Firefly synchrony: a behavioral strategy to minimize visual clutter. Science. 2010;329:181. doi: 10.1126/science.1190421. [DOI] [PubMed] [Google Scholar]
- Mueller C, Klega A, Buchholz H-G, Rolke R, Magerl W, Schirrmacher R, Schreckenberger M, et al. Basal opioid receptor binding is associated with differences in sensory perception in healthy human subjects: a [18F]diprenorphine PET study. NeuroImage. 2010;49(1):731–7. doi: 10.1016/j.neuroimage.2009.08.033. [DOI] [PubMed] [Google Scholar]
- Nuechterlein GL, Storer RW. The Pair-Formation Displays of the Western Grebe. The Condor. 1982;84(4):351–369. [Google Scholar]
- Nummenmaa L, Manninen S, Tuominen L, Hirvonen J, Kalliokoski KK, Nuutila P, Sams M, et al. Adult attachment style is associated with cerebral μ-opioid receptor availability in humans. Human Brain Mapp. 2015;36(9) doi: 10.1002/hbm.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch N, Dunbar RIM. Influence of Kin Network on Maternal and Infant Health and Illness. Journal of Pregnancy and Child Health. 2015;2(2) doi: 10.4172/2376-127X.1000146. [DOI] [Google Scholar]
- Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of lesions of various CNS sites. Behavioral Neuroscience. 1997;111(6):1313–23. doi: 10.1037//0735-7044.111.6.1313. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9438800. [DOI] [PubMed] [Google Scholar]
- Overy K, Molnar-Szakacs I. Musical experience and the mirror neuron system. Music Perception: An Interdisciplinary Journal. 2009;26(5):489–504. [Google Scholar]
- Panksepp J. Affective Neuroscience. Oxford University Press; Oxford: 1999. [Google Scholar]
- Patel AD, Iversen JR, Bregman MR, Schulz I. Experimental evidence for synchronization to a musical beat in a nonhuman animal. Current Biology. 2009;19(10):827–30. doi: 10.1016/j.cub.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Patel AD, Iversen JR, Bregman MR, Schulz I, Schulz C, San C. Investigating the human-specificity of synchronization to music. Proceedings of the 10th International Conference on Music Perception and Cognition (ICMPC10); Sapporo, Japan. 2008. pp. 100–4. [Google Scholar]
- Patel AD, Iversen JR, Chen Y, Repp BH. The influence of metricality and modality on synchronization with a beat. Experimental Brain Research. 2005;163(2):226–38. doi: 10.1007/s00221-004-2159-8. [DOI] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Jarcho MR, Bales KL. Presence of a pair-mate regulates the behavioral and physiological effects of opioid manipulation in the monogamous titi monkey (Callicebus cupreus) Psychoneuroendocrinology. 2013;38(11):2448–61. doi: 10.1016/j.psyneuen.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddish P, Bulbulia J, Fischer R. Does synchrony promote generalized prosociality? Religion Brain & Behavior. 2014;4(1):3–19. doi: 10.1080/2153599X.2013.764545. [DOI] [Google Scholar]
- Reddish P, Fischer R, Bulbulia J. Let’s dance together: synchrony, shared intentionality and cooperation. PloS ONE. 2013;8(8):e71182. doi: 10.1371/journal.pone.0071182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner A, Garvin L. Does synchrony really affect social variables? Effects on cooperation, conformity may not be robust. International Conference on Music Perception and Cognition; Seattle Washington. 2010. [Google Scholar]
- Silk JB. Social components of fitness in primate groups. Science. 2007;317:1347–51. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–4. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Small C. Musicking: The Meaning of Performing and Listening. Wesleyan/University Press of New England; Hanover: 1998. [Google Scholar]
- Spence J. One Thousand Families in Newcastle. Oxford University Press; Oxford: 1954. [Google Scholar]
- Strürmer S, Snyder M, Kropp A, Siem B. Empathy-motivated helping: the moderating role of group membership. Personality & Social Psychology Bulletin. 2006;32(7):943–56. doi: 10.1177/0146167206287363. [DOI] [PubMed] [Google Scholar]
- Sullivan P, Rickers K. The effect of behavioral synchrony in groups of teammates and strangers. International Journal of Sport andExercise Psychology. 2013;11(3):1–6. doi: 10.1080/1612197X.2013.750139. [DOI] [Google Scholar]
- Sullivan P, Rickers K, Gagnon M, Gammage KL, Peters SJ. The synchrony effect in treadmill running. Journal of Sport and Exercise Psychology. 2011;33:S188. [Google Scholar]
- Sullivan P, Rickers K, Gammage KL. The effect of different phases of synchrony on pain threshold. Group Dynamics: Theory, Research, and Practice. 2014;18(2):122–8. [Google Scholar]
- Tarr B, Launay J, Cohen E, Dunbar RIM. Synchrony and exertion during dance independently raise pain threshold and encourage social bonding. Biology Letters. 2015;11(20150767) doi: 10.1098/rsbl.2015.0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr B, Launay J, Dunbar RIM. Music and social bonding: “self-other” merging and neurohormonal mechanisms. Frontiers in Psychology: Auditory Cognitive Neuroscience. 2014;5(1096):1–10. doi: 10.3389/fpsyg.2014.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdesolo P, Desteno D. Synchrony and the social tuning of compassion. Emotion. 2011;11(2):262–6. doi: 10.1037/a0021302. [DOI] [PubMed] [Google Scholar]
- Valdesolo P, Ouyang J, DeSteno D. The rhythm of joint action: Synchrony promotes cooperative ability. Journal of Experimental SocialPsychology. 2010;46(4):693–695. doi: 10.1016/j.jesp.2010.03.004. [DOI] [Google Scholar]
- van Huyck JB, Battalio RC, Beil RO. Tacit Coordination Games, Strategic Uncertainty, and Coordination Failure. The American Economic Review. 1990;80(1):234–48. [Google Scholar]
- Wilson M, Knoblich G. The case for motor involvement in perceiving conspecifics. Psychological Bulletin. 2005;131(3):460–73. doi: 10.1037/0033-2909.131.3.460. [DOI] [PubMed] [Google Scholar]
- Wiltermuth SS, Heath C. Synchrony and Cooperation. Psychological Science. 2009;20(1):1–5. doi: 10.1111/j.1467-9280.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- Wittig RM, Crockford C, Lehmann J, Whitten PL, Seyfarth RM, Cheney DL. Focused grooming networks and stress alleviation in wild female baboons. Hormones and Behavior. 2008;54:170–7. doi: 10.1016/j.yhbeh.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse M, Tidhar D. Group dancing leads to increased person-perception. In: Demorest S, Morrison S, Campbell P, editors. Proceedings of the 11th International Conference on Music Perception and Cogntiion (ICMPC 11); Seattle, Washington. 2010. pp. 605–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.