Abstract
When using immunocytochemistry, investigators may not know how to optimize staining or how to troubleshoot the method when staining fails. Lacking are guides for comparing techniques and applying information derived from one staining method to another. Newer methods amplify signal detection, but will not necessarily work at the same primary antibody concentrations used for less sensitive reactions. Recommendations of optimal titers are often not accurate and are not usually accompanied by information on the method used to test those antibodies or the specifics of the assay. When the staining does not work, the investigators do not know how to determine if the antiserum is bad, the tissue is bad, or the method is inappropriate for their staining. This unit describes detailed procedures for determining optimal staining and applying that information to three common immunofluorescence methods. Lastly, a formula is provided for converting among the different methods.
Keywords: immunoperoxidase, ABC technique, TSA amplification, immunofluorescence, immunocytochemistry, antibody dilution, microwave oven
INTRODUCTION
Immunocytochemistry (ICC) has become common in neuroscience research, with more than 40,000 published articles using this technique. Kits are available for many of the procedures to allow simple execution by investigators with little staining experience. However, a shortcoming in staining tissue with ICC is that investigators typically have no information concerning how optimal staining is achieved or do not know how to troubleshoot the method when staining fails. Moreover, advanced technology that uses confocal microscopy has led to preferential use of immunofluorescence with little understanding of how immunofluorescence techniques compare to each other and to nonfluorescent immunocytochemical techniques. Few investigators know how to take information derived from one staining method and apply it to another, and most are unaware that the sensitivity of antigen detection varies greatly across methods. Even if investigators are aware that newer methods can amplify signal detection, they do not realize that the amplification strategies will not work well at the same primary antibody concentrations used for less sensitive reactions. Recommendations of optimal titers of purchased antisera provided by companies are not usually accompanied by a description of the method(s) used to test those antibodies. Thus, when the staining experiments do not produce expected results, the investigator does not know where to begin to determine if the problem lies with the antiserum, the tissue, or the method. This unit presents detailed procedures for achieving optimal immunocytochemical staining, and then applying that information to three common immunofluorescence methods. In addition, a formula is provided in Table 2.12.1 for converting between the different methods.
Table 2.12.1.
Comparison of ICC Methods
| Method | Concentration/ABC NiDAB | Comments |
|---|---|---|
| ABC–NiDAB | 1 | Most sensitive; strongest staining at lowest concentration |
| Direct tagged secondary antibody | 20- to 100-fold higher concentration of primary antibody to get maximal staining | Least sensitive of the fluorescence methods |
| ABC–streptavidin fluorescence | 10- to 30-fold higher concentration of primary needed to obtain maximal staining | Approximately 3 times more sensitive than the direct tagged secondary fluorescence |
| Biotinylated TSA-amplified streptavidin fluorescence | 2-fold higher concentration of primary antibody yields equal sensitivity to the NiDAB method | Most sensitive of the fluorescence methods |
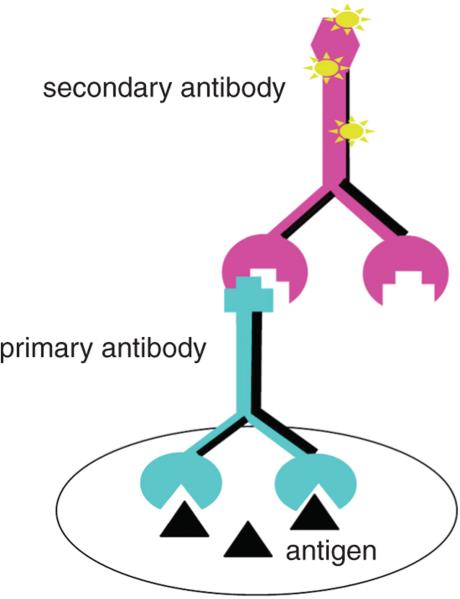
The most widely used ICC techniques are “indirect” in that detection is achieved not by labeling the primary antibody, but by tagging the Fc region (the species-specific portion) of the secondary (2°) antibody either with a molecule that can be visualized (fluorescent, colored, or electron-opaque), with an enzyme whose reacted substrate is visible, or with biotin, which can easily be complexed further to either a fluorophore or an enzyme whose products are colored. Tagged secondary antibodies are readily available from multiple companies. Conceptually, the simplest of the indirect methods uses a fluorophore-tagged secondary antibody (Fig. 2.12.1). There are many fluorophore-tagged secondary antibodies commercially available, and selection of an appropriate fluorophore can affect the brightness and stability of the fluorescent signal. While it is beyond the scope of this unit to review fluorophore selection, for simplicity, data are presented using one of the stable, green fluorescent molecules, Cy-2 (GE Healthcare). It should be noted that the basic principles of the methods used here apply equally well to other green fluorophores, as well as to orange or red fluorescent molecules. Blue or far-red fluorophores are often weaker than those in the visible green-orange-red range.
Figure 2.12.1.
Immunocytochemical method that employs a directly tagged secondary antibody (pink). As illustrated, the secondary antibody is labeled with a fluorescent molecule, but this same approach can be used with enzymes attached. For the color version of this figure go to http://www.currentprotocols.com.c
In 1970, Dr. Ludwig Sternberger (Sternberger et al., 1970) described a method of immunohistochemistry called the peroxidase-anti peroxidase technique (PAP), which involved an antibody complex generated in the same species as the test primary antibody joined to the peroxidase enzyme (usually three per complex). With that method, the secondary antibody served as a bridge to both the primary antibody and the anti-peroxidase complex. Use of a chromogen and hydrogen peroxide led to detection of the complex, and the entire procedure was ~3 to 4 times more sensitive than peroxidase linked directly to the secondary antibody. A cumbersome aspect of this method was that both the anti-peroxidase and the primary antibody had to be generated in the same species.
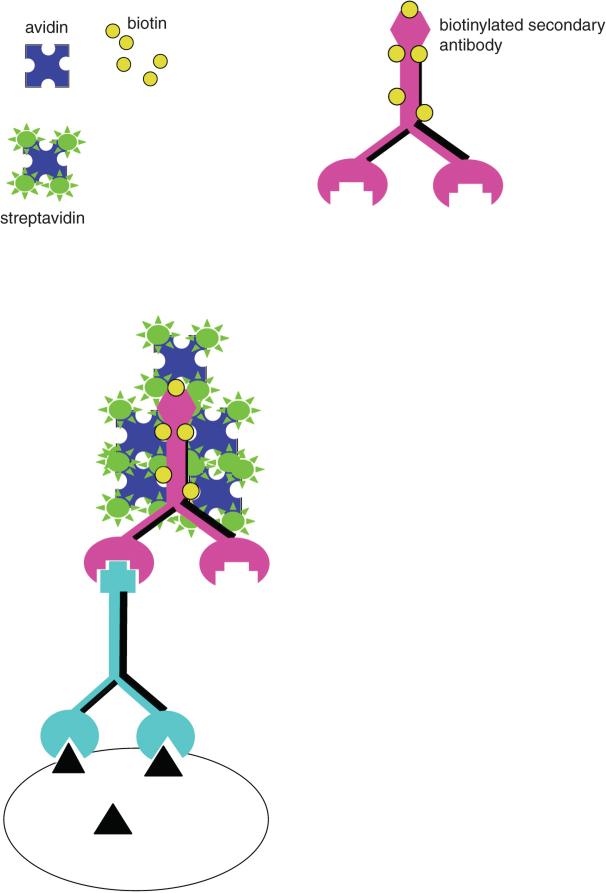
Later, in 1981, indirect ICC took another quantum leap when Hsu and coworkers (Hsu et al., 1981) introduced a method for ICC that used strong binding between an egg white protein (avidin) and a small molecule termed biotin to link the colored complex or enzyme to the secondary antibody. In its fluorescent variant, the secondary antibody is biotinylated and an avidin analog (streptavidin) is bound to any of a number of fluorophores (Fig. 2.12.2). By use of avidin-biotin methods, the signals for fluorescence are increased 20-fold over those seen with directly tagged fluorescent probes (estimated by the relative amount of primary antibody needed to detect antigen in a tissue). For enzymatic detection, biotinylated horseradish peroxidase joins to a biotinylated secondary antibody via an avidin bridge. This method is termed the ABC (for avidin-biotin-complex) peroxidase method (Fig. 2.12.3), and is one of the most widely used ICC methods today. It provides approximately a 100-fold increase in sensitivity compared with fluorescent methods using a direct tagged secondary antibody.
Figure 2.12.2.
The avidin-biotin complex (ABC) method for immunofluorescence. Note that, compared to the use of a direct fluorophore-linked secondary (Fig. 2.12.1), this method enables more molecules of fluorophore to be attached to the complex. For the color version of this figure go to http://www.currentprotocols.com.
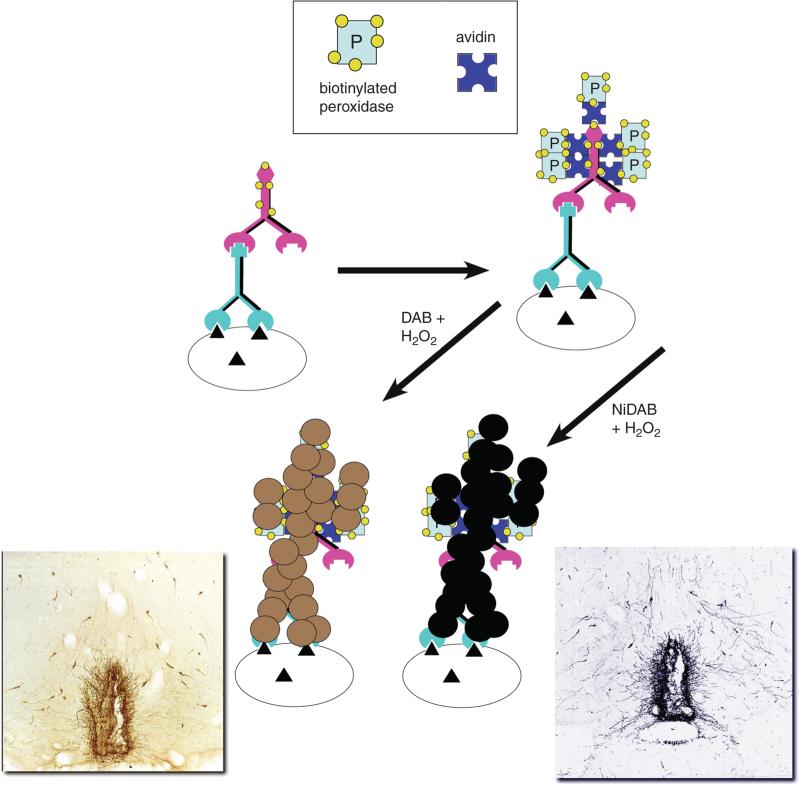
Figure 2.12.3.
The ABC peroxidase technique. This approach is similar to that shown in Figure 2.12.2, but substitutes a biotinylated peroxidase. When incubated with a substrate such as DAB (lower left) or NiDAB (lower right), the colored insoluble product is deposited to sites near the enzyme. The micrographs (insets) show an example of each of the chromogens (staining for luteinizing hormone releasing hormone/gonadotrophin releasing hormone). For the color version of this figure go to http://www.currentprotocols.com.
When applying strategies that use enzymes to generate a colored product, the choice of enzyme and substrate can greatly affect the sensitivity of the method. Some enzymes, especially alkaline phosphatase, accumulate product such that the longer the reaction is run, the more product is generated, whereas others, like horseradish peroxidase, when used with diaminobenzidine (DAB) as the substrate, deposit an insoluble product. Peroxidase reactions of this type are thus self-limiting in terms of how much product can be generated. On the other hand, 4-Cl naphthol generates a blue-gray peroxidase product that is not a precipitate. It will accumulate in the cell but has the disadvantage that it can fade. With DAB-based products, as more and more product is deposited, the enzyme and the antibodies linked to it are eventually covered by the precipitate and no further product can be generated. When used appropriately, precipitating products provide more accurate information concerning which subcellular compartment contains the antigen and how much antigen is actually present. The DAB product is normally brown in color but, if generated in the presence of metal salts (cobalt or nickel), can be blue/black in color. It is the peroxidase-DAB or nickel-DAB (NiDAB) combination that is used for most simple enzymatic assays in this unit (Fig. 2.12.3).
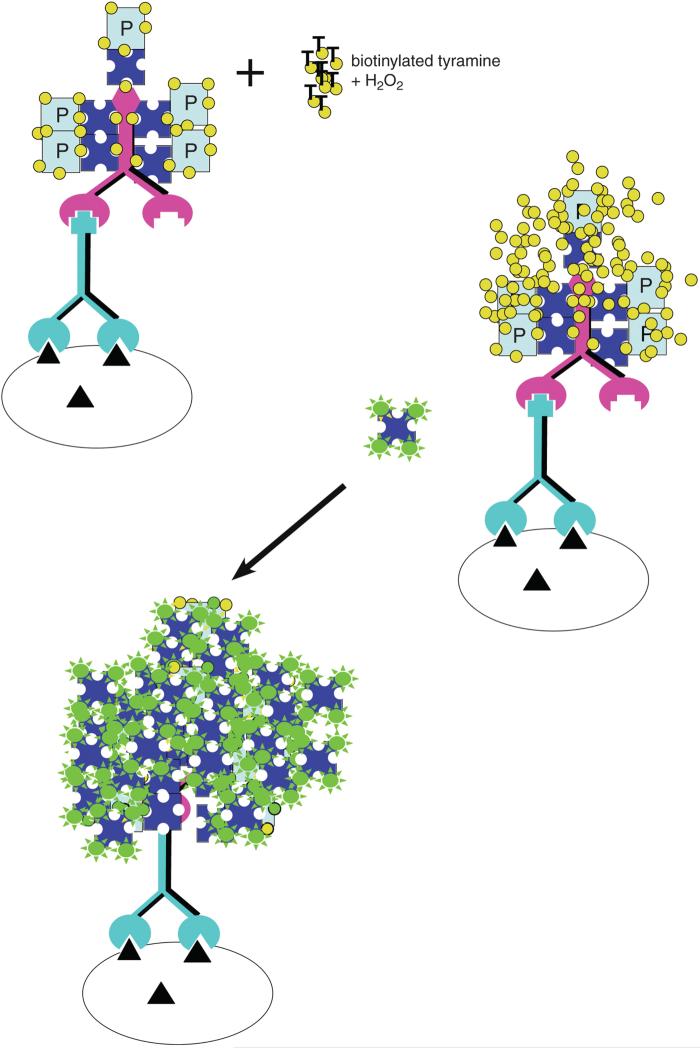
Lastly, an amplified fluorescence assay is presented in which the ABC complex's peroxidase generates products which deposit biotin over the enzyme, and the biotin is then coupled to a streptavidin-linked fluorophore (Fig. 2.12.4; Berghorn et al., 1994). The amount of fluorescence generated is two orders of magnitude greater than that obtained with the other fluorescent methods. For each of the methods presented, it must be recognized that the ability to detect a particular antigen will be a function of (1) how much antigen is present, (2) the affinity of the antibodies used to bind to the antigen, (3) how much accumulated product is deposited by the reaction, and (4) the strategy used for visualization. It is critical to keep in mind that the optimal titer differs predictably with each method. One goal in this unit is to stress that a simple formula enables an investigator to successfully use other methods after conducting a full titration with only one method (ABC peroxidase). To best compare results across methods, results are presented using the same antiserum for each method, all conducted on the same set of brains. The antibody used in the displayed results was generated against melanin-concentrating hormone (MCH) and was the gift of Dr. Wylie Vale and Joan Vaughan at the Peptide Biology Laboratory at The Salk Institute. The basic principles illustrated have been tested on over 200 antibodies from multiple sources, both commercial and private.
Figure 2.12.4.
TSA-amplified fluorescence using biotinylated tyramine and streptavidin fluorophore. Note the greatly increased number of fluorescent molecules compared with that seen for either fluorophore-tagged secondaries or ABC streptavidin methods. P = peroxidase. For the color version of this figure go to http://www.currentprotocols.com.
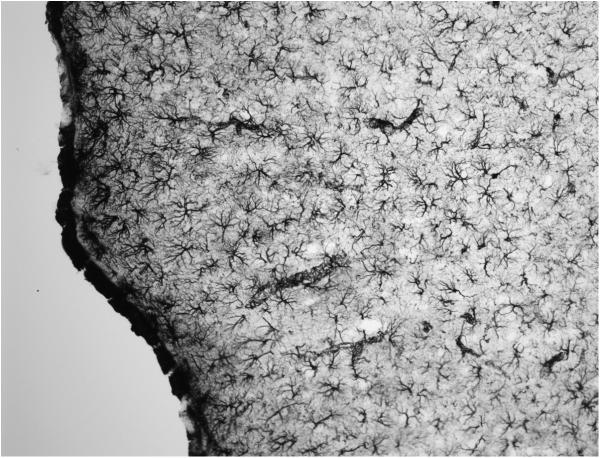
The immunoperoxidase ABC method is recommended for providing the baseline titers of antibodies. ABC reactions of peroxidase using precipitating chromogens such as diaminobenzidine (DAB), alone or with nickel salts (NiDAB), provide up to 100× to 200× the sensitivity of direct tagged fluorescence methods. These offer the distinct advantage of providing permanent products that do not fade. The majority of published studies use DAB as the chromogen for the enzymatic reaction, which yields a brown reaction product when incubated with peroxide. As mentioned previously, a blue-black product results when the peroxidase reactions are run in the presence of nickel or cobalt salts. There is essentially no difference in the sensitivity of the enzymatic reactions with DAB versus nickel-DAB, although the black product is sometimes more obvious. A light brown structure can be more difficult to see than a light gray one. Under dark-field optics, visibility of the DAB (but not nickel-DAB) is striking, since the precipitate glows yellow-orange. There is, however, one major difference: nickel salts are more informative in titrating antibodies because, when the primary antibody is too concentrated, what is seen is not only less product, but a product whose color is inappropriately brown. In so doing, the investigator has a reliable indicator that the primary antibody needs to be further diluted to obtain optimal staining. Figure 2.12.5 shows the progressive changes in staining when titrating an antibody using NiDAB.
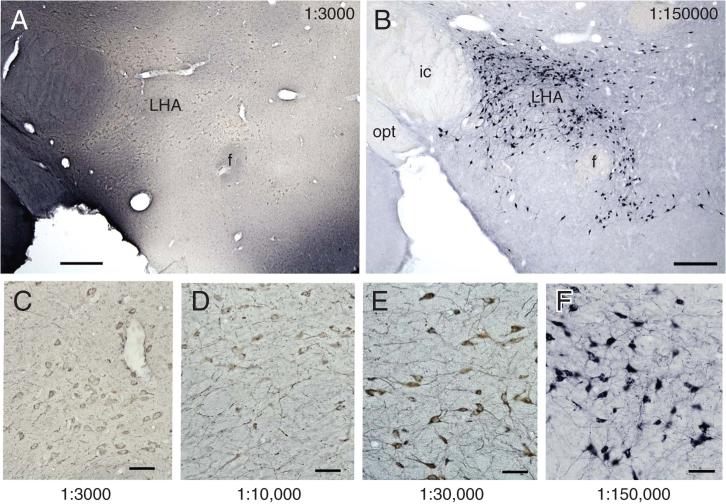
Figure 2.12.5.
Variation in the color of the NiDAB product when high versus low concentrations of the primary antibody are used. Low-power micrographs show melanin-concentrating hormone (MCH) in the lateral hypothalamic area (LHA) using the anti-MCH at 1:3,000 and 1:150,000. Note that at the high concentration of the antibody (A), the staining is uneven and in some regions has very high background compared with that seen when the primary antibody concentration is reduced (B). The series along the bottom (C to F) provides higher magnification and a more complete titration series that illustrates how the signal-to-noise ratio increases and the color shifts from brown to blue-black as the antibody is diluted to its optimum. Scale bar for A and B = 250 μm; C-F = 50 μm. Abbreviations: ic = internal capsule, f = fornix, opt = optic tract. For the color version of this figure go to http://www.currentprotocols.com.
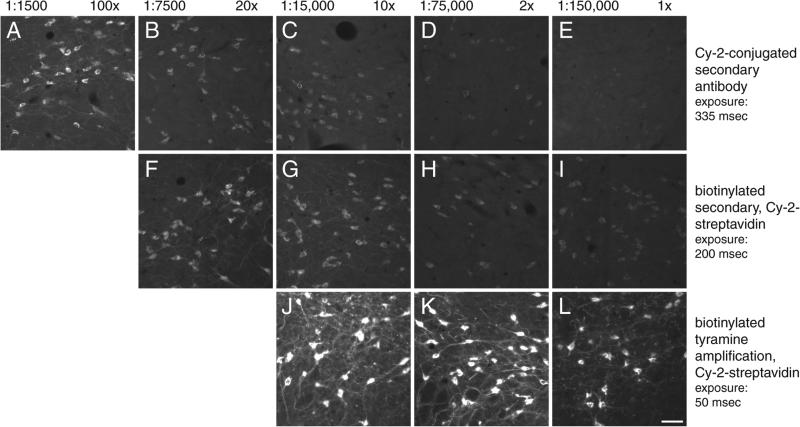
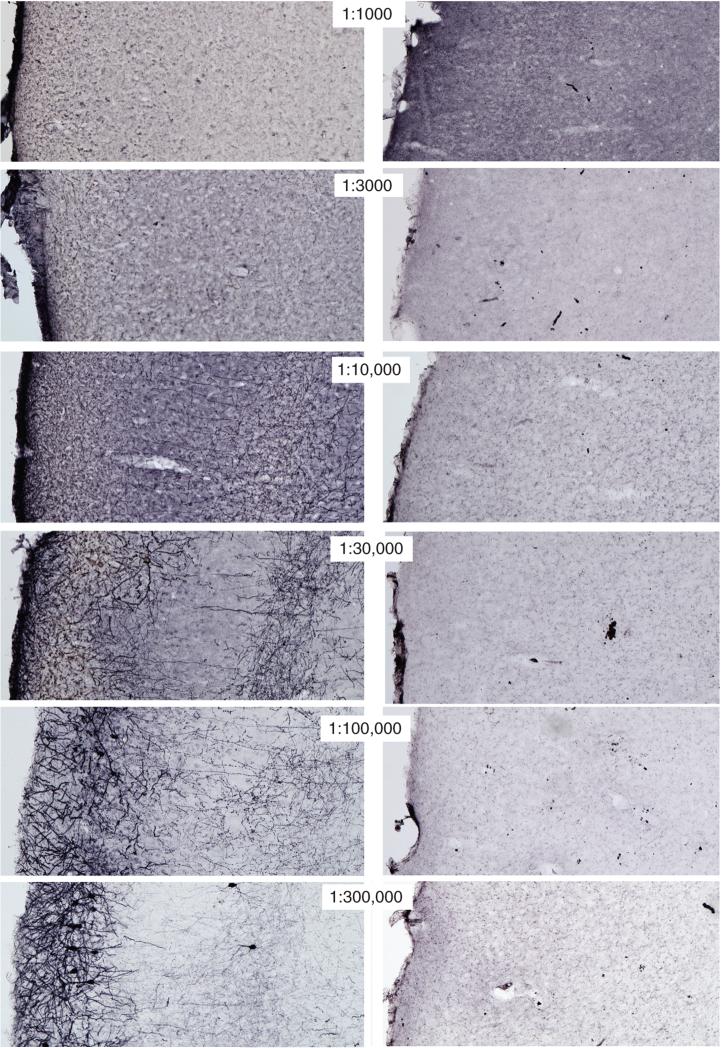
Comparison of titrations using three fluorescence techniques is shown in Figure 2.12.6. Translated into practical terms, as more sensitive methods are used, primary antibody concentrations must be REDUCED to obtain optimal staining, and the amount of product deposited increases with the sensitivity of the method. Table 2.12.1 summarizes the changes in primary antibody concentrations at the point of optimal staining for the four methods compared in this unit. By selecting the most sensitive method, the concentrations of primary antibodies used can be greatly reduced while producing stronger and clearer staining.
Figure 2.12.6.
Titrations of anti-MCH (melanin-concentrating hormone) using different immunofluorescence methods. Fluorescence photomicrographs show MCH-immunoreactive cells in the lateral hypothalamic area using Cy-2 conjugated directly to the secondary antibody (A-E), a biotinylated secondary antibody plus streptavidin-Cy-2 (F-I), and TSA amplification of biotinylated tyramine followed by the same Cy-2-conjugated streptavidin (J-L). The values above the pictures refer to the absolute concentrations of the primary antibody used (left) and the concentrations of the primary antibody relative to the optimal concentration determined with ABC immunoperoxidase and NiDAB as shown in Figure 2.12.5. The values on the right indicate the exposure times in milliseconds for acquiring the images in the series. Some increased detection could have been obtained if longer times were used, but times in a series were held constant to stress signal-intensity changes. All labeling was conducted from tissue obtained from the same animal. Scale bar = 50 μm. For the color version of this figure go to http://www.currentprotocols.com.
Newer technology has posited that valuable time for processing tissue can be shortened by use of microwave oven technology that enables even heating of specimens, control of power and temperature. However, a major caveat may be that high antibody concentrations will be initially required (Munoz et al., 2004) making the cost of reagents prohibitive. This document describes a hybrid approach using microwave technology that spares the cost of antisera while still shortening processing times. The approach is applied to all the standard techniques for ICC staining.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
BASIC PROTOCOL 1
TITRATION OF ANTIBODIES USING IMMUNOCYTOCHEMISTRY
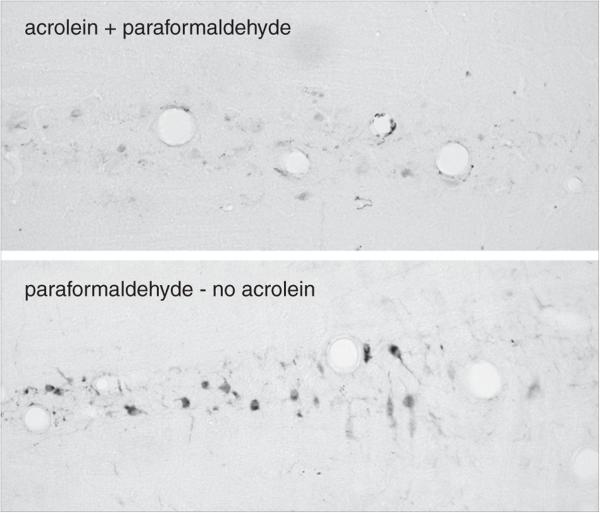
This protocol outlines the standard ABC peroxidase method to titrate antibodies using NiDAB as chromogen in rat brain sections fixed with 4% paraformaldehyde plus 2.5% acrolein, but other kinds of tissue can also be used. If tissue samples are very small, they can be processed free-floating by first embedding them into an egg yolk/gelatin matrix, fixing the block, and then treating it in exactly the same way as larger tissues. This approach has been used for pituitaries of mice and rats, intestinal samples, ovaries, and smaller parts of the brain (e.g., cerebellum, unpub. observ.). That embedding procedure is described in Alternate Protocol 4. The best results are obtained if animals are perfused rather than immersion-fixed and, for most antigens, the acrolein:paraformaldehyde mixture is the best fixative. For many antigens, standard buffered 4% paraformaldehyde fixatives are also acceptable. Specialized fixatives, such as carbodiimide or polylysine periodate, can also be applied using the modifications of the staining procedure indicated for 4% paraformaldehyde without added acrolein. The essential approach to titrating antibodies is based on the methods described in Berghorn et al., 1994, and Hoffman, et al., 1992.
Materials
Animal of choice: The protocols for animal perfusion have been applied to adult animals (young and old) that include rats, mice, hamsters, guinea pigs, chickens, and voles. For very large species we have euthanized the animal and used carotid perfusions (sheep, for example – adult or fetal; baboons - adult and fetal). Very young animals (fetuses and small newborn animals) may be immersion fixed but then treatment for blood will be necessary. {AU: Is there a specific age range and can older animals be used? Also if other species etc can be used perhaps do not provide the specific detail of the animal, as is described in step 1 note}
1 U/μl heparin
0.9% (w/v) NaCl containing 2% (w/v) sodium nitrite
10% sodium bisulfite
4% (w/v) paraformaldehyde containing 2.5% acrolein, in potassium phosphate buffer, pH 6.8 (see recipe)
30% (w/v) sucrose, cold
Antifreeze cryoprotectant (see recipe)
0.05 M KPBS (see recipe)
0.1% (w/v) sodium borohydride in 0.05 M KPBS
0.014% (w/v) phenylhydrazine hydrochloride or 1% (v/v) hydrogen peroxide in 0.05 M KPBS
Primary antibody
0.05 M KPBS (see recipe) containing 0.4% (v/v) Triton X-100
Biotinylated secondary antibody
Vectastain Elite ABC Kit (Standard; Vector Laboratories, cat. no. PK-6100), including Solution A and Solution B
0.175 M sodium acetate
NiDAB chromogen solution (see recipe)
3% (v/v) H2O2
50%, 70%, and 95% (v/v) ethanol
Absolute ethanol
Xylene or Histoclear (National Diagnostics)
Mounting medium: e.g., Permount (Fisher Scientific), Histomount (National Diagnostics), Krystalon (Harleco, cat. no. 64969, available from Voigt Global Distribution LLC, http://www.voigtglobal.com), or D.P.X. (Aldrich)
Surgical equipment
15-G needle
Peristaltic pump
Freezing sliding microtome or cryostat (also see UNIT 1.1)
Large petri dishes
Subbed glass slides (see recipe) or Fisher Superfrost Plus electrostatically charged slides
Glass coverslips
Additional reagents and equipment for anesthesia of rodents (APPENDIX 4B) and sectioning of brain tissue (UNIT 1.1)
CAUTION: Acrolein is a potent volatile eye and respiratory irritant and must be handled with care. See the comments on acrolein use in step 7. Whole-animal perfusion should be performed in a hood with the animal on a dissecting tray or rack such that a “capture” tray can be placed below. The hood should be checked by the Institution's Health and Safety office to ensure proper air flow for use of acrolein. Availability of 10% sodium bisulfite solutions is important for neutralizing any acrolein that spills, escapes the animal or is left over after perfusion. [AU: May want to also add a statement for proper PPE as acrolein gives off very potent fumes as also discussed in step 7]
Perfuse the animals and prepare tissue sections
- Anesthetize the animal with 100 mg/kg pentobarbital, i.p., or use veterinarian recommendations for rodent anesthesia if pentobarbital is unavailable.When using adult rats, the age/weight of the animal does not greatly influence the perfusion procedure, provided that the descending aorta is clamped with a hemostat. If whole-body perfusion is required, then the volume of perfusion solution is increased by 100 ml for a 225-g rat; larger animals would require a proportional increase in this volume. For young animals or smaller species, the gauge of the perfusion cannula is changed to fit the diameter of the aorta, and the proper volume of fixative will be that delivered in a 20-min period. Rates of flow are adjusted to be just below systolic pressure in the animal.
- Open chest cavity by making an inverted “T” incision of the skin over the chest and cutting into the peritoneal cavity to expose the diaphragm (Fig. 2.12.7A). Cut the diaphragm to expose the heart. Cut both sides of the rib cage and lift the sternum and ribs over the heart so the entire heart is visible (Figs. 2.12.7B and 2.12.7C). Using a 1-ml syringe, inject 0.1 ml (100 U) of heparin into the heart apex.The heparin will prevent blood clots from impairing flow of fixative to the brain tissue.
- Clamp descending aorta to eliminate whole-body perfusion.If spinal cord or visceral tissue will be needed, do not clamp the descending aorta. In this case, a greater volume of fixative will be required.
- Insert a needle (connected to a tubing system of a peristaltic pump) into the heart apex (Figs. 2.12.7C and 2.12.7D).The proper needle gauge when using animals of different ages, or when perfusing other species, is that which approximates the diameter of the animal's aorta.
Cut a hole in the right atrium to allow fluids to drain from the circulatory system.
- Pump 0.9% NaCl containing 2% sodium nitrite at room temperature at a rate that approximates systolic blood pressure until the effluent runs clear (about 200 to 250 ml for an adult rat). If whole-body perfusion is necessary, double the volume. [AU: would you consider this speed sufficient even if one were to perform electron microscopy experiments? As I recall, our perfusion rate was slower to prevent, as much as possible, blowout of vessels]In general if cannula approximates the aortic size, rate of perfusion is selected by adjustment to a flow just below that which produces a steady stream. This rule of thumb would apply for use of other species or ages. For very old animals one might need to lower the rate if vessels are more brittle. Pressure in the aorta should not exceed systolic pressure. Sodium nitrite is a vasodilator, helping the fixative flow through the vessels. However, there may be some dilated vessels observed in the sections. If vessel caliber is to be measured as a part of the study, use 0.9% saline without sodium nitrite. If tissue is being prepared for electron microscopy, acrolein-paraformaldehyde fixation provides excellent tissue preservation, but the investigator may need to pay greater attention to perfusion pressure (reducing it and staying below systolic pressure) and should use pH 7.2 for fixative pH. Most ultrastructural studies use higher acrolein concentrations (3.75-5.0%) along with 2 or 4% paraformaldehyde and shortening the fixation time to maintain maximal antigenicity (Chan et al., 1990; Felten and Olschowka, 1987).
- Clean or replace the “fluid capture” tray and add 10% sodium bisulfite to deactivate acrolein, which will be present in the effluent. Switch pump feed to the 4% paraformaldehyde containing 2.5% acrolein, pH 6.8 (250 ml for an adult rat) at room temperature. Slow the rate to about 75% of initial ml/min. Note the animal should become hard.CAUTION: Fixation with acrolein-containing fixatives must be done in a hood (approved for proper air flow). Since acrolein is a respiratory and lacrimal gland irritant, in case of a spill, large volumes of a 10% sodium bisulfite solution should be readily available to pour over the spill.The saline solutions are used effectively at refrigerator temperatures; cold fixation is used for antigens that are highly diffusible but can retard tissue preservation requiring longer fixation times. Post-fixation is then needed for as long as up to 1 week. The addition of sucrose to the fixative reduces the time needed for the block to sink prior to cutting with a cryostat or freezing microtome; sucrose infiltration is not necessary if a Vibratome is used to section the tissue.
- Rinse with 150 ml (for an adult rat) of 0.9% NaCl containing 2% sodium nitrite to remove acrolein from the animal's blood at the end of the perfusion period.See annotation to step 6 regarding use or omission of nitrite in the saline.
- Remove the brain from the skull and immerse it in 30% sucrose at 4°C. Change the sucrose solution several times until the brain sinks. Keep refrigerated until cutting is initiated.The brain can be kept refrigerated for as long as 2 months (changing the sucrose every couple of weeks) if the laboratory environment does not promote growth of fungus or bacteria. In locations that are tropical, it is best to cut the brain soon after it has sunk.Post-fixation is not necessary, but after fixation it is essential to completely infiltrate the tissue with the sucrose solution to protect it from ice-crystal damage when it is later frozen for sectioning. If this is not done correctly, the tissue will look like Swiss cheese upon sectioning with a freezing microtome or a cryostat.
- After the brain sinks in the 30% sucrose (about 2 days later), cut the brain into a series of 25-μm sections with a sliding microtome (freezing microtome) or cryostat (UNIT 1.1). Place the sections immediately into the antifreeze cryoprotectant at −20°C to prevent freezing during tissue section storage.The authors typically cut sections and place them in tissue culture wells (24/plate) filled with the antifreeze solution. For whole forebrain, as the sections are cut, they are consecutively placed into 12 tissue culture wells. At the end of this process, each well of tissue contains every 12th section (and the series is thus termed a 1-in-12 series of sections). For brainstem or smaller structures, a 1-in-6 series is typically generated. Sections can be stored indefinitely in cryoprotectant at −20°C. If a Vibratome is used, sections should ideally be no more than 30 μm thick if fixed with acrolein/paraformaldehyde, or 50 μm thick if fixed in paraformaldehyde without acrolein. Thicker sections are possible, but all rinses must be lengthened and incubation times with the various antibodies may need to be longer to avoid problems with penetration of reagents; staining may be weaker toward the center of the section. Sections mounted on slides (e.g., normal cryostat sections) will require higher concentrations of antibodies.
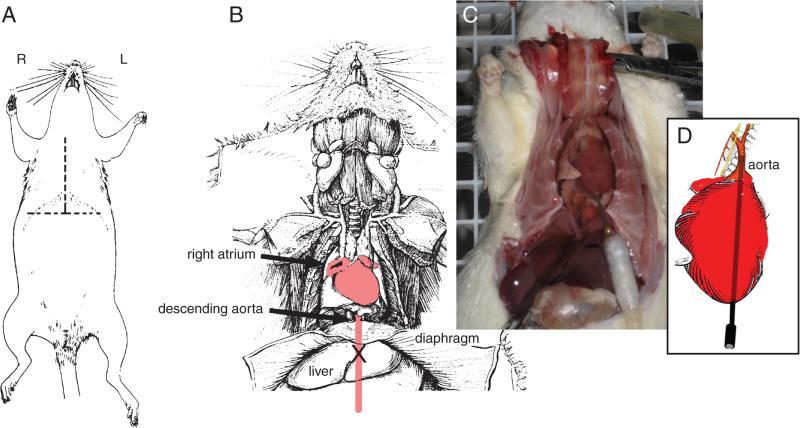
Figure 2.12.7.
The procedures used to perfuse a rat. The rat is anesthetized and an inverted “T”-shaped incision made (A). The diaphragm is cut and the rib cage opened to expose the heart (B and C). After injecting heparin into the heart at its apex, a catheter is inserted into the heart as shown in C. The tip of the catheter is eased into the proximal portion of the aorta as shown in D. The right atrium is cut (as illustrated in B to enable the perfusate to drain from the animal. (Photograph courtesy of Emmanuel Díaz, a student at the Ricardo Miledi Neuroscience Course, Universidad Nacional Autónoma de México, 2008).
Perform immunohistochemistry
Day 1
-
11.Remove sections from the antifreeze solution and place them into a large petri dish filled with 0.05 M KPBS at room temperature.If the antigen to be stained is present throughout the tissue, then a single well of tissue can be used for testing multiple dilutions. Be sure to have at least two sections per concentration.
-
12.Thoroughly rinse out cryoprotectant with six 10-min washes in 0.05 M KPBS.Slight changes in the molarity of the KPBS (e.g., use of 0.02 M) typically do not alter the final labeling. Other common buffers such as sodium phosphate buffer (0.1 M) also can be used. The most important thing is to keep the pH between 7.3 and 7.4.
-
13.Incubate 20 min in 1% sodium borohydride diluted in 0.05 M KPBS.This is an important step for tissue fixed with acrolein, glutaraldehyde, or picric acid. These chemicals improve the fixation, but accumulate free aldehydes in the tissue. The incubation in sodium borohydride removes these free aldehydes; sodium borohydride should not be used for tissue fixed only with paraformaldehyde.
-
14.
Rinse ~10 times with KPBS, until bubbles are gone.
-
15.If blood is still present in the tissue, incubate 15 min in 0.014% phenylhydrazine in 0.05 M KPBS or 1% hydrogen peroxide in 0.05 M KPBS. Rinse multiple times.Selection of phenylhydrazine (an inhibitor of peroxidase activity) or peroxide depends upon the stability of the antigen. For some proteins, antigenicity is compromised with peroxide, whereas phenylhydrazine may not affect primary antibody binding. Selection of the best blocker is done empirically.
-
16.Incubate sections in primary antibody in KPBS containing 0.4% Triton X-100, first for 1 hr at room temperature on a shaker, then for 48 hr at 4°C, at the following antibody dilutions: 1:1000; 1:3000; 1:10,000; 1:30,000; 1:100,000; 1:300,000. [AU: Should the sections be continuously agitated during incubation?]Table 2.12.2 indicates the exact volumes of the antibody solutions and Triton X-100 buffer needed for the titration. Proteins such as bovine serum albumin, thyroglobulin, or hemocyanin are often used to increase immunogenicity when generating the primary antibody. If the antibody being tested was generated from a protein conjugate of this type, add 1% (w/v) of the protein used for conjugation to the primary antibody to block any antibodies generated against the linking protein. Some investigators continuously agitate their sections during incubation with the primary antibody. In our experience this procedure is unnecessary if the sections are well separated in the incubation cup. If agitation is used for either the one hr incubation at room temperature or the long incubation in the cold, care must be taken to avoid sections moving up the side of the vessel and drying out.As the amount of specific antigen-antibody binding follows an exponential dissociation, always incubate sections in a series of solutions that spans a 3-log scale of antibody concentrations in half-log increments. In the authors’ experience, the optimal concentration that yields specific staining with low background with most antibodies is far more dilute than others recommend or have used, and it will almost never be optimal at concentrations higher than those recommended/published. After the first test, intermediate concentrations can be tested to determine an optimum result.The best incubation duration is usually 48 hr at 4°C, but there is generally no problem allowing incubation for longer times—up to 72 hr. Incubation periods shorter than 48 hr are not generally recommended, but an overnight incubation to facilitate antibody penetration may be used if the tissue is kept at room temperature. Shortening of the time will produce a shift in the titration curve to the left - 24 hr incubation, for example requires, 2-3x higher concentrations of primary antibody compared to 48 hr. Longer times are important if sections are thicker. Use of lower primary antibody concentrations with prolonged incubation times may also pertain to electron microscopic studies (Felten and Olschowka, 1987); however systematic reports of manipulations of primary antibody concentrations and times for exposure of tissue to the primary antibody have not been reported.The use of polyclonal and monoclonal antibodies for staining in species other than the host species can yield excellent results, and no advantage is typically seen using one in contrast to the other, nor does the type of antibody predict the titer. As examples, the monoclonal antibody from Chemicon against tyrosine hydroxylase is optimal at 1:250,000 with ABC peroxidase, and the sheep polyclonal anti–tyrosine hydroxylase from Pel-Freeze had a similar titer years ago, but currently displays optimal staining at 1:10,000. A polyclonal antibody from Chemicon against lamin C has an optimal titer of 1:3000 and a monoclonal antibody against microtubule-associated proteins, also from Chemicon, has an optimal titer of 1:1,000,000. A rabbit polyclonal from RBI against the enzyme nitric oxide synthase had an optimal titer of 1:300,000; a monoclonal antibody against the same antigen had a titer that was optimal at 1:3,000. With each, the intensity and pattern of staining at the optimum dilution was identical. (see RBI, 1998)
Table 2.12.2.
Making Dilutions of the Primary Antibody for a Titrationa
| Final concentration | Final volume | Antibody (μl) | Buffer added (KPBS + 0.4% Triton X-100) |
|---|---|---|---|
| 1:1000 | 3 ml | 3 μl of 1:1 | 2997 μl |
| 1:3000 | 2 ml | 666.7 μl of 1:1000 | 1333.3 μl |
| 1:10,000 | 2 ml | 200 μl of 1:1000 | 1800 μl |
| 1:30,000 | 2 ml | 66.7 μl of 1:1000 | 1933.3 μl |
| 1:100,000 | 2 ml | 20 μl of 1:1000 | 1980 μl |
| 1:300,000 | 2 ml | 6.7 μl of 1:1000 | 1993.3 μl |
The chart assumes that the starting material is pure serum (1:1). Immunoglobulins are normally present in blood at a concentration of ~100 mg/ml. If purchased antibodies are provided as mg or μg rather than a volume, prepare a starting concentration of 100 mg/ml in distilled water and term that solution 1:1. Storage of stock solutions is at −70°C (small aliquots can be prepared and stored this way, if desired). When using the antibodies on a regular basis, dilute an aliquot of the stock solution to either 1:10 or 1:100 in a glycerol solution consisting of 1 g bovine serum albumin, 50 g 100% glycerol, and 0.05 g sodium azide in 100 ml of 0.05 M KPBS, and keep this solution at −20°C.
48 hr later
-
17.
Rinse sections 10 times over 60 min in KPBS at room temperature.
-
18.Incubate tissue sections 1 hr at room temperature with biotinylated secondary antibody diluted 1:600 in KPBS containing 0.4% Triton X-100.This secondary antibody must be raised against the host of the primary antibody. Be careful to use a specific antibody from a reliable source and, in setting up an assay, it may be necessary to test a few concentrations lower and higher than those recommended by the supplier to achieve the lowest background and optimal signal-to-noise ratios. This concentration may vary from 1:500 to 1:1000. High concentrations may provide strong labeling, but with higher background.
-
19.
Rinse sections five times for 10 min each in KPBS at room temperature.
-
20.Prepare the next solution (A/B solution from Vectastain Elite ABC Kit Standard) at the beginning of the rinses by combining 45 μl of solution A and 45 μl of solution B in 10 ml of KPBS containing 0.4% Triton X. Let stand at least 30 min before use.This kit provides avidin and biotinylated horseradish peroxidase, which are combined in the form of the A/B solution. They have high affinity and bind to the biotinylated secondary antibody. Use of a different fixative for the tissue may require some adjustment of the concentration of the A/B solution. In the authors’ experience, the recommendations for amounts of solution A and B in the kits are higher than are necessary and may generate higher background staining.
-
21.
Incubate sections in A/B solution for 1 hr at room temperature.
-
22.
Rinse sections three times, each time for 5 min in KPBS.
-
23.Rinse three times, each time for 5 min in 0.175 M sodium acetate.Do not adjust the pH of this solution. Use it as is, but be aware that the correct pH is critical for successful staining in the next step. The molarity can range from 0.1 to 0.2 (pH about 7.0).
-
24.Prepare the NiDAB chromogen solution fresh, and, as quickly as possible, mix, and incubate the sections in this solution for 20 min.The 20 min staining time is critical. Shorter times may not permit completion of the enzymatic reactions and may lessen antigen detection. Longer times could evoke nonspecific background staining. It is important to recognize that NiDAB will precipitate in chloride solutions, so no NaCl should be included in any buffer exposed to NiDABFor titrating antibodies, always run the ICC with NiDAB because the color of product is only clearly blue/black when approaching the optimal concentration (see Fig. 2.12.5). When the primary antibody is too concentrated, the product color is a brown, unlike the optimal blue/black color. Background will become white at the optimal concentrations, yielding the maximal signal-to-noise ratio. At very dilute concentrations, the color remains black but drops out of the structures having the lowest antigen concentration. Staining time is held constant at 20 min to guarantee complete staining throughout the section. This feature becomes critical when comparing treatments across animals and for determining differences in antigen amount.
-
25.
Rinse three times, each time for 5 min in 0.175 M sodium acetate, to stop the reaction.
-
26.
Rinse three times, each time for 5 min in KPBS.
-
27.Mount the sections onto subbed glass slides (or Fisher Superfrost Plus electrostatically charged slides) and let them air dry overnight (also see UNIT 1.1).Note that only the electrostatically charged version of Superfrost slide is an alternative to the subbed slides; uncharged slides (Superfrost or others) should be subbed as described in Reagents and Solutions.
-
28.Dehydrate and clear the slide-mounted sections as by immersing in Coplin jars containing the indicated reagents, as follows:
- 5 min in distilled water
- 5 min in 50% ethanol
- 5 min in 70% ethanol
- 5 min in 95% ethanol
- 5 min in fresh 95% ethanol
- 10 min in absolute ethanol
- 10 min in fresh absolute ethanol
- 10 min in either xylene or Histoclear (for clearing)
- 10 min in fresh xylene or Histoclear.If the solution turns cloudy and does not clear immediately, there is too much water in the absolute ethanol. Repeat the absolute ethanol dehydrations with fresh absolute ethanol. If the tissue is not transparent when placed in the xylene or Histoclear, then repeat the 100%, 95% and 70% ethanol incubations with fresh alcohol solutions and clear again with fresh xylene or Histoclear, using longer times for each step.
-
29.
Overlay the tissue with an adequate amount of mounting medium and cover with coverslip.
-
30.Let the slides air dry. Observe slide-mounted sections using a light microscope. Determine the optimum antibody concentration (the one that produces strong signals with low noise).In other words, the best concentration is that one in which cells are black and the background is absent.
ALTERNATE PROTOCOL 1
IMMUNOHISTOCHEMISTRY USING ENZYMATIC PEROXIDE GENERATION WITH GLUCOSE AND A GLUCOSE OXIDASE CHROMOGEN
The ICC staining in Basic Protocol 1 involves a chemical reaction between horseradish peroxidase conjugated to the avidin-biotin complex (ABC Kit), a chromogen (DAB), and hydrogen peroxide. This protocol employs a variation in the chromogen solution using glucose oxidase as an enzyme that catalyzes the oxidation of β-D-glucose to D-gluconic acid, thus generating hydrogen peroxide. As such, this protocol is somewhat more complicated, but yields lower background, which is a significant advantage when the background signal is high upon initial testing. However, it does not affect results using antibodies that already generate high signal-to-noise ratios.
Additional Materials (also see Basic Protocol 1)
β-D (+)-glucose (C6H12O6)
3,3′-diaminobenzidine tetrahydrochloride (DAB; Fluka, cat. no. 32750)
Nickel (II) sulfate hexahydrate (NiSO4·6H2O; Sigma, cat. no. N-4882)
Sodium acetate–imidizole solution (see recipe)
7.5 U/ml glucose oxidase (Sigma, cat. no. G-0543)
Follow Basic Protocol 1, modifying only the chromogen solution in step 24.
-
24a.Prepare the chromogen solution by dissolving 20 mg of β-D-glucose, 2 mg of DAB, and 250 mg of nickel (II) sulfate hexahydrate in 10 ml of sodium acetate-imidazole solution. Add 1.5 μl of glucose oxidase and, as quickly as possible, mix, and incubate the sections in this solution for 20 min at room temperature.Immediately after addition of the glucose oxidase, it begins to oxidize the glucose, supplying the solution with hydrogen peroxide. It is therefore very important to incubate the sections immediately after addition of the glucose oxidase.
ALTERNATE PROTOCOL 2
FIXATION OF BRAIN TISSUE USING BUFFERED 4% PARAFORMALDEHYDE WITHOUT ACROLEIN
Basic Protocol 1 describes the antibody titration procedure using brain tissue fixed in 4% paraformaldehyde plus 2.5% acrolein. Although this fixative provides a number of advantages over more common fixatives (e.g., better antigen retention and optimal signal-to-noise ratios), which is a decisive factor for achieving high-quality ICC staining, it has a number of disadvantages. Acrolein is expensive and very toxic, and requires a tested fume hood. In some countries, a specific license for its use is required, or the compound may not be available for purchase. Fixation with other solutions is effective but can result in higher background staining that must be controlled. This alternate protocol describes ICC using brain tissue fixed in a buffered paraformaldehyde fixative without acrolein, made in the same buffer solution as the acrolein-containing fixative.
Additional Materials (also see Basic Protocol 1)
4% paraformaldehyde in phosphate buffer pH 6.8 (see recipe for 4% paraformaldehyde plus 2.5% acrolein, but do not add the acrolein)
Perform steps 1 to 6 of Basic Protocol 1.
In step 7, switch the pump to the paraformaldehyde fixative solution without acrolein (at room temperature) and slow the rate to 15 to 18 ml/min.
- Perfuse with 400 ml of fixative (at room temperature).Note that the animal will become hard.
- Remove the brain from the skull and immerse it in the same fixative for 2 to 48 hr at 4°C.The length of post-fixation required will depend on the antigen being examined. For most antigens, post-fixation overnight is adequate.
Change solution to cold 30% sucrose until it sinks as described in step 9 of the Basic Protocol 1. Keep refrigerated until cutting is initiated.
Cut the brain in 12 series of 25- to 50-μm sections using a sliding microtome (freezing microtome) or a cryostat. Place the sections immediately into antifreeze cryoprotectant to prevent freezing during tissue section storage.
Proceed with step 11 and 12 of Basic Protocol 1.
Skip steps 13 and 14 of Basic Protocol 1.
Follow steps 15 to 30 of Basic Protocol 1.
ALTERNATE PROTOCOL 3
FIXATION OF BRAIN TISSUE USING BORATE BUFFER (pH 9.5) WITHOUT ACROLEIN
This alternate protocol describes ICC using brain tissue fixed in a buffered paraformaldehyde fixative without acrolein using borate buffer at pH 9.5. Procedural modifications are described to reduce background and nonspecific labeling.
Additional Materials (also see Basic Protocol 1)
0.9% (w/v) NaCl at 4°C (not room temperature)
4% paraformaldehyde in borate buffer, pH 9.5 (see recipe), 4°C
20% sucrose diluted in 4% paraformaldehyde in borate buffer, 4°C
20% sucrose diluted in 0.05 M KPBS, 4°C
Normal serum from the same host as the biotinylated secondary antibody
Perform steps 1 to 5 of Basic Protocol 1.
In step 6, use cold 0.9% NaCl for the perfusion (omit nitrite).
- Perfuse with 900 ml of fixative (cold 4% paraformaldehyde in borate buffer, pH 9.5).Note that the animal will become hard.
- Remove the brain from the skull and immerse it in the post-fixation solution (20% sucrose diluted in 4% paraformaldehyde/borate buffer) for 2 hr at 4°C.Post-fixation of tissue blocks for at least 2 hr is necessary to improve fixation. The addition of sucrose to the fixative reduces the time needed to fully infiltrate the tissue with sucrose and sink the block prior to cutting with a cryostat or freezing microtome. Sucrose infiltration is not necessary if a Vibratome will be used to section the tissue.
Change solution to 20% sucrose in 0.05 M KPBS (no paraformaldehyde) and keep overnight at 4°C until the brain sinks in this solution (overnight or longer, if necessary).
Cut the brain in 12 series of 25- to 50-μm sections using a sliding microtome (freezing microtome) or a cryostat.
Place the sections immediately into antifreeze cryoprotectant to prevent freezing during tissue section storage.
Proceed starting at step 11 of Basic Protocol 1, modifying the following steps as described below.
- Do not perform the sodium borohydride incubation described in step 13 of Basic Protocol 1.Free aldehydes are not significantly present in tissue fixed only with buffered paraformaldehyde fixative.
- In step 15 of Basic Protocol 1, incubate the sections in 0.3% hydrogen peroxide diluted in 0.05 M KPBS for 15 min.Fixing the tissue in basic solutions of 4% paraformaldehyde (without acrolein) does not eliminate all the endogenous peroxidases present in the fresh tissue in macrophages and blood cells. The use of hydrogen peroxide neutralizes it, avoiding the emergence of background.
- In step 16 of Basic Protocol 1, add the appropriate normal serum (from the same host as the biotinylated secondary antibody) at a concentration of 3% (v/v) to the primary antibody solution.The normal serum is obtained from nonimmunized healthy animals and it is used as a blocking agent to reduce background from nonspecific binding of the secondary antibody.
In step 18 of Basic Protocol 1, dilute the biotinylated secondary antibody to 1:800. Rinse as described in step 19.
In step 20 of Basic Protocol 1, add only 30 μl of solution A and 30 μl of solution B in 10 ml, to reduce background.
Follow steps 21 to 30 in Basic Protocol 1.
ALTERNATE PROTOCOL 4
EGG YOLK/GELATIN EMBEDDING OF BRAIN TISSUE FOR IMMUNOHISTOCHEMISTRY
In some instances, tissues are too small or too fragile to be processed freely floating. In particular, when small pieces of the brain like the olfactory bulbs or ganglia are studied, when fetal tissue is used, or when lesions are placed into the brain, it may be more difficult to process the tissue. In such instances, having the tissue embedded in a matrix enables optimal staining. The use of traditional embedding with paraffin or water-soluble waxes does not permit unmounted tissue to be processed, and, in addition, the dehydration and heating that is required for wax embedding can extract the antigens from the tissue. Use of egg yolk/gelatin obviates these problems.
Additional Materials (also see Basic Protocol 1)
12% and 6% gelatin (see recipe), prepared fresh
Fresh eggs at room temperature (remove from refrigerator several hours before use)
Peel-A-Way molds (Ted Pella, cat. no. 27116 for small tissues, or 27110 for larger samples)
40°C water bath
Whatman no. 1 filter paper
Pin
Smooth-tipped forceps
Prepare tissue
Perfuse/fix tissue and post-fix as required for the application (see Basic Protocol 1 and Alternate Protocols 2 and 3). Sink tissue in sucrose as described in step 9 of Basic Protocol 1.
Several hours before embedding, rinse the tissue through several changes of 0.05 M KPBS.
Embed tissue in egg yolk/gelatin
-
3.
Freshly prepare 12% and 6% gelatin as described in Reagents and Solutions.
-
4.Pour a small amount of 12% gelatin into the bottom of each mold (~0.5 ml) and place on a flat surface at room temperature to harden. For each of four samples, use ~2 ml of the 12% gelatin for making the base of clear gelatin at the bottom of each mold.The hardened layer will be the first part cut and allows the investigator to see where the tissue is located.
-
5.Incubate the brain in 6% gelatin for 5 min at 40°C, then gently place in warm 12% gelatin in the oven for 5 min at 40°C to completely coat the tissue with 12% gelatin.At this time, prepare the egg yolk/gelatin solution as described in the steps below.
-
6.Separate the egg yolk from the white by carefully cracking the egg open and pouring the egg back and forth (as when cooking) in the two egg shell halves, being careful not to break the yolk on the sharp edges.One egg will provide ~10 ml of yolk and thus produces 50 ml of egg yolk/gelatin mixture.
-
7.Carefully place the intact egg yolk onto a piece of filter paper (Whatman no. 1) and tip the paper to “pour” the yolk toward the edge. Pierce the yolk with a pin and let the yolk run into a beaker.This maneuver is designed to allow the yolk to be harvested with no contamination by the white, a procedure essential for use with ABC staining. If yolk should break prematurely or egg white should get into the yolk, discard and start over.
-
8.Without introducing any bubbles, quickly mix 1 part egg yolk and 4 parts of 12% gelatin in the beaker on a warming plate, and, when thoroughly mixed, use immediately.The left-over mixture of egg yolk/gelatin can be stored up to 2 to 4 weeks at −20°C.
-
9.
Remove tissue from 12% gelatin using smooth-tipped forceps and place onto the hardened base in the mold.
-
10.
Apply egg yolk/gelatin mixture from step 8 around the tissue with a disposable transfer pipet, taking care to avoid trapping air bubbles. Cover the tissue entirely with the egg yolk/gelatin mixture.
-
11.
Let harden at room temperature for 1 hr.
Fix and section tissue
-
12.
Place into 4% paraformaldehyde (in potassium phosphate buffer, pH 6.8, no acrolein or borate buffer; see Reagents and Solutions) overnight at 4°C.
-
13.
Peel off the mold, remove the tissue block, and trim the block to reduce the amount of embedding medium around the specimen.
-
14.
Dilute 100 ml of 4% paraformaldehyde solution with 200 ml distilled water and slowly add 120 g sucrose in small portions, waiting until each addition has dissolved before adding the next. Adjust the final volume to 400 ml with distilled water to achieve a 1% paraformaldehyde solution in 30% sucrose.
-
15.Transfer the embedded tissue block to the 1% paraformaldehyde 30% sucrose solution (prepared in step 14) and incubate for several more days at 4°C.When the tissue has sunk, it is ready to cut. If it cannot be sectioned immediately, place into 30% sucrose as in step 9 of Basic Protocol 1.
-
16.Cut the tissue as described in step 10 of Basic Protocol 1 and store in antifreeze cryoprotectant until staining is performed.Any staining method can be used for the sections.
BASIC PROTOCOL 2
IMMUNOFLUORESCENCE DETECTION USING FLUOROPHORE-TAGGED SECONDARY ANTIBODIES
This protocol outlines an immunofluorescence method with low amplification, but which is very useful for double immunofluorescence labeling. In this protocol, the concentration of the primary antibody must be 20- to 100-fold greater than that used in the NiDAB immunocytochemistry.
Materials
Brain sections as obtained in Basic Protocol 1, steps 1 to 15
Primary antibody
Fluorophore-conjugated secondary antibody
Additional reagents and equipment for titration of antibodies using immunocytochemistry (Basic Protocol 1)
Day 1
Incubate sections (obtained as in Basic Protocols 1 to 15) with primary antibody as described in step 16 of Basic Protocol 1 using 10×, 30×, and 100× greater concentrations of primary antibody than were determined in Basic Protocol 1.
48 hours later
-
2.
Rinse 10 times over 60 min in 0.05 M KPBS.
-
3.
Incubate in fluorophore-conjugated secondary antibody diluted 1:200 in 0.05 M KPBS containing 0.4% Triton X-100 at 37°C for 3 hr. Keep out of direct light.
-
4.
Rinse 10 times over 60 min in 0.05 M KPBS, continuing to avoid direct light.
-
5.
Mount the sections onto subbed glass slides and let them air dry overnight out of direct light.
-
6.
Dehydrate through ascending ethanol series (see step 28 of Basic Protocol 1), moving more rapidly at the lower concentrations (2-3 dips at 50% and 70%; ~2 min each in the 95% and then 5-10 min in absolute ethanol), clear in xylenes, and coverslip the slides as described in steps 28 to 30 of Basic Protocol 1.
BASIC PROTOCOL 3
IMMUNOFLUORESCENCE USING BIOTIN-TAGGED SECONDARY ANTIBODIES AND STREPTAVIDIN-CONJUGATED FLUOROPHORES
This protocol outlines an immunofluorescence method with some amplification using biotin-tagged secondary antibodies and fluorophore-conjugated streptavidin. In this protocol, the concentration of the primary antibody must be 10- to 30-fold more concentrated than what is used for NiDAB immunocytochemistry (Basic Protocol 1).
Materials
Brain sections as obtained in Basic Protocol 1, steps 1 to 15
Primary antibody
Biotinylated secondary antibody
Fluorophore-conjugated streptavidin
Additional reagents and equipment for titration of antibodies using immunocytochemistry (Basic Protocol 1)
Day 1
Incubate sections with primary antibody as described in step 16 of Basic Protocol 1 using a 20-fold more concentrated primary antibody than was determined in Basic Protocol 1.
48 hours later
-
2.
Rinse 10 times over 60 min in 0.05 M KPBS.
-
3.
Incubate sections with biotinylated secondary antibody diluted 1:600 in 0.05 M KPBS containing 0.4% Triton X-100 for 1 hr at room temperature.
-
4.
Rinse 10 times over 60 min in 0.05 M KPBS.
-
5.
Incubate with fluorophore-conjugated streptavidin diluted 1:200 in KPBS containing 0.4% Triton-X for 2 to 3 hr at 37°C. Keep out of direct light.
-
6.
Rinse 10 times over 60 min in 0.05 M KPBS, continuing to avoid direct light.
-
7.
Mount the sections onto subbed glass slides and let them air dry overnight out of direct light.
-
8.
Dehydrate through ascending ethanol series, clear in xylenes, and coverslip the slides as described in step 6 of Basic Protocol 2.
BASIC PROTOCOL 4
BIOTINYLATED TYRAMINE AMPLIFICATION
This protocol outlines an immunofluorescence method using biotinylated tyramine amplification, which enables use of the primary antibody at a concentration only 2-fold greater than is used in the NiDAB immunocytochemistry (Basic Protocol 1). It is the most sensitive of the fluorescence methods. Biotinylated tyramine was described for ICC by Adams (1992) and later adapted for fluorescence by Berghorn et al. (1994).
Materials
Brain sections as obtained in Basic Protocol 1, steps 1 to 15
Primary antibody
Biotinylated secondary antibody
Vectastain Elite ABC Kit (Standard; Vector Laboratories, cat. no. PK-6100) including solution A and Solution B
Biotinylated tyramine produced as outlined in Adams (1992). This reagent can be purchased from a number of suppliers (such as ThermoFisher Scientific) but concentrations must be determined empirically.
3% H2O2
Fluorophore-conjugated streptavidin
Additional reagents and equipment for titration of antibodies using immunocytochemistry (Basic Protocol 1)
Day 1
Incubate sections with primary antibody as described in step 16 of Basic Protocol 1 using a 2-fold more concentrated primary antibody than was determined in Basic Protocol 1.
48 hours later
-
2.
Rinse 10 times over 60 min in 0.05 M KPBS.
-
3.Incubate sections with biotinylated secondary antibody diluted 1:5000 in 0.05 M KPBS containing 0.4% Triton X-100 for 1 hr at room temperature.This antibody must be made against the host of the primary antibody. Note that it is a very dilute solution.
-
4.
Rinse 8 times in 0.05 M KPBS over 45 min.
-
5.At the beginning of the KPBS rinses, prepare the A/B solution (from Vectastain Elite ABC Kit, Standard) by combining 11.25 μl of solution A and 11.25 μl of solution B in 10 ml of KPBS containing 0.4% Triton-X. Let stand at least 30 min before use.Note this is one-fourth the amount normally used for ICC. Recent samples of the ABC reagents have produced intermittent variation in the “correct” concentration to be used. If problems with the stated concentrations do not enable optimal staining, refer to the section on Trouble Shooting: Secondary antibody and ABC reagents
-
6.
Incubate sections in A/B solution for 45 min at room temperature.
-
7.
Prepare the next solution (0.5% biotinylated tyramine plus 0.005% H2O2): add 50 μl of biotinylated tyramine (Berghorn et. al 1994) and 16.6 μl of 3% H2O2 to 10 ml of 0.05 M KPBS.
-
8.
Rinse 8 times in 0.05 M KPBS over 45 min.
-
9.
Incubate sections in 0.5% biotinylated tyramine plus 0.005% H2O2 for 20 min at room temperature.
-
10.
Rinse 8 times in 0.05 M KPBS over 45 min.
-
11.Incubate sections in fluorophore-conjugated streptavidin diluted 1:200 in KPBS containing 0.4% Triton-X at 37°C for 3 hr. Keep out of direct light.Selection of the fluorophore for this step can be important if double labeling is going to be performed after the amplification and if standard fluorescence microscopes are used. When the TSA amplification is used with fluorophores that emit in the green range (around 500 to 550 nm; e.g., Cy-2, Oregon Green, or Alexa 488), the concentrations of the fluorophore may be sufficient to display excitation that extends to that of the red fluorophore (and produces false positives). Thus, the products of this first reaction must be checked for bleed-through into the red channels. Substituting the red fluorophores for green-emitting fluorophores in the amplification step obviates this problem.
-
12.
Rinse 8 times over 45 min in 0.05 M KPBS. Keep out of direct light.
-
13.
Mount the sections onto subbed glass slides and let them air dry overnight. Keep out of direct light.
-
14.Dehydrate, clear, and coverslip the slides as described in step 6- of Basic Protocol 2.This procedure can be adapted for chromagen assays by substituting A/B reagent in step 10 in place of fluorophore-conjugated streptavidin. 11.25μl A and 11.2μl B in 10 ml of PBS are used for the incubation for 3 hr at 37°C. Development of product then uses steps 22-30 of Basic protocol 1. Use of TSA provides comparable staining at a level of primary antibody 3-5 times more dilute than was found with conventional ABC processing.
BASIC PROTOCOL 5
IMMUNOHISTOCHEMISTRY USING THE PELCO BIOWAVE PRO® HISTOLOGICAL MICROWAVE WITH STEADYTEMP™ RECIRCULATING WATER BATH
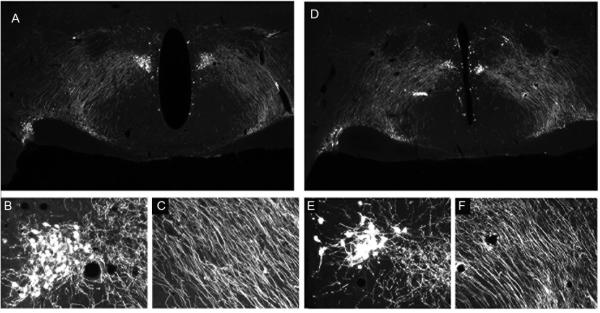
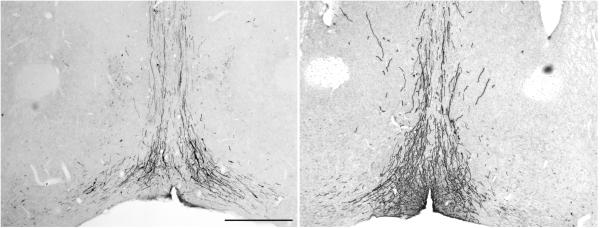
This protocol outlines method that can be adapted to any of the staining protocols noted above for either chromogen or immunofluorescent staining. Earlier publication of this methodology on freely floating material (Munoz et al. 2004; Ferris et al, 2009), while producing high quality staining in < 1.5 hr., required prohibitively high concentrations of primary antibody, thereby making the approach unduly expensive. Our laboratory devised a modification of the Ferris microwave protocol wherein incubation of the samples with the primary antibody is conducted in the conventional way, and processing of sections in the BioWave oven was performed with increased incubation times of secondary antibodies and ABC reagents or fluorescent tagged approaches in order to retain method sensitivity comparable to the basic bench protocols. A clear advantage of the BioWave oven is that the user has the ability to set power level, temperature, and time and generate programs for each protocol. A stage, which has a temperature-controlled water circulating through it, ensures that sample heating is constant thereby eliminating the hot spots of conventional microwave ovens. We have programmed our microwave with a series of step-specific MW Programs in order to be able to turn the microwave off during the 48-hour incubation of the primary antibody, as well as to allow us to mix-and-match the protocols to fit the needs of our experiment. An overview of setting up the BioWave unit for both manual and MW Programs, as well as a decision tree demonstrating choices of protocols to use to perform an experiment, is included in Figure 2.12.10 at the end of this protocol. Figure 2.12.11 shows an example of a section of rat brain stained for one of the same antigens presented in the Munoz and Ferris studies (GFAP). Note that very strong immunoreactivity is seen despite the fact that the primary antibody concentration is 70 times more dilute than the other studies used. Figure 2.12.12 shows an example of fluorescent detection using the same primary antibody and using TSA to increase signal strength employing either the conventional (Basic Protocol 4) or microwave-assisted (Basic Protocol 8) methodology, and Figure 2.12.13 demonstrates the amplification of signal strength achieved using TSA (Basic Protocol 8) over regular ABC peroxidase (Basic Protocol 5) detection in microwave-assisted immunocytochemistry.
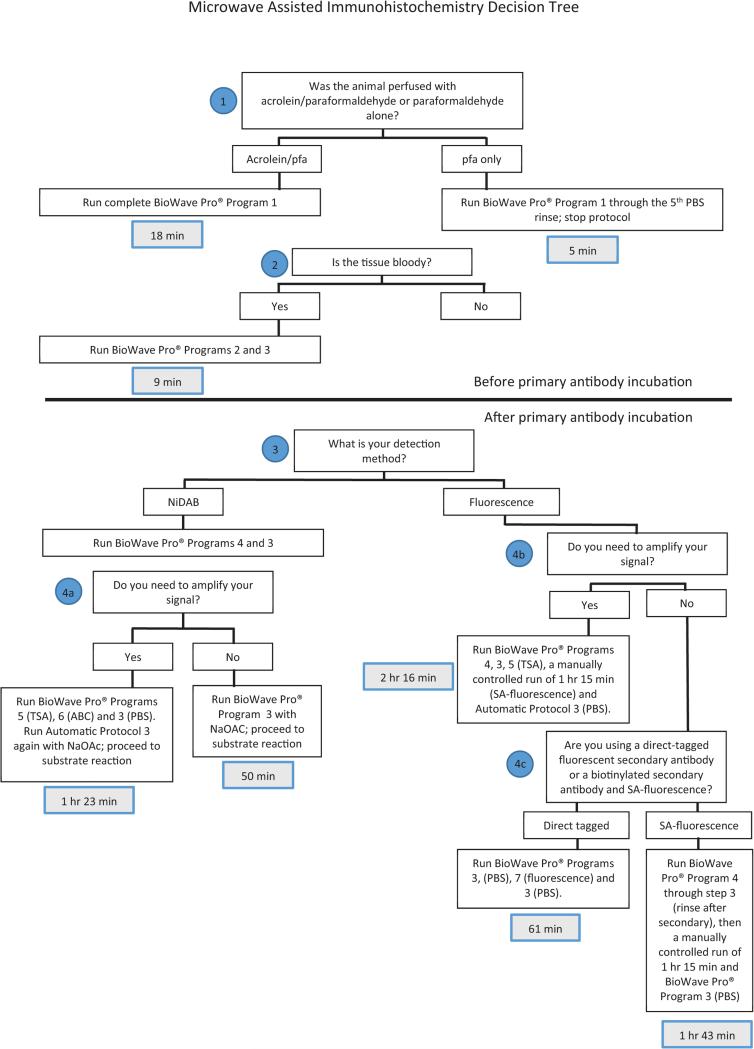
Figure 2.12.10.
Decision tree for application of MW-assisted Immunocytochemistry. Included in this flow-chart are times of processing for comparison with those of the conventional methods.
Figure 2.12.11.
Example of a section of rat brain stained for glial fibrillary acidic protein (GFAP) with a polyclonal GFAP antibody from DAKO (catalog # Z0334) used at a concentration of 1:70,000 with Basic MW Protocol 5 for ABC immunoperoxidase staining. Note that a similar primary antibody against GFAP from DAKO used in the Munoz publication with the BIOWAVE oven required a concentration of 1:100.
Figure 2.12.12.
Comparison of fluorescent detection using the conventional method/TSA (Basic Protocol 4) and microwave-assisted ICC/TSA (Basic Protocol 8).
Figure 2.12.13.
Comparison of NiDAB detection using microwave-assisted immunocytochemistry with and without TSA (Basic Protocols 5 and 8).
Additional Materials
Brain sections as obtained in Basic Protocol 1, steps 1 to 15
Primary antibody
Biotinylated secondary antibody
Vectastain Elite ABC Kit (Standard; Vector Laboratories, kcat. No. PK-6100) including solution A and Solution B
BioWave® Pro Laboratory Microwave System (Ted Pella Instruments, cat. no. 36500)
PELCO SteadyTemp™ Pro Digital Thermoelectric Recirculator (Ted Pella Instruments cat. no. 50062)
5 mL plastic beaker cups (VWR, cat. no. 13915-985)
Nets to fit inside beaker cups (Costar, cat. no. 3478)
Holder/tray for beaker cups
Paint brushes of various sizes
Transfer pipets (Fisher, cat. no. 13-711-7M)
Perform microwave (MW)-assisted immunohistochemistry
Day 1
Prepare the microwave by immersing the temperature probe inside the oven in a beaker cup filled with water and turning on the SteadyTemp™ digital water bath and warming it up to 30°C. Once the water bath has reached that temperature, use the manual protocol setting (see the section on programming the BioWave unit at the end of this protocol) to turn on the BioWave unit for at least 20 minutes at 35°C using a power level of 150 watts to bring the water-filled stage and interior of the microwave to temperature.
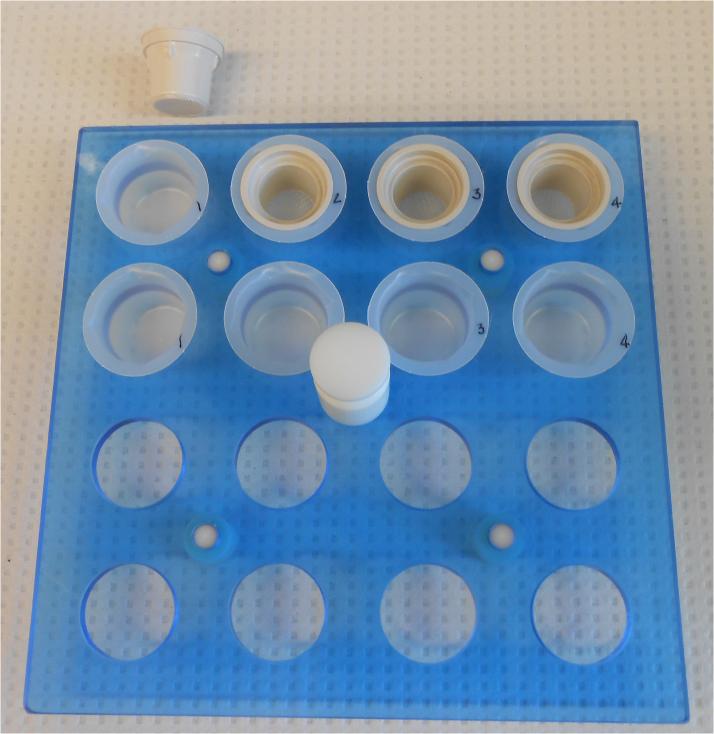
- Place nets into cups (in an IHC tray or holder) that will receive the sections and add approximately 3 ml/cup PBS using a transfer pipet. Label a matching second set of cups, place them in the holder and add PBS. (See Figure 2.12.14)The nets are used only during wash steps in order to quickly manually transfer all of the sections with minimal manipulation between microwave rinse steps. Two cups are used for each set of sections in order for the solution to prewarm before the sections are placed into it.
Remove sections from the antifreeze solution and place them into a large petri dish filled with PBS at room temperature for sorting (as in Basic Protocol step 11). Using a paintbrush, transfer sections into a cup with a net (maximum of 15 sections of rat/20 sections of mouse brain).
- Rinse the antifreeze solution out of the sections, treat them with NaBH4 and rinse by running MW Program 1 in the microwave. This step replaces steps 12 – 14 in Basic Protocol 1.
Summary of MW Program 1 Rinsing out cryoprotectant and NaBH4 treatment Microwave Settings Time Wattage 1. PBS rinse 5 × 1 mina, c 150 2. NaBH4 treatment 8 minb 150 3. PBS rinse 5 × 1 mina 150 aUnit will pause between each rinse. Move the net to the fresh buffer, pour out the old buffer and replace with fresh buffer so that it can prewarm. After the fourth rinse, if the tissue was fixed with acrolein add the NaBH4 to the cups so that it can prewarm.b3 min on, 2 min off, 3 min oncIf sections were fixed in 4% paraformaldehyde only, end the program after the first five rinses and proceed to Step 5 (if tissue is bloody) or 6.Whenever the sections are to be placed into a solution other than a rinse buffer, they are washed out of the net and into the cup containing, e.g., NaBH4. This is accomplished by aspirating the warmed solution with a transfer pipet, picking up the net containing the sections and inverting it over the cup that the sections will be washed into, and squirting the warmed solutions through the bottom of the net in order to wash the sections into the cup. It is most effective if the cup is held at a 45° angle to the top of the cup, and the tip of the transfer pipet placed against the highest edge of the bottom of the net so that the sections can be washed out at an oblique angle. Any sections remaining inside the cup can be picked up with a paintbrush and transferred to the new solution. Alternatively, the sections can all be moved over to one side of the net with a paintbrush, picked up, and manually moved to the cup containing the new solution.It is crucial to rinse off as much of the new solution from the nets (or paintbrush) before placing them back into the rinse buffer. After washing the sections out of the net, drop the net into a medium-sized weigh boat containing approximately 80 mL of PBS in order to rinse off the NaBH4 and to ensure that all sections have transferred (any sections that were missed will float in the PBS and can be easily picked up with a brush and transferred).To transfer the sections back into the net for the second set of washes, pick up the net and, holding it over a waste container, pour the solution containing the sections through the net; rinse the cup 2 – 3 times with PBS, pouring the PBS through the net each time, to rinse the cup and ensure that all of the sections are in the net. Place the now-empty cup back into the tray/holder, fill the cup with buffer, and proceed with the rinses. After the NaBH4 step the sections will be very bunched up; use the transfer pipet to “puff” or stir the sections with a little extra PBS to swirl them at the beginning of each rinse. Jiggling the net using forceps may also help to separate the sections and allow them to rinse properly.While it is available on the BioWave, we do not use vacuum on any of our immunohistochemistry protocols. - If blood is present in the tissue, incubate the tissues in 1% hydrogen peroxide in PBS for 6-min (MW Program 2) followed by three 1-min washes in PBS (MW Program 3). If perfusion left no blood in the tissue skip this step and move to step 6.
Summary of MW Program 2 1% H2O2 Treatment for bloody sections Microwave Settings Time Wattage 1. H2O2 6 mina 150 a2 min on, 2 min off, 2 min onRinse the sections out of the nets and into cups containing warmed hydrogen peroxide as in step 4. The microwave will beep as the power cycles on and off during protocol 2, but no action will need to be taken until it is finished. At that point, pour the sections back into the nets and wash them three times in PBS.aNote in a later step, sodium acetate will be used with this program prior to incubation with NiDAB. In this step, only PBS is used.bUnit will pause between each rinse to allow moving the nets between cups - Wash the sections out of the nets into fresh cups by squirting primary antibody diluted in PBS/0.4% Triton X-100 prepared as in step 16 of Basic Protocol 1 (1.5 mL/cup) through the bottom of the nets; always start with the highest dilution of antibody, moving toward the highest concentration if you are performing a titration series, and place the net in rinse buffer immediately after tissue transfer. Incubate the sections for 1 hour at room temperature on a rotator at 55 rpm. Transfer the tray with the cups to a refrigerator and incubate the primary antibody for 48 hours at 4°C. Agitation is in the refrigerator is unnecessary provided the sections are well separated in the cups. [AU: should the sections be agitated?]It is crucial that the nets not be allowed to dry out at any time, and that fresh nets are used for the subsequent incubation steps for the detection reagents. The nets should be washed with dish detergent after each experiment. Alternatively, if you have a relatively small number of primary antibodies, you can label a set of nets and dedicate them for use with only one primary.
Figure 2.12.14.
Typical tray set up for microwave-assisted immunocytochemistry, demonstrating the duplicate beaker cups for pre-warming solutions and the nets used to transfer section between cups during rinse steps.
48 hours later
-
7.
Prepare the microwave as in step 1
-
8Pour the sections out of the cup and into fresh nets. Run MW Program 4 to expose tissue to the AB reagents. This step replaces steps 17 – 21 in Basic Protocol 1.
Summary of MW Program 4 Rinsing, secondary antibody and ABC Microwave Settings Time Wattage 1. PBS rinse 3 × 1 mina 150 2. Secondary antibody 19 minb 150 3. PBS rinse 3 × 1 mina 150 4. A/B reagent 19 minb 150 aUnit will pause between each rinse to allow moving the nets between cupsb5 min on, 2 min off, 5 min on, 2 min off, 5 min onThe secondary antibody and the A/B reagent are prepared as in Basic Protocol 1, but it is important to hold them at room temperature (rather than on ice) before they are applied to the sections. As in step 4, transfer the sections in the nets during the wash steps and wash them out of the nets and into the buckets for the secondary antibody and ABC incubations.Make the secondary antibody dilution(s) and A/B reagent while the microwave is warming up so that the ABC has time to form complexes before being applied to the sections -
9Pour the sections out of the cup and back into nets. Run Protocol 3 (three 1-min washes) twice, the first time rinsing with PBS and the second time equilibrating the tissues with 0.175M sodium acetate. This step replaces steps 22 and 23 of Basic Protocol 1.
Summary of MW Program 3 Rinsing with buffer Microwave Settings Time Wattage 1. PBS or NaOAc 3 × 1 mina 150 aUnit will pause between each rinse to allow moving the nets between cups -
10.
Prepare the NiDAB chromagen solution as in step 24 of Basic Protocol 1. Wash the sections out of the nets into fresh cups by squirting NiDAB substrate solution through the bottom of the nets and incubate the sections in this solution for 20 min.
-
11.
Proceed starting at step 25 of Basic Protocol 1. The sections can be poured back into the nets to move them between rinses, or simply use a paintbrush as in Basic Protocol 1.
BASIC PROTOCOL 6
MICROWAVE-ASSISTED IMMUNOFLUORESCENCE DETECTION USING FLUOROPHORE-TAGGED SECONDARY ANTIBODIES
This protocol outlines the use of the BioWave Pro® Histological Microwave to perform immunofluorescence detection. The signal obtained in the microwave is equivalent to that obtained using Basic Protocol 2, so the concentration of the primary antibody must be 20- to 100-fold greater than that used in the NiDAB immunocytochemistry.
Materials
Brain sections are obtained as in Basic Protocol 1, steps 1 to 10
Primary antibody
Fluorophore-conjugated secondary antibody
Additional reagents and equipment for titration of antibodies using microwave-assisted immunocytochemistry (Basic Protocol 5)
Day 1
Rinse tissue and incubate sections with primary antibody as described in steps 1 - 6 of Basic Protocol 5.
48 hours later
-
2.
Prepare the microwave as in step 1 of Basic Protocol 5.
-
3Pour the sections out of the cup and into fresh nets. Run MW Program 3 to rinse the primary antibody off of the sections
Summary of MW Program 3: Rinsing with buffer Microwave Settings Time Wattage 1. PBS 3 × 1 mina 150 aUnit will pause between each rinse to allow moving the nets between cups -
4Wash the sections out of the nets into fresh cups to label the sections with a direct-tagged secondary antibody in MW Program 7
Summary of MW Program 7: Direct-tagged fluorescent antibodies Microwave Settings Time Wattage 1. Secondary antibody 55 mina 150 a10 min on, 5 min off, 10 min on, 5 min off, 10 min on, 5 min off, 10 min on -
5Pour the sections out of the cup and back into nets. Run MW Protocol 3 with PBS.
Summary of MW Program 3: Rinsing with buffer Microwave Settings Time Wattage 1. PBS 3 × 1 mina 150 aUnit will pause between each rinse to allow moving the nets between cups -
6.
Proceed as in steps 5 and 6 of Basic Protocol 2.
BASIC PROTOCOL 7
MICROWAVE-ASSISTED IMMUNOFLUORESCENCE DETECTION USING BIOTIN-TAGGED SECONDARY ANTIBODIES AND STREPTAVIDIN-CONJUGATED FLUOROPHORES
This protocol outlines an immunofluorescence method with some amplification using biotin-tagged secondary antibodies and fluorophore-conjugated streptavidin. In this protocol, the concentration of the primary antibody must be 10- to 30-times more concentrated than what is used for microwave-assisted NiDAB immunocytochemistry (Basic Protocol 5).
Materials
Brain sections as obtained in Basic Protocol, steps 1 to 15
Primary antibody
Biotinylated secondary antibody
Fluorophore-conjugated streptavidin
Additional reagents and equipment for titration of antibodies using microwave-assisted immunocytochemistry (Basic Protocol 5)
Day 1
Incubate sections with primary antibody as described in steps 1 to 6 of Basic Protocol 5.
48 hours later
-
2.
Prepare the microwave as in step 1 of Basic Protocol 5.
-
3Pour the sections out of the cup and into fresh nets. Run MW Program 4 through the end of step 3 (rinses after the secondary antibody) to label the sections with a biotinylated secondary antibody.
Summary of MW Program 4 Rinsing, secondary antibody and ABC Microwave Settings Time Wattage 1. PBS rinse 3 × 1 mina 150 2. Secondary antibody 19 minb 150 3. PBS rinse 3 × 1 mina, c 150 4. A/B reagent 19 minb 150 aUnit will pause between each rinse to allow moving the nets between cupsb5 min on, 2 min off, 5 min on, 2 min off, 5 min oncEnd the program after this stepNote: step 4 is grayed out; the investigator will terminate the program after step 3. -
4.
Wash the sections out of the net and into cups containing fluorophore-conjugated streptavidin. Set the BioWave manually to run for 1 hr 15 min (temperature 35°C; power 150W).
-
5Pour the sections out of the cup and back into nets. Run MW Program 3 with PBS.
Summary of MW Program 3: Rinsing with buffer Microwave Settings Time Wattage 1. PBS 3 × 1 mina 150 aUnit will pause between each rinse to allow moving the nets between cups -
6.
Proceed as in steps 5 and 6 of Basic Protocol 2.
BASIC PROTOCOL 8
MICROWAVE-ASSISTED BIOTINYLATED TYRAMINE AMPLIFICATION
This protocol outlines a method that can be used with either NiDAB or immunofluorescence to enable the use of primary antibody at a higher dilution and to amplify signal to increase sensitivity of detection. Two options are given after the amplification step: a second reaction with A/B reagent for NiDAB detection, or incubation with streptavidin-fluorophore for fluorescent detection.
Day 1
Incubate sections with primary antibody as described in steps 1 – 6 of Basic Protocol 5.
48 hours later
-
2.
Prepare the microwave as in step 1 of Basic Protocol 5.
-
3Pour the sections out of the cup and into fresh nets. Run MW Program 4
Summary of MW Program 4 Rinsing, secondary antibody and ABC Microwave Settings Time Wattage 1. PBS rinse 3 × 1 mina 150 2. Secondary antibody 19 minb 150 3. PBS rinse 3 × 1 mina 150 4. A/B reagent 19 minb 150 aUnit will pause between each rinse to allow moving the nets between cupsb5 min on, 2 min off, 5 min on, 2 min off, 5 min onFor Fluorescence: The secondary antibody is prepared at a concentration of 1:2000-1:5000; the A/B complex reagents for this step are prepared at normal strength (45 μl each of A and B per 10 mL PBS/0.4% Triton X-100) but with some ABC kits, if quenching occurs, the A and B reagents will need to be diluted to 25% of normal . It is important to hold reagents at room temperature (rather than on ice) before they are applied to the sections. As in step 8 of Basic Protocol 5, transfer the sections in the nets during the wash steps and wash them out of the nets and into the buckets for the secondary antibody and ABC incubations.For NiDAB staining: Make the secondary antibody dilution(s) and two A/B reagent solutions (normal concentration) while the microwave is warming up so that the A/B has time to form complexes before being applied to the sections. If quenching of stainining is noted and is not eliminated as the primary antibody is reduced, then reduce the A and B reagents to 11.25μl each). The reason for making the A/B reagent more dilute in this step is that, in the authors’ experience, using normal strength A/B can lead to either high levels of background or quenching of product and the kits lately have been variable in their strength. -
4Pour the sections out of the cup and back into nets. Run MW Program 3 (three 1-min washes) with PBS.
Summary of MW Program 3: Rinsing with buffer Microwave Settings Time Wattage 1. PBS 3 × 1 mina 150 aUnit will pause between each rinse to allow moving the nets between cups -
5Wash the sections out of the net and into cups containing biotinylated tyramine (BT) prepared as in step 7 of Basic Protocol 4. Run MW Program 5 (biotinylated tyramine incubation followed by rinsing in PBS).
Summary of MW Program 5: Tyramide Signal Amplification with BT followed by rinsing Microwave Settings Time Wattage 1. BT incubation 7 mina 150 2. PBS rinse 4 × 1 minb 150 a3 min on, 1 min off, 3 min onbUnit will pause between each rinse to allow moving the nets between cups -
6a(NiDAB detection) Wash the sections out of the nets and into cups containing normal strength A/B reagent prepared in step 3. Run MW Program 6 (A/B reagent after TSA).
Summary of MW Program 6: ABC incubation after TSA Microwave Settings Time Wattage 1. A/B reagent 19 mina 150 a5 min on, 2 min off, 5 min on, 2 min off, 5 min on -
6b.
(SA-fluorescent detection) Wash the sections out of the nets and into cups containing the streptavidin-conjugated fluorophore. Run a manually controlled incubation of 1 hr 15 minutes at 35°C/150 watts.
-
7a.
(NiDAB detection) Pour the sections out of the cups and back into nets. Run MW Program 3 twice, first with PBS and then with sodium acetate.
-
7b(SA-fluorescent detection) Pour the sections out of the cups and back into nets. Run MW Program 3 with PBS.
Summary of MW Program 3: Rinsing with buffer Microwave Settings Time Wattage 1. PBS or NaOAc 3 × 1 mina 150 aUnit will pause between each rinse to allow moving the nets between cups -
8a.
(NiDAB detection) Proceed starting at step 10 of Basic Protocol 5.
-
8b.
(SA-fluorescent detection) Proceed starting with step 5 of Basic Protocol 2.
Programming the BioWave Pro® Histological Microwave
The BioWave unit can be programmed using either the touchscreen or through importation of protocols in Excel and transferred using an adaptor. The authors have the manual control set to maintain a temperature of 35°C at 150 watts of power and vacuum at 20 mm Hg. PDFs of MW Programs loaded from Microsoft Excel are presented in Supplemental Materials.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see SUPPLIERS APPENDIX.
Antifreeze cryoprotectant
500 ml 0.1 M sodium phosphate buffer pH 7.2 (APPENDIX 2A)
300 g sucrose
300 ml ethylene glycol
10 g polyvinylpyrrolidone (PVP-40)
Adjust volume to 1000 ml with distilled water
Store indefinitely at 4°C
An unexplained feature of storing sections in antifreeze solutions is that the staining often improves with longer storage times. Use of sections that are processed right after the sections are cut provides weaker and more uneven staining than is seen with the same sections after antifreeze storage. Our recommendation is that sections be kept for a minimum of 5 days in the antifreeze at −20°C before initiating staining.
Gelatin, 12% and 6%
Heat 100 ml distilled water on a heat/stir plate at a setting of low and slowly add small amounts of gelatin (type A, 200 to 300 bloom; Sigma) with stirring, waiting for the gelatin to dissolve between each addition. Use 12 g gelatin for a 12% solution, and 6 g for a 6% solution. Stir slowly (to avoid bubbles) until totally dissolved. Monitor the temperature so as not to exceed 50°C. Keep in an oven at 40°C until use. Prepare fresh.
Imidazole, pH 6.8, 0.2 M
13.62 g imidazole
1000 ml distilled H2O
Adjust pH to 6.8
Store indefinitely at 4°C
KPBS, 0.05%
9 g NaCl
80 ml 0.5 M K2HPO4 (dibasic potassium phosphate)
20 ml 0.5 M KH2PO4 (monobasic potassium phosphate)
Add distilled water to a total volume of 1000 ml
If not in a tropical climate, months of storage at 4°C is fine.
Nickel sulfate stock solution
Prepare a 5% (w/v) solution of nickel (II) sulfate hexahydrate (NiSO4·6H2O; Sigma, cat. no. N4882) in 0.175 M sodium acetate. Store indefinitely at 4°C protected from light. Immediately before preparing chromogen solution, mix with an equal volume of 0.175 M sodium acetate buffer to yield a 2.5% solution.
NiDAB chromogen solution
250 mg nickel (II) sulfate hexahydrate (add from nickel sulfate stock solution, see recipe)
2 mg DAB
10 ml 0.175 M sodium acetate
8.3 μl 3% H2O2 (3% peroxide solutions are more stable than 30% solutions, and can be purchased at most drug stores)
Prepare immediately before use
Some researchers use ammonium nickel (II) sulfate hexahydrate instead of nickel (II) sulfate hexahydrate, but this substitution will slightly increase the background.
The authors do not recommend the use of DAB in tablets.
Some investigators may choose to generate stock DAB solutions Prepare a 2% (w/v) solution (in Milli-Q water) in a hood, making sure that the DAB is completely dissolved (use a vortex and filter if necessary). Aliquot 100 μl per vial (amber vials preferred). Store at −20°C. To prepare the final chromogen solution, thaw at room temperature and add one aliquot to 10 ml of acetate/NiSO4 solution. You can store at −20 °C indefinitely, but never put the aliquot back in the freezer once thawed. The aliquots are exactly the amount needed for one recipe of 10ml.
DAB solutions, whether prepared with nickel or not, should be disposed of as hazardous waste, not with bleach as this creates waste which is even more toxic than DAB/NiDAB alone. The authors have also noted that any bleach present after washing glassware will cause NiDAB to fade over time, therefore glassware and nets used for microwave-assisted immunostaining should be washed in normal detergent rather than lab detergents such as Alconox. The reason for this is that any bleach in contact with tissue (and Alconox behaves like bleach) causes the NiDAB to be reduced and fade.
Paraformaldehyde, 4%, containing acrolein, 2.5%, in potassium phosphate buffer, pH 6.8
Dissolve 40g of paraformaldehyde in 500 ml of distilled water. Stir and heat to 53° to 55°C (do not exceed 58°C).
Add 12 N NaOH dropwise with stirring to clear.
Cool to room temperature.
Add 100 ml of 0.5 M dibasic potassium phosphate, 100 ml of 0.5 M monobasic potassium phosphate, and 300 ml of distilled water. If adding acrolein, reduce this volume to 275 ml.
Filter through Whatman filter paper size 0.
- Check and adjust pH to 6.8 with either 12 N NaOH or 10 N HCl using a pH meter.This fixative solution is stable for 2 months at 4°C in most laboratories. Bring to room temperature before use. In some sites with tropical climates, mold and bacteria can be problematic, so storage of the fixative, even in the refrigerator, is discouraged. The pH of this fixative is slightly more acidic than most investigators use. Solutions of paraformaldehyde buffered to pH 7.4 can be substituted, but may yield more nonspecific background, particularly around blood vessels and around the circumventricular organs (median eminence, organum vasculosum of the lamina terminalis, area postrema, and sub-fornical organ). The fixative can be used as is, or acrolein can be added to improve fixation.
- If using this fixative with added acrolein, just before the perfusion, add 25 ml of acrolein (Polysciences) for each 1000 ml buffered paraformaldehyde solution.After adding acrolein, use the fixative immediately.
Paraformaldehyde, 4%, in borate buffer, pH 9.5
Dissolve 4 g of NaOH in 1000 ml of distilled water.
Add 40 g of paraformaldehyde. Stir and heat to 53° to 55°C (do not exceed 58°C).
Add 38.14 g of sodium tetraborate decahydrate (Na2B4O7·10H2O) and stir.
Store at 4°C overnight.
On the next day, filter through Whatman no. 0 filter paper, then check pH and adjust to 9.5 with 5 N NaOH or 10 N HCl using a pH meter.
- Keep refrigerated until the following day then use.On the day before perfusion, prepare 1000 ml of this fixative for each rat. After preparing the fixative, also prepare the post-fixation solution (20% sucrose in this fixative). The high pH of this fixative preserves the morphology of cell structures, but slows the fixation.
Sodium acetate–imidazole solution
50 ml 0.2 M imidazole, pH 6.8 (see recipe)
175 ml 1.0 M sodium acetate
775 ml distilled H2O
Store indefinitely at 4°C
Subbed glass slides
Standard precleaned microscope slides are subbed by immersing slides for 1 min in a 55°C solution consisting of 0.10% (w/v) gelatin (ovine type B) and 0.01% (w/v) chromic potassium sulfate in distilled water. Subbed slides are allowed to dry thoroughly at room temperature prior to use. They can be returned to boxes and stored indefinitely.
Alternatively, Fisher Superfrost Plus electrostatically charged slides may be used.
COMMENTARY
Critical Parameters and Troubleshooting
The greatest challenge in using immunocytochemistry well is to determine if the antibody has activity and, if so, whether that activity truly identifies the antigen against which the antibody was generated. The method outlined in this unit describes how to test an antibody effectively. In addition, some aspects of the protocols described herein facilitate testing antibodies for future studies. Over the past 35 years, the authors’ laboratory has tested a wide variety of antisera, and the laboratory maintains tissue banks for testing antibodies. In most instances, tissue that will stain well for one antigen will also stain for others. This feature provides a way of testing if the tissue preparation is adequate and reliable. One of the major advantages of using cryoprotectant antifreeze for tissue storage is that sections that worked well for ICC are available years later for testing new antibodies. A good control for the fixation quality and staining reagents is to use stored tissue, react it with a known antibody that worked in the past (generated in the same species as the test antibody), and stain it in parallel with the titration of new tissue and new antibody. If the new tissue reacts poorly (with the known antibody) under those conditions, while the old tissue reacts as expected, then fixation may be the problem. One good example of this is in preparation of paraformaldehyde. If the temperature of the solution exceeds 58°C, the paraformaldehyde can turn to formic acid and destroy the tissue. Also, long-term storage of acrolein, even under refrigerated conditions, can ruin the fixation. Preparation of small amounts that are not stored for more than a few months is best.
If a tested and validated antibody will be used extensively in future studies, it is important that a sufficient amount be purchased to complete all the studies. If a delay is imposed, a second lot of an antibody that has previously worked well may have lost substantial activity. This feature is true for both monoclonal and polyclonal antisera, particularly if titers are low. In attempting to convey this problem to the company from which the serum was purchased, having photographic evidence that an adjacent section stained well and the current one did not is of great benefit.
If the titration is conducted as indicated in this unit, on reliable tissue, and no immunoreactivity is detected, the first thing to check should be whether the most dilute concentration yielded a total absence of both background and specific staining. If the tissue color is still brown, then continue to dilute the antibody. Some recent tests of new antibodies (both monoclonal and polyclonal) reveal optimal titers in the range of 1:2,000,000. Others can be 1:500, so there is no way of predicting what is going to work best. If specific staining is still not observed, then other procedures, such as antigen retrieval (boiling the sections in a citrate buffer), should be pursued. If using antibodies successfully applied by others, obtaining similarly treated, fixed, and processed tissue is the next requirement. Contact the laboratory that originally supplied the antibody and ask if it is still working well for them. If embarking upon new territory for a new antibody, it is important to recognize that there are some antibodies that do not work for ICC, but it is useful to let the supplier know that you have had a problem. If they recommend the antibody for ICC, ask them when the last quality-control check was conducted and how it was performed. Sometimes they may send an alternative batch for you to try. The important thing to remember is that some antisera sold today have no discernible activity. An example of this is shown in Figure 2.12.8, which compares two antisera against the same antigen (green fluorescent protein). When testing antibodies, if two are available from different companies, test both. It is equally important to test new lots of detection reagents to ensure that results remain consistent; before using up the last of an aliquot of secondary or ABC reagent, be sure to run the new reagent in parallel with old reagents before switching completely over to the new ones.
Figure 2.12.8.
Example of titrations of two purchased antisera (both rabbit polyclonal antisera) against green fluorescent protein (GFP). Both are tested in the same tissue. Note that the antiserum used in the series on the left (purchased from Novus) shows specific staining for GFP in neurons of the cortex, whereas only background labeling is found in the series on the right that used the other antiserum. For the color version of this figure go to http://www.currentprotocols.com.
The authors recommend storing tissue fixed with a number of different fixatives to facilitate testing. Fixation can affect the ability of an antigen to be localized. While most laboratories will use only buffered paraformaldehyde solutions, for some antibodies, this fixation may not generate positive or optimal results. The reasons for this are varied. In some instances, the portion of the antigen molecule that the antibody recognizes may be masked when the tissue is fixed, and the particular fixative used can affect the degree of masking. The investigator should be aware that some antisera are generated by using special methods to present the antigen to the antibody-generating host animal. For example, when generating antisera against serotonin, a monoaminergic transmitter, the tissue serotonin must be converted to a β-carboline molecule, accomplished by reacting serotonin with paraformaldehyde. As a result, fixation of tissue for localization studies must also use paraformaldehyde to similarly convert the tissue serotonin to a recognizable form (Fig. 2.12.9). Similarly, histamine (another amine transmitter) is not antigenic itself, but when reacted with carbodiimide, it becomes antigenic. Tissue fixed with standard fixatives will not reveal any histamine, but if the animal is perfused with carbodiimide followed by formaldehyde, the transmitter is localized and is abundant.
Figure 2.12.9.
Comparisons of staining for serotonin in horizontal sections in brainstem tissue that was fixed with buffered 4% paraformaldehyde with added 2.5% acrolein, versus that fixed with only buffered 4% paraformaldehyde. The inclusion of acrolein in the fixative impairs recognition of the tissue serotonin by the antibody. For the color version of this figure go to http://www.currentprotocols.com.
Anticipated Results
Figures 2.12.5 and 2.12.6 show some representative ICC-staining titration results. In evaluating the results of titrations conducted with NiDAB as described in this unit, a number of features should be noted: (1) in examining sections across the range of concentrations of primary antibody recommended in this unit, the staining should progress from high background, with little obvious signal, to strong clear product with low background, and then eventually the signal should fade with further primary antibody dilution. If no change across all concentrations is noted, nonspecific reactivity should be suspected. As mentioned previously, there may be antisera that have no activity for immunocytochemical staining. (2) DAB staining products are precipitates, and thus any staining should be particulate. Nongranular weak staining can be misinterpreted as positive. (3) When describing new sites of reactivity, be prepared to validate staining. Will the staining be blocked by preadsorption of the antibody with purified antigen from a source other than the company that produced the antibody? Will a second antibody against the same antigen also stain the tissue? Can the tissue synthesize the antigen? Use in situ hybridization to verify that the molecule's mRNA is made by the cells, or use biochemical measures to show that the antigen is present in the tissue. One must be skeptical and prove that not only is positive staining present, but that this staining is due to the reaction of the primary antibody with the antigen. Will similar staining be observed if normal serum from the same species at the same concentrations as the primary antibody is substituted (if so, is the staining with the primary antibody nonspecific)? Elimination of primary antibody will only determine whether there is endogenous peroxidase reactivity (and this is rare if the tissue is properly perfused), or if immunoglobulins of the secondary antibody complex bind nonspecifically to the tissue. It does not control for selectivity of the primary antibody.
Time Considerations
Basic Protocol 1
It generally takes at least 4 hr to prepare fixatives, since warm solutions have to cool before filtering, and, after that, the solution pH needs to be adjusted. After animals are perfused with fixative, 2 to 3 days are required for the tissue to become infiltrated with sucrose (thus protecting the tissue from ice crystal formation). After cutting, immunoreactivity in the tissue is improved if it remains in the antifreeze solution for a minimum of 5 days. Approximately 2 days are required for conducting a titration as described. The time needed to mount the sections will depend on the experience of the individual, but normally will not take more than 2 hr to complete. The tissue must then dry overnight and be dehydrated, cleared, and coverslipped. These last procedures take approximately 2 hr.
Alternate Protocol 1
This protocol requires the same amount of time as Basic Protocol 1.
Alternate Protocol 2
This protocol takes ~1 hr less than Basic Protocol 1.
Alternate Protocol 3
This protocol requires ~1 hr less than Basic Protocol 1, but note that the time needed for perfusion of the animal must be longer to accommodate the increased volume of fixative.
Alternate Protocol 4 (egg yolk)
This procedure requires ~4 hr, since it takes a couple of hours to dissolve the gelatin. The egg yolk/gelatin solution once made, can be stored up to 2 to 4 weeks at −20°C. It can then be thawed and warmed in the oven at 40°C, but once thawed, the solution should not be refrozen, and any excess should be discarded.
Timing of Basic Protocols 2-8 with that of Basic Protocol 1
Table 2.12.3 includes a comparison of the timing of all the protocols to each other. Note that the table separates the time of preparation of tissue before and after primary antibody incubation. For all the protocols, incubation of tissue with primary antibody is held constant so as to provide maximal technique sensitivity.
Table 2.12.3.
Time Required for each Basic Protocol Before and After Primary Antibody Incubation
| Basic Protocol Number | Before Primary Antibodya | After Primary Antibodyb |
|---|---|---|
| 1 | 1 hr 40 min | 4 hr 20 min |
| 2 | 1 hr 40 min | 5 hr |
| 3 | 1 hr 40 min | 7 hr |
| 4 | 1 hr 40 min | 9 hr 5 min |
| 5 | 18 minc or 5 mind | 50 min |
| 6 | 18 minc or 5 mind | 58 min |
| 7 | 18 minc or 5 mind | 1 hr 43 min |
| 8 | 18 minc or 5 mind | 1 hr 23 mine or 2 hr 16 minf |
All times are calculated without treatment for endogenous peroxidase. If sections need to be treated with with phenylhydrazine or hydrogen peroxide, add 25 minutes for Basic Protocol 1 or 9 minutes for Basic Protocol 5.
Times do not include substrate development, mounting sections onto slides, or dehydrating and coverslipping.
For tissues fixed in paraformaldehyde:acrolein.
For tissues fixed in paraformaldehyde alone.
For detection using NiDAB (or DAB) substrate
For detection using SA-fluorescence
Supplementary Material
Significance Statement.
Immunocytochemical approaches use the specificity of antibody-antigen reactions to enable localization of molecules in tissue. Approaches to do this vary in terms of tissue preparation (fixation, sectioning, storage), method of detection, and lengths of time tissue is incubated in each reagent. This article presents a systematic way of establishing effective immunocytochemical assays through protocols for antibody titration. It includes comparisons of the common detection techniques (fluorophore-tagged secondary antibodies, biotinylated secondary antibodies followed by streptavidin-linked fluorophores, ABC immunoperoxidase technology, and tyramine-amplified chromagen and fluorophore detection). The addition of microwave-assisted technology for tissue incubation in that employs programmable, temperature- and power-controlled microwave ovens enables an investigator to select working protocols that optimize antibody use, effective assay conditions and processing times.
ACKNOWLEGEMENT
This article is dedicated to the memories of Dr. Wei Wei Le, who pioneered much of the work in this paper and Mr Ziqiang Zhang who provided technical assistance on the work included here. Supported by NIH grants UO1 HD066435, RO1 HD068777, U54 GM009635, R21 HD071873 and FAPESP #11/09816-8
Footnotes
Excel spreadsheets for downloading of microwave programs to the BioWave Unit are presented as Supplementary Data.
Literature Cited
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J. Histochem. Cytochem. 1992;40:1457–1463. doi: 10.1177/40.10.1527370. [DOI] [PubMed] [Google Scholar]
- Berghorn KA, Bonnett JH, Hoffman GE. cFos immunoreactivity is enhanced with biotin amplification. J Histochem. Cytochem. 1994;42:1635–1642. doi: 10.1177/42.12.7983364. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- Ferris AM, Giberson RT, Sanders MA, Day JR. Advanced laboratory techniques for sample processing and immunolabeling using microwave radiation. J Neurosci Methods. 2009;182:157–164. doi: 10.1016/j.jneumeth.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Fitzsimmons MD. Detecting steroidal effects on immediate early gene expression in the hypothalamus. Neuroprotocols. 1992;1:52–66. [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem. Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Munoz TE, Giberson RT, Demaree R, Day JR. Microwave-assisted immunostaining: a new approach yields fast and consistent results. J Neurosci Methods. 2004;137:133–139. doi: 10.1016/j.jneumeth.2004.02.020. [DOI] [PubMed] [Google Scholar]
- RBI (Research Biochemicals International) Too much of a good thing. Neurotransmissions. 1998;14:14–17. [Google Scholar]
- Sternberger L, Hardy P, Cuculus J, Meyer H. The unlabeled antibody-enzyme method of immunochemistry: Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase) and its use in identification of spirochetes. J. Histochem. Cytochem. 1970;18:315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.