Abstract
Objectives. To determine if a widely available weight-management program (Weight Watchers) could achieve sufficient weight loss in persons with prediabetes compared with a Diabetes Prevention Program–based individual counseling program supported by National Diabetes Education Program materials.
Methods. We conducted an individual, randomized intervention trial in Indianapolis, Indiana, in 2013 to 2014, in 225 persons with prediabetes. We compared the Weight Watchers weight-management program (n = 112) with Your Game Plan to Prevent Type 2 Diabetes, a program developed by the National Diabetes Education Program. Outcomes were weight and metabolic markers measured at baseline, 6 months, and 12 months.
Results. Intervention participants lost significantly more weight than controls at 6 months (5.5% vs 0.8%) and 12 months (5.5% vs 0.2%; both P < .001). The intervention group also had significantly greater improvements in hemoglobin A1c and high-density lipoprotein cholesterol level than did controls.
Conclusions. A large weight-management program is effective for achieving lifestyle changes associated with diabetes prevention. Such programs could significantly increase the availability of diabetes prevention programs worldwide making an immediate and significant public health impact.
Type 2 diabetes is one of the most significant public health crises of our time. Both incidence and prevalence have reached epidemic proportions, affecting more than 29 million Americans1 with costs exceeding $245 billion annually.2 Of particular concern is the estimated 86 million persons with prediabetes in the United States, a metabolic condition that significantly increases the risk for developing type 2 diabetes.2,3 If left unchallenged, there will be a significant increase in the number of persons burdened by type 2 diabetes with concomitant social and fiscal costs associated with the disease.
Fortunately, the Diabetes Prevention Program (DPP) demonstrated that type 2 diabetes risk can be reduced through modest weight loss and increased physical activity achieved through lifestyle change programs.4 Unfortunately, the DPP addressed efficacy, with little regard to the feasibility or costs required to implement prevention programs into the broader public health. There are programs targeting the translation of the DPP to the broader public health.5–9 These programs have been shown to be effective in addressing the primary risk factor for developing type 2 diabetes—weight reduction in persons with prediabetes.5–14 In spite of more than a decade of evidence that diabetes prevention is feasible, the speed at which programs have become available is still relatively slow.
There are limits to the scalability of programs that use the DPP curriculum format. They are “disease specific” (i.e., they target only persons with prediabetes). Although this is consistent with the evidence base that demonstrates the effectiveness of the DPP format curriculum only for those with prediabetes, given that excessive weight is the prime risk factor, this may limit interest and availability for persons who do not know they have prediabetes—a condition that is still not routinely assessed in primary care.15 Thus, disease-specific programs may fail to reach the critical mass required to start and scale a cohort-based program (i.e., all participants have to start at same time).
In addition, the DPP-based curriculum has some inherent limitations that potentially have an impact on the development of the program on a broad public health scale. The DPP uses a 16 weekly session sequential curriculum in which each session is only offered once. If a session is missed, it is difficult to make it up. Increasing evidence illustrates that it is difficult for many persons to commit to a 4-month weekly program.5–9 By contrast, many weight-loss programs use “loop” models in which sessions are repeated frequently and at a wide range of times and locations, often within the same week. Such programs are easy to restart when needed if life events or scheduling conflicts interrupt program attendance. This feature may offer important alternatives to consumers in terms of “user friendliness.”
There is clearly a need to identify mechanisms that can more quickly implement cost-effective, evidence-based intervention programs. In this context, the role that widely available commercial weight-loss programs might play has not been fully explored. There are many scalable weight-management programs available in the commercial space. Most incorporate the central components used in the DPP—monitoring of food intake, modification in food selection to reduce calories, focus on social and psychological factors that influence eating behavior, and the use of regular physical activity to help with weight loss and maintenance. None of these programs, however, has been tested among those with prediabetes to determine if they can achieve weight loss consistent with risk reduction. If such programs can produce weight loss among persons with prediabetes comparable to that seen in the DPP and derivative programs, and do so at a reasonable cost, they could provide a valuable approach to diabetes prevention without the need for creating additional infrastructure, disseminating complex treatment programs, and training new treatment providers.
To explore the potential of a widely available weight-loss program to contribute to the primary prevention of type 2 diabetes, we investigated if the Weight Watchers program, when combined with a single session to address prediabetes, could effectively reduce weight at levels similar to the DPP-based community programs.
METHODS
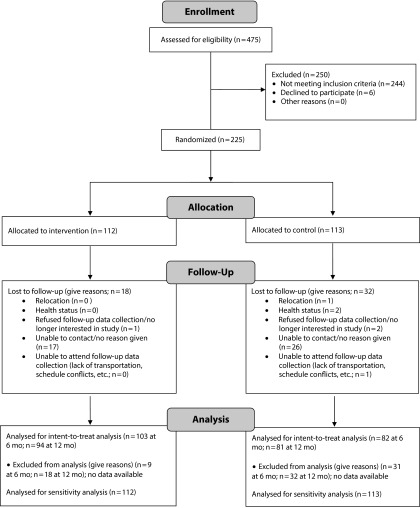
This was an individual, randomized intervention trial to evaluate the effects of a modified version of the Weight Watchers program compared with a self-initiated program developed by the National Diabetes Education Program on weight and glucose control as measured by hemoglobin A1c (Figure 1).
FIGURE 1—
Consort Flow Diagram for Randomized Controlled Trial of Weight Watchers Versus Counseling With National Diabetes Education Program Materials: Indianapolis, IN, 2013–2014
Participants
We screened potential participants for eligibility in community settings. To be eligible, a person had to be aged 18 years or older, have a body mass index (BMI; defined as weight in kilograms divided by the square of height in meters) of 24 or higher (persons of Asian descent BMI ≥ 23), and to complete the 7-item American Diabetes Association (ADA) Diabetes Risk Assessment with a score of 5 or greater. In addition, they had to have prediabetes, which was determined by a hemoglobin A1c value between 5.7% and 6.5%. Women with a self-reported history of gestational diabetes with a hemoglobin A1c value less than 6.5% or causal capillary blood glucose (CCBG) less than 199 milligrams per deciliter were also included. Persons were noneligible if they had no evidence of prediabetes; were currently pregnant or planning to become pregnant during the study; had any condition or used any medication that could alter glucose metabolism; suffered heart attack, stroke, or transient ischemic attack in the past 6 months; had uncontrolled hypertension (systolic blood pressure > 180 mm Hg or diastolic blood pressure > 105 mm Hg); received treatment of cancer (excluding surgery alone) within the past 2 years (excluding skin cancer); reported chest pain, shortness of breath with minimal activity or at rest, or unexplained dizziness or fainting with physical activity; had chronic lung disease (chronic obstructive pulmonary disease or asthma requiring home oxygen therapy); current use of antidiabetes medications for the treatment of diagnosed diabetes; were unable to communicate with research staff; were unable to read written English; and were unable or unwilling to provide consent.
Multiple screening sessions were held at 8 community sites, including recreation centers, churches, and community clubhouses. Hemoglobin A1c was determined by using a DCA Vantage analyzer (Siemens, Malvern, PA).16 The CCBG level was determined by using a One-Touch Ultra handheld glucose meter (Roche Diagnostics, Indianapolis, IN).17 The ADA risk screener was used.18 People with an ADA risk score of 5 or greater, a hemoglobin A1c from 5.7% to 6.4%, and CCBG of 110 to 199 milligrams per deciliter (100–109 mg/dL if fasting 8 or more hours) were informed that they were at increased risk for developing diabetes and were potentially eligible for the study. People with a hemoglobin A1c of 6.5% or greater and a CCBG of 200 milligrams per deciliter or greater were informed that they were at high risk for diabetes and should see a health care provider immediately to undergo formal confirmatory testing and follow-up.
Measures
Measures were collected by an Indiana University School of Medicine research team from April 2013 to April 2014 at community sites at baseline, 6 months, and 12 months of study enrollment. The primary outcome was percent change in body weight after 6 and 12 months. Body weight (and height) was measured with a calibrated Detecto ProDoc Series PD300MHR Digital Physician scale (Cardinal, Webb City, MO) with mechanical height rod and a Detecto 758C Weight Indicator with a Seca 213 Portable Stadiometer (Cardinal, Webb City, MO).
Secondary outcomes included changes in blood pressure, hemoglobin A1c, total cholesterol, and HDL-cholesterol (HDL-c). Total cholesterol and HDL-c were measured with a Cholestech LDX lipid analyzer (Alere, Providence, RI).19 Blood pressure was assessed with an aneroid sphygmomanometer (Welch Allyn, Skaneateles Falls, NY) and with an Accutorr V vital signs monitor (Mindray, Mahwah, NJ) with appropriate-sized cuff after participants were seated and relaxed for at least 5 minutes. All research staff were trained in use of this technology. This included proper sample collection, calibration of the equipment, and data transfer to study forms.
Eligible and consented participants were randomly assigned either to the intervention or the control group in a 1-to-1 ratio with a computer-generated randomization list with a block size of 4. Assignment was done by use of REDCap version 6.5.20 (Vanderbilt University, Nashville, TN), a secure Web-based application that automated the computer-based randomization list generated by the biostatistician. Participants were informed of treatment assignment at the conclusion of the baseline assessment.
Intervention and Controls
All screening participants received personalized advice about their risk for developing diabetes, and those without contraindications were advised that modest weight loss (5%–10%) via caloric restriction and the adoption of moderate physical activity were generally safe and effective in preventing or delaying the onset of diabetes. This advice typically took 5 minutes via an individual session with a trained research assistant and was supplemented by use of Small Steps, Big Rewards educational materials available from the National Diabetes Education Program.20,21
The intervention was the lifestyle modification program offered by Weight Watchers International. The Weight Watchers core curriculum is evidence-based15,22–24 and covers the same behavioral topics used in the DPP:
self-monitoring of weight, intake, and activity;
dietary modification;
physical activity;
stimulus control; and
relapse prevention.
The curriculum is delivered in a supportive, weekly group environment by appropriately trained group leaders.
Before beginning the lifestyle intervention program, participants attended a 45-minute “activation” session at 1 of 5 WW facilities in the Indianapolis area. This was done within 1 week of the baseline assessment. This session was conducted by existing WW coaches who were trained for this study-specific session. It focused on educating participants about the meaning of prediabetes, how the condition increases risk for developing type 2 diabetes, and the role of lifestyle modification to reduce their risk. A weight loss goal of 7% was also assigned. Following this activation session, participants were enrolled into the already existing Weight Watchers program in the community. Participants were free to choose a Weight Watchers group session time and location that was convenient for them. Participants attended already existing meetings that were not study-specific and included those without prediabetes. They were encouraged to attend the weekly meetings and told they were free to switch both time and location as best fit their schedules. They were also given access to the Weight Watchers e-tools, which includes digital tools to track weight, intake, and activity as well as tips to facilitate adherence. All Weight Watchers services and sessions were provided free of charge for the duration of the study.
Participants assigned to the control condition were provided a review of how they could initiate a weight loss and activity program with Your Game Plan to Prevent Type 2 Diabetes educational materials developed by the National Diabetes Education Program.20,21 These materials review the meaning and implications of prediabetes, the results of the DPP study, an overview of how to initiate a risk-reducing lifestyle program, a reproducible tracker to help monitor their food intake, and a booklet with fat gram and calorie content for common foods. Emphasis was placed on strategies for tracking food intake and calculating fat grams by using the food tracker and calorie fat gram guide provided in the materials. This individual counseling, which took approximately 15 minutes, was provided by trained research staff and conducted during the screening event after randomization.
The primary efficacy outcome was the percent weight change from baseline to 6 months and 12 months. In our Diabetes Education and Prevention with a Lifestyle Intervention Offered at the YMCA (DEPLOY) study,9 the intervention group had a 6.0% loss of weight (95% confidence interval [CI] = 3.8%, 8.3%) with a variability of 4.3%, and the control group experienced a mean weight reduction of 1.8% (95% CI = −0.3%, 3.9%) with a variability of 4.1% at 12 months. There was a 15% dropout rate at 6 months and 18% at 12 months. Assuming a common variability of 4.3%, there was a 90% probability that the half width of the 95% CI for estimating the mean percent weight change would be no more than 1.1% for a sample size of 85 per group. Also, with a conservative estimate of 3% weight reduction for the intervention group and 1% weight reduction for the control group, a sample size of 85 per group had 85% power at 5% type I error rate. To account for a 15% dropout rate, we planned to recruit 100 participants per group.
Statistical Analyses
We summarized all baseline continuous variables by using descriptive statistics and presented them by treatment group. We summarized categorical variables with frequency counts and percentages. We compared baseline clinical and demographic data among the 2 groups. We examined dichotomous and ordinal variables by using either χ2 test or Fisher exact test and continuous measures with 2-sample t test or 2 sample nonparametric Wilcoxon Rank Sum Test (if normality assumptions are not met).
The primary outcomes were the percent weight change at 6 and 12 months from baseline. We modeled the longitudinal data on percent weight change at 6 and 12 months by using repeated-measures analysis of covariance. Secondary outcomes included changes in hemoglobin A1c, blood pressure, glucose level, total cholesterol, and HDL-c. Again, we used repeated-measures analysis of covariance to analyze all the secondary outcomes. We also analyzed the proportion of participants with at least 5% weight loss as well as the proportion of participants with at least 7% weight loss at 6 and 12 months. We used logistic regression models with generalized estimating equations. All models included baseline value of the outcome variable, total cholesterol, and diastolic blood pressure. We used an unstructured covariance matrix in all models. To account for missing weight data (27% at 6 months and 28% at 12 months in control group; 8% at 6 months and 16% at 12 months in the intervention group), we performed sensitivity analysis with multiple imputation method with the assumption that missing data at follow-up was a function of treatment group and baseline weight.
RESULTS
The study sample was primarily female (85%), non-Hispanic (94%), and White (64%) with 88% having at least 1 year of college and 70% having an annual household income of at least $35 000. Participants were aged on average 52 (SD = 11) years with a BMI of 36.8 (SD = 7.1) and baseline weight 100.63 (SD = 20.8) kilograms. Table 1 shows baseline characteristics by treatment group. There were no differences between groups on baseline characteristics, except that control participants had slightly higher total cholesterol and lower diastolic blood pressure than the intervention group.
TABLE 1—
Baseline Characteristics of Study Participants in Randomized Controlled Trial of Weight Watchers Versus Counseling With National Diabetes Education Program Materials: Indianapolis, IN, 2013–2014
| Characteristics | WW Intervention (n = 112), Mean (SD) or % | Control (n = 113), Mean (SD) or % | P |
| Demographics | |||
| Age, y | 51.5 (11.5) | 51.7 (11.0) | .89 |
| Gender, female | 83.0 | 86.7 | .44 |
| Ethnicity, non-Hispanic | 93.7 | 93.7 | .45 |
| Race | .64 | ||
| White | 63.4 | 65.5 | |
| African American | 28.6 | 22.1 | |
| Asian/Pacific Islander | 5.4 | 8.0 | |
| Multiracial | 1.8 | 1.8 | |
| Other | 0.9 | 2.6 | |
| Education | .98 | ||
| Some high school | 1.8 | 2.8 | |
| High-school graduate or GED | 9.8 | 10.1 | |
| Some college or 2-y college degree | 34.8 | 33.0 | |
| 4-y college graduate | 17.9 | 20.2 | |
| > 4-y college degree | 35.7 | 33.9 | |
| Marital status | .54 | ||
| Married | 59.8 | 53.6 | |
| Divorced | 19.6 | 26.4 | |
| Never married | 14.3 | 11.8 | |
| Widowed | 4.5 | 2.7 | |
| Separated | 0.9 | 2.7 | |
| Living with partner | 0.9 | 2.7 | |
| Income, $ | .58 | ||
| < 10 000 | 1.8 | 0.9 | |
| 10 000–15 000 | 2.7 | 4.5 | |
| > 15 000–25 000 | 7.1 | 1.8 | |
| > 25 000–35 000 | 10.7 | 14.5 | |
| > 35 000–50 000 | 17.0 | 16.4 | |
| > 50 000–75 000 | 19.6 | 19.1 | |
| > 75 000 | 33.0 | 35.4 | |
| Didn’t know | 4.5 | 1.8 | |
| Refused | 3.6 | 5.4 | |
| Clinical variables | |||
| Baseline weight in kg | 100.9 (21.7) | 100.0 (19.9) | .74 |
| Body mass index, kg/m2 | 36.9 (7.3) | 36.7 (7.0) | .89 |
| Hemoglobin A1c, % | 5.9 (0.3) | 5.8 (0.3) | .11 |
| Glucose, mg/dL | 107.6 (21.3) | 110.0 (21.6) | .41 |
| Total cholesterol, mg/dL | 186.2 (31.5) | 195.6 (38.3) | .047 |
| HDL-c, mg/dL | 47.8 (14.2) | 47.6 (14.1) | .91 |
| Diastolic blood pressure, mm Hg | 82.6 (8.3) | 80.2 (8.7) | .032 |
| Systolic blood pressure, mm Hg | 130.9 (12.7) | 129.5 (15.1) | .45 |
Note. GED = general equivalency diploma; HDL-c = high-density lipoprotein cholesterol; WW = Weight Watchers.
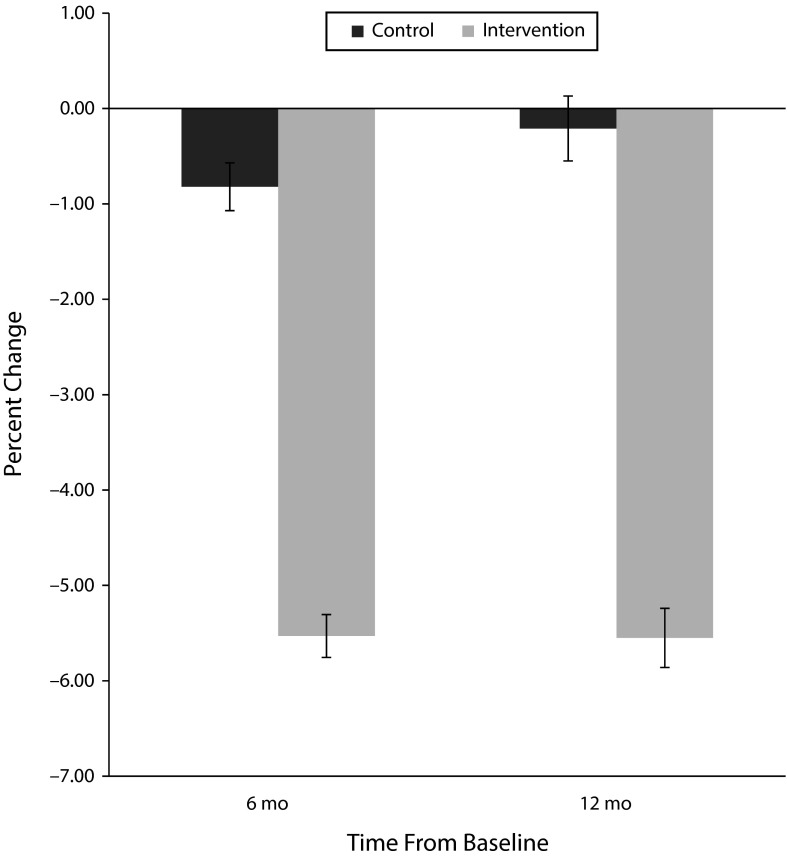
Changes in body weight and clinical outcomes are shown in Table 2. The intervention group lost significantly more weight than controls at both 6 and 12 months (P values < .001). On average, controls lost 0.8% (SE = 0.5%) at 6 months and 0.2% (SE = 0.7%) at 12 months and intervention participants lost 5.5% (SE = 0.5%–0.6%) both at 6 and 12 months (Figure 2). This translated, on average, to 4.6 (SE = 0.70) kilograms more weight loss at 6 months and 5.3 (SE = 0.94) kilograms more weight loss at 12 months for intervention participants than controls. The sensitivity analyses for treatment difference showed either identical or almost the same results at 6 months and 12 months; that is, mean treatment differences were 4.7% (SE = 0.68%) at 6 months and 5.3% (SE = 0.93%) at 12 months when we analyzed data without any imputation for missing values versus 4.7% (SE = 0.68%) at 6 months and 5.5% (SE = 1.00%) at 12 months when were analyzed data with multiple imputations for missing values.
TABLE 2—
Changes in Body Weight and Clinical Outcomes in Randomized Controlled Trial of Weight Watchers Versus Counseling With National Diabetes Education Program Materials: Indianapolis, IN, 2013–2014
| Outcomes | WW Intervention, No. or Mean (SD) | Control, No. or Mean (SD) | P |
| At 6 mo from baseline | 103 | 82 | |
| Baseline weight in kg | 100.9 (10.58) | 100.0 (10.63) | .74 |
| Percent weight change | −5.53 (0.45) | −0.82 (0.50) | <.001 |
| Weight in kg | −5.49 (0.46) | −0.91 (0.51) | <.001 |
| Body mass index, kg/m2 | −2.03 (0.17) | −0.30 (0.19) | <.001 |
| Hemoglobin A1c, % | −0.22 (0.02) | −0.14 (0.03) | .032 |
| Glucose, mg/dL | −5.47 (1.78) | −2.06 (1.99) | .21 |
| Total cholesterol, mg/dL | −4.33 (2.52) | −1.75 (2.80) | .499 |
| HDL-c, mg/dL | 2.48 (0.96) | −0.05 (1.07) | .084 |
| Systolic blood pressure, mm Hg | −3.31 (1.11) | −2.99 (1.24) | .85 |
| Diastolic blood pressure, mm Hg | −4.39 (0.89) | −4.80 (0.99) | .76 |
| At 12 mo from baseline | 94 | 81 | |
| Baseline weight in kg | 100.9 (10.58) | 100.0 (10.63) | .74 |
| Percent weight change | −5.55 (0.62) | −0.21 (0.68) | <.001 |
| Weight in kg | −5.51 (0.63) | −0.22 (0.69) | <.001 |
| Body mass index, kg/m2 | −2.06 (0.23) | −0.07 (0.25) | <.001 |
| Hemoglobin A1c, % | −0.25 (0.03) | −0.18 (0.03) | .068 |
| Glucose, mg/dL | −2.77 (2.10) | −1.97 (2.27) | .80 |
| Total cholesterol, mg/dL | 0.45 (2.46) | −0.61 (2.66) | .77 |
| HDL-c, mg/dL | 6.29 (1.05) | 2.99 (1.14) | .036 |
| Systolic blood pressure, mm Hg | −3.30 (1.18) | −3.39 (1.27) | .96 |
| Diastolic blood pressure, mm Hg | −5.39 (0.92) | −5.77 (0.99) | .78 |
Note. HDL-c = high-density lipoprotein cholesterol; WW = Weight Watchers.
FIGURE 2—
Mean Percent Weight Change in Randomized Controlled Trial of Weight Watchers Versus Counseling With National Diabetes Education Program Materials: Indianapolis, IN, 2013–2014
There was a greater proportion in the intervention group who had 5% or greater weight loss, as well as 7% or greater weight loss, which defines the range in which risk reduction is felt to occur.4 The odds of achieving 5% or greater weight loss for the intervention group was 6.9 times greater (P<.001) than the control group at 6 months and 4.7 times greater (P < .001) at 12 months. In a similar way, the odds of achieving 7% or greater weight loss for the intervention group were 6.2 times greater (P < .001) than controls at 6 months and 5.0 times greater (P < .001) at 12 months.
Among secondary outcomes, the intervention group showed significantly greater improvements than controls at 6 and 12 months, in hemoglobin A1c (-0.22 vs −0.14; P = .032) and 3.3 milligrams per deciliter greater increases in HDL-c (P = .036). There were no significant differences between groups on any of the other secondary outcomes. We also calculated the average number of group sessions that participants in the Weight Watchers condition attended to determine what the “dose” of the intervention was. The average number of group sessions attended during the study over a 1-year period was 21.6 (range = 1–55). Also, 63% reported using the online app; however, we did not assess how many used both live and app opportunities. Anecdotal evidence suggests that many users attended groups and used the app to record dietary intake and calculate points.
DISCUSSION
The incidence of diabetes has reached epidemic proportions and illuminates the need to rapidly expand community access to evidence-based prevention programs. Although such programs continue to increase, there are still far too few to meet the current and expanding public health need. The data from this study show that a widely available weight management program results in changes in body weight after 6 and 12 months that are comparable to those observed in the DPP study and community adaptations.6–14,25–28
The program studied here, Weight Watchers, is a widely available, evidence-based lifestyle intervention that combines face-to-face and digital offerings. There are more than 25 000 Weight Watchers meetings held each week in the United States, led by trained facilitators in a variety of times and locations, including more than 5000 in workplace settings. Thus, Weight Watchers has the potential for rapidly expanding access to a lifestyle intervention for those with prediabetes that significantly exceeds the reach of current efforts. The potential to achieve weight-loss levels could translate into considerable reductions in diabetes risk.
An important consideration when one is assessing models for scaling up diabetes prevention programs is cost. A recent meta-analysis of 1-year randomized trials of various commercial weight-loss approaches found that Weight Watchers was the most cost-effective.29 The cost of participating in Weight Watchers is $515.40 per year for unlimited access to group sessions anywhere in the country and includes access to their eTools and mobile app. The online program alone is $227.40 per year. This compares favorably with $429 charged by the national YMCA Diabetes Prevention Program, which offers 24 rigidly scheduled sessions in a year.30 It is important to note, however, that the YMCA offers income-based fee scaling. Eligible individuals pay anywhere from $0 to $429 out of pocket. When one is considering costs, there is growing acceptance by the insurance industry to pay the costs of participating in evidence-based prevention programs.31–33 If this expands to weight-management programs that can demonstrate effectiveness delivered with fidelity, the ability to expand access to a broad public health scale quickly is a probable outcome with significant impacts on diabetes prevention.
The lack of differences between the 2 groups in lipids and blood pressure is surprising in light of the well-documented effects of weight loss on cardiovascular disease risk factors. Unlike the DPP-based intervention, Weight Watchers does not specifically target fat gram control, which may explain why there were no changes in lipids observed. It is more likely, however, that the baseline values within normal limits (Table 1) provided a restricted range to see weight-loss effects. There was, however, a significantly greater reduction in hemoglobin A1c among intervention versus controls. This is consistent with the effects of weight loss on hemoglobin A1c among those with prediabetes. This was also the case in the DEPLOY study of adapting the DPP for delivery in the YMCA where weight loss was observed but not differences in lipids in the control group.10
One aspect of the intervention described here is the use of an activation session. We did not design this study to assess the importance of this to the overall impact. We chose to use the activation session as a means to better acquaint participants with their risk status and to assign a 7% weight-loss goal. We believe that this session helped to motivate the participants and should be considered as a feature of a national Weight Watchers prevention effort.
Limitations
There are limitations to this study that must be considered. There was a loss of participants to follow-up at 12 months. Thus, for the primary weight-loss outcome, the effectiveness of the intervention may have been overestimated if weight loss was lower in nonrespondents. There were, however, fewer participants lost to follow up in the intervention (16%) versus control (28%) group. As loss to follow up was greater in the control group, the group differences observed might have been even greater if all participants were represented. Sensitivity analyses showed the same results—mean treatment differences were 4.7% (SE = 0.68%) at 6 months and 5.5% (SE = 1.0%) at 12 months with statistical adjustment for baseline differences in confounders, total cholesterol, and diastolic blood pressure. However, it was not possible to adjust for all potential confounders in this study.
In addition, the majority of the sample were women and the Weight Watchers sample also had higher socioeconomic status and education status. Recruitment targeted all residents of the Indianapolis metropolitan area. Thus, there was no attempt to exclude men or any group on the basis of their socioeconomic status. This may reflect a tendency of women to gravitate toward diet-modification programs.34 It does caution against generalizing the results of this study to low-socioeconomic status populations and men.
Conclusions
These data suggest that Weight Watchers, a widely available, empirically validated weight-management program, could offer a potential tool to significantly expand access to diabetes prevention programs in community settings and produce weight-loss levels that translate into considerable reductions in diabetes risk. The role that commercial programs can play in diabetes prevention among those at high risk for diabetes deserves future study.35,36
ACKNOWLEDGMENTS
This study was funded by Weight Watchers International.
This study was presented at the International Diabetes Federation Meeting in 2014.
We wish to thank all of the Weight Watchers coaches who participated in this study. We also want to thank Stephanie Rost and Angela Fredrick for their invaluable assistance in coordinating the Weight Watchers programs and for providing access to Weight Watchers data. We thank the Diabetes Translational Research Center staff who worked on this study, the community sites that allowed us to collect outcome data, and all study participants.
Note. K. Miller-Kovach and G. D. Foster are both employed by Weight Watchers International.
HUMAN PARTICIPANT PROTECTION
The Indiana University–Purdue University Indianapolis institutional review board approved the study protocol. The Clinical Trials Registration number is NCT02000024. All participants provided written informed consent and were provided copies of the consent forms stating study objectives.
REFERENCES
- 1. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2014.
- 2.Dall TM, Zhang Y, Chen YJ, Quick WW, Yang WG, Fogli J. The economic burden of diabetes. Health Aff (Millwood) 2010;29(2):297–303. doi: 10.1377/hlthaff.2009.0155. [DOI] [PubMed] [Google Scholar]
- 3.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes Res Clin Pract. 2011;91(1):1–12. doi: 10.1016/j.diabres.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Dunkley AJ, Bodicoat DH, Greaves CJ et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37(4):922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 7.Whittemore R. A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med. 2011;1(3):480–491. doi: 10.1007/s13142-011-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson M, Jones R, Freeman C et al. Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence. Diabet Med. 2013;30(1):3–15. doi: 10.1111/dme.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann RT, Liss DT, Finch EA et al. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2105;105(11):2328–2334. doi: 10.2105/AJPH.2015.302641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: the DEPLOY Pilot Study. Am J Prev Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macaulay AC, Paradis G, Potvin L et al. The Kahnawake Schools Diabetes Prevention Project: intervention, evaluation, and baseline results of a diabetes primary prevention program with a native community in Canada. Prev Med. 1997;26(6):779–790. doi: 10.1006/pmed.1997.0241. [DOI] [PubMed] [Google Scholar]
- 12.Katula JA, Vitolins MZ, Rosenberger EL et al. One-year results of a community-based translation of the diabetes prevention program Healthy-Living Partnerships to Prevent Diabetes (HELP PD) project [erratum in Diabetes Care. 2012;35(2):455] Diabetes Care. 2011;34(7):1451–1457. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer MK, McWilliams JR, Chen HY, Siminerio LM. A community-based diabetes prevention program evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Educ. 2011;37(5):659–668. doi: 10.1177/0145721711411930. [DOI] [PubMed] [Google Scholar]
- 14.Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care. 2008;31(4):684–689. doi: 10.2337/dc07-1869. [DOI] [PubMed] [Google Scholar]
- 15.Geiss LS, James C, Gregg EW, Albright A, Williamson DF, Cowie CC. Diabetes risk reduction behaviors among US adults with prediabetes. Am J Prev Med. 2010;38(4):403–409. doi: 10.1016/j.amepre.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56(1):44–52. doi: 10.1373/clinchem.2009.130641. [DOI] [PubMed] [Google Scholar]
- 17.Dai K-S, Tai D-Y, Ho P et al. Accuracy of the EasyTouch blood glucose self-monitoring system: a study of 516 cases. Clin Chim Acta. 2004;349(1-2):135–141. doi: 10.1016/j.cccn.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Herman WH, Smith PJ, Thompson TJ, Engelgau MM, Aubert RE. A new and simple questionnaire to identify people at increased risk for undiagnosed diabetes. Diabetes Care. 1995;18(3):382–387. doi: 10.2337/diacare.18.3.382. [DOI] [PubMed] [Google Scholar]
- 19.Shemesh T, Rowley KG, Shephard M, Piers LS, O’Dea K. Agreement between laboratory results and on-site pathology testing using Bayer DCA2000+ and Cholestech LDX point-of-care methods in remote Australian Aboriginal communities. Clin Chim Acta. 2006;367(1-2):69–76. doi: 10.1016/j.cca.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Clark CM, Fradkin JE, Hiss RG, Lorenz RA, Vinicor F, Warren-Boulton E. The National Diabetes Education Program, changing the way diabetes is treated: comprehensive diabetes care. Diabetes Care. 2001;24(4):617–618. doi: 10.2337/diacare.24.4.617. [DOI] [PubMed] [Google Scholar]
- 21.National Diabetes Education Program. Available at: http://ndep.nih.gov. Accessed August 20, 2014.
- 22.Johnston CA, Rost S, Miller-Kovach K, Moreno JP, Foreyt JP. A randomized controlled trial of a community-based behavioral counseling program. Am J Med. 2013;126(12):1143.e19–1143.e24. doi: 10.1016/j.amjmed.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 23.Pinto AM, Fava JL, Hoffmann DA, Wing R. Combining behavioral weight loss treatment and a commercial program: a randomized clinical trial. Obesity (Silver Spring) 2013;21(4):673–680. doi: 10.1002/oby.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jebb SA, Ahern AL, Olson AD et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomized controlled trial. Lancet. 2011;378(9801):1485–1492. doi: 10.1016/S0140-6736(11)61344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33(1):69–78. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 26.Ali MK, Echouffo-Tcheugui JB, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 27.Amundson HA, Butcher MK, Gohdes D et al. Translating the Diabetes Prevention Program into practice in the general community: findings from the Montana Cardiovascular Disease and Diabetes Prevention Program. Diabetes Educ. 2009;35(2):209–210. doi: 10.1177/0145721709333269. 213–214, 216–220. [DOI] [PubMed] [Google Scholar]
- 28.Jackson L. Translating the Diabetes Prevention Program into practice: a review of community interventions. Diabetes Educ. 2009;35(2):309–320. doi: 10.1177/0145721708330153. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein EA, Kruger E. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity (Silver Spring). 2014;22(9):1942–1951. doi: 10.1002/oby.20824. [DOI] [PubMed] [Google Scholar]
- 30.YMCA’s Diabetes Prevention Program. 2014. Available at: http://www.ymca.net/diabetes-prevention. Accessed August 20, 2014.
- 31.Gold R, DeVoe J, Shah A, Chauvie S. Insurance continuity and receipt of diabetes preventive care in a network of federally qualified health centers. Med Care. 2009;47(4):431–439. doi: 10.1097/mlr.0b013e318190ccac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride PE, Einerson J, Grant H et al. Putting the Diabetes Prevention Program into practice: a program for weight loss and cardiovascular risk reduction for patients with metabolic syndrome or type 2 diabetes mellitus. J Nutr Health Aging. 2008;12(10):745S–749S. doi: 10.1007/BF03028624. [DOI] [PubMed] [Google Scholar]
- 33.Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma Y. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychol. 2008;27(1 suppl):S91–S98. doi: 10.1037/0278-6133.27.1.S91. [DOI] [PubMed] [Google Scholar]
- 34.Millstein RA, Carlson SA, Fulton JE et al. Relationships between body size satisfaction and weight control practices among US adults. Medscape J Med. 2008;10(5):119. [PMC free article] [PubMed] [Google Scholar]
- 35.Wing RR. Treatment options for obesity: do commercial weight loss programs have a role? JAMA. 2010;304(16):1837–1838. doi: 10.1001/jama.2010.1529. [DOI] [PubMed] [Google Scholar]
- 36.Ackermann RT, Sandy LG, Beauregard T, Coblitz M, Norton KF, Vojta D. A randomized comparative effectiveness trial of using cable television to deliver diabetes prevention programming. Obesity (Silver Spring) 2014;22(7):1601–1607. doi: 10.1002/oby.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]