Abstract
Parkinson’s disease (PD) is a multifactorial neurodegenerative disease involving oxidative stress, neuroinflammation and apoptosis. Epoxyeicosatrienoic acids (EETs) are arachidonic acid metabolites and they play a role in cytoprotection by modulating various cell signaling pathways. This cytoprotective role of EETs are well established in cerebral stroke, cardiac failure, and hypertension, and it is due to their ability to attenuate oxidative stress, endoplasmic reticulum stress, inflammation, caspase activation and apoptosis. The actions of EETs in brain closely parallel the effects which is observed in the peripheral tissues. Since many of these effects could potentially contribute to neuroprotection, EETs are, therefore, one of the potential therapeutic candidates in PD. Therefore, by increasing the half life of endogenous EETs in vivo via inhibition of sEH, its metabolizing enzyme can, therefore, constitutes an important therapeutic strategy in PD.
Keywords: sEH, EETs, PD, Neuroprotection, Cytochrome P450, Neuroinflammation, Oxidative stress, Apoptosis
Introduction
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disorder with a characteristic symptoms such as bradykinesia, rigidity, resting tremor and posture instability [1–4]. It is a multifactorial disease involving age, genetic and environment factors. Aging is associated with mitochondrial dys-function, increased free radical production and oxidative stress, which may lead to genomic instability and DNA mutations, with reduced survival [5–7]. For the past 15 years, genetic characterization of PD has shown sequence or copy number variants in at least six genes [(synuclein alpha (SNCA), leucine-rich repeat kinase 2 (LRRK2), E3 ubiquitin-protein ligase parkin (PARK2), PTEN induced putative kinase 1 (PINK1), PARK7 – protein deglycase DJ-1, ATPase Type 13A2 (ATP13A2)] which have been identified to cause monogenic forms of PD [7,8]. Environmental factors such as head trauma and exposure to pesticides (rotenone, paraquat, dieldrin, etc.), solvents (trichloroethylene, carbon tetrachloride, n-hexane etc.), and metals (lead, iron, manganese etc.) are reported to cause destruction of dopaminergic neurons through oxidative and inflammatory reactions [9]. The morphologic hallmark of PD is the presence of alpha-synuclein (α-syn)-rich Lewy bodies within the dopaminergic neurons, which are mainly formed due to mutations in α-syn gene, leading to protein aggregation. Lewy body formation is observed both in familial and sporadic forms of PD [10–14]. Molecular level analysis of PD confirm that oxidative stress, mitochondrial dysfunction and neuroinflammation are the major contributing factors [15].
Plasma membrane arachidonic acid (AA) released by phospholipids by phospholipase A2 (PLA2) is metabolized to prostaglandins and thromboxane by cyclooxygenase (COX), to leukotrienes by lipoxygenase (LOX), and to Epoxyeicosatrienoic acid (EETs) by cytochrome P450 (CYP450) oxidases (Fig. 1) [16,17]. Four EETs regioisomers, 5,6-, 8,9-, 11,12-, and 14,15-EET are produced by CYP450 epoxygenases pathway. These regioisomers are quickly metabolized to inactive or less active metabolites by soluble or to a lesser degree the microsomal epoxide hydroxylases (sEH or mEH) and their estimated in vivo half-life is of few seconds to minutes [18] (Figs. 1 and 2). EETs are present in heart, lungs, kidneys, gastro-intestinal tract and in brain [19,20]. Sura et al., reported the preferential expression sEH in neuronal cell bodies, oligodendrocytes, astrocytes, meningeal blood vessels, and in choroid plexus of human brain [20]. EET’s are reported to exhibit anti-inflammatory and antioxidant properties which protect against mitochondrial dysfunction and apoptosis and play an important role in regulation of cerebral blood flow [21]. A diverse class of agents such as amides, ureas, thioamides, thioureas, carbamates, acyl hydrazones, chalcone oxides, etc., has been reported to possess sEH inhibitory potential [22]. Some of the sEH inhibitors have been extensively studied for their cytoprotective benefits in hypertension, ischemic heart disease, heart failure, renocardiac failure, diabetic neuropathy, cancer and obesity [23–31]. Although EETs are widely distributed in the brain, little research has been carried out to exploit their cytoprotective benefits in the treatment/prevention of PD [25,32].
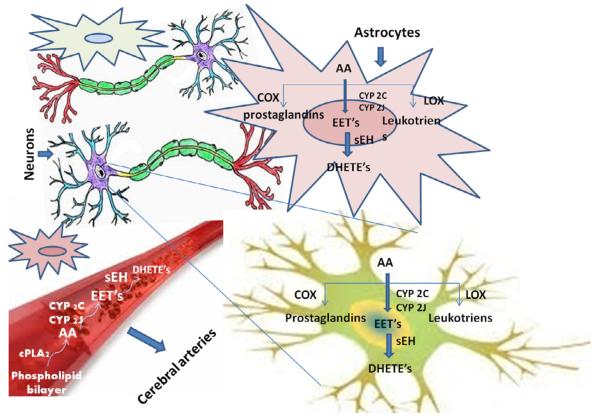
Fig. 1.
Arachidonic acid pathway in astrocytes, dopaminergic neurons, and cerebral blood vessels. AA – archidonic acid, COX-2 – cyloclooxygenase-2, LOX – lipoxygenase, EET’s – Epoxyeicosatrienoic acids, sEH – soluble epoxide hydrolase, CYP2C – cytochrome p450 2C, CYP2J – cytochrome p450 2J, DHETs – dihydroxyeicosatrienoic acids, cPLA – phospholipase A.
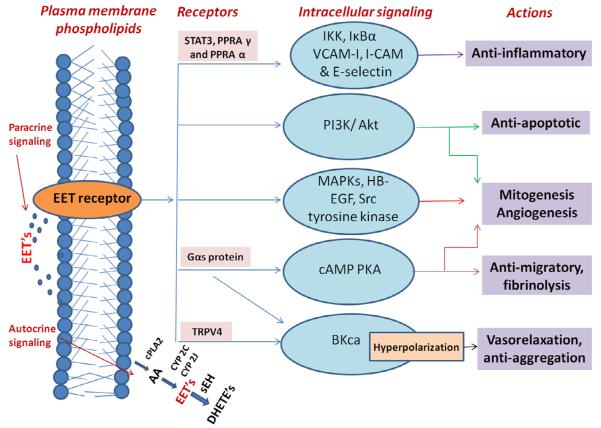
Fig. 2.
Intracellular signaling pathways of EET’s. Epoxyeicosatrienoic acids exhibit both autocrine and paracrine signaling mechanism, and they are either directly released from the phospholipids stores or synthesized from the archidonic acids (AA) via a cytochrome P450 pathway. The actions of EET’s are mediated by various intracellular signaling pathways as shown in blue color bubbles, which get activated at specific conditions in different tissues. The activation of large-conductance Ca-activated K (BKCa) channels occurs through a Gαs protein coupled to the putative receptor or due to activation of transient receptor potential vanilloid type 1. The cAMP-PKA, phosphatidylinositol 3-kinase (PI3K)-Akt, mitogen activated protein kinase (MAPK) pathways produce response by activation of gene expression. Src and tyrosine kinase promote phosphorylation of specific proteins and promote various intracellular signalings such as mitognesis. The activation of PPRAαυ inhibits the NF-kappa B action via IKK, IκBα and also reduce the expression of the leukocyte adhesion proteins such as V-CAM, I-CAM and E-selectin. STAT3 tyrosine promotes phosphorylation and nuclear translocation which inhibits IL-10-mediated inflammatory signaling and gene expression. Heparin-binding EGF-like growth factor (HB-EGF) is the protein encoded by HBEGF gene. AA – archidonic acid, EET’s – Epoxyeicosatrienoic acids, sEH – soluble epoxide hydrolase, CYP2C – cytochrome p450 2C, CYP2J – cytochrome p450 2J, DHETs – dihydroxyeicosatrienoic acids, cPLA – phospholipase A.
The hypothesis proposed
The cytoprotective properties of EETs have been well established in various peripheral disorders and they may play a similar role in the brain cells. The cytoprotective effect of EETs, however, is limited by their metabolism via soluble epoxide hydrolase [23,25,33–38]. Therefore, we hypothesize that increasing the half life of endogenous EET’s through inhibition of its major metabolizing enzyme, soluble epoxide hydrolase [39], is, therefore a novel approach to prevent/treat the PD [21,40–44].
Justification of proposed hypothesis
The pathogenesis of PD is associated with oxidative stress, mitochondrial dysfunction, protein aggregation, misfolding, inflammation, excitotoxicity, and apoptosis [45]. Oxidative stress results due to overproduction of reactive species or a failure of cell buffering mechanisms that normally limit their accumulation. This oxidative stress results in damage to proteins, lipids, and nucleic acids has been found in the substantia nigra (SN) of PD patients [46]. The ROS production occurs due to a variety of factors including dopamine metabolism, exposure to environmental toxins, mitochondrial dysfunction, probably all of which can result in inhibition of mitochondrial Complex I activity in the SN of PD patients [45–47]. PD patients display impairment of endogenous protective mechanism such as lowered antioxidants such as glutathione, superoxide dismutase, etc. EETs have been reported to promote endogenous mechanisms to buffer free radicals, thereby reducing the oxidative damage to sub-cellular organelles [21,48]. A study by Liu et al. demonstrated that EETs attenuate oxidative stress, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide (ATO) [49]. The results showed that pretreatment with 11,12-EET increased the expression of the antioxidant enzymes superoxide dismutase and catalase and inhibited ATO-induced apoptosis and activation p38 mitogen-activated protein kinase, c-Jun NH2-terminal kinase, caspase-3, and caspase-9, which could have potential neuroprotective and therapeutic implications for PD [49]. However the specific signaling mechanisms by which EETs exert their direct protective effects in astrocytes and neurons still remain unclear.
Neuroinflammation secondary to oxidative stress is one of the primary mechanisms involved in PD. Elevated pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α have been reported in the SN of PD patients [50]. The accumulation of alpha-synuclein (α-syn)-rich Lewy bodies has been reported to directly trigger a microglial response and release of cytotoxic factors [51]. Anamitra Ghosh and co-workers have reported that selective inhibition of NF-κB prevents dopaminergic neuronal loss in a mouse model of PD. The study evaluated the ability of a peptide corresponding to the NF-κB essential modifier-binding domain (NBD) of IκB kinase (IKK) or IKKβ to prevent nigrostriatal degeneration in the MPTP mouse model of PD [52]. The NBD peptide reduced nigral activation of NF-κB, which in turn suppressed the nigral microglial activation, protected both the nigrostriatal axis and neurotransmitters, and improved motor functions in the MPTP model [52]. The anti-inflammatory actions of EETs have also been attributed to cytokine-activated nuclear factor-κB (NF-κB)-mediated transcription. This in turn results in inhibition of IKK phosphorylation of IκBα, resulting in reduction in the plasma levels of pro-inflammatory cytokines and nitric oxide metabolites [38,53].
In addition to NF-κB inhibition, the EETs are also reported to activate the STAT3 receptor, thereby promoting STAT3 tyrosine-705 phosphorylation and nuclear translocation which plays an important role in IL-10-mediated anti-inflammatory signaling and gene expression [54]. EETs are also reported to reduce the expression of the leukocyte adhesion proteins such as vascular cell adhesion molecule (V-CAM), intercellular adhesion molecule (I-CAM) and E-selectin [37], thereby reducing the number of leukocytes induced activation of microglial cells mediated inflammatory damage.
The anti-inflammatory effects of EETs could also be caused via transient receptor potential vanilloid type 1 (TRPV1), which is activated in brain due to heat, endogenous lipid molecules and oxidative stimuli [55] and also due to exogenous agonists such as evodiamine (an active ingredient of the evodia fruit) and capsaicin (an active ingredient of hot pepper). Activation of TRPV1 permits calcium (Ca2+) entry, which results in an elevated level of intracellular Ca2+ which serves as a signal to elicit anti-inflammatory responses in neurons [56,57]. In addition to anti-inflammation, EETs are reported to activate PPAR-γ thereby promoting various physiological processes such as fatty acid and glucose metabolism, angiogenesis, cellular proliferation and differentiation, [58]. The activation of PPAR-γ are reported to suppresses the NF-κB mediated expression of molecules such as VCAM-1, ICAM-1, and endothelins that are involved in the inflammatory response [56]. They also increase the endogenous antioxidant levels of glutathione, SOD, catalase etc. There are several studies which have reported the neuroprotective properties of PPAR-γ agonists in PD [59].
The dopaminergic neurons in the SN of PD patients have reported increased glutamate receptors, and receive glutamatergic innervations from the subthalamic nucleus and cortex. The excessive NMDA receptor activation by glutamate increases intracellular Ca2+ levels which in turn activate cell death pathway [60]. The intracellular Ca2+ is sequestered regularly into the endoplasmic reticulum and mitochondria to prevent the activation of cell death pathways. Due to increased oxidative stress, mitochondrial dysfunction there is imbalance in Ca2+ levels [61]. The Blockade of L-type Cav1.3 calcium channels are reported to decrease the severity of PD. The excitotoxicity of glutamate is reported to be mediated by sustained increase in the cytosolic Ca2+ concentration. EETs have been reported to inhibit cardiac L-type calcium channels which play an important role in regulating cardiac contractility, heart rate etc [62]. Similar mechanisms, may sequester the excessive Ca2+ overload and Ca2+ mediated glutamate excitotoxicity in PD [25,37]. Isradipine a L-type calcium channel inhibitor is in the Phase III clinical trial, which has therapeutic potential in slowing the progression of the PD in pre-clinical studies [63]. The role of EETs in the brain and CNS appears to closely parallel the functions as described in other peripheral tissues [21].
Apart from the above proposed mechanisms, the signaling of by EETs is involved in the process that is distinct to CNS functions. EETs modulate neuronal pain processing in the brainstem. The CYP oxidase metabolic pathway interacts with the neuroactive endocannabinoid pathway [48], which plays an important role in regulating neurohormone release from neuroendocrine regions of the brain. The function of EETs in the neurogenic regulation of cerebral blood flow suggests that EETs may be key regulators of synaptic transmission, a function which is distinct to CNS [37]. Strauss et al., evaluated the effect of traumatic brain injury (TBI) on behavioral phenotypes in soluble Epoxide Hydrolase Knockout Mice (Ephx2-KO). They report that the Ephx2-KO mice showed improved motor coordination in beam walk test when compared to wild-type and a minor impairment in working spatial memory independent of TBI in Morris water maze test. The results of this study show that sEH deficiency interacted both with neurologic and cognitive performance, independent of brain injury [64].
Experimental works carried out by Xiaocui Qin et al., on MPTP model of mice and primary cortical neuronal cell cultures, have reported the increased expression of sEH in MPTP-treated mice. The study reported sEH deficiency and inhibition significantly attenuated tyrosine hydroxylase (TH)-positive cell loss and improved rotarod performance in mice. Data suggested that sEH inhibition might be a powerful tool to protect dopaminergic neurons in PD [65]. Terashvili et al., performed mechanistic studies on co-cultures of astrocytes and N27 dopaminergic neuron and reported that EETs (released from astrocytes and neurons) enhanced cell viability against ROS induced injury. Further, sEH inhibitor (12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) increase the EET levels, thereby increasing the neuronal viability and projecting the cytoprotective ability of EETs against hydrogen peroxide induced oxidative stress in brain [40].
One of the drawbacks associated with EETs is their promitogenic action which may result in uncontrolled cell division and cancer progression. However, Emun Abdu et al., have reported the administration of regioisomeric mixture of EETs resulted in a concentration dependent increase in axon outgrowth in primary sensory and cortical neuronal cell cultures. This suggested a novel therapeutic use of EET’s in promoting nerve regeneration [66]. Munzenmaier et al., have proved that the promitogenic actions of EETs resulted in the formation of endothelium tube, when the cerebral microvascular endothelial cells were cocultured with astrocytes [67]. EETs have also been reported to play an important role in promoting angiogenesis in cerebral vasculature which is due to its promitogenic potential [21,36,48,68]. Thus the promitogenic property of EET exhibit positive or negative actions on CNS is still under research.
In peripheral vasculature, sEH inhibitors are reported to exert vasodilatory effects [24]. however, in the cardiovascular regions of brain such as brainstem they are reported to increase blood pressure (BP) and heart rate (HR) in spontaneously hypertensive rats but not in Wistar Kyoto rats [69]. Hence the central role of sEH inhibitors in hypertension is not yet clear.
Conclusion
Since the pathogenesis in PD is multifactorial, novel compound (s) that simultaneously target multiple degenerate pathways are required. In this regard, our hypothesis on the potential therapeutic effects of EETs is viable since EETs protect against oxidative stress, mitochondrial dysfunction, neuroinflammation and apoptosis. Administration of sEH inhibitors should be tested in various PD models, such as LPS, MPTP, and rotenone. Further, since EETs are naturally occurring endogenous compounds, their elevation might not pose neurotoxicity or systemic toxicity. However, additional experiments are required to understand the solubility of sEH inhibitors, their ability to cross the BBB and bioavailability in the brain. However, it needs to be seen whether such approaches could be tested as independent or adjunct therapy along with existing drug therapy with dopamine replacement.
Acknowledgments
Funding
This work was partially supported by National Institute of Environmental Health Sciences (NIEHS), United States, (Grant number: ES002710). Bruce D. Hammock is a George and Judy Marcus senior fellow of the American Asthma Foundation.
Abbreviations
- sEH
soluble epoxide hydrolase
- mEH
microsomal epoxide hydroxylases
- EETs
Epoxyeicosatrienoic acids
- PD
Parkinson’s disease
- SNCA
synuclein alpha
- LRRK2
leucine-rich repeat kinase 2
- PARK2
E3 ubiquitin-protein ligase parkin
- PINK1
PTEN induced putative kinase 1
- DJ-1
PARK7 – protein deglycase
- ATP13A2
ATPase Type 13A2
- COX
cyclooxygenase
- LOX
lipoxygenase
- CYP 450
cytochrome P450
- PLA2
phospholipase A2
- DiHETEs
dihydroxye-icosatrienoic acids
- ROS
reactive oxygen species
- AUDA
(12-(3-adamantan-1-yl-ureido)-dodecanoic acid
- GC–MS
gas chromatography–mass spectrometry
- LC–MS
liquid chromatography–mass spectrometry
- HPLC
high-performance liquid chromatography
- MCA
middle cerebral artery
- PPAR
peroxisome proliferator-activated receptor
- TRPV4
transient receptor potential cation channel
- mitoKATP
mitochondrial ATP-sensitive K+ channels
- MAPK
mitogen-activated protein kinase
- PI3K
phosphatidylinositol 3kinase
- EDHF
endothelium-derived hyperpolarizing factor
- SN
substantia nigra
- ATO
arsenic trioxide
- IL
interleukins
- TNF
tumor necrosis factor
- NF-κB
nuclear factor κB
- IKK
IκB kinase
- STAT3
signal transducer and activator of transcription 3
- V-CAM
vascular cell adhesion molecule
- I-CAM
intercellular adhesion molecule
- TRPV1
transient receptor potential vanilloid type 1
- CNS
central nervous system
Footnotes
Conflict of interest
Disclosures
The author declares that there is no conflict of interest.
References
- [1].Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurol Clin. 1996;14:317–35. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- [3].Chen S-Y, Tsai S-T. The epidemiology of Parkinson’s disease. Tzu Chi Med J. 2010;22:73–81. [Google Scholar]
- [4].Hague S, Klaffke S, Bandmann O. Neurodegenerative disorders: Parkinson’s disease and Huntington’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1058–63. doi: 10.1136/jnnp.2004.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hindle JV. Ageing, neurodegeneration and Parkinson’s disease. Age Ageing. 2010;39:156–61. doi: 10.1093/ageing/afp223. [DOI] [PubMed] [Google Scholar]
- [7].Antony P, Diederich NJ, Krüger R, Balling R. The hallmarks of Parkinson’s disease. FEBS J. 2013;280:5981–93. doi: 10.1111/febs.12335. [DOI] [PubMed] [Google Scholar]
- [8].Crosiers D, Theuns J, Cras P, Van Broeckhoven C. Parkinson disease: insights in clinical, genetic and pathological features of monogenic disease subtypes. J Chem Neuroanat. 2011;42:131–41. doi: 10.1016/j.jchemneu.2011.07.003. [DOI] [PubMed] [Google Scholar]
- [9].und Halbach OVB, Schober A, Krieglstein K. Genes, proteins, and neurotoxins involved in Parkinson’s disease. Prog Neurobiol. 2004;73:151–77. doi: 10.1016/j.pneurobio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- [10].Eriksen JL, Wszolek Z, Petrucelli L. Molecular pathogenesis of Parkinson disease. Arch Neurol. 2005;62:353–7. doi: 10.1001/archneur.62.3.353. [DOI] [PubMed] [Google Scholar]
- [11].Bonnet A, Houeto J. Pathophysiology of Parkinson’s disease. Biomed Pharmacother. 1999;53:117–21. doi: 10.1016/S0753-3322(99)80076-6. [DOI] [PubMed] [Google Scholar]
- [12].Fernandez-Espejo E. Pathogenesis of Parkinson’s disease. Mol Neurobiol. 2004;29:15–30. doi: 10.1385/MN:29:1:15. [DOI] [PubMed] [Google Scholar]
- [13].Ambhore N, Mali J, Kanhed A, Antony S, Bhalerao A, Bhojraj S. Pharmacological and biochemical interventions of cigarette smoke, alcohol, and sexual mating frequency on idiopathic rat model of Parkinson’s disease. J Young Pharm. 2012;4:177–83. doi: 10.4103/0975-1483.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dalfó E, Portero-Otín M, Ayala V, Martínez A, Pamplona R, Ferrer I. Evidence of oxidative stress in the neocortex in incidental Lewy body disease. J Neuropathol Exp Neurol. 2005;64:816–30. doi: 10.1097/01.jnen.0000179050.54522.5a. [DOI] [PubMed] [Google Scholar]
- [15].Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–72. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- [16].Lee H-J, Bazinet RP, Rapoport SI, Bhattacharjee AK. Brain arachidonic acid cascade enzymes are upregulated in a rat model of unilateral Parkinson disease. Neurochem Res. 2010;35:613–9. doi: 10.1007/s11064-009-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Samuelsson B. An elucidation of the arachidonic acid cascade. Drugs. 1987;33:2–9. doi: 10.2165/00003495-198700331-00003. [DOI] [PubMed] [Google Scholar]
- [18].Falck J, Kodela R, Manne R, et al. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14, 15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–75. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marowsky A, Burgener J, Falck J, Fritschy J-M, Arand M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 2009;163:646–61. doi: 10.1016/j.neuroscience.2009.06.033. [DOI] [PubMed] [Google Scholar]
- [20].Sura P, Sura R, EnayetAllah AE, Grant DF. Distribution and expression of soluble epoxide hydrolase in human brain. J Histochem Cytochem. 2008;56:551–9. doi: 10.1369/jhc.2008.950659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010;91:68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shen HC. Soluble epoxide hydrolase inhibitors: a patent review. Expert Opin Ther Pat. 2010;20:941–56. doi: 10.1517/13543776.2010.484804. [DOI] [PubMed] [Google Scholar]
- [23].Jiang H, Anderson GD, McGiff JC. The red blood cell participates in regulation of the circulation by producing and releasing epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat. 2012;98:91–3. doi: 10.1016/j.prostaglandins.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khan MAH, Pavlov TS, Christain SV, et al. Epoxyeicosatrienoic acid analogue lowers blood pressure through vasodilation and sodium channel inhibition. Clin Sci. 2014;127:463–74. doi: 10.1042/CS20130479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shrestha A, Krishnamurthy PT, Thomas P, Hammock BD, Hwang SH. Soluble epoxide hydrolase inhibitor, t-TUCB, protects against myocardial ischaemic injury in rats. J Pharm Pharmacol. 2014;66:1251–8. doi: 10.1111/jphp.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Qiu H, Li N, Liu JY, Harris TR, Hammock BD, Chiamvimonvat N. Soluble epoxide hydrolase inhibitors and heart failure. Cardiovasc Ther. 2011;29:99–111. doi: 10.1111/j.1755-5922.2010.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang H, Chen J, Lin T, et al. Epoxyeicosatrienoic acids – novel mechanism and pharmacological therapy of chronic renocardiac syndrome. Med Hypotheses. 2011;76:550–2. doi: 10.1016/j.mehy.2010.12.015. [DOI] [PubMed] [Google Scholar]
- [28].Lee KSS, Liu J-Y, Wagner KM, et al. Optimized inhibitors of soluble epoxide hydrolase improve in vitro target residence time and in vivo efficacy. J Med Chem. 2014;57:7016–30. doi: 10.1021/jm500694p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wagner K, Yang J, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition is antinociceptive in a mouse model of diabetic neuropathy. J Pain. 2014;15:907–14. doi: 10.1016/j.jpain.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang G, Panigrahy D, Hwang SH, et al. Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc Natl Acad Sci. 2014;111:11127–32. doi: 10.1073/pnas.1410432111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].López-Vicario C, Alcaraz-Quiles J, García-Alonso V, et al. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci. 2015;112:536–41. doi: 10.1073/pnas.1422590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang W, Koerner IP, Noppens R, et al. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–40. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev. 2011;63:597–609. doi: 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [34].Nithipatikom K, Gross GJ. Epoxyeicosatrienoic acids: novel mediators of cardioprotection. J Cardiovasc Pharmacol Ther. 2010 doi: 10.1177/1074248409358408. [DOI] [PubMed] [Google Scholar]
- [35].Yang B, Graham L, Dikalov S, et al. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60:310–20. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]
- [36].Iliff JJ, Alkayed NJ. Soluble epoxide hydrolase inhibition: targeting multiple mechanisms of ischemic brain injury with a single agent. Future Neurol. 2009;4:179–99. doi: 10.2217/14796708.4.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol-Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- [38].Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433:413–20. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- [39].Qin X, Wu Q, Lin L, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates MPTP-induced parkinsonism. Mol Neurobiol. 2014:1–9. doi: 10.1007/s12035-014-8833-3. [DOI] [PubMed] [Google Scholar]
- [40].Terashvili M, Sarkar P, Van Nostrand M, Falck JR, Harder DR. The protective effect of astrocyte-derived 14, 15-EET on H2O2-induced cell injury in Astrocyte-dopaminergic neuronal cell line co-culture. Neuroscience. 2012;223:68. doi: 10.1016/j.neuroscience.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hammock BD, Wagner K, Inceoglu B. The soluble epoxide hydrolase as a pharmaceutical target for pain management. Pain Manage. 2011;1:383–6. doi: 10.2217/pmt.11.47. [DOI] [PubMed] [Google Scholar]
- [42].Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA. 2005;102:2186–91. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–9. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schmelzer KR, Kubala L, Newman JW, Kim I-H, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–7. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yacoubian TA, Standaert DG. Targets for neuroprotection in Parkinson’s disease. Biochim Biophys Acta (BBA) – Mol Basis Dis. 2009;1792:676–87. doi: 10.1016/j.bbadis.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Alam Z, Jenner A, Daniel S, et al. Oxidative DNA damage in the Parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- [47].Dexter D, Sian J, Rose S, et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 1994;35:38–44. doi: 10.1002/ana.410350107. [DOI] [PubMed] [Google Scholar]
- [48].Zhang G, Kodani S, Hammock BD. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog Lipid Res. 2014;53:108–23. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liu L, Chen C, Gong W, et al. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J Pharmacol Exp Ther. 2011;339:451–63. doi: 10.1124/jpet.111.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–97. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- [51].Zhang W, Wang T, Pei Z, et al. Aggregated α-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- [52].Ghosh A, Roy A, Liu X, et al. Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci. 2007;104:18754–9. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Node K, Huo Y, Ruan X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–9. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–76. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- [55].Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-γ activators. Circulation. 2000;101:235–8. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- [57].Zhao J-F, Ching L-C, Kou YR, et al. Activation of TRPV1 prevents OxLDL-induced lipid accumulation and TNF-α-induced inflammation in macrophages: role of liver X receptor α. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/925171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu Y, Zhang Y, Schmelzer K, et al. The antiinflammatory effect of laminar flow: the role of PPARγ, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–52. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Swanson CR, Du E, Johnson DA, Johnson JA, Emborg ME. Neuroprotective properties of a novel non-thiazoledinedione partial PPAR-γ agonist against MPTP. PPAR Res. 2013;2013 doi: 10.1155/2013/582809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kannurpatti SS, Joshi PG, Joshi NB. Calcium sequestering ability of mitochondria modulates influx of calcium through glutamate receptor channel. Neurochem Res. 2000;25:1527–36. doi: 10.1023/a:1026602100160. [DOI] [PubMed] [Google Scholar]
- [61].Surmeier DJ. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol. 2007;6:933–8. doi: 10.1016/S1474-4422(07)70246-6. [DOI] [PubMed] [Google Scholar]
- [62].Chen J, Capdevila JH, Zeldin DC, Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol Pharmacol. 1999;55:288–95. doi: 10.1124/mol.55.2.288. [DOI] [PubMed] [Google Scholar]
- [63].Ilijic E, Guzman J, Surmeier D. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 2011;43:364–71. doi: 10.1016/j.nbd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Strauss KI, Gruzdev A, Zeldin DC. Altered behavioral phenotypes in soluble epoxide hydrolase knockout mice: effects of traumatic brain injury. Prostaglandins Other Lipid Mediat. 2013;104:18–24. doi: 10.1016/j.prostaglandins.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Qin X, Wu Q, Lin L, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates MPTP-induced parkinsonism. Mol Neurobiol. 2015;52:187–95. doi: 10.1007/s12035-014-8833-3. [DOI] [PubMed] [Google Scholar]
- [66].Abdu E, Bruun DA, Yang D, et al. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2011;117:632–42. doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol – Heart Circ Physiol. 2000;278:H1163–7. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- [68].Wang Y, Wei X, Xiao X, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther. 2005;314:522–32. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- [69].Sellers KW, Sun C, Diez-Freire C, et al. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J. 2005;19:626–8. doi: 10.1096/fj.04-3128fje. [DOI] [PubMed] [Google Scholar]