Abstract
Recently, catheter ablation (CA) has become a therapeutic option to target focal triggers of polymorphic ventricular tachycardia and ventricular fibrillation (VF) in the setting of electrical storm (ES). This strategy was first described in subjects without organic heart disease (i.e. idiopathic VF) and subsequently in other conditions, especially in patients with ischaemic heart disease. In the majority of cases, the triggering focus originates in the ventricular Purkinje system. In patients with Brugada syndrome, besides ablation of focal trigger in the right ventricular outflow tract, modification of a substrate in this region has been described to prevent recurrences of VF. In conclusion, CA appears to be a reasonable strategy for intractable cases of ES due to focally triggered polymorphic ventricular tachycardia and VF. Therefore, early transport of the patient into the experience centre for CA should be considered since the procedure could be in some cases life-saving. Therefore, the awareness of this entity and link to the nearest expert centre are important.
Keywords: Ventricular fibrillation, polymorphic ventricular tachycardia, catheter ablation, ventricular premature beats, Brugada syndrome, long QT syndrome, ischaemic heart disease
Ventricular fibrillation (VF) is a complex arrhythmia that leads invariably to cardiac arrest. Its mechanisms remain largely unclear. Similar to atrial fibrillation, the mother rotor hypothesis is one plausible alternative.1,2 In larger animals, some authors reported that the dominant frequency of VF could be recorded at a junction of the left ventricular posterior wall and the septum.3–6 Others have shown that the posterior papillary muscle could be the major anchoring structure of VF reentrant wavelets, and the site harboring prominent Purkinje potentials and the dominant domain.7 Some studies suggest that the dominant domain in this region reflects both focal firing from the Purkinje network and reentry around the posterior papillary muscle.8,9 In the clinical arena, a bulk of experience has accumulated on catheter ablation (CA) of focal sources of VF. It confirms the important role of focal triggers in driving VF in different clinical settings.10–12 In addition, recent reports have suggested that CA may modify a substrate for polymorphic ventricular tachycardia (VT) or VF, at least in conditions such as Brugada syndrome.13,14 Therefore, it appears that different mechanisms are not mutually exclusive in the large animal or human heart.15 The role of this paper is to review available data on CA of polymorphic VT and VF in a human.
Pioneering Period
The first cases of CA of focal triggers in polymorphic VT or VF were performed in several centres in the late 1990s. In 1998, we observed a young patient with history of resuscitated cardiac arrest due to idiopathic VF who presented with electrical storm (ES) following replacement of his implantable cardioverter defibrillator (ICD).16 It was apparent that every episode of polymorphic VT and VF was triggered by a short-coupled, monotopic ventricular premature beat. Its electrocardiogram (ECG) morphology (right bundle branch block with left axis deviation and QRS duration around 130 milliseconds [ms]) suggested possible origin in the conduction system of the left posterior fascicle. The coupling interval of ectopic beat was 240 ms. After a series of shocks due to ES, the decision was made to perform CA of the trigger. Mapping of this focus at the left ventricular septum revealed the origin in the distal Purkinje network of the posterior fascicle with P potential preceding local ventricular activation during ectopy by 60–80 ms. CA completely suppressed ectopic activity and terminated ES without subsequent recurrences (see Figure 1). Similar anecdotal cases have initiated an interest and led finally to a cooperative study under a leadership of Michel Haïssaguerre.10,11
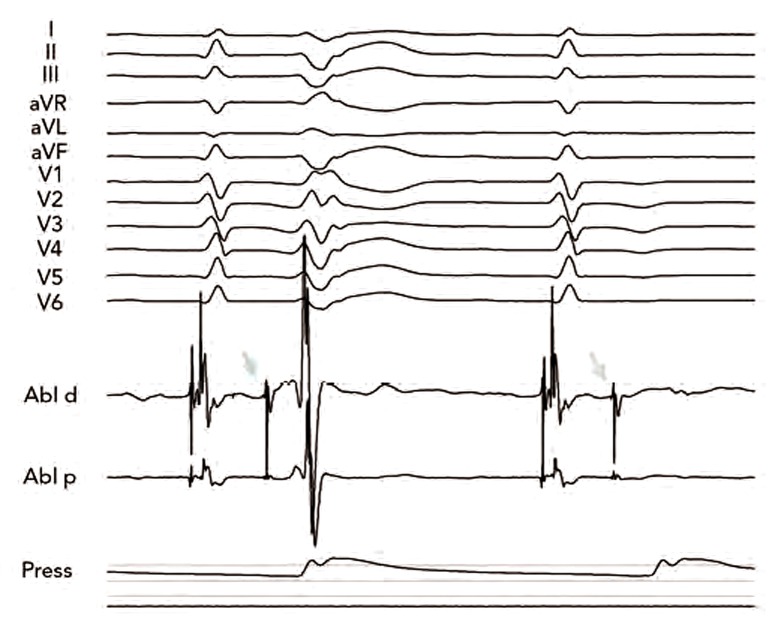
Figure 1: Twelve-lead Electrocardiogram with Intracardiac Electrograms from Ablation Catheter (Abl-d and Abl-p) and Arterial Pressure (Press) During Our First Catheter Ablation of Focally Triggered Idiopathic Ventricular Fibrillation.
The first ectopic beat (arrow) is preceded by a sharp deflection from the Purkinje system. Note that the second sinus rhythm beat is followed by the same sharp signal, which is not conducted to the surrounding myocardium. Application at that site abolished ectopy and prevented recurrence of ventricular arrhythmias.
Focally Triggered Ventricular Fibrillation Without Organic Heart Disease
Idiopathic Ventricular Fibrillation
An initial pilot report and later analysis of a series of 27 patients published by Haïssaguerre et al.10,11 showed that predominant site of triggering foci for idiopathic VF is in the His-Purkinje network of the left or right ventricle. The first initiating beat of VF had typically an identical ECG morphology and coupling interval of 297 ± 41 ms. Location of these triggers was determined by mapping the earliest electrical activity. Importantly, the foci occurred in the Purkinje conducting system in 23 patients – from the left ventricular septum in 10, from the anterior right ventricle in nine and from both in four. Only in the remaining four patients, the premature beats originated from the right ventricular outflow tract musculature, without any relation to the conduction system. After radiofrequency CA, 24 patients (89 %) had no recurrence of VF without drug during a follow-up of 24 ± 28 months.
Long-term follow-up of patients after CA of idiopathic VF has recently been published by Knecht et al.17 It comprises 38 patients (21 men) age 42 ± 13 years, refractory to a median of two antiarrhythmic drugs. In this larger cohort, triggering ventricular premature beats originated from the right (n=16), the left (n=14) or both (n=3) Purkinje network systems. During a median post-procedural follow-up of 63 months, seven (18 %) of 38 patients experienced some recurrence of VF at a median of four months. Five of these seven patients underwent repeated ablation with subsequent survival without VF recurrences. Survival free of VF was predicted only by transient bundle branch block in the originating ventricle during the electrophysiological study (p<0.0001). The number of significant events (confirmed VF or aborted sudden death) was reduced from four (interquartile range 3–9) before to 0 (interquartile range 0–4) after ablation (p<0.01).
Our experience over the last 15 years covers a series of four subjects with focally triggered idiopathic VF. Two presented with a focus in the left ventricle and two had right ventricular location. One of them presented with two foci in the right ventricle. Long-term prognosis is very good without recurrences of VF.
Long QT Syndrome
Both patients with inherited and acquired long QT interval syndrome are at risk of developing polymorphic VTs and/or VF. In high-risk subjects with inherited long QT syndrome (LQTS), ICD appears to be the best means to prevent sudden cardiac death.18 However, patients are at risk of ES that necessitates complex management. In some cases, arrhythmias could be triggered by a focal source and thus amenable to CA. To date, there have been few reports on CA in LQTS patients – all have involved cases of congenital LQTS.19–21 The largest series described VF ablation in four patients with LQTS.19 The triggering ectopy was monomorphic in two patients and polymorphic in the other two. Interestingly, the site of ablation was again the distal Purkinje network in three patients and the right ventricular outflow tract in one patient. The patients were followed up for a mean period of 24 months with no recurrences of VF (an ICD was inserted in two of the patients).
Brugada Syndrome
Brugada syndrome may present with recurrent VF that may or may not be initiated by premature ventricular beats. Compared with most cases of idiopathic VF, ectopic activity in patients with Brugada syndrome appears to originate invariably from the right ventricular outflow tract. The first report on CA of a triggering focus of polymorphic VT or VF was presented again by Haïssaguerre et al.19 Subsequently, other anecdotal cases were published on successful ablation of triggering ectopy within the right ventricular outflow tract sites in patients with this condition.22,23
More interestingly, other authors explored the possibility of modifying a substrate for VF in patients with Brugada syndrome. The first prospective study of VF ablation in Brugada syndrome patients was reported by Nademanee et al.13 This group evaluated nine patients (all male; median age 38 years) with recurrent VF that required multiple ICD shocks. Endocardial and epicardial electroanatomic mapping of both ventricles together with computed tomography (CT) image integration were performed. Interestingly, abnormal low-voltage areas with prolonged duration and fractionated late potentials around the anterior aspect of the right ventricular outflow tract epicardium were found of all subjects. CA targeted at the abnormal arrhythmogenic substrate in this location successfully abolished further arrhythmic episodes in all but one patient during a follow-up period of 20 ± 6 months. The most interesting observation was that CA resulted in normalisation of the Brugada ECG pattern (all had type 1 pattern pre-ablation). A similar finding was described by the Bordeaux group in a case report24 and confirms previous pilot experimental evidence.25
More recently, a group from Thailand14 reported on observations in 10 patients with Brugada syndrome (all men; median age 36.5 years). Four subjects presented with ES while the remaining group had no arrhythmias. All patients underwent electrophysiological study using noncontact mapping with the multielectrode array placed in the right ventricular outflow tract. The isopotential map was analysed during sinus rhythm and the region with electrical activity occurring during J point to +60 (J+60) milliseconds interval of the V1 or V2 of surface ECG was considered as the late activation zone. Interestingly, such a late activation zone was always found in the right ventricular outflow tract with variable distribution in both groups. Endocardial CA of the late activated areas modified Brugada ECG pattern in three of four patients (75 %) and suppressed ES in all four patients during long-term follow-up (12–30 months). One patient had complete right bundle branch block from the ablation procedure.
All the above studies provide important evidence that the increasingly recognised subtle structural abnormalities observed in the right ventricular outflow tract region of patients with Brugada syndrome26 may be a potential target to treat recurrent VF in this condition. They also open up the possibility of ‘substrate modification’ to treat recurrent VF, even if pathological premature ectopic beats are not present at the time of the study.
Focally Triggered Ventricular Fibrillation in Organic Heart Disease
Ischaemic Heart Disease
Bansch et al.12 reported for the first time their experience of CA in four patients with incessant VT and VF triggered by monomorphic premature ectopic beats after acute myocardial infarction. Again, similar to idiopathic polymorphic VT or VF, the triggering foci were located within the Purkinje system, specifically in the left posterior fascicle. CA of the triggering premature ectopic beats successfully controlled ES and none of the patients experienced further episodes of VF within the follow-up period ranging between 5 and 33 months. The authors estimated that the scenario occurs relatively rarely in patients following acute myocardial infarction. In their experience, CA was only required in four reported patients out of a total of 2,340 post-infarction patients (i.e. 0.17 % of cases). Similarly, Enjoji et al.27 reported their experience with CA of triggering premature ventricular contractions in four patients with acute coronary syndrome and low ejection fraction who suffered from multiple VF or VT episodes, despite successful revascularisation. The premature ectopic beats originated again in the Purkinje fiber network, and were located in the left ventricular posteroinferior region of the left ventricle. Szumowski et al.28 performed CA of triggering ectopy in a small series of patients both early and late after myocardial infarction. Using the three-dimensional (3D) electroanatomical mapping system, they documented the site of origin of the triggering foci in Purkinje arborisation, close to the border zone of the necrosis or scar. They also observed repetitive activation of the Purkinje system during polymorphic VT, and persistent Purkinje activity despite the absence of propagation to the ventricular myocardium. These findings implicate the role of Purkinje arborisation in the scar border zone after previous myocardial infarction not only in the initiation, but also in the maintenance of initial beats of polymorphic VT and VF. Marrouche et al.29 investigated the mode of initiation of ES in patients with ischaemic cardiomyopathy who had suffered their myocardial infarction more than six months earlier. Eight patients required CA to suppress ES. Using electroanatomical mapping, the authors demonstrated that in five cases the culprit ectopic activity originated from the scar border zone, often preceded by Purkinje potentials. In three subjects without frequent ventricular ectopy, the ablation strategy consisted of linear lesion along the length of the border zone in order to eliminate all detected Purkinje potentials. This appeared to be successful and over a 10 ± 6 month follow-up period, VF only recurred in one patient.
Recently, we published our experience with CA of triggering foci of VF in ischaemic heart disease, reporting on nine subjects (mean age 62 ± 7 years, two females, all after myocardial infarction between three days to 171 months, mean left ventricular ejection fraction (LVEF) 25 ± 7 %).30 In six of them (67 %), the ablation procedure was performed on mechanical ventilation. CA was successful in eight patients. During a follow-up of 13 ± 7 months, two patients died of progressive heart failure without any recurrence of ventricular arrhythmias. Another patient had recurrence of focally triggered VF from the other fascicle. The other had recurrence of ES due to monomorphic VT that was successfully re-ablated by substrate modification. Our more recent unpublished experience comprises 19 subjects with focally triggered VF after myocardial infarction and/or after cardiac surgery. Thirteen of them were admitted early after infarction, four remotely and two following coronary artery bypass surgery. CA was successful in suppressing the ES in 17 out of 19 patients. Mean procedural time reached 171 ± 53 minutes (min) with fluoroscopic time of 12 ± 9 min. Interestingly, we observed six early recurrences of ectopy from a different region than originally ablated. Three of them were transient and disappeared spontaneously. Three patients underwent successful re-ablation of the newly manifested focus (see Figure 2). Four patients deceased early after the procedure, two due to heart failure, one due to multiorgan failure (after multiple direct current [DC] shocks before transfer for CA) and the other due to electromechanical dissociation and pericardial effusion after emergency introduction of temporary pacing catheter. Importantly, 80 % (12/15) of acute survivors had no recurrence of ES during 26 ± 21 months of follow-up.
Figure 2: Electroanatomical Maps of the Left Ventricle in a Patient After Previous Myocardial Infarction, Depicting Location of the Triggering Foci From Different Regions of the Purkinje System (Right Anterior Oblique Views).
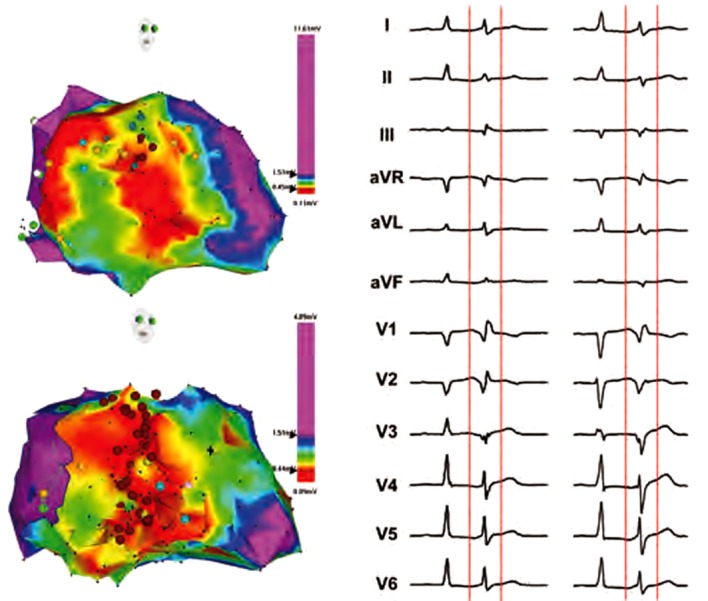
Red colour depicts region of myocardial necrosis with bipolar voltage below 0.45 mV, green color represents transitional zone. Yellow tags annotate conduction system and dark red tags correspond to site of ablation. Corresponding 12-lead electrocardiograms are on right panels. Initially, the patient had monotopic ectopy (I) that was successfully abolished by focal ablation at the high septum (upper panel). However, the patient had next day recurrence of ES due to occurrence of new triggering ectopy (II) originating from the posterior fascicle. The re-ablation was performed more extensively across the whole middle part of septum (lower panel), which successfully prevented VF recurrences.
The above mentioned two clinical scenarios (i.e. ES early and late after myocardial infarction) appear to have different mechanisms. In early post-infarction period, the trigger appears to originate in Purkinje fibers surviving in the region of myocardial necrosis and/or scar. This view is supported by some experimental data showing survival of Purkinje fibers in the region of myocardial infarction.31 These cells are more resistant and therefore can survive even severe ischaemia. They can also be nourished by retrograde perfusion through various ventricular sinusoidal channels,32 through the left atrial venous system33 or simply by diffusion of oxygen from ventricular cavity blood through the endocardium.34 These surviving Purkinje fibers crossing the border-zone of the myocardial infarction demonstrate heightened automaticity, triggered activity and supernormal excitability.35–37 On the other hand, the reason for sudden appearance of ectopic activity from Purkinje fibers in the late post-infarction period is not clear. Subclinical infarction may be the underlying cause that promotes ectopic activity from the surviving Purkinje cells. Anecdotal cases suggest that patients with focally triggered VF may have some anatomical abnormalities that promote arrhythmogenesis. For instance, Nogami et al.38 described autopsy specimens from a patient with ischaemic cardiomyopathy who underwent radiofrequency CA for VF. They revealed fibromuscular bands connecting the posterior papillary muscle and ventricular septum at the successful ablation sites of the trigger ectopic activity. Microscopic examinations showed Purkinje cells in the centre of that fibromuscular band. It is also less known why ectopic activity starts to trigger VF late after myocardial infarction.
Non-ischaemic Cardiomyopathy
Some authors reported successful CA of focal VF in patients with non-ischaemic dilated cardiomyopathy and refractory ES despite optimal heart failure medication and the use of antiarrhythmic drugs.39,40 In one case, non-contact mapping was employed to localise triggering focus outside of the conduction system. Subsequent ablation abolished ES.39 In a series of five patients, 3D electroanatomical mapping was used to tag the site of focus and Purkinje-like potentials.40 Limited endocardial scar was revealed near the mitral annulus with Purkinje-like potentials close to scar zone in four subjects who underwent CA. During one-year follow-up, only one patient had recurrence of ES.
Aortic Valve Disease
Focally triggered VF was also described in an adolescent patient after aortic valve repair of a perforated non-coronary cusp with resulting severe aortic regurgitation.41 During electrophysiological study, frequent short runs of VF initiated by ventricular ectopic beats with a narrow QRS complex were observed. After extensive mapping of the right and left ventricles, two distinct sources of ectopy originating from anteroseptal and inferoseptal areas of the left ventricle could be successfully ablated. Ectopic beats were preceded by distinct Purkinje potentials with intervals from the Purkinje potential to QRS onset of ventricular premature beats (VPBs) of 68 and 30 ms at effective sites, respectively. Another patient with ES after aortic valve replacement was reported by a group from Leipzig.42 Using the electroanatomic mapping system, the authors were able to abolish the triggering focus at mid-inferior septum of the left ventricle. Again, Purkinje system was the site of origin of this ectopy.
Cardiac Amyloidosis
Anecdotal reports showed that CA might be curative in focally triggered VF in cardiac amyloidosis.43 Interestingly, electrophysiological testing revealed that the sites of earliest activation were localised endocardially within the left ventricle in the absence of significant scar tissue. In the first case, no Purkinje potentials were recorded at spot of trigger in inferolateral apical region. In the other case, the earliest activation during ectopy was recorded within the left posterior fascicle. After CA, ventricular ectopy subsided in both cases and there were no further VF recurrences.
Technique of Catheter Ablation
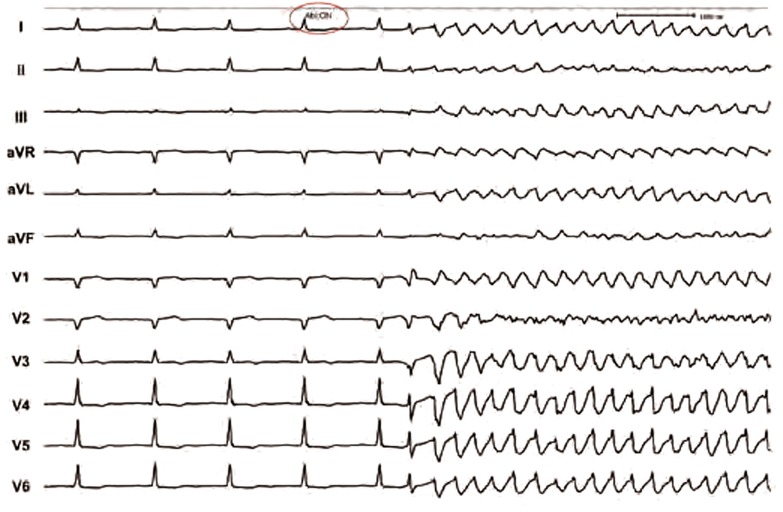
From the above section, it can be appreciated that CA targets predominantly ectopic focus that triggers ES due to polymorphic VT and VF. In subjects with idiopathic form, ablation site is usually contained within a relatively small area.10,11 CA of triggering ectopy appears to have a favourable outcome, despite occasional presence of other foci without documented initiation of VF. As a result of the possibility of disappearance of ectopy during mapping (‘bumping’ the ectopic focus with the catheter), it appears to be useful to employ the electroanatomical mapping system for tagging the sites of interest. This allows both 3D reconstruction of left ventricular endocardial surface with annotation of location of the conduction system and delineation of myocardial necrosis or scar as low voltage areas. Patients with ischaemic cardiomyopathy often present with more than one ectopic focus. In our experience the risk of early recurrences of ES after successful ablation of one trigger supports the strategy to ablate all ectopic foci. It emphasises the need for ECG recording of ectopy on 12-lead ECG. Such recordings serve as roadmaps for subsequent ablation (see Figure 3). More important than in idiopathic VF, the use of the electroanatomical mapping system is recommendable. Besides annotation of the earliest activation during the ectopy, it allows displaying the extent of myocardial necrosis and/or scar, and CA may address more Purkinje tissue along the margin of the affected tissue. In addition, this strategy could be used when no ectopy is present during the mapping or if catheter manipulation induces left bundle branch block, making analysis of conduction system difficult. Electroanatomical system can also support modification of substrate for monomorphic VTs, provided they occur at the same time. On the other hand, a subendocardial location of Purkinje tissue allows very rapid stabilisation of the clinical status when necessary. Few applications of radiofrequency current after rapid mapping usually terminate ES quickly and provide more time for detailed mapping and ablation. It is also important to emphasise that delivery of radiofrequency current at ectopic source sites often leads to acceleration of ectopy (see Figure 4) and may trigger runs of polymorphic VT or VF that have to be terminated by DC shock. Sometimes, even after stabilisation of ES, other extremes may be encountered – absence of ectopic activity. In such cases, some authors recommend the use of isoproterenol to induce ventricular ectopic beats for more focused mapping when absent during ablation procedure.
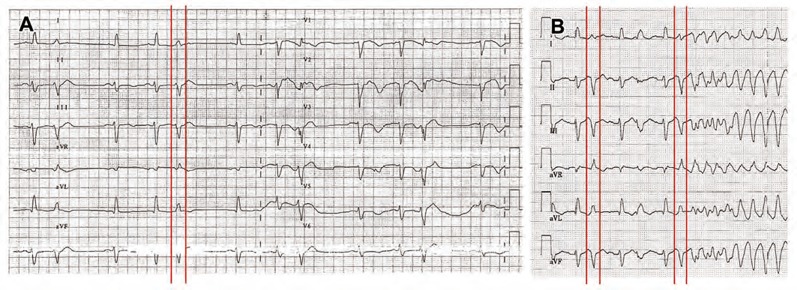
Figure 3: Twelve-lead Electrocardiogram at the Time of Electrical Storm After Acute Myocardial Infarction.
The panel A shows frequent monotopic ectopy with relatively narrow QRS complex. The panel B depicts initiation of VF by ectopic beat of the same morphology as the preceding ectopy.
Figure 4: Electrocardiogram Recording During Delivery of Radiofrequency Current in the Region of Focal Trigger. It Depicts Thermally-induced Polymorphic Ventricular Tachycardia.
Interestingly, a different strategy has been used for CA in the majority of cases with ES in Brugada syndrome. As mentioned above, mapping and ablation were performed in the right ventricular outflow tract, either epicardially or endocardially.13,14 The main target was zones of delayed activation and late potentials.
Unresolved Problems
We believe that the situation is analogous to pathophysiology of atrial fibrillation. Although ECG pattern of atrial fibrillation may be similar in different patients, many factors influence individual manifestation of the arrhythmia. Yet, we reached the agreement that pulmonary venous isolation is considered a technique of choice to keep triggering foci at bay and treat paroxysmal atrial fibrillation with a high degree of success. In analogy to this development, better understanding of mechanisms of VF and critical structures for its maintenance may help to use more effectively CA. At this stage we have more questions than answers.
In any case, it is clear that CA cannot be considered as a curative procedure for focally triggered VF, both in idiopathic cases and in patients with structural heart disease. Despite the fact that some patients may be without recurrences for certain period, VF can still reappear.11,17,19 Therefore, an ICD will remain a necessity in these subjects, especially when LVEF is depressed. Given the number of potential underlying causes and mechanisms, the need for particular expertise and relative scarcity of referrals (which does not necessarily reflect scarcity of cases but more probably lack of awareness) it is unlikely that we will have large randomised trials. Instead, observational studies using modifications of strategies designed based on empirical experience and/or some novel discoveries in experimental models will serve as a source of scientific evidence.
Conclusions
Within the last 15 years, CA has emerged as a potentially important treatment strategy to target clearly identifiable focal triggers of polymorphic VT and VF in the setting of ES. It has been used in a limited number of centres both in patients with idiopathic and structural heart disease-related VF with very favourable results. In view of the invasive nature of CA for VF, potential complications and expertise required, patients presenting with ES should still be managed along conventional lines in the first instance. These measures, which include deep sedation, antiarrhythmic medication and/or overdrive ventricular pacing, may be effective in the majority of cases. However, CA could be a reasonable therapeutic option for intractable cases and early transport of the patient into the experience centre for CA should always be considered. Therefore, the awareness of this entity and link to the expert centre are important.
Acknowledgements
Supported by Ministry of Health, Czech Republic – conceptual development of research organization (Institute for Clinical and Experimental Medicine – IKEM, IN 00023001”)
References
- 1.Jalife J. Ventricular fibrillation: Mechanisms of initiation and maintenance. Annu Rev Physiol. 2000;62:25–50. doi: 10.1146/annurev.physiol.62.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Zaitsev AV, Berenfeld O, Mironov SF et al. Distribution of excitation frequencies on the epicardial and endocardial surfaces of fibrillating ventricular wall of the sheep heart. Circ Res. 2000;86:408–17. doi: 10.1161/01.res.86.4.408. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Mandapati R, Berenfeld O et al. High-frequency periodic sources underlie ventricular fibrillation in the isolated rabbit heart. Circ Res. 2000;86:86–93. doi: 10.1161/01.res.86.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Newton JC, Smith WM, Ideker RE. Estimated global transmural distribution of activation rate and conduction block during porcine and canine ventricular fibrillation. Circ Res. 2004;94:836–42. doi: 10.1161/01.RES.0000120860.01645.17. [DOI] [PubMed] [Google Scholar]
- 5.Nanthakumar K, Huang J, Rogers JM et al. Regional differences in ventricular fibrillation in the open-chest porcine left ventricle. Circ Res. 2002;91:733–40. doi: 10.1161/01.res.0000038945.66661.21. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Walcott GP, Killingsworth CR et al. Quantification of activation patterns during ventricular fibrillation in open-chest porcine left ventricle and septum. Heart Rhythm. 2005;2:720–8. doi: 10.1016/j.hrthm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Pak HN, Kim YH, Lim HE et al. Role of the posterior papillary muscle and purkinje potentials in the mechanism of ventricular fibrillation in open chest dogs and Swine: Effects of catheter ablation. J Cardiovasc Electrophysiol. 2006;17:777–83. doi: 10.1111/j.1540-8167.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- 8.Pak HN, Oh YS, Liu YB et al. Catheter ablation of ventricular fibrillation in rabbit ventricles treated with beta-blockers. Circulation. 2003;100:3149–56. doi: 10.1161/01.CIR.0000104563.12408.12. [DOI] [PubMed] [Google Scholar]
- 9.Pak HN, Kim GI, Lim HE et al. Both Purkinje cells and left ventricular posteroseptal reentry contribute to the aintenance of ventricular fibrillation in open-chest dogs and swine: effects of catheter ablation and the ventricular cut-and-sew operation. Circ J. 2008;72:1185–19. doi: 10.1253/circj.72.1185. [DOI] [PubMed] [Google Scholar]
- 10.Haïssaguerre M, Shah DC, Jaïs P et al. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet. 2002;359:677–8. doi: 10.1016/S0140-6736(02)07807-8. [DOI] [PubMed] [Google Scholar]
- 11.Haïssaguerre M, Shoda M, Jaïs P et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–7. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 12.Bänsch D, Oyang F, Antz M et al. Successful catheter ablation of electrical storm after myocardial infarction. Circulation. 2003;108:3011–6. doi: 10.1161/01.CIR.0000103701.30662.5C. [DOI] [PubMed] [Google Scholar]
- 13.Nademanee K, Veerakul G, Chandanamattha P et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–9. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 14.Sunsaneewitayakul B, Yao Y, Thamaree S, Zhang S. Endocardial mapping and catheter ablation for ventricular fibrillation prevention in Brugada syndrome. J Cardiovasc Electrophysiol. 2012;23(Suppl 1):S10–6. doi: 10.1111/j.1540-8167.2012.02433.x. [DOI] [PubMed] [Google Scholar]
- 15.Nash MP, Mourad A, Clayton RH et al. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation. 2006;111:536–42. doi: 10.1161/CIRCULATIONAHA.105.602870. [DOI] [PubMed] [Google Scholar]
- 16.Kautzner J, Bytešník J. Catheterablationofarrhythmogenicfocus in “short-coupled“ variant ofTorsade de Pointes (abstract) Pacing Clin Electrophysiol. 2000;23:717. [Google Scholar]
- 17.Knecht S, Sacher F, Wright M et al. Long-term follow-up of idiopathic ventricular fibrillation ablation: a multicenter study. J Am Coll Cardiol. 2009;54:522–8. doi: 10.1016/j.jacc.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 18.Olde Nordkamp LR, Wilde AA, Tijssen JG et al. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. 2013;6:91–100. doi: 10.1161/CIRCEP.112.975268. [DOI] [PubMed] [Google Scholar]
- 19.Haïssaguerre M, Extramiana F, Hocini M et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003;108:925–8. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 20.Srivathsan K, Gami AS, Ackerman MJ, Asirvatham SJ. Treatment of ventricular fibrillation in a patient with prior diagnosis of long QT syndrome: importance of precise electrophysiologic diagnosis to successfully ablate the trigger. Heart Rhythm. 2007;4:1090–3. doi: 10.1016/j.hrthm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Z, Gao P, Cheng K et al. Elimination of fatal arrhythmias through ablation of triggering premature ventricular contraction in type 3 long QT syndrome. Ann Noninvasive Electrocardiol. 2012;17:394–7. doi: 10.1111/j.1542-474X.2012.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darmon JP, Bettouche S, Deswardt P et al. Radiofrequency ablation of ventricular fibrillation and multiple right and left atrial tachycardia in a patient with Brugada syndrome. J Interv Card Electrophysiol. 2004;11:205–9. doi: 10.1023/B:JICE.0000048571.19462.54. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa E, Takagi M, Tatsumi H, Yoshiyama M. Successful radiofrequency catheter ablation for electrical storm of ventricular fibrillation in a patient with Brugada syndrome. Circ J. 2008;72:1025–9. doi: 10.1253/circj.72.1025. [DOI] [PubMed] [Google Scholar]
- 24.Shah AJ, Hocini M, Lamaison D et al. Regional substrate ablation abolishes Brugada syndrome. J Cardiovasc Electrophysiol. 2011;22:1290–1. doi: 10.1111/j.1540-8167.2011.02054.x. [DOI] [PubMed] [Google Scholar]
- 25.Morita H, Zipes DP, Morita ST et al. Epicardial ablation eliminates ventricular arrhythmias in an experimental model of Brugada syndrome. Heart Rhythm. 2009;6:665–71. doi: 10.1016/j.hrthm.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Papavassiliu T, Veltmann C, Doesch C. Spontaneous type 1 electrocardiographic pattern is associated with cardiovascular magnetic resonance imaging changes in Brugada syndrome. Heart Rhythm. 2010;7:1790–6. doi: 10.1016/j.hrthm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Enjoji Y, Mizobuchi M, Muranishi H et al. Catheter ablation of fatal ventricular tachyarrhythmias storm in acute coronary syndrome–role of Purkinje fiber network. J Interv Card Electrophysiol. 2009;26:207–15. doi: 10.1007/s10840-009-9394-7. [DOI] [PubMed] [Google Scholar]
- 28.Szumowski L, Sanders P, Walczak F et al. Mapping and ablation of polymorphic ventricular tachycardia after myocardial infarction. J Am Coll Cardiol. 2004;44:1700–6. doi: 10.1016/j.jacc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 29.Marrouche NF, Verma A, Wazni O et al. Mode of initiation and ablation of ventricular fibrillation storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2004;43:1715–20. doi: 10.1016/j.jacc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Peichl P, Cihák R, Kozeluhová M et al. Catheter ablation of arrhythmic storm triggered by monomorphic ectopic beats in patients with coronary artery disease. J Interv Card Electrophysiol. 2010;27:51–9. doi: 10.1007/s10840-009-9443-2. [DOI] [PubMed] [Google Scholar]
- 31.Friedman PL, Stewart JR, Fenoglio JJ, jr, Wit AL. Survival of Subendocardial Purkinje Fibers after Extensive Myocardial Infarction in Dogs: in vitro and in vivo correlations. Circ Res. 1973;33:597–611. doi: 10.1161/01.res.33.5.597. [DOI] [PubMed] [Google Scholar]
- 32.Myers WW, Honic CR. Amount and distribution of Rb transported into myocardium from ventricular lumen. Am J Physiol. 1966;211:739–45. doi: 10.1152/ajplegacy.1966.211.3.739. [DOI] [PubMed] [Google Scholar]
- 33.Moir TW. Study of luminal coronary collateral circulation in the beating canine heart. Circ Res. 1969;24:735–44. doi: 10.1161/01.res.24.5.735. [DOI] [PubMed] [Google Scholar]
- 34.Bagdonas AA, Stuckey JH, Piera J et al. Effects of ischemia and hypoxia on the specialized conduction system of the canine heart. Am Heart J. 1961;61:206–18. doi: 10.1016/0002-8703(61)90577-4. [DOI] [PubMed] [Google Scholar]
- 35.Arnar DO, Bullinga JR, Martins JB. Role of the Purkinje system in spontaneous ventricular tachycardia during acute ischemia in a canine model. Circulation. 1997;96:2421–9. doi: 10.1161/01.cir.96.7.2421. [DOI] [PubMed] [Google Scholar]
- 36.Berenfeld O, Jalife J. Purkinje-muscle reentry as a mechanism of polymorphic ventricular arrhythmias in a three-dimensional model of the ventricles. Circ Res. 1998;82:1063–77. doi: 10.1161/01.res.82.10.1063. [DOI] [PubMed] [Google Scholar]
- 37.Kupersmith J, Li ZY, Maidonado C. Marked action potential prolongation as a source of injury current leading to border zone arrhythmogenesis. Am Heart J. 1994;127:1543–53. doi: 10.1016/0002-8703(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 38.Nogami A, Kubota S, Adachi M, Igawa O. Electrophysiologic and histopathologic findings of the ablation sites for ventricular fibrillation in a patient with ischemic cardiomyopathy. J Interv Card Electrophysiol. 2009;24:133–7. doi: 10.1007/s10840-008-9312-4. [DOI] [PubMed] [Google Scholar]
- 39.Kirubakaran S, Gill J, Rinaldi CA. Successful catheter ablation of focal ventricular fibrillation in a patient with nonischemic dilated cardiomyopathy. Pacing Clin Electrophysiol. 2011;34:e38–42. doi: 10.1111/j.1540-8159.2010.02750.x. [DOI] [PubMed] [Google Scholar]
- 40.Sinha AM, Schmidt M, Marschang H et al. Role of left ventricular scar and Purkinje-like potentials during mapping and ablation of ventricular fibrillation in dilated cardiomyopathy. Pacing Clin Electrophysiol. 2009;32:286–90. doi: 10.1111/j.1540-8159.2008.02233.x. [DOI] [PubMed] [Google Scholar]
- 41.Li YG, Gronefeld G, Israel C, Hohnloser SH. Catheter ablation of frequently recurring ventricular fibrillation in a patient after aortic valve repair. J Cardiovasc Electrophysiol. 2004;15:90–3. doi: 10.1046/j.1540-8167.2004.03386.x. [DOI] [PubMed] [Google Scholar]
- 42.Bode K, Hindricks G, Piorkowski C et al. Ablation of polymorphic ventricular tachycardias in patients with structural heart disease. Pacing Clin Electrophysiol. 2008;31:1585–91. doi: 10.1111/j.1540-8159.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 43.Mlcochova H, Saliba WI, Burkhardt DJ et al. Catheter ablation of ventricular fibrillation storm in patients with infiltrative amyloidosis of the heart. J Cardiovasc Electrophysiol. 2006;17:426–30. doi: 10.1111/j.1540-8167.2005.00321.x. [DOI] [PubMed] [Google Scholar]