Abstract
The ornamental pet trade is often considered a key culprit for conservation problems such as the introduction of invasive species (including infectious diseases) and overharvesting of rare species. Here, we present the first assessment of the biodiversity of freshwater molluscs in the ornamental pet trade in Singapore, one of the most important global hubs of the ornamental aquarium trade, and discuss associated conservation concerns. We recorded freshwater molluscs from ornamental pet shops and major exporters including non-ornamental species (e.g., hitchhikers, molluscs sold as fish feed). We recorded an unexpectedly high diversity—59 species—of freshwater bivalves and gastropods, with the majority (38 species or 64%) being from the Oriental region. In addition to morphological examination, we sequenced the DNA barcode region of mitochondrial CO1 and 16S genes to provide molecular data for the confirmation of the identification and for future re-identification. DNA barcodes were obtained for 50 species, and all but four were separated by > 3% uncorrected pairwise distances. The trade has been considered a main introduction pathway for non-native species to Singapore, and we found that out of 15 species in the trade as well as in the wild in Singapore, 12 are either introduced or of unknown origin, representing almost half of the known non-native freshwater molluscs in Singapore. Particularly prevalent are non-ornamental species: six hitchhikers on aquarium plants and six species sold as fish feed. We found that a quarter of the trade species have a history of introduction, which includes 11 known or potentially invasive species. We conclude that potential overharvesting is difficult to assess because only half of the trade species have been treated by IUCN. Of these, 21 species are of Least Concern and three are Data Deficient. Our checklist, with accompanying DNA barcodes, images, and museum vouchers, provides an important reference library for future monitoring, and constitutes a step toward creating a more sustainable ornamental pet trade.
Introduction
The global aquarium or ornamental fish industry has been valued at US$15 billion per year [1]. While fish in the ornamental pet trade have generally been well catalogued and studied, (e.g., [2, 3, 4], little information exists for invertebrates, including freshwater molluscs, which have seen noticeably increased interest from hobbyists in recent years [5, 6, 7]. Few accounts of non-fish taxa in the trade have been published, and most of these concentrate on the ornamental trade as a source or pathway of introduction of organisms (e.g., [8–15]). Invasive species are often noted in the ornamental pet trade only after they have established populations, or have caused negative impacts in a new range (e.g., [8, 16]). The few freshwater molluscs reported from the trade have mainly been species identified as intermediate hosts of zoonotic parasites [8, 16, 17, 18, 19]. Despite the risks related to the spread of freshwater molluscs via the pet trade, ornamental freshwater molluscs continue to escape in-depth scrutiny.
In addition to being implicated in the introduction of non-native species, the ornamental pet trade has also been associated with overharvesting of species, especially of fish [20, 21, 22]. Overexploitation of freshwater molluscs is a neglected, but genuine issue, because many species have highly-restricted distributions, strict habitat requirements, or both [23, 24, 25]. Non-marine molluscs are recognised as being highly threatened—99% of documented mollusc extinctions are of non-marine species, yet fewer than 2% of mollusc species have been properly assessed [26]. This makes it likely that the correct number of extinctions is much higher than estimated [27]. Demand for narrowly-endemic species in the ornamental pet trade has increased recently [28] and may represent a conservation concern that will remain difficult to detect until there is a concerted effort to document the freshwater molluscs in the trade.
While freshwater molluscs in the ornamental pet trade have largely been overlooked, countries have been implementing measures to monitor other commonly-traded animals for 1) biosecurity to prevent the introduction of potentially harmful organisms, including vectors of infectious diseases, or 2) law enforcement to protect against illegal wildlife trade [29–32]. One of the main challenges in monitoring organisms that are imported or exported is the difficulty in reliably identifying those species that are of biosecurity or conservation concern. To address this problem, molecular tools such as DNA barcoding are increasingly being used for rapid and accurate identification of ornamental organisms, especially the employment of DNA barcoding for fish [33, 34]. The mitochondrial cytochrome oxidase I (CO1) and 16S rRNA genes have been used for this purpose and for tracing source populations of species in the ornamental trade [33–39].
Biosecurity measures are of primary importance to countries involved in the ornamental pet industry. Singapore is the top exporter of ornamental fish in the world, with annual trade of ornamental freshwater fish alone averaging over US$60 million [40, 41]. The United States of America and the United Kingdom receive 40% of Singapore’s exports, and among the top 20 trading partners are countries from across Asia, Europe, Africa, and Australasia [41]. Biosecurity efforts are usually focusing on preventing entry and the spread of infectious diseases [31, 42]. A related concern is that shipments of ornamental fish or plants could also carry hitchhiking organisms. Snails are regularly intercepted by border control worldwide, including in shipments originating from Singapore [19, 43, 44]. Although the ornamental pet trade has been suspected to be the main source of the many non-native freshwater molluscs established in Singapore [45–48], the link remains speculative [47].
We present here the first assessment of freshwater mollusc species acquired from the ornamental pet trade. We included both intentionally imported ornamental species as well as species that are accidentally brought via the trade. As Singapore is a global hub for the ornamental pet trade, the resulting species list is potentially representative of species that could be traded and potentially introduced worldwide. Additionally, we provide the first set of CO1 and 16S DNA barcodes of freshwater molluscs present in the ornamental trade for a nascent DNA reference library of freshwater molluscs in the ornamental pet trade. Such a library could provide critical information for the conservation of freshwater molluscs (e.g., to monitor if any threatened species are being traded [38]), and to aid in biosecurity monitoring and prevention of the introduction of harmful alien species [34].
Material and Methods
Sample and data collection
We recorded freshwater mollusc species encountered in the Singapore ornamental pet trade—local ornamental pet retail shops and major ornamental exporters (see S1 Table)—between 2008 and 2014. “Ornamental exporters” refers to major distributors that import stock from various sources (especially from within the surrounding region), and re-package the animals for export to customers abroad [49]. Species were considered as hitchhiking species when they were found in shipments or tanks that did not specifically contain those molluscs for sale. This includes hitchhikers from tanks holding other mollusc or fish species, and hitchhikers on ornamental aquatic plants in home aquariums or nurseries. Specimens that were being sold primarily as fish feed were also included in the study. Voucher specimens are deposited in the Zoological Reference Collection (ZRC) of the Lee Kong Chian Natural History Museum at the National University of Singapore (ZRC.MOL.5904–5952, 6284–6333, 6752–6754; see Results). At least two individuals per lot (except for hitchhikers, in which case there were mostly one individual per lot) were examined, and identified based on descriptions and figures in [47, 48, 50–75], and checked against original descriptions of the taxa. The distribution, introduction history, and associated parasites of all species were recorded based on available literature (see Results). When necessary, we consulted malacological experts of respective taxa, for advice and assistance with identification.
DNA extraction, amplification, and sequencing
We extracted total genomic DNA from the tissue samples (foot tissue of gastropods or adductor muscle of bivalves) of freshwater molluscs obtained from the aquarium trade using a phenol-chloroform extraction protocol. The DNA barcodes (mitochondrial CO1 and 16S rRNA) were amplified in polymerase chain reactions (PCR) with a total volume of 23–24μl PCR rection mixture (2.5μl of Taq 10× buffer, 2mM dNTPs, 1μl of 10μM primers (Table 1), 0.25μl of BioReady rTaq DNA Polymerase (Bulldog Bio), and DNase-free sterile water), at 95°C for 5min, 34 cycles of 95°C for 30sec, 45–48°C for 30sec, and 72°C for 30sec, and a final extension of 72°C for 10min. Fragments of CO1 were obtained using three different pairs of primers that enabled us to assess the wide range of taxa involved (Table 1). The primer pairs LCO1490/HCO2198 and GASF1_t1/GASR1_t1 amplified approximately 600 base pairs (bp) in the barcode region, while mlCOintF/jgHCO2198 amplified a shorter fragment (313 bp) in the 3’ region. The latter pair was used in specimens that failed to amplify the standard barcode region. The size of 16S fragments amplified ranged from 320 to 476 bp. The PCR products were checked visually on a 1% agarose gel. Post PCR clean-ups were performed on successfully amplified products using SureClean reagent (Bioline Inc.) following the manufacturer’s recommendations. The purified products were sequenced with BigDye Terminator reactions and analysed on the ABI PRISM 3130XL sequencer (Applied Biosystems) at the DNA Sequencing Laboratory of the National University of Singapore.
Table 1. Primers used to obtain sequences.
| Primers | Sequences | References |
|---|---|---|
| LCO1490 | 5′ GGTCAACAAATCATAAAGATATTGG 3′ | [120] |
| HCO2198 | 5′ TAAACTTCAGGGTGACCAAAAAATCA 3′ | [120] |
| GASF1_t1 | 5′ TGTAAAACGACGGCCAGTTTTCAACAAACCATAARGATATTGG 3′ | [121] |
| GAS R1_t1 | 5′ CAGGAAACAGCTATGACACTTCWGGRTGHCCRAARAATCARAA 3′ | [121] |
| jgHCO2198 | 5’ TAIACYTCIGGRTGICCRAARAAYCA 3’ | [122] |
| mICOintF | 5’ GGWACWGGWTGAACWGTWTAYCCYCC 3’ | [123] |
| 16Sar-L | 5’ CGCCTGTTTATCAAAAACAT 3’ | [124] |
| 16Sbr-H | 5’ CCGGTCTGAACTCAGATCACGT 3’ | [124] |
Data analysis
We visually inspected and trimmed sequences using Sequencher ver. 4.6 (Genecodes). The CO1 and 16S genes were aligned using MAFFT version 7 [76] with default settings. Aligned CO1 sequences were checked for translatability into amino acids and were gap free. DNA sequences were inspected using objective clustering in SpeciesIdentifier version 1.7.9 [77], with species delimitation thresholds of 1–4% uncorrected pairwise distances [78, 79]. Objective clusters are groups of sequences which have at least one other sequence below the threshold. Each cluster is considered as a molecular operational taxonomic unit (mOTU) [77, 80]. Previous studies [78, 81] have shown the intraspecific genetic distance for majority of gastropods is <2% for CO1. Here, we employed 1–4% thresholds to assess for stability of genetic clusters. A BLASTn search (highly similar sequences (megablast)) [82] was carried out on GenBank in order to confirm the species delimited in SpeciesIdentifier. If identified sequences from GenBank were 97–100% identical to the query sequence, but the species identity differed from our morphology-based identification, we re-examined the specimens and compared it to the original descriptions. Wherever possible, we contacted the contributors of the GenBank sequences for comparative material (photographs or material deposited in collections). All sequences were deposited in GenBank and BOLD (S2 Table).
Results
Source of freshwater molluscs in the trade
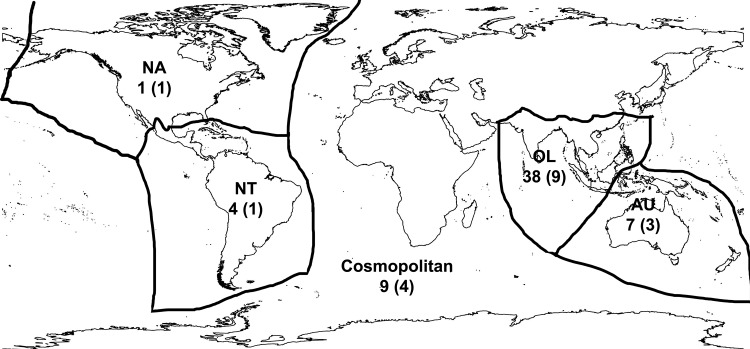
We obtained 148 lots of freshwater molluscs, and identified 59 species from 13 families in the ornamental pet trade based on morphology (Table 2, Fig 1). Fifty species in the trade originate from one of four zoogeographic regions (Fig 2)—38 species from the Oriental region (including Sundaic Southeast Asia, Indo-Burma, and the Indian subcontinent), seven from the Australasian region (including Sulawesi), four from the Neotropics, and one from the Nearctic—while nine species are regarded as being native to more than one region (cosmopolitan). Fourteen species from seven families have been introduced to regions beyond their native distributions, with 11 of these recorded to cause (or potentially cause) negative impacts in their invaded habitats (Table 3). Twenty-four species have been assessed for conservation status by the IUCN, with 21 being species of Least Concern (LC), and three species being Data Deficient (DD) [83].
Table 2. Checklist of freshwater molluscs recorded from the Singapore ornamental pet trade from 2008 to 2014.
| Family | Species | Geographic Distribution | Source | IUCN Status | DNA barcode | Catalogue No. | Remarks | References | |
|---|---|---|---|---|---|---|---|---|---|
| E | L | ||||||||
| Cyrenidae | Batissa similis Prime, 1862 | OL | 1 | DD | ZRC.MOL.6752 | [58] | |||
| Batissa violacea (Lamarck, 1818) | OL | 1 | CO1; 16S | ZRC.MOL.5904 | [51] | ||||
| Corbicula fluminea (Müller, 1774)† | OL | 1 | 1 | LC | CO1; 16S | ZRC.MOL.5905 | Hitchhiker and feed. | [56] | |
| Corbicula moltkiana Prime, 1878 | OL | 1 | DD | CO1; 16S | ZRC.MOL.5907 | [61] | |||
| Unionidae | Hyriopsis bialata (Simpson, 1800) | OL | 1 | 1 | LC | CO1 | ZRC.MOL.5908 | [55] | |
| Hyriopsis desowitzi Brandt, 1974 | OL | 1 | DD | 16S | ZRC.MOL.2195 | [55] | |||
| Parreysia burmana (Blandford, 1869) | OL | 1 | LC | CO1; 16S | ZRC.MOL.5909 | [55, 58] | |||
| Parreysia tavoyensis (Gould, 1843) | OL | 1 | CO1; 16S | ZRC.MOL.5910 | [58] | ||||
| Pilsbryoconcha exilis (Lea, 1856)† | OL | 1 | LC | CO1 | ZRC.MOL.5911 | [47, 55, 125] | |||
| Scabies crispata (Gould, 1843) | OL | 1 | LC | 16S | ZRC.MOL.5913 | [55, 58] | |||
| Sinanodonta woodiana (Lea, 1834)† | OL | 1 | LC | CO1 | ZRC.MOL.2914 | [47, 56, 126, 127] | |||
| Unionetta fabagina (Deshayes & Jullien, 1874) | OL | 1 | LC | ZRC.MOL.67523 | [55, 128] | ||||
| Ampullariidae | Marisa cornuarietis (Linnaeus, 1758) | NT | 1 | LC | CO1 | ZRC.MOL.5914 | [66, 129, 130] | ||
| Pomacea canaliculata (Lamarck, 1822)† | NT | 1 | 1 | LC | CO1 | ZRC.MOL.5915 | Feed. | [67, 130] | |
| Pomacea maculata Perry, 1810† | NT | 1 | Collection of K.A. Hayes | [48, 66] (as Pomacea insularum), [67] (as Pomacea insularum), [72] | |||||
| Pomacea diffusa Blume, 1957 | NT | 1 | 1 | CO1 | ZRC.MOL.5916 | [66, 67, 129, 130] | |||
| Bithynidae | Bithynia sp.† | OL, PA, AU, AT | 1 | CO1; 16S | ZRC.MOL.5917 | Feed. | [47] | ||
| Lymnaeidae | Radix rubiginosa (Michelin, 1831) | OL | 1 | CO1; 16S | ZRC.MOL.5920 | [55] | |||
| Nassariidae | Anentome bockii (Brot, 1881) | OL | 1 | CO1 | ZRC.MOL.5918 | [131] | |||
| Anenetome helena (Philippi, 1847) | OL | 1 | CO1 | ZRC.MOL.5919 | [55] | ||||
| Neritidae | Clithon corona (Linnaeus, 1758) | OL | 1 | 1 | LC | CO1 | ZRC.MOL.5921 | [53, 75] | |
| Clithon diadema (Récluz, 1841) | OL | 1 | CO1; 16S | ZRC.MOL.5922 | [58, 75] | ||||
| Clithon lentiginosum (Reeve, 1855) | OL, AU | 1 | CO1; 16S | ZRC.MOL.5923 | [75] | ||||
| Clithon mertoniana (Récluz, 1843) | OL | 1 | CO1; 16S | ZRC.MOL.5924 | [75] | ||||
| Neripteron auriculata (Lamarck, 1816) | OL, AU | 1 | CO1; 16S | ZRC.MOL.5925 | [53, 75] | ||||
| Neritina iris (Mousson, 1849) | OL, AU | 1 | CO1 | ZRC.MOL.5926 | T. Eichhorst, pers. comm. | ||||
| Neritina juttingae Mienis, 1973 | OL | 1 | CO1; 16S | ZRC.MOL.5927 | [132] | ||||
| Neritina violacea (Gmelin, 1791)† B | OL, AU, PA | 1 | LC | CO1 | ZRC.MOL.5928 | [53] | |||
| Neritodryas cornea (Linnaeus, 1758) | OL to PAC | 1 | CO1 | ZRC.MOL.5929 | [55] | ||||
| Septaria porcellana (Linnaeus, 1758) | OL | 1 | 16S | ZRC.MOL.5933 | [110] | ||||
| Vittina coromandeliana (Sowerby, 1932)† B | OL | 1 | CO1 | ZRC.MOL.5930 | [55] | ||||
| Vittina turrita (Gmelin, 1791) | OL to PAC | 1 | CO1 | ZRC.MOL.5931 | [53] | ||||
| Vittina waigiensis (Lesson, 1831) | OL | 1 | CO1; 16S | ZRC.MOL.5932 | [53] | ||||
| Pachychilidae | Brotia armata (Brandt, 1968) | OL | 1 | LC | 16S | ZRC.MOL.2935 | Narrowly-endemic | [64] | |
| Brotia binodosa (Blanford, 1903) | OL | 1 | LC | 16S | ZRC.MOL.2934 | Narrowly-endemic | [55] | ||
| Brotia herculea (Gould, 1846) | OL | 1 | LC | CO1 | ZRC.MOL.5934 | [64] | |||
| Brotia pagodula (Gould, 1847) | OL | 1 | ZRC.MOL.5935 | Narrowly-endemic | [55] | ||||
| Sulcospira tonkiniana (Morlet, 1887) | OL | 1 | LC | CO1 | ZRC.MOL.5936 | [70] | |||
| Tylomelania towutica (Kruimel, 1913) | AU | 1 | CO1 | ZRC.MOL.5939 | Narrowly-endemic | [133] | |||
| Tylomelania sp. | AU | 1 | CO1, 16S | ZRC.MOL.2397 | Narrowly-endemic | T. von Rintelen, pers. comm. | |||
| Tylomelania sp. | AU | 1 | ZRC.MOL.5939 | Narrowly-endemic | T. von Rintelen, pers. comm. | ||||
| Tylomelania sp. | AU | 1 | ZRC.MOL.2933 | Narrowly-endemic | T. von Rintelen, pers. comm. | ||||
| Physidae | Physa sp. | NA | 1 | 1 | CO1 | ZRC.MOL.5773 | Hitchhiker. | [74, 134] | |
| Planorbidae | Amerianna carinata (H. Adams, 1861)† | AU | 1 | ZRC.MOL.5952 | Feed and hitchhiker. | [47, 53, 55, 135, 136] | |||
| Gyraulus convexiusculus (Hutton, 1849) | OL, PA | 1 | LC | CO1 | ZRC.MOL.5953 | Hitchhiker. | [137] | ||
| Indoplanorbis exustus (Deshayes, 1834)† | OL | 1 | LC | CO1 | ZRC.MOL.5940 | Feed. | [53, 57] | ||
| Semisulcospiridae | Semisulcospira sp. | OL | 1 | CO1 | ZRC.MOL.5942 | [56], F. Köhler pers. comm. | |||
| Thiaridae | Melanoides tuberculata (Müller, 1774)† | OL, AU, PA | 1 | LC | CO1 | ZRC.MOL.5943 | [19, 55, 138] | ||
| Stenomelania offachiensis Lesson, 1831 | AU | 1 | CO1; 16S | ZRC.MOL.5944 | [112] | ||||
| Stenomelania plicaria (Born, 1778) | OL | 1 | LC | CO1 | ZRC.MOL.5945 | [112] | |||
| Stenomelania cf. plicaria | OL | 1 | CO1 | ZRC.MOL. 2195 | [112] | ||||
| Stenomelania sp. | OL, AU, PA, PAC | 1 | CO1; 16S | ZRC.MOL. 5946 | M. Glaubrecht, pers. comm. | ||||
| Thiara cancellata Röding, 1798 | OL | 1 | CO1; 16S | ZRC.MOL.5947 | [53] | ||||
| Viviparidae | Celetaia persculpta (Sarasin & Sarasin, 1898) | AU | 1 | ZRC.MOL.6752 | Narrowly-endemic | [139] | |||
| Filopaludina cambodjensis (Mabile & Le Mesle, 1866) | OL | 1 | LC | CO1 | ZRC.MOL.5948 | Hitchhiker. | [55] | ||
| Filopaludina peninsularis Brandt, 1974† | OL | 1 | LC | CO1; 16S | ZRC.MOL.5949 | Hitchhiker. | [47, 55] | ||
| Filopaludina polygramma (Martens, 1897)† | OL | 1 | ZRC.MOL.2721 | [47, 55] | |||||
| Sinotaia guangdungensis (von Frauenfeld, 1862)† | OL | 1 | CO1 | ZRC.MOL.5950 | Feed. | [47] (as Taia polyzonata), [73, 140] (as Bellamya heudei guangdungensis) | |||
| Taia pseudoshanensis Zilch, 1955 | OL | 1 | 16S | ZRC.MOL.5951 | [52] | ||||
† indicates species found in Singapore
B indicates brackish water species. Geographic distribution: NA—Nearctic; NT—Neotropical; PA—Palaearctic; AT—Afrotropical; OL—Oriental; AU—Australasian; PAC—Pacific Oceanic Islands. Source (of specimens): E—export; obtained from ornamental exporters; L—local; obtained from local pet shops or aquatic plant nurseries. IUCN status (if assessed): LC—Least Concern; DD—Data Deficient. DNA barcode: CO1—mitochondrial cytochrome oxidase subunit 1 gene; 16S—16S ribosomal RNA gene; refer to S2 Table for further details. Catalogue no.: ZRC—Zoological Reference Collection of the Lee Kong Chian Natural History Museum. Remarks include: hitchhiker—hitchhikers among other ornamental animals or plants; feed—sold as fish food; narrowly-endemic—species that are endemic to a particular river system or lake.
Fig 1. Freshwater molluscs in the ornamental pet trade.
Unless indicated differently, scale bars = 10mm. 1. Batissa similis; 2. Batissa violacea; 3. Corbicula fluminea; 4. Corbicula moltkiana; 5. Hyriopsis bialata; 6. Hyriopsis desowitzi; 7. Parreysia burmana; 8. Parreysia tavoyensis; 9. Pilsbryoconcha exilis; 10. Scabies crispata; 11. Sinanodonta woodiana; 12. Unionetta fabagina; 13. Marisa cornuarietis; 14. Pomacea canaliculata; 15. Pomacea diffusa; 16. Pomacea maculata (photograph by K.A. Hayes); 17. Bithynia sp.; 18. Clea bockii; 19. Clea helena; 20. Radix rubiginosa; 21. Clithon corona; 22. Clithon diadema; 23. Clithon lentiginosum; 24. Clithon mertoniana; 25. Neripteron auriculata; 26. Neritina iris; 27. Neritina juttingae; 28. Neritina violacea; 29. Neritodryas cornea; 30. Septaria porcellana; 31. Vittina coromandeliana; 32. Vittina turrita; 33. Vittina waigiensis; 34. Brotia armata; 35. Brotia binodosa; 36. Brotia herculea; 37. Brotia pagodula; 38. Sulcospira tonkiniana; 39. Tylomelania towutica; 40. Tylomelania sp.; 41. Tylomelania sp.; 42. Tylomelania sp.; 43. Physa sp.; 44. Amerianna carinata; 45. Indoplanorbis exustus; 46. Gyraulus convexiusculus; 47. Semisulcospira sp.; 48. Melanoides tuberculata; 49. Stenomelania offachiensis; 50. Stenomelania plicaria; 51. Stenomelania cf. plicaria; 52. Stenomelania sp.; 53. Thiara cancellata; 54. Celetaia persculpta; 55. Filopaludina cambodjensis; 56. Filopaludina peninsularis; 57. Filopaludina polygramma; 58. Sinotaia guangdungensis; 59. Taia pseudoshanensis.
Fig 2. Native distribution of freshwater mollusc species in the ornamental pet trade.
Numbers indicate the number of species from each zoogeographic region; numbers in brackets are the number of families. Zoogeographic regions follow [24].
Table 3. Freshwater molluscs in the aquarium trade with history of introduction.
| Family | Species | Native to | Introduced to | Known or potential impacts | References |
|---|---|---|---|---|---|
| Cyrenidae | Corbicula fluminea* | Unknown | Worldwide | Outcompete native species; biofouling | [141–146] |
| Unionidae | Pilsbryoconcha exilis* | OL | Singapore and Philippines | — | [47, 125] |
| Sinanodonta woodiana | OL | Europe, Indonesia, Singapore, Central America, USA | Displaced native species; compete with native species for food, habitats, fish hosts | [47, 126, 128, 147–150] | |
| Ampullariidae | Marisa cornuarietis* | NT | USA, Caribbean islands, Central America, Africa, Middle East, Spain | Agricultural pest | [66, 129, 130, 151] |
| Pomacea canaliculata* | NT | Worldwide | Agricultural pest; outcompete native species; co-introduction of zoonotic parasite Angistrongyliasis cantonensis resulted in outbreaks of angiostrongyliasis when snail consumed by humans | [67, 72, 130, 152–155] | |
| Pomacea diffusa* | NT | USA, Brazil, Colombia, Panama, French Guiana, Israel, South Africa, Australia, New Zealand, India, Sri Lanka | Potential predator and competitor of native species; known to feed on aquatic macrophytes, snail eggs. | [44, 66, 67, 129] (as Pomacea bridgesii), [130, 156] (as Pomacea bridgesii) | |
| Pomacea maculata* | NT | Southeast Asia, South Korea, USA | Agricultural pest; outcompete native species; co-introduction of zoonotic parasite Angistrongyliasis cantonensis | [67] (as Pomacea insularum), [72, 130] (as Pomacea insularum), [157] (as Pomacea insularum), [158, 159 | |
| Lymnaeidae | Radix rubiginosa* | OL | UK, Israel, South Africa | Known host of various parasites that affect humans and livestock | 44, 160–162] |
| Planorbidae | Amerianna carinata* | AU | India, Java, Martinique island, Nigeria, Singapore, Thailand, Japan | — | [47, 135, 163] |
| Gyraulus convexiusculus* | OL | Japan, Middle East | Known host of zoonotic parasites Echinostomatidae | [71, 137] | |
| Indoplanorbis exustus* | OL | Nigeria, Java and Sulawesi, of unknown origin in Singapore | Known host of zoonotic parasite Schistosoma spindale | [47, 50, 53, 57, 164] | |
| Thiaridae | Melanoides tuberculata* | OL, AU, PA | Worldwide | Outcompeted native species; co-introduction of various parasites including zoonotic parasites and Centrocestus formosanus that has resulted in losses in commercial fisheries and infected wild fish populations | [19, 89, 138, 165, 166] |
| Viviparidae | Filopaludina polygramma* | OL | Singapore | Known host of zoonotic parasite Angiostrongyliasis cantonensis | [47, 71] |
| Sinotaia guangdungensis | OL | Australia, Singapore, Malaysia | — | [47] (as Taia polyzonata), [73, 140] (as Bellamya heudei guangdungensis) |
*Purportedly introduced via the ornamental pet trade.
Twenty-one of the species were recorded from aquarium shops or ornamental plant nurseries in Singapore, while 45 were recorded from ornamental exporters. Of these, five species were recorded from both sources. Six species were found as hitchhikers on aquatic plants or incidentally transported with ornamental fish or other freshwater molluscs (“hitchhiker”). Fifteen of the species are found in the wild in Singapore—three are native species (Melanoides tuberculata, Neritina violacea, Vittina coromandeliana), eight introduced (Amerianna carinata, Corbicula fluminea, Filopaludina polygramma, Pilsbryoconcha exilis, Pomacea canaliculata, Pomacea maculata, Sinanodonta woodiana, Sinotaia guangdungensis), and four species are of unknown origin (Bithynia sp., Indoplanorbis exustus, Filopaludina peninsularis, Radix rubiginosa). Two of the native species, Neritina violacea and Vittina coromandeliana, are only found in brackish water habitats in Singapore [69], and are excluded from further discussion. Among the introduced species that are established in Singapore, seven are sold as fish feed (Amerianna carinata, Bithynia sp., Corbicula fluminea, Indoplanorbis exustus, Melanoides tuberculata, Pomacea canaliculata, Sinotaia guangdungensis). They are sold cheaply in mixed-species bags (<US$1/bag), and appear to have been collected from locally-established populations (i.e., not imported).
DNA barcodes
DNA barcodes were successfully amplified for 50 of the 59 recorded species (17 species have both CO1 and 16S sequences, 27 only CO1, and seven only 16S) (Table 4, S2 Table). Fresh samples could not be obtained for some species, and they could thus not be sequenced. Overall, there is high congruency between the species identity based on morphology, and mOTU (Table 4). For CO1, all 44 morphologically-identified species were congruent with molecular data at 1–3% thresholds (Table 4). Within the same threshold, the 24 16S morphological species were grouped into 22 clusters (i.e., mOTU) (Table 4). Most of the mOTUs remained stable for CO1 until up to 8–9% thresholds, except for Clithon corona and Clithon lentiginosum that were separated by 3.5% uncorrected pairwise distance. Morphology and genetic data were incongruent for two 16S mOTUs, with two morphological species each being lumped into one genetic cluster, even at 1% threshold: Brotia armata and Brotia binodosa were separated by 0.5% uncorrected pairwise distance, while Corbicula fluminea and Corbicula moltkiana were separated by 0.8%.
Table 4. Comparison of morphologically-identified species with sequence clusters (mOTU) for CO1 and 16S at 1–4% thresholds, and corresponding top hits on BLAST above 96% identity.
| Identity Based on Morphology | CO1 | 16S | |||
|---|---|---|---|---|---|
| Family | Species | Congruence with sequence clusters | Top Hits on BLAST (> 96% identity) | Congruence with sequence clusters | Top Hits on BLAST (> 96% identity) |
| Cyrenidae | Batissa violacea | Yes (1–4%) | Batissa violacea DQ837727 (97) | Yes (1–4%) | — |
| Corbicula cf. fluminea | Yes (1–4%) | Corbicula sp. GU781082 (100) | No (1–4%) | Corbicula colorata JX399588 (99) | |
| Corbicula sp. | Yes (1–4%) | Corbicula moltkiana AY275660.1 (100) | No (1–4%) | Corbicula largillierti AB522658 (99) | |
| Unionidae | Hyriopsis bialata | Yes (1–4%) | — | N/A | N/A |
| Hyriopsis desowitzi | N/A | N/A | Yes (1–4%) | — | |
| Parreysia burmana | Yes (1–4%) | — | Yes (1–3%), no (4%) | Parreysia olivacea KP795044 (96) | |
| Parreysia sp. | Yes (1–4%) | Parreysia tavoyensis JN243901 (99) | Yes (1–3%), no (4%) | Parreysia tavoyensis KP795043 (99) | |
| Pilsbryoconcha exilis | Yes (1–4%) | — (matched to same species KP795024 at 90%) | N/A | N/A | |
| Scabies crispata | N/A | N/A | Yes (1–4%) | Scabies crispata KP795048 (97) | |
| Sinanodonta woodiana | Yes (1–4%) | Sinanodonta woodiana KJ434487 (99) | N/A | N/A | |
| Ampullariidae | Marisa cornuarietis | Yes (1–4%) | Marisa cornuarietis KM100140 (98) | N/A | N/A |
| Pomacea canaliculata | Yes (1–4%) | Pomacea canaliculata KJ739609 (99) | N/A | N/A | |
| Pomacea diffusa | Yes (1–4%) | Pomacea diffusa EF515065 (100) | N/A | N/A | |
| Bithyniidae | Bithynia sp. | Yes (1–4%) | — | Yes (1–4%) | — |
| Lymnaeidae | Radix rubiginosa | Yes (1–4%) | — (matched to same species GU451737 at 95%) | Yes (1–4%) | Radix rubiginosa U82076.2 (100) |
| Nassariidae | Anentome bockii | Yes (1–4%) | — | N/A | N/A |
| Anentome helena | Yes (1–4%) | — | N/A | N/A | |
| Neritidae | Clithon corona | Yes (1–3%), no (4%) | — (matched to same species EU732362 at 93%) | N/A | N/A |
| Clithon diadema | Yes (1–4%) | — | Yes (1–3%), no (4%) | Clithon spinosus AY771224 (96) | |
| Clithon lentiginosum | Yes (1–3%), no (4%) | — | Yes (1–4%) | — | |
| Clithon mertoniana | Yes (1–4%) | — | Yes (1–3%), no (4%) | — | |
| Neripteron auriculata | Yes (1–4%) | — | Yes (1–4%) | — | |
| Neritina iris | Yes (1–4%) | — | N/A | N/A | |
| Neritina juttingae | Yes (1–4%) | — | Yes (1–4%) | — | |
| Neritina violacea | Yes (1–4%) | Neritina violacea JX411691 (99) | N/A | N/A | |
| Neritodryas cornea | Yes (1–4%) | — | N/A | N/A | |
| Septaria porcellana | N/A | N/A | Yes (1–4%) | Septaria porcellana AY771228 (98) | |
| Vittina coromandeliana | Yes (1–4%) | Neritina turrita JX411698 (99) | N/A | N/A | |
| Vittina turrita | Yes (1–4%) | — | N/A | N/A | |
| Vittina waigiensis | Yes (1–4%) | Neritina turrita AY820497 (100) | Yes (1–4%) | — | |
| Pachychilidae | Brotia armata | N/A | N/A | No (1–4%) | Brotia armata AY330810 (100) |
| Brotia binodosa | N/A | N/A | No (1–4%) | Brotia sp. FJ377079 (100) | |
| Brotia herculea | Yes (1–4%) | — | N/A | N/A | |
| Sulcospira tonkiniana | Yes (1–4%) | Sulcospira tonkiniana FJ377297 (99) | N/A | N/A | |
| Tylomelania towutica | Yes (1–4%) | Tylomelania towutica KJ850891 (99) | N/A | N/A | |
| Tylomelania sp. | Yes (1–4%) | Tylomelania sp. KJ850861 (99) | Yes (1–4%) | Tylomelania centaurus AY311829 (99) | |
| Physidae | Physa sp. | Yes (1–4%) | — | N/A | N/A |
| Planorbidae | Gyraulus convexiusculus | Yes (1–4%) | — | N/A | N/A |
| Indoplanorbis exustus | Yes (1–4%) | Indoplanorbis exustus GU451743 (99) | N/A | N/A | |
| Semisulcospiridae | Semisulcospira sp. | Yes (1–4%) | — | N/A | N/A |
| Thiaridae | Melanoides tuberculata | Yes (1–4%) | Melanoides tuberculata KP284138 (100) | N/A | N/A |
| Stenomelania offachiensis | Yes (1–4%) | — | Yes (1–4%) | Stenomelania sp. AY010518 (97) | |
| Stenomelania plicaria | Yes (1–4%) | — | N/A | N/A | |
| Stenomelania cf. plicaria | Yes (1–4%) | — | N/A | N/A | |
| Stenomelania sp. | Yes (1–4%) | — | Yes (1–4%) | — | |
| Thiara cancellata | Yes (1–4%) | — | Yes (1–4%) | — | |
| Viviparidae | Filopaludina cambodjensis | Yes (1–4%) | — | N/A | N/A |
| Filopaludina peninsularis | Yes (1–4%) | — | Yes (1–4%) | — | |
| Sinotaia guangdungensis | Yes (1–4%) | *Bellamya heudei guangdungensis AY296827 (99) | N/A | N/A | |
| Taia pseudoshanensis | N/A | — | Yes (1–4%) | — | |
“N/A” indicates barcodes were not obtained; “—”indicate that top hits on BLAST were <96%.
*Bellamya heudei guangdungensis is a synonym of Sinotaia guangdugensis [73].
For matches to GenBank, only 17 species had 97–100% identity matches to available CO1 sequences in GenBank (Table 4), and 13 of those had species names that matched those assigned based on morphology. For the other four species that did not match the morphology-based identities—Corbicula moltkiana and Parreysia tavoyensis were only identified to genus level based on morphology, and were confirmed based on the GenBank hits [61, 84], with additional comparison to the original descriptions; two individuals identified by morphology as Vittina coromandeliana and Vittina waigiensis were 99–100% matched to two separate submissions on GenBank that were identified as Vittina turrita. Neither study included photographs of the species, nor could the sequenced individuals be located ([85], [86], M.B. Goodwin, pers. comm.); because the two GenBank sequences for Vittina turrita were separated by a 4.5% uncorrected pairwise distance, we retained our morphology-based identifications for Vittina coromandeliana and Vittina waigiensis. Among the 27 CO1 sequences that fell outside the 3% species delimitation threshold, three had top hits with species that matched the morphology-based identification, but were only 90–95% matches—Pilsbryoconcha exilis, Radix rubiginosa, and Clithon corona (Table 4). For the other 24 sequences, the top hits had between 78 and 94% identity matches, and all the top hits were for the same family as the original (morphology-based) identification (S2 Table). For 16S, 10 species had 97–100% identity matches to sequences on GenBank (Table 4)—five of which exactly matched the species identified based on morphology, while the other five were matched to congeners. For the remaining 14 sequences, the top hits had between 86 and 96% identity matches, and all were of the same family as the original identification.
Discussion
This study represents the first comprehensive survey of freshwater mollusc species in the ornamental pet trade. We found an unexpectedly high diversity of species in the trade over the six-year period, which suggests an increase in interest in ornamental freshwater molluscs, mirroring the increased demand for invertebrates elsewhere [5, 7]. Our results highlight the role of the ornamental pet trade as a key anthropogenic factor influencing both introductions (of non-native molluscs, and associated parasites), and the conservation of freshwater molluscs.
Invasion potential
The aquarium or ornamental pet trade has been suspected to be the main pathway for freshwater alien species introductions into Singapore [45, 87]. The results of the present survey indicate that nearly half of known introduced and species of unknown origin in Singapore are in the trade [47, 48,72, 73, 88]. More importantly, the study found evidence for the indirect introduction of species via other ornamental taxa [89, 90]—more than half of the species recorded as hitchikers in the trade are established in Singapore. Singapore is also a hub for the import and export of ornamental aquatic plants, and freshwater snails have been detected among shipments of plants from the country [19, 43, 44, 90]. It is likely that even species that are not attractive to hobbyists (e.g., species <10 mm in size) could still be imported, and subsequently introduced into the environment through indiscriminate disposal of aquarium water [14], or planting of ornamental plants around waterbodies.
Among the 14 species with a history of introduction elsewhere, 12 were purportedly introduced via the ornamental pet trade (Table 3). The two species with no prior records of introduction through the trade, Sinanodonta woodiana and Sinotaia guangdungensis, were likely co-introduced with fish [47, 73, 91, 92]. The ornamental species with recorded or potential impacts include some of the world’s most notorious invasive species—Corbicula fluminea and Pomacea canaliculata (likely Pomacea maculata too) [93, 94]. These species have caused environmental as well as economic damage throughout their introduced ranges, including habitat modification, agricultural losses, and possible displacement of native species (Table 3). Recognising the threat of Pomacea spp., commonly known as golden apple snails, the European Commission recently banned their import into the European Union [95]. Our results show that the authorities may need to expand their monitoring efforts to include other similarly invasive species like Sinanodonta woodiana and Corbicula fluminea.
Additionally, the spread of snail-mediated zoonotic diseases such as schistosomiasis with the introduction of freshwater molluscs via the ornamental pet trade has been a cause for concern for many decades [19, 96, 97], and our results highlight that the threat remains—half of the species with introduction impacts are also known intermediate hosts of parasites that can cause diseases in humans and livestock, with disease outbreaks impacting humans or fisheries being recorded following the introduction of Pomacea spp. and Melanoides tuberculata (Table 3). Besides the species with introduction history that are known intermediate hosts of zoonotic parasites, snails of the family Bithyniidae, of which at least one species is in the trade, are also known parasite vectors [98].
Origins of molluscs in the trade and conservation concerns
Of the 59 species recorded, more than half are naturally distributed in the Oriental region, while almost a fifth have a more widespread distribution that includes the Oriental region. This could be because the Oriental region has the highest diversity for snails of the families Neritidae, Thiaridae, Pachychilidae, and Viviparidae [24], which made up almost 90% of the recorded ornamental mollusc species. The Oriental region is also one of only two global hotspots of Unionidae bivalve diversity and endemism (the other being the southeastern United States) [23]. The higher proportion of species from the Oriental region contrasts with the freshwater fishes in the Singapore ornamental pet trade, which mostly originate from the Neotropics [99].
The high diversity of freshwater molluscs in the trade originating from the Oriental region may be of conservation concern, especially considering the freshwater molluscs in the region are understudied—with taxonomic status and distributions of many species being uncertain [25]. Fewer than half of the species in the ornamental pet trade (22 Oriental species and two Neotropical species) have been assessed by the IUCN. Three species are listed as Data Deficient, while many of the 21 Least Concern species lack updated population information [83]. An exception among the Least Concern species is Hyriopsis bialata, which has a decreasing population trend in Thailand [100]. Although population trends are unknown for the other Least Concern species, there have been accounts of localized threats, for e.g., degradation of Corbicula moltkiana habitat in Indonesia, and multiple threats of habitat loss, pollution, and overharvesting potentially impacting the Vietnamese populations of Pilsbryoconcha exilis [83].
Overharvesting for human consumption is a known threat to freshwater molluscs in the Oriental region, especially for narrowly endemic species [25, 83]. Although harvesting for the ornamental pet trade has thus far not been documented to be a threat, we recorded seven out of nine pachychilid and one viviparid species in the trade with restricted ranges; specifically, Brotia armata, Brotia binodosa, Brotia pagodula which are confined to particular river systems in Indo-Burma [63, 101], and all Tylomelania spp. and Celetaia persculpta, which are endemic to Lake Poso of Sulawesi ([102], T. von Rintelen pers. comm.). These species appeared to fetch higher prices compared to more common species (up to US$10 per individual Tylomelania sp. compared to <US$5 per individual Thiaridae or Neritidae, THN pers. obs.). The rarity of the species may drive increased demand, which may ultimately lead to a decline of the species [103].
Besides the huge knowledge gaps in population trends, taxonomic uncertainty is also a factor that may mask the true distribution of species [104]. Clithon corona is one of the species in the trade that has been assessed as Least Concern and is assumed to be widely distributed; however, its taxonomy, and thus its true distribution, remains unresolved [75, 83]. The limited knowledge of species distributions, restricted ranges, and unresolved taxonomy of freshwater molluscs in the ornamental pet trade makes it essential that the trade be monitored more closely lest the harvesting of species for the trade becomes unsustainable.
Utility of DNA barcodes
Introduction pathways are difficult to track; many introductions fail to be detected because in many cases, the appropriate expertise is not available for identifying molluscs to species. While it is currently not feasible to rely wholly on molecular methods for species identification and delimitation [105] (see below regarding limitations), the fact remains that there is a global decline in trained taxonomists, and funding for necessary resources and educational support [106]. This lack of expertise affects all taxa, including molluscs [25, 26], and is especially crucial in relation to the ornamental pet trade. Border security officials are given the insurmountable task to inspect large volumes of often poorly-categorised or wrongly identified shipments [13, 107].
It is here that DNA barcodes provide useful information. In general, identification of species via DNA barcodes can be viewed as a two-stop process. First, the samples can be delimited into mOTUs via genetic distance and it is generally found that most mOTUs are stable across a large range of distances. Next, the mOTUs are identified to species either by matching sequences to an existing database or by expert morphological examination. It is at this stage that conflict between data sources can be detected and often resolved. Our results support the use of DNA barcoding for rapid species delimitation, especially for species in the ornamental pet trade, which originate from multiple (and often unknown) sources. However, we acknowledge our DNA database is currently still too incomplete for routine identification of many species that lack coverage. This has been a problem for many taxa, and researchers around the world are tackling this issue via rapid generation of name-matched DNA barcodes [108, 109].
The process of amplifying and extracting DNA from freshwater molluscs is often difficult owing to the presence of additional mucopolysaccharides in the slime that inhibits PCR [110]. However, we show that even with short DNA fragments (≤ 313 bp), species can be reliably matched to existing sequences on GenBank, e.g., Melanoides tuberculata (CO1, 239 bp), or distinguished from congeners, e.g., Vittina turrita and Vittina waigiensis which were separated by 10% genetic divergence (CO1, both 313 bp). DNA barcodes also allowed for more accurate identification of some species (e.g., confirmation of identification for Corbicula moltkiana and Parreysia tavoyensis). In addition, DNA barcodes are usually less variable than the morphology of wild and cultured individuals, which makes rapid identification based solely on external morphology difficult and sometimes unreliable. For example, two morphologically distinct forms of Indoplanorbis sp. were collected from the trade (Fig 3), but shared identical DNA barcodes (GenBank Accession numbers KU318341–42), and were instead then considered conspecific (Indoplanorbis exustus).
Fig 3. Two morphs of Indoplanorbis exustus in the aquarium trade.
DNA barcodes work well when the chosen molecular markers are sufficiently diagnostic to delimit species [111] and the species taxonomy of species is well resolved [111]; most species in our study satisfied these conditions. Some exceptions, however, were closely-related, sympatric species (Tylomelania spp., Brotia armata, Brotia binodosa) that failed to have diagnostic barcodes, possibly due to either recent speciation or to introgression [63]. Our study also included species with poorly understood species boundaries; a good example is in the case of three Stenomelania spp. in the trade which belong to the Stenomelania plicaria species complex ([112], M. Glaubrecht pers. comm.). DNA barcodes can here suggest species boundaries, but they have to be confirmed via taxonomic revision. Completing this task will be particularly important for some invasive molluscs with high genetic variability [113]. Another problem with DNA barcodes are misidentified sequences in barcode databases [114–117], which was encountered here for two Vittina species that matched two separate sequences identified as Vittina turrita on GenBank. The lack of supporting voucher specimens or photographs made it impossible to resolve the conflict and is characteristic of many misidentifications in barcode databases.
Because of these known problems, we made sure to identify specimens using both morphology and DNA sequences [118], and deposit voucher specimens in the Lee Kong Chian Natural History Museum for future validation or reconfirmation. In any case, despite the discussed limitations in the utility of mitochondrial DNA, it presently remains the most suitable marker for rapid identification, especially in the case of monitoring the ornamental pet trade [33, 34]. It is hoped that this study would serve as a start for building a more complete DNA barcode library for freshwater molluscs in the ornamental pet trade.
Conclusions
We believe that our assessment is representative of freshwater molluscs that are currently in the trade, but the ornamental pet trade changes over time [6, 7] and it would be important to monitor the trade on a regular basis. It would be prudent to continue building up a reliable DNA barcode library, as barcodes would be extremely useful to rapidly identify species in limited availability or absence of taxonomic expertise. Future work should take advantage of new amplification techniques [119], and cheap NGS barcodes obtained with next-generation sequencing [109], which lower the cost for obtaining barcodes, and will allow for the inclusion of population genomics for tracing the origin and spread of species that have been widely introduced. Also, as indirect introductions via the importation of aquatic plants appear to be overlooked, this pathway warrants more in-depth attention. In light of the recent European Union regulations against Pomacea spp., it would be in the interest of major ornamental distributors, such as Singapore, to prevent the export of unwanted molluscs with aquatic plants. Current knowledge gaps in the autecology of freshwater molluscs (especially those in the Oriental region) need to be filled to identify high invasive risk species that are commonly translocated via this pathway. For example, it would be important to know how long mollusc eggs remain viable on plants. Besides helping the industry to prevent import and monitor or manage the spread of potentially invasive species, such ecological information would also help inform management and conservation of endemic freshwater molluscs, thereby creating a less harmful and more sustainable ornamental pet trade.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
We would like to thank colleagues and friends, particularly: Kelvin KP Lim for assistance in obtaining molluscs from the trade and providing important references; Swee Hee Tan for first suggesting the idea for an earlier version of this study; Thomas Eichhorst, Matthias Glaubrecht, Frank Köhler, and Thomas von Rintelen for help in species identifications; Kenneth Hayes and Ravindra C Joshi for photographs and information of Pomacea maculata; Wendy Y Wang, Darren Yeo and other members of the Evolutionary Biology Laboratory, members of the Freshwater and Invasion Biology Laboratory, and Amanda Windsor of the Smithsonian Institution, for assistance in various aspects of the molecular work and data collection process. We acknowledge financial support from the Department of Biological Sciences of the National University of Singapore, the Singapore National Research Foundation and the Economic Development Board, SPORE, COY-15-EWI-RCFSA/N197-1, and Wildlife Reserves Singapore Ah Meng Memorial Conservation Fund, National University of Singapore grant number R-154-000-617-720. Finally, we would like to thank three anonymous reviewers and the academic editor for their helpful recommendations that have greatly improved the manuscript.
Data Availability
DNA sequences are available from GenBank (accession numbers KU318325-KU318393), and BOLD. Voucher specimens are available from the Zoological Reference Collection (ZRC) of the Lee Kong Chian Natural History Museum, National University of Singapore (accession numbers ZRC.MOL.5904-5951, 6284–6333, 6752-6754).
Funding Statement
The authors acknowledge financial support from the Department of Biological Sciences of the National University of Singapore, the Singapore National Research Foundation and the Economic Development Board, SPORE, COY-15-EWI-RCFSA/N197-1, http://www.nrf.gov.sg/, THN; and Wildlife Reserves Singapore Ah Meng Memorial Conservation Fund, National University of Singapore grant number R-154-000-617-720, http://www.wrscf.org.sg/index.html, to DCJY. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.FAO (Food and Agricutural Organization of the United Nations) 2005. Fisheries and Aquaculture Topics: Ornamental Fish: Topics Fact Sheets. Available: http://www.fao.org/fishery/topic/13611/en.
- 2.Liang SH, Chuang LC, Chang MH. The pet trade as a source of invasive fish in Taiwan. Taiwania 2006; 51(2): 93–98. [Google Scholar]
- 3.Rhyne AL, Tlusty MF, Schofield PJ, Kaufman L, Morris JA Jr., Bruckner AW. Revealing the appetite of the marine aquarium trade: the volume and biodiversity of fish imported into the United States. PLoS ONE 2012; 7(5): e35808 10.1371/journal.pone.035808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maceda-Veiga A, Escribano-Alacid J, de Sosfoa A, García-Berthou E. The aquarium trade as potential source of fish introductions in southwestern Europe. Biological Invasions 2013; 15: 2707–2816. [Google Scholar]
- 5.Lukhaup C. The next generation. Practical Fishkeeping 2009; December 2009: 31–35. [Google Scholar]
- 6.Rhyne A, Rotjan R, Bruckner A, Tlusty M. Crawling to collapse: ecologically unsound ornamental invertebrate fisheries. PLoS ONE 2009; 4: e8413 10.1371/journal.pone.0008413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Dutta D, Banerjee S. Changing trend in ornamental fish markets of Kolkata, West Bengal, India. Fishing Chimes 2012; 32: 61–63 [Google Scholar]
- 8.Mackie GL. Molluscs introductions through the aquarium trade In: Claudi R, Leach JH, editors. Nonindigenous freshwater organisms: vectors, biology and impacts. Boca Raton, Florida: Lewis Publishers; 2000. pp. 135–149. [Google Scholar]
- 9.Cowie RH, Robinson DG. Pathways of introduction of nonindigenous land and freshwater snails and slugs In: Ruiz G, Carlton JT, editors. Invasive species: vectors and management strategies. Washington, D.C.: Island Press; 2003. pp. 93–122. [Google Scholar]
- 10.Maki K, Galatowitsch S. Movement of invasive aquatic plants into Minnesota (USA) through horticultural trade. Biological Conservation 2004; 118: 389–396. [Google Scholar]
- 11.Padilla DK, Williams SL. Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Frontiers in Ecology and the Environment. 2004; 2(3): 131–138. [Google Scholar]
- 12.Cohen J, Mirotchnick N, Leung B. Thousands introduced annually: the aquarium pathway for non-indigenous plants to the St Lawrence Seaway. Frontiers in Ecology and the Environment 2007; 5(10): 528–532. [Google Scholar]
- 13.Keller RP, Lodge DM. Species invasions from commerce in live aquatic organisms: problems and possible solutions. Bioscience 2007; 57(5): 428–436. [Google Scholar]
- 14.Duggan IC. The freshwater aquarium trade as a vector for incidental invertebrate fauna. Biological Invasions 2010; 12: 3757–3770. [Google Scholar]
- 15.Strecker AL, Campbell PM, Olden JD. The aquarium trade as an invasion pathway in the Pacific Northwest. Fisheries 2011; 36(2): 74–85. [Google Scholar]
- 16.Karatayev AY, Burlakova LE, Karatayev VA, Padilla DK. Introduction, distribution, spread, and impacts of freshwater gastropods in Texas. Hydrobiologia 2009; 619: 181–194. [Google Scholar]
- 17.Salisbury JR, Harkin JT, Smith BJ. Lymnaea columella in aquariums. Australian Veterinary Journal 1976; 52: 487. [DOI] [PubMed] [Google Scholar]
- 18.Dudgeon D, Yipp MW. A report on the gastropod fauna of aquarium fish farms in Hong Kong, with special reference to an introduced human schistosome host species, Biomphalaria straminea (Pulmonata: Planorbidae). Malacological Review 1983; 16: 93–94. [Google Scholar]
- 19.Madsen H, Frandsen F. The spread of freshwater snails including those of medical and veterinary importance. Acta Tropica 1989; 46: 139–146. [DOI] [PubMed] [Google Scholar]
- 20.Andrews C. The ornamental fish trade and fish conservation. Journal of Fish Biology 1990; 37(supplement A): 53–59. [Google Scholar]
- 21.Ng PKL, Tan HH. Freshwater fishes of Southeast Asia: potential for the aquarium fish trade and conservation issues. Aquarium Sciences and Conservation 1997; 1: 79–90. [Google Scholar]
- 22.Raghavan R, Dahanukar N, Tlusty MF, Rhyne AL, Kumar KK, Molur S, et al. Uncovering an obscure trade: threatened freshwater fishes and the aquarium pet markets. Biological Conservation 2013; 164: 158–169. [Google Scholar]
- 23.Bogan AE. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia 2008; 595: 139–147. [Google Scholar]
- 24.Strong EE, Gargomimy O, Ponder WF, Bouchet P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 2008; 595: 149–166. [Google Scholar]
- 25.Köhler F, Seddon M, Bogan AE, Do VT, Sri-Aroon P, Allen D. The status and distribution of freshwater molluscs of the Indo-Burma region In: Allen DJ, Smith KG, Darwall WRT, compilers. The status and distribution of freshwater biodiversity in Indo-Burma. Cambridge, UK and Gland, Switzerland: IUCN; pp. 67–89. [Google Scholar]
- 26.Lydeard C, Cowie RH, Ponder WF, Bogan AE, Bouchet P, Clark SA, et al. The global decline of nonmarine mollusks. BioScience 2004; 54: 321–330. [Google Scholar]
- 27.Regnier C, Fontaine B, Bouchet P. Not knowing, not recording, not listing: numerous unnoticed mollusk extinctions. Conservation Biology 2009; 23: 1214–1221. 10.1111/j.1523-1739.2009.01245.x [DOI] [PubMed] [Google Scholar]
- 28.Lukhaup C, Hummel S. Treasures of Pirate Island. Practical Fishkeeping 2012; 6: 30–39. [Google Scholar]
- 29.McDowall RM. Shoot first, and then ask questions: a look at aquarium fish imports and invasiveness in New Zealand. New Zealand Journal of Marine and Freshwater Research 2004; 38(3): 503–510. [Google Scholar]
- 30.Alacs E, Georges A. Wildlife across our borders: a review of the illegal trade in Australia. Ausralian Journal of Forensic Sciences 2008; 40(2): 147–160. [Google Scholar]
- 31.AVA (Agri-Food and Veterinary Authority of Singapore). Current aquatic animal diseases under surveillance. Ornamental Fish Newsletter 2011; 2(3): 1–3. [Google Scholar]
- 32.Welton LJ, Siler CD, Linkern CW, Diesmos AC, Diesmos ML, Sy E, et al. Dragons in our midst: phyloforensics of illegally traded Southeast Asian monitor lizards. Biological Conservation 2013; 159: 7–15. [Google Scholar]
- 33.Steinke D, Zemlak TS, Hebert PDN. Barcoding Nemo: DNA-based identifications of the ornamental fish trade. PLoS ONE 2009; 4(7): e6300 10.1371/journal.pone.0006300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins RA, Armstrong KF, Meier R, Yi Y, Brown SDJ, Cruickshank RH, et al. Barcoding and border biosecurity: identifying cyprinid fishes in the aquarium trade. PLoS ONE 2012; 7(1): e28381 10.1371/journal.pone.0028381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vences M, Thomas M, van der Meijden A, Chiari Y, Vieites DR. Comparative performance of the 16S rRNA gene in DNA barcoding of amphibians. Frontiers in Zoology 2005; 2: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weese DA, Santos SR. Genetic identification of source populations for an aquarium-traded invertebrate. Animal Conservation 2009; 12: 13–19. [Google Scholar]
- 37.Filipová L, Grandjean F, Chucholl C, Soes DM, Petrusek A. Identification of exotic North American crayfish in Europe by DNA barcoding. Knowledge and Management of Aquatic Ecosystems 2011; 401: 11. [Google Scholar]
- 38.Pečnikar ZF, Buzan EV. 20 years since the introduction of DNA barcoding: from theory to application. Journal of Applied Genetics 2014; 55: 43–52. 10.1007/s13353-013-0180-y [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Tan Z, Wang D, Zue L, Guan M, Huang T et al. Species identification through mitochondrial rRNA genetic analysis. Scientific Reports 2014; 4: 4089 10.1038/srep04089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.FAO (Food and Agricutural Organization of the United Nations). FAO Yearbook: Fishery and Aquaculture Statistics 2009. Rome: Food and Agriculture Organization of the United Nations; 2011. p. 127. [Google Scholar]
- 41.International Enterprise Singapore 2016. Singapore Trade Statistics—Singapore's Annual Export to World, Live Freshwater Ornamental Fish (HS 030111) 2013–2015. Available: https://statlink.iesingapore.gov.sg.
- 42.Evans BB, Lester RJG. Parasites of ornamental fish imported into Australia. Bull. Eur. Ass. Fish Pathol. 2001; 21(2): 51–55. [Google Scholar]
- 43.Brown DS, Gracio MAA, Meier-Brook C. The Asian freshwater snail Gyraulus chinensis (Dunker, 1848) (Planorbidae) in West Africa and Europe. Journal of African Zoology 1998; 112: 1–11. [Google Scholar]
- 44.Roll U, Dayan T, Simberloff D, Mienis HK. Non-indigenous land and freshwater gastropods in Israel. Biological Invasions 2009; 11: 1963–1972. [Google Scholar]
- 45.Ng PKL, Chou LM, Lam TJ. The status and impact of introduced freshwater animals in Singapore. Biological Conservation 1993; 64: 29–24. [Google Scholar]
- 46.Yeo DCJ, Chia CS. Introduced species in Singapore: an overview. COSMOS 2010; 6: 23–37. [Google Scholar]
- 47.Tan SK, Chan SY, Clements GR. A Guide to Snails and Other Non-Marine Mollucs of Singapore. Singapore: Science Centre Singapore; 2012. 176 pp. [Google Scholar]
- 48.Ng TH, Tan SK, Low MEY. Singapore Mollusca: 7. The family Ampullariidae (Gastropoda: Caenogastropoda: Ampullarioidea). Nature in Singapore 2014; 7: 31–47. [Google Scholar]
- 49.Ling KH, Lim LY. The status of ornamental fish industry in Singapore. Singapore Journal of Primary Industries 2005; 32: 59–69. [Google Scholar]
- 50.van Benthem Jutting WSS. Planorbis exustus Desh. in Java. Journal of Conchology 1946; 22: 221 [Google Scholar]
- 51.van Benthem Jutting WSS. Systematic studies on the non-marine Mollusca of the Indo-Australian Archipelago: IV. Critical revision of the freshwater bivalves of Java. Treubia 1953; 22: 19–73. [Google Scholar]
- 52.Zilch A. Die typen und typoide des Natur-Museums Senckenberg, 14: Mollusca, Viviparidae. Archiv für Molluskenkunde 1955; 64: 1–41. [Google Scholar]
- 53.van Benthem Jutting WSS. Systematic studies on the non-marine Mollusca of the Indo-Australian archipelago: V. Critical revision of the Javanese freshwater gastropods. Treubia 1956; 23: 259–477. [Google Scholar]
- 54.Habe T. Freshwater molluscan fauna of Thailand In: Kira T, Umesao T, editors. Nature and Life in Southeast Asia, Vol. III Kyoto, Japan: Flora and Fauna Research Society; 1964. pp. 45–66, pls. I–II. [Google Scholar]
- 55.Brandt RAM. The Non-Marine Aquatic Mollusca of Thailand. Archiv für Molluskenkunde 1974; 105: 1–423. [Google Scholar]
- 56.Liu Y, Zhang W, Wang Y, Wang E. Economic Fauna of China—Freshwater Molluscs. Beijing: Science Press; 1979. 134 pp. [Google Scholar]
- 57.Kristensen TK, Oggunnowo O. Indoplanorbis exustus (Deshayes, 1834), a freshwater snail new for Africa, found in Nigeria (Pulmonata: Planorbidae). Journal of Molluscan Studies 1987; 53: 243–246. [Google Scholar]
- 58.Subba Rao NV. Handbook of Freshwater Molluscs of India. Calcutta: Zoological Survey of India; 1989. 289 pp. [Google Scholar]
- 59.Haynes A. Freshwater snails of the tropical Pacific islands Suva: Institute of Applied Sciences; 2001. 116 pp. [Google Scholar]
- 60.Köhler F, Glaubrecht M. Towards a systematic revision of the Southeast Asian freshwater gastropod Brotia H. Adams, 1866 (Cerithioidea: Pachychilidae): An account of species from around the South China Sea. Journal of Molluscan Studies 2001; 67: 281–318. [Google Scholar]
- 61.Glaubrecht M, von Rintelen T, Korniushin AV. Towards a systematic revision of brooding freshwater Corbiculidae in Southeast Asia (Bivalvia, Veneroida): on shell morphology, anatomy and molecular phylogenetics of endemic taxa from islands in Indonesia. Malacologia 2003; 45(1): 1–40. [Google Scholar]
- 62.von Rintelen T, Glaubrecht M. New discoveries in old lakes: Three new species of Tylomelania Sarasin & Sarasin, 1897 (Gastropoda: Cerithioidea: Pachychilidae) from the Malili Lake System on Sulawesi, Indonesia. Journal of Molluscan Studies 2003; 69: 3–17. [Google Scholar]
- 63.Glaubrecht M, Köhler F. Radiating in a river: systematics, molecular genetics and morphological differentiation of viviparous freshwater gastropods endemic to the Kaek River, central Thailand (Cerithioidea, Pachychilidae). Biological Journal of the Linnean Society 2004; 82: 275–311. [Google Scholar]
- 64.Köhler F, Glaubrecht M. A systematic revision of the Southeast Asian freshwater gastropod Brotia (Cerithioidea: Pachychilidae). Malacologia 2006; 48: 1–93. [Google Scholar]
- 65.von Rintelen T, Bouchet P, Glaubrecht M. Ancient lakes as hotspots of diversity: a morphological review of an endemic species flock of Tylomelania (Gastropoda: Cerithioidea: Pachychilidae) in the Malili lake system on Sulawesi, Indonesia. Hydrobiologia 2007; 592: 11–94. [Google Scholar]
- 66.Rawlings TA, Hayes KA, Cowie RH, Collins TM. The identity, distribution, and impacts of non-native apple snails in the continental United States. BMC Evolutionary Biology 2007; 7: 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayes KA, Joshi RC, Thiengo SC, Cowie RH. Out of South America: multiple origins of non-native apple snails in Asia. Diversity and Distributions 2008; 14: 701–712. [Google Scholar]
- 68.von Rintelen T, Glaubrecht M. Three new species of the freshwater snail genus Tylomelania (Caenogastropoda: Pachychilidae) from the Malili lake system, Sulawesi, Indonesia. Zootaxa 2008; 1852: 37–49. [Google Scholar]
- 69.Tan SK, Clements GR. Taxonomy and distribution of the Neritidae (Mollusca: Gastropoda) in Singapore. Zoological Studies 2008; 47: 481–494. [Google Scholar]
- 70.Köhler F, Holford M, Do VT, Hai HT. Exploring a largely unknown fauna: On the diversity of pachychilid freshwater gastropods in Vietnam (Caenogastropoda: Cerithioidea). Molluscan Research 2009; 29: 121–146. [Google Scholar]
- 71.Sri-Aroon P. Freshwater snails of medical importance in Thailand Bangkok: Faculty of Tropical Medicine, Mahidol University; 2010. 20 pp. [Google Scholar]
- 72.Hayes KA, Cowie RH, Thiengo SC, Strong EE. Comparing apples with apples: clarifying the identities of two highly invasive Neotropical Ampullariidae (Caenogastropoda). Zoological Journal of the Linnean Society 2012; 166: 723–753. [Google Scholar]
- 73.Ng TH, Tan SK, Yeo DCJ. On the taxonomy, status, and introduction of the earliest reported alien freshwater mollusc in Singapore—Sinotaia guangdungensis (Gastropoda: Viviparidae). Malacologia 2014; 57: 401–408. [Google Scholar]
- 74.Ng TH, Tan SK, Yeo DCJ. Clarifying the identity of the long-established, globally-invasive Physa acuta Draparnaud, 1805 (Gastropoda: Physidae) in Singapore. BioInvasions Records 2015; 4(3): 189–194. [Google Scholar]
- 75.Eichhorst TE. Neritidae of the World. Volume One Harxheim: ConchBooks; 2016. 694 pp. [Google Scholar]
- 76.Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software Version 7: improvements in performance and usability. Molecular Biology and Evolution 2013; 30(4): 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meier R, Kwong S, Vaidya G, Ng PKL. DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Systematic Biology 2006; 55: 715–728. 10.1080/10635150600969864 [DOI] [PubMed] [Google Scholar]
- 78.Meier R, Zhang GY, Ali F. The use of mean instead of smallest interspecific distances exaggerates the size of the "barcoding gap" and leads to misidentification. Systematic Biology 2008; 57(5): 809–813. 10.1080/10635150802406343 [DOI] [PubMed] [Google Scholar]
- 79.Srivathsan A, Meier R. On the inappropriate use of Kimura-2-parameter (K2P) divergences in the DNA-barcoding literature. Cladistics 2012; 28(2): 190–194. [DOI] [PubMed] [Google Scholar]
- 80.Blaxter ML (2004) The promise of a DNA taxonomy. Philosophical Transactions of the Royal Society B: Biological Sciences 359:669–679. 10.1098/rstb.2003.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Layton KK, Martel AL, Hebert PD. Patterns of DNA barcode variation in Canadian marine molluscs. PLoS ONE 2014; 9(4): e95003 10.1371/journal.pone.0095003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 2000; 7(1–2): 203–214. 10.1089/10665270050081478 [DOI] [PubMed] [Google Scholar]
- 83.IUCN (International Union for Conservation of Nature) 2015. IUCN Red List of Threatened Species. Version 2015.4. Available: www.iucnredlist.org
- 84.Whelan NV, Geneva AJ, Graf DL. Molecular phylogenetic analysis of tropical freshwater mussels (Mollusca: Bivalvia: Unionida) resolves the position of Coelatura and supports a monophyletic Unionidae. Molecular Phylogenetics and Evolution 2011; 61: 504–514. 10.1016/j.ympev.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 85.Bunje PMJ. Diversification and comparative phylogeography of freshwater neritid gastropods. PhD. Thesis, University of California, Berkeley. 2004. 90pp.
- 86.Chee SY, Siti Azizah MN. DNA barcoding reveals neritid diversity (Mollusca: Gastropoda) diversity in Malaysian waters. Mitochondrial DNA 2016; 27: 2282–2284. 10.3109/19401736.2014.987237 [DOI] [PubMed] [Google Scholar]
- 87.Ng HH, Tan HH. An annotated checklist of the non-native freshwater fish species in the reservoirs of Singapore. COSMOS 2010; 6: 95–116. [Google Scholar]
- 88.Clements R, Koh LP, Lee TM, Meier R, Li D. Importance of reservoirs for the conservation of freshwater molluscs in a tropical urban landscape. Biological Conservation 2006; 128: 136–146. [Google Scholar]
- 89.Pointier JP. Invading freshwater gastropods: some conflicting aspects for public health. Malacologia 1999; 41: 403–411. [Google Scholar]
- 90.Robinson DG. Alien invasions: the effects of the global economy on non-marine gastropod introductions into the United States. Malacologia 1999; 41: 413–438. [Google Scholar]
- 91.Yang SL. Record of a freshwater bivalve, Pseudodon vondembuschianus in Singapore. Raffles Bulletin of Zoology 1990; 38: 1–2. [Google Scholar]
- 92.Wong YT, Lim YM, Chiam-Tai YC. A Guide to Freshwater Phytoplankton in Singapore Reservoirs. Singapore: Science Centre Singapore; 2011. p. 11. [Google Scholar]
- 93.Global Invasive Species Database 2005. Corbicula fluminea. Available: http://www.issg.org/database/species/ecology.asp?si=537&fr=1&sts=sss&lang=EN.
- 94.Global Invasive Species Database 2005. Pomacea canaliculata. Available: http://www.issg.org/database/species/ecology.asp?si=135&fr=1&sts=sss&lang=EN.
- 95.The European Commission. Commission implementing decision of 8 November 2012 as regards measures to prevent the introduction into and the spread within the Union of the genus Pomacea (Perry). Official Journal of the European Union 2012; L311: 14–17.
- 96.Bastert J, Sing A, Wollenberg A, Korling HC. Aquarium dermatitis: cercarial dermatitis in an aquarist. Dermatology 1998; 197: 84–86. [DOI] [PubMed] [Google Scholar]
- 97.Poulin R, Paterson RA, Townsend CR, Tompkins DM, Kelly DW. Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshwater Biology 2011; 56: 676–688. [Google Scholar]
- 98.Petney T, Sithithaworn P, Andrews R, Kiatsopit N, Tesana S, Grundy-Warr C, et al. The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitology International 2012; 61: 38–45. 10.1016/j.parint.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 99.Lee, S. Z. L., 2008. The ornamental fish trade and invasive freshwater fish species in Singapore. B.Sc. (Hons.) Thesis, National University of Singapore. 82pp.
- 100.Areekijseree M, Engkagul A, Kovitvadhi U, Thongpan A, Mingmuang M, Pakkong P, et al. Temperature and pH characteristics of amylase and proteinase of adult freshwater pearl mussel, Hyriopsis (Hyriopsis) bialatus Simpson 1900. Aquaculture. 2004; 234: 575–587. 10.1016/j.aquaculture.2003.12.008 [DOI] [Google Scholar]
- 101.Simonis J, Köhler F. Brotia pagodula. The IUCN Red List of Threatened Species 2012: e.T184824A1755214. Available: 10.2305/IUCN.UK.2012-1.RLTS.T184824A1755214.en. [DOI]
- 102.Sarasin P, Sarasin F. Über die molluskenfauna der grofsen süfswasser-seen von Central-Celebes. Zoologischer Anzeiger 1897; 539: 308–312. [Google Scholar]
- 103.Courchamp F, Angulo E, Rivalan P, Hall RJ, Signoret L, Bull L, et al. Rarity value and species extinction: the anthropogenic allee effect. PLoS Biology 2006; 4: e415 10.1371/journal.pbio.0040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iglésias SP, Toulhoat L, Sellos DY. Taxonomic confusion and market mislabelling of threatened skates: important consequences for their conservation status Aquatic Conservation: Marine and Freshwater Ecosystems. John Wiley & Sons, Ltd; 2010; 20: 319–333. 10.1002/aqc.1083 [DOI] [Google Scholar]
- 105.de Carvalho MR, Bockmann FA, Amorim DS, et al. (2007) Taxonomic impediment or impediment to taxonomy? A commentary on systematics and the cybertaxonomic-automation paradigm. Evolutionary Biology 34:140–143. 10.1007/s11692-007-9011-6 [DOI] [Google Scholar]
- 106.Kim KC, Byrne LB. Biodiversity loss and the taxonomic bottleneck: emerging biodiversity science. Ecological Research. 2006; 21: 794–810. [Google Scholar]
- 107.Smith KF, Behrens MD, Max LM, Daszak P (2008) U.S. drowning in unidentified fishes: scope, implications, and regulation of live fish import. Conservation Letters 1:103–109. 10.1111/j.1755-263X.2008.00014.x/pdf [DOI] [Google Scholar]
- 108.Shokralla S, Gibson JF, Nikbakht H, et al. (2014) Next‐generation DNA barcoding: using next‐generation sequencing to enhance and accelerate DNA barcode capture from single specimens. Molecular Ecology Resources 14:892–901. 10.1111/1755-0998.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meier R, Wong WH, Srivathsan A, Foo M. $1 DNA barcodes for reconstructing complex phenomes and finding rare species in specimen‐rich samples. Cladistics 2016; 32(1): 100–110. [DOI] [PubMed] [Google Scholar]
- 110.Winnepenninckx B, Backeljau T, De Wachter R. Extraction of high molecular weight DNA from molluscs. Trends in Genetics 1993; 9(12): 407 [DOI] [PubMed] [Google Scholar]
- 111.Meier R. DNA sequences in taxonomy—Opportunities and challenges In: Wheeler QD, editor. New Taxonomy. New York: CRC Press; 2008. 95–127. [Google Scholar]
- 112.Glaubrecht M, Podlacha K. Freshwater gastropods from early voyages into the Indo-West Pacific: the ‘melaniids’ (Cerithioidea, Thiaridae) from the French ‘La Coquille’ circumnavigation, 1822–1825. Zoosystematics and Evolution 2010; 86(2): 185–211. [Google Scholar]
- 113.Wong YT, Meier R, Tan KS. High haplotype variability in established Asian populations of the invasive Caribbean bivalve Mytilopsis sallei (Dreissenidae). Biological Invasions 2011; 13(2), 341–348. [Google Scholar]
- 114.Bortolus A. Error cascades in the biological sciences: the unwanted consequences of using bad taxonomy in ecology. Ambio 2008; 37(2): 114–118. [DOI] [PubMed] [Google Scholar]
- 115.Becker S, Hanner R, Steinke D. Five years of FISH-BOL: brief status report. Mitochondrial DNA 2011; 22(supplement 1): 3–9. [DOI] [PubMed] [Google Scholar]
- 116.Vink CJ, Paquin P, Cruickshank RH. Taxonomy and irreproducible biological science. BioScience 2012; 62(5): 451–452. [Google Scholar]
- 117.Collins RA, Cruickshank RH. The seven deadly sins of DNA barcoding. Molecular Ecology Resources 2013; 13: 969–975. 10.1111/1755-0998.12046 [DOI] [PubMed] [Google Scholar]
- 118.Steinke D, Hanner R. The FISH-BOL collaborators’ protocol. Mitochondrial DNA 2011; 22(supplement 1): 10–14. [DOI] [PubMed] [Google Scholar]
- 119.Wong WH, Tay YC, Puniamoorthy J, Balke M, Cranston PS, Meier R. ‘Direct PCR’ optimization yields a rapid, cost-effective, nondestructive and efficient method for obtaining DNA barcodes without DNA extraction. Molecular Ecology Resources 2014; 14(6): 1271–1280. 10.1111/1755-0998.12275 [DOI] [PubMed] [Google Scholar]
- 120.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse invertebrates. Molecular Marine Biology and Biotechnology 1994; 3(5): 294–299. [PubMed] [Google Scholar]
- 121.Kulsantiwong J, Prasopdee S, Ruangsittichai J, Ruangjirachuporn W, Boonmars T, Viyanant V, et al. DNA barcode identification of freshwater snails in the family Bithyniidae from Thailand. PLoS ONE 2013; 8(11): e79144 10.1371/journal.pone.0079144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geller J, Meyer C, Parker M, Hawk H. Redesign of PCR primers for mitochondrial cytochrome C oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Molecular Ecology Resources 2013; 13: 851–861. 10.1111/1755-0998.12138 [DOI] [PubMed] [Google Scholar]
- 123.Leray M, Yang JY, Meyer CP, Mills SC, Agudelo N, Ranwez V, Boehm JT, Machida RJ. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Frontiers in Zoology 2013; 10: 34 10.1186/1742-9994-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G. The simple fool’s guide to PCR. Version 2.0 Honolulu: University of Hawaii; 2002. 45 pp. [Google Scholar]
- 125.Do VT. Pilsbryoconcha exilis. The IUCN Red List of Threatened Species 2013: e.T171874A6808653. Available: 10.2305/IUCN.UK.2011-1.RLTS.T171874A6808653.en [DOI]
- 126.Djajasasmita M. The occurrence of Anodonta woodiana Lea, 1837 in Indonesia (Pelecypoda: Unionidae). The Veliger 1982; 25: 1–1. [Google Scholar]
- 127.Douda K, Vrtílek M, Slavík O, Reichard M. The role of host specificity in explaining the invasion success of the freshwater mussel Anodonta woodiana in Europe. Biological Invasions 2012; 14: 127–137. [Google Scholar]
- 128.Chan SY. Some aquaria-limited clams from Singapore (Unionidae—Hyriopsis bialatus Simpson, 1900). Ellipsaria 2008; 10: 10. [Google Scholar]
- 129.Appleton CC. Alien and invasive fresh water Gastropoda in South Africa. African Journal of Aquatic Science 2003; 28: 69–81. [Google Scholar]
- 130.Cowie RH, Hayes KA. Apple snails In: Francis RA (editor). A handbook of global freshwater invasive species. Oxon: Earthscan; 2012. pp. 207–221. [Google Scholar]
- 131.van Benthem Jutting WSS. Catalogue of the non-marine Mollusca of Sumatra and of its satellite islands. Beaufortia 1959; 7:41–191. [Google Scholar]
- 132.Mienis HK. Notes on recent and fossil Neritidae 3. Neritina juttingae, new name for Nerita aculeata Gmelin, 1791, non Müller, 1774 (Mollusca, Gastropoda). Basteria 1973; 37: 1–2. [Google Scholar]
- 133.von Rintelen T, Stelbrink B, Marwoto RM, Glaubrecht M. A snail perspective on the biogeography of Sulawesi, Indonesia: origin and intra-island dispersal of the viviparous freshwater gastropod Tylomelania. PLoS ONE 2014; 9(6): e98917 10.1371/journal.pone.0098917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wethington AR, Lydeard C. A molecular phylogeny of Physidae (Gastropoda: Basommatophora) based on mitochondrial DNA sequences. Journal of Molluscan Studies 2007; 73: 241–257. 10.1093/mollus/eym021 [DOI] [Google Scholar]
- 135.Brown DS. A freshwater snail new for Africa: Amerianna carinata (Planorbidae) found in Nigeria. Journal of Molluscan Studies 1983; 49: 77–79. [Google Scholar]
- 136.Pointier JP. Invading freshwater snails and biological control in Martinique Island, French West Indies. Memórias do Instituto Oswaldo Cruz 2001; 96: 67–74. [DOI] [PubMed] [Google Scholar]
- 137.Van Damme D. Gyraulus convexiusculus. The IUCN Red List of Threatened Species 2014: e.T166681A42421590. Available: 10.2305/IUCN.UK.2014-1.RLTS.T166681A42421590.en [DOI] [Google Scholar]
- 138.Cowie RH, Dillon RT Jr., Robinson DG, Smith JW. Alien non-marine snails and slugs of priority quarantine importance in the United States: a preliminary risk assessment. American Malacological Bulletin 2009; 27: 113–132. [Google Scholar]
- 139.Sarasin P, Sarasin F. Materialien Zur Naturgeschichte Der Insel Celebes. Weisbaden: C.W. Kreidel's Verlag; 1898. pp. 62–63. [Google Scholar]
- 140.Shea M. The Chinese viviparid snail Bellamya heudei guangdungensis (Kobelt, 1906) in Australia (Prosobranchia: Viviparidae). Molluscan Research 1994; 15: 3–11. [Google Scholar]
- 141.McMahon RF. The occurrence and spread of the introduced Asiatic freshwater clam, Corbicula fluminea (Müller), in North America: 1924–1982. The Nautilus 1982; 96(4): 134–141. [Google Scholar]
- 142.Ishibashi R, Komaru A. Invasion of Corbicula fluminea into the Lake Biwa-Yodo River System. VENUS: Journal of the Malacological Society of Japan 2003; 62(1–2): 65–70. [Google Scholar]
- 143.Sousa R, Gutiérrez JL, Aldridge DC. Non-indigenous invasive bivalves as ecosystem engineers. Biological Invasions 2009; 11: 2367–2385. [Google Scholar]
- 144.Pigneur L-M, Marescaux J, Roland K, Etoundi E, Descy J-P, Van Doninck K. Phylogeny and androgenesis in the invasive Corbicula clams (Bivalvia, Corbiculidae) in Western Europe. BMC Evolutionary Biology 2011; 11: 147 10.1186/1471-2148-11-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ilarri MI, Sousa R. Corbicula fluminea Müller (Asian clam) In: Francis RA (editor). A handbook of global freshwater invasive species. Oxon: Earthscan; 2012. pp. 171–183. [Google Scholar]
- 146.Foster AM, Fuller P, Benson A, Constant S, Raikow D, Larson J, Fusaro A. Corbicula fluminea Gainesville, Florida: USGS Nonindigenous Aquatic Species Database; 2015. Available: http://nas.er.usgs.gov/queries/factsheet.aspx?speciesid=92 [Google Scholar]
- 147.Watters GT. A synthesis and review of the expanding range of the Asian freshwater mussel Anodonta woodiana (Lea, 1834) (Bivalvia: Unionidae). The Veliger 1997; 40: 152–256. [Google Scholar]
- 148.Cianfanelli S, Lori E, Bodon M. Non-indigenous freshwater molluscs and their distribution in Italy In: Gherardi F (editor). Biological invaders in inland waters: profiles, distribution, and threats. Dordrecht: Springer; 2007. pp. 103–122. [Google Scholar]
- 149.Latjner J, Crnčan P. Distribution of the invasive bivalve Sinanodonta woodiana (Lea, 1834) in Croatia. Aquatic Invasions 2011; 6(supplement 1): S119–S124. [Google Scholar]
- 150.Benson AJ. Sinanodonta woodiana Gainesville, Florida: USGS Nonindigenous Aquatic Species Database; 2016http://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=2824 [Google Scholar]
- 151.Arias A, Torralba-Burrial A. First European record of the giant ramshorn snail Marisa cornuarietis (Linnaeus, 1758) (Gastropoda: Ampullariidae) from northern Spain. Limnetica 2014: 33(1): 65–72. [Google Scholar]
- 152.Halwart M. The golden apple snail Pomacea canaliculata in Asian rice farming systems: present impact and future threat. International Journal of Pest Management 1994; 40: 1–8. [Google Scholar]
- 153.Naylor R. Invasions in agriculture: assessing the cost of the golden apple snail in Asia. Ambio 1996; 25: 443–448. [Google Scholar]
- 154.Joshi RC, Sebastian LS. Global Advances in Ecology And Management of Golden Apple Snails. Nueva Ecija: Philippine Rice Research Institute; 2006. 588 pp. [Google Scholar]
- 155.Deng ZH, Lv S, Lin JY, Lin RX, Pei FQ. An outbreak of angiostrongyliasis in Guanging, People’s Republic of China: migrants vulnerable to an emerging disease. Southeast Asian Journal of Tropical Medicine and Public Health 2011; 42(5): 1047–1053. [PubMed] [Google Scholar]
- 156.Raut SK, Aditya G. Occurrence of Golden Mystery Snail Pomacea bridgesi (Gastropoda: Ampullariidae) in West Bengal, India. Current Science 1999; 77: 1389–1390. [Google Scholar]
- 157.Marwoto RM, Isnaningsih NR. Notes on the distribution of the invasive freshwater snail Pomacea canaliculata (Lamarck, 1822) and P. insularum (D’Orbigny, 1835) in Indonesia. BIOTROPIA 2011; 18(2): 123–128. [Google Scholar]
- 158.Posch H, Garr AL, Reynolds E. The presence of an exotic snail, Pomacea maculata, inhibits growth of juvenile Florida apple snails, Pomacea paludosa. Journal of Molluscan Studies 2013; 79: 383–385. [Google Scholar]
- 159.Teem JL, Qvarnstrom Y, Bishop HS, da Silva AJ, Carter J, White-Mclean J, et al. The occurrence of the rat lungworm, Angiostrongylus cantonensis, in nonindigenous snails in the Gulf of Mexico Region of the United States. Hawai’i Journal of Medicine and Public Health 2013; 72: 11–14. [PMC free article] [PubMed] [Google Scholar]
- 160.Charoenchai A, Tesana S, Pholpark M. Natural infection of trematodes in Lymnaea (Radix) auricularia rubiginosa in water reservoirs in Amphoe Muang, Khon Kaen Province. Southeast Asian Journal of Tropical Medicine and Public Health 1997; 28(supplement 1): 209–212. [PubMed] [Google Scholar]
- 161.Anderson R. 2008. Annotated list of the non-marine Mollusca of Britain and Ireland. Available: http://www.conchsoc.org/sites/default/files/MolluscWorld/Anderson-2008.pdf
- 162.Appleton CC, Miranda NAF. Two Asian freshwater snails newly introduced into South Africa and an analysis of alien species reported to date. African Invertebrates 2015; 56(1): 1–17. [Google Scholar]
- 163.Butot LJM. Planorbis exustus Desh. and Amerianna carinata (Adams) in Java. Basteria 1954; 18(4): 65. [Google Scholar]
- 164.Singh KI, Krishnasamy M, Ambu S, Rasul R, Chong NL. Studies on animal schistosomes in Peninsular Malaysia: record of naturally infected animals and additional hosts of Schistosoma spindale. Southeast Asian Journal of Tropical Medicine and Public Health 1997; 28(2): 303–307. [PubMed] [Google Scholar]
- 165.Mitchell AJ, Hobbs MS, Brandt TM. The effect of chemical treatments on red-rimmed melania Melanoides tuberculata, an exotic aquatic snail that serves as a vector of trematodes to fish and other species in the USA. North American Journal of Fisheries Management 2007; 27(4): 1287–1293. [Google Scholar]
- 166.Clausen JH, Madsen H, Murrell KD, Van PT, Hung NM, Khue NV, et al. Relationship between snail population density and infection status of snails and fish with zoonotic trematodes in Vietnamese carp nurseries. PloS Neglected Tropical Diseases 2012; 6(12): e1945 10.1371/journal.pntd.0001945 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
DNA sequences are available from GenBank (accession numbers KU318325-KU318393), and BOLD. Voucher specimens are available from the Zoological Reference Collection (ZRC) of the Lee Kong Chian Natural History Museum, National University of Singapore (accession numbers ZRC.MOL.5904-5951, 6284–6333, 6752-6754).