Abstract
Studies about changes in hippocampal volumes in subjects with bipolar disorder (BD) have been contradictory. Since the number of manic episodes and hospitalization has been associated with brain changes and poor cognitive outcomes among BD patients, we have hypothesized that these variables could clarify this issue. We stratified subjects with BD in early (BD-Early), intermediate (BD-intermediate) and late (BD-Late) stages as a function of number of manic episodes and prior hospitalization. Then, we compared their hippocampal volumes and California Verbal Learning Test-II (CVLT-II) scores with healthy controls (HC) using the general linear model. A total of 173 subjects were included in the study (112 HC, 15 BD-Early, 30 BD-Intermediate, and 16 BD-Late). We found a significant group effect on hippocampus volume (F(3,167) = 3.227, p = 0.024). Post-hoc analysis showed that BD-Late subjects had smaller hippocampus than HC (p = 0.017). BD-Early and BD-Intermediate subjects showed no significant difference in hippocampus volume compared to HC and BD-Late subjects. The CVLT trial 1 to 5 scores were significantly different across the groups (F(3,167) = 6.371, p < 0.001). Post-hoc analysis showed that BD-Intermediate (p = 0.006) and BD-Late (p = 0.017) subjects had worse memory performance during immediate recall than HC, while the performance difference between BD-Early subjects and HC was not significant (p = 0.208). These findings add to the notion that BD is a neuroprogressive disorder with brain changes and cognitive impairment according to prior morbidity (number of manic episodes and hospitalization). Also, they suggest that hippocampus is a brain marker and a potential therapeutic target for patients at late stage.
Keywords: Bipolar disorder, Hippocampus, Verbal memory, Staging, Neuroprogression
1. Introduction
Lifetime prevalence of bipolar disorder (BD) is 2.1% worldwide, with subthreshold forms affecting another 2.4% (Merikangas et al., 2007). BD is associated with cognitive impairment even during periods of euthymia (Martínez-Arán et al., 2004; Barrett et al., 2009). Emergent evidence from systematic reviews in the field suggests an association between the number of manic episodes as well as psychiatric hospitalizations with neurocognitive decline (Robinson and Ferrier, 2006), particularly verbal memory impairment (Martínez-Arán et al., 2004). Not only cognition seems to be impaired in portion as a function of number of mood episodes in BD. There is evidence of overall brain atrophy in bipolar patients with multiple-episodes and a cross-sectional study showed that lateral ventricles were significantly larger in bipolar patients with multiple-episodes as compared to first-episode patients or healthy controls (Strakowski et al., 2002). Also, a recent study showed decreased volume of corpus callosum in BD women with more than 10 episodes and at least one psychiatric hospitalization (Lavagnino et al., 2015).
Hippocampus is essential for the acquisition, consolidation and retrieval of memory (Eichenbaum, 2000), which places it as an interesting structure to study the relations among cognitive impairment, brain changes, and number of manic episodes. However, studies of hippocampal volumes in BD patients have been contradictory so far, showing no changes (Brambilla et al., 2003; Altshuler et al., 2000; Bertolino et al., 2003), smaller volumes (Blumberg et al., 2003; Bearden et al., 2008), and even larger volumes in BD patients as compared to controls (Javadapour et al., 2010; van Erp et al., 2012). In addition, six meta-analyses did not show changes in hippocampal volumes in patients with BD (McDonald et al., 2004; Videbech and Ravnkilde, 2004; Kempton et al., 2008; Arnone et al., 2009; Bora et al., 2010; Ellison-Wright and Bullmore, 2010).
Thus we hypothesize that reduced hippocampal volumes and poorer verbal memory performance can be identified in patients with multiple episodes and hospitalizations but not in patients with fewer and less severe episodes. We set forth to assess hippocampal volume and verbal memory according to number of manic episodes and hospitalization in BD.
2. Methods
We performed a cross-sectional study to assess hippocampal volume and verbal memory in subjects with BD type 1 according to prior number of episodes and hospitalizations. Because the distribution of the number of episodes was not normal, we previously stratified subjects into subgroups. A similar approach was used in a recent paper in major depressive disorder (Treadway et al., 2015). Also, the grouping approach is less sensitive to variability in the retrospective report of number of episodes, especially when the number of episodes experienced by the subject are high (Treadway et al., 2015). Therefore, we classified subjects as “BD-Late” if they had 10 or more manic episodes and 1 or more hospitalizations due to manic or depressive episodes. Similar definitions were used in previous studies (Magalhães et al., 2012; Lavagnino et al., 2015). We classified subjects as “BD-Early” when subjects had 3 or less manic episodes. The remaining subjects were classified as “intermediate-stage” (BD-Intermediate). The study was approved by the Institutional Review Boards of the University of Texas Health Science Center at San Antonio. Subjects signed informed consent before any study-related procedures after a complete description of the study with ample time for questions.
2.1. Participants
Subjects were recruited from the community and psychiatric clinics through flyers, radio, and newspaper advertisements. Inclusion criteria were subjects with BD type I according to DSM-IV, and age between 18 and 65. Exclusion criteria were head trauma with residual effects, neurological disorder, and uncontrolled major medical conditions. Healthy controls (HC) with a history of any Axis I disorder or with any first-degree relative with any Axis I disorder or use of psychoactive medication less than two-weeks prior to the study were also excluded. Subjects were evaluated through a socio-demographic history form to assess age, gender, years of education, and occupational status. Axis-I diagnoses and clinical characteristics were assessed with the Structured Clinical Interview for DSM-IV axis-I Disorders (SCID-I), which was administered by fully trained staff. Current dimensional mood symptoms were assessed with the Hamilton Depression Scale (HAM-D) (HAMILTON, 1960) and the Young Mania Rating Scale (YMRS) (Young et al., 1978). Verbal memory was measured using California Verbal Learning Test-II (CVLT-II) (Elwood, 1995).
2.2. MRI data acquisition
We acquired structural T1-weighted scans using a Philips 1.5 T MRI scanner (Philips Medical System, Andover, MA, USA) with a three-dimensional axial fast field echo sequence. The parameters are as follows: repetition time (TR) = 24 ms, echo time (TE) = 5 ms, flip angle = 40°, field of view (FOV) = 256 mm, slice thickness = 1 mm, matrix size = 256 × 256 and 150 slices.
2.3. MRI data preprocessing
All scans were visually inspected to rule out gross artifacts. Cortical and subcortical reconstruction and segmentation were performed with the Freesurfer software suite version 5.3.0 (http://surfer.nmr.mgh.harvard.edu). The whole procedure including motion correction, intensity normalization, automated topology corrections and automatic segmentations of cortical and subcortical regions was documented elsewhere (Dale and Sereno, 1993; Dale et al., 1999; Fischl et al., 1999a, 1999b, 2002; Fischl and Dale, 2000; Ségonne et al., 2004; Jovicich et al., 2006). Regions labeled as left and right hippocampus were extracted, and the corresponding volumes were calculated according to the voxel numbers contained within the regions and the voxel volume. Six subjects (4 HC and 2 BD-Early) were excluded as outliers in either of the hippocampus volumes (threshold: 3 standard deviations) and a total number of 173 subjects were further analyzed. The left and right hippocampus volumes were then averaged, because we did not find significant laterality effect in the preliminary analysis (F = 0.191, p = 0.663). The average hippocampus volume was then scaled by the estimated total intracranial volume (ICV) for each subject.
2.4. Memory performance
All participants were administered the Wechsler Abbreviated Scale of Intelligence (WASI), which is a screener of verbal, non-verbal, and general cognitive ability and the Wechsler Test of Adult Reading (WTAR), which is a measure of premorbid intellectual quotient (IQ) (Friedmann, 2013). All participants were administered a revised version of the CVLT – a standardized test measuring verbal learning and declarative memory via a trial list-learning paradigm (Donders, 2008). This version of the CVLT was part of the South Texas Assessment of Neurocognition (STAN) which includes both standardized and computerized neurocognitive tasks (Glahn et al., 2007). In the CVLT task participants are presented orally with 16 words for 5 times and asked to recall as many words as possible in any order. The total number of correctly recalled words from trial 1 to 5 was used to evaluate the memory performance. Furthermore, semantic clustering and series clustering scores were used to evaluate the memory strategy.
2.5. Statistical analyses
Statistical analyses were conducted using SPSS software (Version 21.0). Descriptive analyses were reported as means (standard deviations), median (interquartile range) or absolute and relative frequencies. We have used analysis of variance (ANOVA) to compare demographic and clinical variables. For each hippocampus measurement, we have used a general linear model with one way ANCOVA. Group (BD-Late, BD-Intermediate, BD-Early, and HC) was entered as an independent variable, while hippocampus volume was entered as a dependent outcome variable. We used age and gender as covariates. The education level was significantly different across the groups. However, in a preliminary analysis, we found no significant effect of education level using ANCOVA to test the group effect on hippocampus volumes and CVLT-II scores with age, gender and education as the covariates (p > 0.1). In addition, 22 subjects did not have valid education level data (14 HC, 5 BD-Intermediate and 3 BD-Late subjects), which would significantly affect the statistical power for BD subjects, especially for BD-Late subjects, as 18.8% (3 out of 16) BD-Late subjects would be missing in the analysis. Thus, we did not include education as a covariate in the final analysis. The multiple pairwise comparisons were performed using a Bonferroni correction if the ANOVA was significant. We considered p-values < 0.05 significant.
3. Results
3.1. Demographics
A total of 173 subjects were included in the study (112 HC,15 BD-Early, 30 BD-Intermediate, and 16 BD-Late). Table 1 shows demographics and clinical characteristics. Of note, illness duration (p = 0.690), current use of lithium (p = 0.592), YMRS (p = 0.207) and HAM-D (p = 0.584) scores were not different between BD stages.
Table 1.
Demographics and clinical characteristics.
| Characteristics | Healthy controls (112) | BD-Early (15) | BD-Intermediate (30) | BD-Late (16) | P valuea |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 41.1% (46) | 26.7% (23) | 33.3% (10) | 25% (4) | 0.455 |
| Female | 58.9% (66) | 73.3% (11) | 66.7% (20) | 75% (12) | |
| Age (years) | 33.75 ± 11.99 | 40.47 ± 12.83 | 35.10 ± 10.25 | 39.56 ± 10.65 | 0.075 |
| Years of education | 15.89 ± 3.05 | 14.60 ± 3.72 | 14.04 ± 2.09 | 13.00 ± 3.67 | 0.002 |
| Handedness (Right-handed) | 90.5% | 100% | 86.7% | 100% | 0.672 |
| Illness Duration | – | 22.15 ± 12.78 | 18.81 ± 9.19 | 22.47 ± 9.04 | 0.441 |
| Medications | |||||
| Anticonvulsant | – | 40% (6) | 23% (7) | 50% (8) | 0.308 |
| Antidepressant | – | 27% (4) | 30% (9) | 25% (4) | 0.961 |
| Benzodiazepine | – | 13% (2) | 17% (5) | 38% (6) | 0.341 |
| Lithium | – | 20% (3) | 20% (6) | 7% (1) | 0.592 |
| Stimulant | – | 7% (1) | 0 (0) | 6% (1) | 0.625 |
| Atypical antipsychotic | – | 33% (5) | 17% (5) | 25% (4) | 0.686 |
| YMRS | 0.34 ± 0.83 | 5.47 ± 9.06 | 7.77 ± 6.47 | 6.38 ± 6.90 | <0.001 |
| HAMD | 0.76 ± 1.16 | 13.13 ± 7.62 | 12.07 ± 8.07 | 16.69 ± 9.47 | <0.001 |
BD-Early, bipolar disorder type I at early stage; BD-Intermediate, bipolar disorder at intermediate stage; BD-Late, bipolar disorder at late stage.
Chi-square was used to compare gender, handedness and medications among groups, while ANOVA was used to age and years of education.
3.2. Hippocampal volume
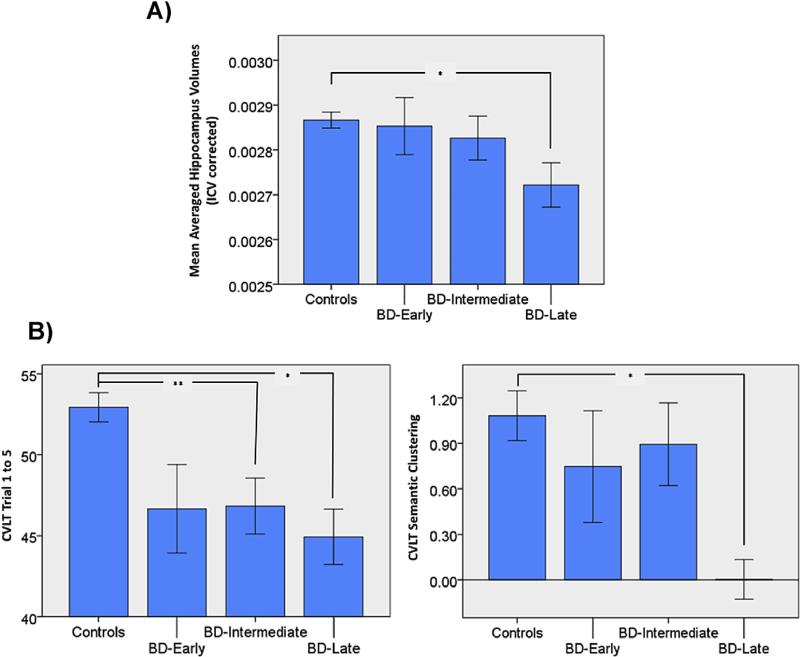
We found a significant group effect on hippocampus volume (F(3,167) = 3.227, p = 0.024, partial η2 = 0.055). Further post-hoc analysis with Bonferroni correction showed that BD-Late subjects had significantly smaller hippocampus than HC (p = 0.017). BD-Early (p = 1) and BD-Intermediate (p = 1) subjects showed no significant difference in hippocampus volume compared to HC (Fig. 1a). These results indicated an atrophic effect on hippocampus volumes as a function of number of episodes and hospitalization.
Fig. 1.
Reduced hippocampal volume and worse memory performance and strategy in BD-Late subjects. A) BD-Late subjects showed significantly reduced hippocampal volumes compared to HC. B) Both BD-Intermediate and BD-Late subjects showed significantly worse memory recall performance compared to HC, while only BD-Late subjects showed worse semantic clustering score than HC. BD-Early, bipolar disorder type I at early stage; BD-Intermediate, bipolar disorder at intermediate stage; BD-Late, bipolar disorder at late stage. *p < 0.05; **p < 0.01.
3.3. California Verbal Learning Test – verbal memory performance
The CVLT trial 1 to 5 scores were significantly different across the groups (F(3,167) = 6.371, p < 0.001, partial η2 = 0.103). Further Post-hoc analysis with Bonferroni correction showed that both BD-Intermediate (p = 0.006) and BD-Late (p = 0.017) subjects had worse memory performance during recall than HC, while the performance difference between BD-Early subjects and healthy controls was not significant (p = 0.208) (Fig. 1b).
We also found a marginally significant group difference on CVLT semantic clustering scores (F(3,167) = 2.481, p = 0.063, partial η2 = 0.043). Post-hoc analysis with Bonferroni correction showed that BD-Late subjects had significantly worse semantic clustering scores than HC (p = 0.049), while BD-Early (p = 1) and BD-Intermediate (p = 1) subjects showed no significant difference in the semantic clustering score compared to HC (Fig. 1b).
However, we did not find a direct correlation between any CVLT score and hippocampus volume within each patient group (p > 0.05), which may imply that there are other pathways besides hippocampus to explain memory performance impairment as a function of prior manic episodes.
4. Discussion
The present study showed that subjects at late-stage BD had a decreased hippocampal volume when compared to controls. In addition, we showed that both subjects at intermediate and late-stage BD showed worse verbal memory recall as compared to healthy controls. Moreover, late-stage subjects showed worse semantic clustering scores than controls. These findings add to the notion that BD is a neuroprogressive disorder with brain changes and cognitive impairment according to prior morbidity (number of manic episodes and hospitalization in our study). Of note, illness duration and current use of lithium were not different between BD stages in our sample.
These results suggest that changes in the volume of hippocampus may be a brain marker of neuroprogression. In this same vein, a recent study showed that patients with major depressive disorder present reductions in hippocampal volume as a function of number of episodes (Treadway et al., 2015). Moreover, a recent study showed neuroinflammation in the hippocampus of patients of BD (Haarman et al., 2014), which may provide a mechanistic link between staging and inflammatory changes that occur during mood episodes of BD (Réus et al., 2015). Manic episodes are particularly associated with increased levels of inflammatory markers (Munkholm et al., 2013) and the level of proinflammatory cytokines are negatively correlated with hippocampal volumes (Monje et al., 2003).
Our findings also suggest that memory recall impairment occur in intermediate stages. Memory recall impairments become more evident in late stages of BD (Fig. 1). Consistently, in a five-year follow-up study of neurocognitive functioning in BD, a dysfunction in verbal memory became more evident in patients at late stages (Santos et al., 2014). In addition, our study showed that only subjects at later stages of illness present worse semantic clustering scores as compared to controls. Also, the cognitive impairment as a function of number of mood episodes might be explained by other mechanisms besides the reductions in the hippocampus.
The present findings have important clinical implications since they may offer objective biological measures to identify late-stage BD subjects, who may benefit from differential therapeutic strategies as compared to early-stage subjects (Grande et al., 2014). This is an important step towards a personalized medicine. Therapeutic approaches designed to prevent deleterious changes in hippocampus and recover memory function may provide valuable help to prevent and reverse changes associated with late stage in BD. For instance, a recent study in treatment-resistant BD patients showed that erythropoietin was associated with memory improvement and reversal of brain matter loss in the left hippocampus (Miskowiak et al., 2015). Preclinical studies using mesenchymal stem cells have increased hippocampal neurogenesis, improved cognitive impairment, and counteracted depressive-like behavior (Tfilin et al., 2010). In addition, treatment with lithium has been shown to prevent hippocampal reduction (Hajek et al., 2012) among BD patients and poor treatment adherence has been correlated with cognitive impairment (Martinez-Aran et al., 2009).
The present study has a cross-sectional design and reverse causality cannot be discarded. In addition, recall bias and the influence of current symptoms may have interfered with our findings. Specifically, our reliance on retrospective self-report for the number of manic episodes may be influence of biases such as the fact that patients with greater severity of illness may have been more likely to identify previous manic episodes. Longitudinal studies are needed to clarify the extent to which number of manic episodes is a result or a consequence of changes in brain volume and neurocognitive performance. Moreover, we were not able to control for some confounders, such as medication status and education. Although current lithium use was not different between the groups, we do not know the impact of other psychotropic medications. This analysis was not possible because of the number of missing data.
In conclusion, this study provides evidence of neuroprogression and the usefulness of staging models in BD. In addition, it points to pathophysiologic associations between number of episodes/hospitalization, brain changes, and cognitive impairment. Also, the characterization of late-stage as a cluster of bipolar patients with more pronounced memory impairment has potential implications for treatment. In particular, they contribute to the growing literature suggesting that hippocampal volume may be a potential biomarker and treatment target for BD.
Acknowledgment
Supported in part by NIMH grant R01 085667, the Dunn Research foundation, and the Pat Rutherford, Jr. Endowed Chair in Psychiatry (Jair C. Soares). Dr Passos is supported by scholarship from “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES), Brazil.
Footnotes
Contributors
Dr. Cao processed and analyzed the neuroimaging and cognitive data, drafted the manuscript and critically edited the draft of the manuscript.
Dr. Passos drafted the manuscript and critically edited the draft of the manuscript.
Dr. Mwangi processed the neuroimaging data and critically edited the draft of the manuscript.
Dr. Bauer organized and analyzed the cognitive data and critically edited the draft of the manuscript.
Dr. Zunta-Soares coordinated the subject enrollment and data collection.
Dr. Kapczinski supervised the study and critically edited the draft of the manuscript.
Dr. Soares supervised the study, provided financial and instrumental support, collected the data and critically edited the draft of the manuscript.
Conflicts of interest
Dr. Cao reported no biomedical financial interests or potential conflicts of interest. Dr. Passos reported no biomedical financial interests or potential conflicts of interest. Dr. Mwangi reported no biomedical financial interests or potential conflicts of interest. Dr. Bauer reported no biomedical financial interests or potential conflicts of interest. Dr. Zunta-Soares reported no biomedical financial interests or potential conflicts of interest. Dr. Kapczinski has received grants/research support from AstraZeneca, Eli Lilly, Janssen-Cilag, Servier, CNPq, CAPES, NARSAD, and the Stanley Medical Research Institute; has been a member of speakers’ boards for AstraZeneca, Eli Lilly, Janssen and Servier; and has served as a consultant for Servier. Dr. Soares has received grants/research support from Forrest, BMS, Merck, Stanley Medical Research Institute, NIH and has been a speaker for Pfizer and Abbott.
References
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol. Psychiatry. 2000 Jul 15;48(2):147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br. J. Psychiatry. 2009 Sep.195(3):194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Barrett SL, Mulholland CC, Cooper SJ, Rushe TM. Patterns of neurocognitive impairment in first-episode bipolar disorder and schizophrenia. Br. J. Psychiatry. 2009 Jul.195(1):67–72. doi: 10.1192/bjp.bp.108.054874. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MAM, Nicoletti M, et al. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008 May;33(6):1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Frye M, Callicott JH, Mattay VS, Rakow R, Shelton-Repella J, et al. Neuronal pathology in the hippocampal area of patients with bipolar disorder: a study with proton magnetic resonance spectroscopic imaging. Biol. Psychiatry. 2003 May 15;53(10):906–913. doi: 10.1016/s0006-3223(02)01911-x. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch. Gen. Psychiatry. 2003 Dec.60(12):1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yücel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol. Psychiatry. 2010 Jun 1;67(11):1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Brambilla Paolo, Harenski Keith, Nicoletti Mark, Sassi Roberto B., Mallinger Alan G., Frank Ellen, Kupfer David J., Keshavan Matcheri S., Soares Jair C. MRI investigation of temporal lobe structures in bipolar patients. J. Psychiatr. Res. 2003;37(4):287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999 Feb.9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cogn. Neurosci. 1993 Jan.5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Donders J. Subtypes of learning and memory on the California Verbal Learning Test-Second Edition (CVLT-II) in the standardization sample. J. Clin. Exp. Neuropsychol. 2008 Oct.30(7):741–748. doi: 10.1080/13803390701689595. [DOI] [PubMed] [Google Scholar]
- Eichenbaum HA. Cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 2000 Oct.1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr. Res. 2010 Mar.117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Elwood RW. The California Verbal Learning Test: psychometric characteristics and clinical application. Neuropsychol. Rev. 1995 Sep.5(3):173–201. doi: 10.1007/BF02214761. [DOI] [PubMed] [Google Scholar]
- van Erp TGM, Thompson PM, Kieseppä T, Bearden CE, Marino AC, Hoftman GD, et al. Hippocampal morphology in lithium and non-lithium-treated bipolar I disorder patients, non-bipolar co-twins, and control twins. Hum. Brain Mapp. 2012 Mar.33(3):501–510. doi: 10.1002/hbm.21239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000 Sep 26;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002 Jan 31;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a Feb.9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution inter-subject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999b Jan.8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD. Alcohol use in adults. N. Engl. J. Med. 2013 Apr 25;368(17):1655–1656. doi: 10.1056/NEJMc1302445. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. The neurocognitive signature of psychotic bipolar disorder. Biol. Psychiatry. 2007 Oct 15;62(8):910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Grande I, Magalhães PV, Chendo I, Stertz L, Panizutti B, Colpo GD, et al. Staging bipolar disorder: clinical, biochemical, and functional correlates. Acta Psychiatr. Scand. 2014 Jun.129(6):437–444. doi: 10.1111/acps.12268. [DOI] [PubMed] [Google Scholar]
- Haarman BCMB, Riemersma-Van der Lek RF, de Groot JC, Ruhé HGE, Klein HC, Zandstra TE, et al. Neuroinflammation in bipolar disorder – a [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav. Immun. 2014 Aug.40:219–225. doi: 10.1016/j.bbi.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Hajek T, Kopecek M, Höschl C, Alda M. Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. J. Psychiatry Neurosci. 2012 Sep.37(5):333–343. doi: 10.1503/jpn.110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960 Feb.23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadapour A, Malhi GS, Ivanovski B, Chen X, Wen W, Sachdev P. Hippocampal volumes in adults with bipolar disorder. J. Neuropsychiatry Clin. Neurosci. 2010 Jan.22(1):55–62. doi: 10.1176/jnp.2010.22.1.55. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006 Apr 1;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SCR, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch. Gen. Psychiatry. 2008 Sep.65(9):1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Lavagnino L, Cao B, Mwangi B, Wu MJ, Sanches M, Zunta-Soares GB, Kapczinski F, Soares J. Changes in the corpus callosum in women with late e stage bipolar disorder. Acta Psychiatr. Scand. 2015;131(6):458–464. doi: 10.1111/acps.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães PV, Dodd S, Nierenberg AA, Berk M. Cumulative morbidity and prognostic staging of illness in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Aust. N. Z. J. Psychiatry. 2012 Nov.46(11):1058–1067. doi: 10.1177/0004867412460593. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Scott J, Colom F, Torrent C, Tabares-Seisdedos R, Daban C, et al. Treatment nonadherence and neurocognitive impairment in bipolar disorder. J. Clin. Psychiatry. 2009 Jul.70(7):1017–1023. doi: 10.4088/JCP.08m04408. [DOI] [PubMed] [Google Scholar]
- Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sánchez-Moreno J, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am. J. Psychiatry. 2004 Feb.161(2):262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol. Psychiatry. 2004 Sep 15;56(6):411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry. 2007 May;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak Kamilla W., Vinberg Maj, Macoveanu Julian, Ehrenreich Hannelore, Køster Nicolai, Inkster Becky, Paulson Olaf B., Kessing Lars V., Skimminge Arnold, Siebner Hartwig R. Effects of erythropoietin on hippocampal volume and memory in mood disorders. Biol. Psychiatry. 2015 Aug 15;78(4):270–277. doi: 10.1016/j.biopsych.2014.12.013. ISSN 0006-3223. http://dx.doi.org/10.1016/j.biopsych. 2014.12.013. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003 Dec 5;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Vinberg M, Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J. Affect Disord. 2013 Jan 10;144(1–2):16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015 May 14;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006 Apr.8(2):103–116. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Santos JL, Aparicio A, Bagney A, Sanchez-Morla EM, Rodríguez-Jiménez R, Mateo J, et al. A five-year follow-up study of neurocognitive functioning in bipolar disorder. Bipolar Disord. 2014 Nov.16(7):722–731. doi: 10.1111/bdi.12215. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004 Jul.22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, et al. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am. J. Psychiatry. 2002 Nov.159(11):1841–1847. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol. Psychiatry. 2010 Dec.15(12):1164–1175. doi: 10.1038/mp.2009.110. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, Chakravarty MM, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol. Psychiatry. 2015 Feb 1;77(3):285–294. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry. 2004 Nov.161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978 Nov.133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]