Abstract
Objective:
To investigate interobserver and inter-CT variations in using the active breath co-ordinate technique in the determination of clinical tumour volume (CTV) and normal organs in post-operative gastric cancer radiotherapy.
Methods:
Ten gastric cancer patients were enrolled in our study, and four radiation oncologists independently determined the CTVs and organs at risk based on the CT simulation data. To determine interobserver and inter-CT variation, we evaluated the maximum dimensions, derived volume and distance between the centres of mass (CMs) of the CTVs. We assessed the reliability in CTV determination among the observers by conformity index (CI).
Results:
The average volumes ± standard deviation (cm3) of the CTV, liver, left kidney and right kidney were 674 ± 138 (range, 332–969), 1000 ± 138 (range, 714–1320), 149 ± 13 (range, 104–183) and 141 ± 21 (range, 110–186) cm3, respectively. The average inter-CT distances between the CMs of the CTV, liver, left kidney and right kidney were 0.40, 0.56, 0.65 and 0.6 cm, respectively; the interobserver values were 0.98, 0.53, 0.16 and 0.15 cm, respectively.
Conclusions:
In the volume size of CTV for post-operative gastric cancer, there were significant variations among multiple observers, whereas there was no variation between different CTs. The slices in which variations more likely occur were the slices of the lower verge of the hilum of the spleen and porta hepatis, then the paraoesophageal lymph nodes region and abdominal aorta, and the inferior vena cava, and the variation in the craniocaudal orientation from the interobserver was more predominant than that from inter-CT.
Advances in knowledge:
First, this is the first study to evaluate the interobserver and inter-CT variations in the determination of the CTV and normal organs in gastric cancer with the use of the active breath co-ordinate technique. Second, we analysed the region where variations most likely occur. Third, we investigated the influence of interobserver variation on the dose distribution.
INTRODUCTION
Post-operative chemoradiotherapy has been recommended as a standard of care for patients with resectable local advanced gastric cancer.1–3 Among the radiotherapy techniques, three-dimensional (3D) conformal radiation therapy (RT) and intensity-modulated RT are widely used for their superior benefits in improving target coverage and sparing organs at risk (OARs) compared with the simple anteroposterior–posteroanterior technique.4,5 However, many uncertainties accompany these precise RT techniques.
One uncertainty in implementing 3D conformal RT is the target volume determination,6 as variations in the determination of target volume have been widely demonstrated in breast cancer,7 lung cancer,8,9 brain cancer,10,11 bladder cancer,12 prostate cancer,13–16 cervical cancer17 and oesophageal cancer.18
For post-operative gastric cancer radiotherapy, Leong et al19 reported that 35% of RT treatment plans contained major or minor protocol violations at an initial pre-treatment central review. However, studies on target volume variation are rare. Jansen et al20 showed that variability in the clinical tumour volume (CTV) determination in post-operative chemoradiotherapy for gastric cancer is large, but they did not provide a complete explanation of the observed variability.
Theoretically, uncertainties exist in the delineation of target volumes and normal tissues. First, a partial volume effect is caused by the resolution of simulated CT images, especially in the perpendicular orientation of transverse sections.21,22 Second, physical movement of organs, especially artefacts induced by breathing, influences image acquisition23,24 and subsequently affects the dose distribution calculation; this issue is particularly common in the target delineation of lung cancer. Third, during the course of target contouring, the radiation oncologists (ROs) also have to deal noise that is similar to the differences in image quality; for example, the same physician may define different targets at different times, which can be called intraobserver variation, whereas a more obvious variation may exist between different observers, which is called interobserver variation.11,13,25 Furthermore, different types and different times of imaging may offer different reference information for certain categories of tissues,26 which can be called volumetric variation (such as that caused by food intake and bowel gas); therefore, only one simulated CT scan cannot represent the volume that requires treatment.
Organ motion in the thorax and upper abdomen during breathing remains problematic. Imaging studies using fluoroscopy and ultrasound have shown that tumours and organs can move by 10 to >30 mm during the breathing cycle, which may influence the variation to a large extent. Active breath co-ordinate (ABC) has been used to reduce the influence of breath movement on the target volume, as it can reduce the breathing motion to approximately 3–5 mm.27–29 The application of ABC can facilitate the study of interfraction CT variation. However, the variations in CTV and normal organ determination with the ABC technique remain unclear, and little information is available regarding the region in which slice uncertainties most likely occur. The purpose of this study was to evaluate the interobserver and inter-CT variations in the determination of CTV and normal organs in gastric cancer using the ABC technique.
METHODS AND MATERIALS
All data were collected from consenting individuals according to the protocols approved by the Ethics Review Board at the Fudan University Shanghai Cancer Center, Shanghai, China. The consents were verbal, and we recorded the participant consents on our follow-up record to confirm that he/she wanted to join the study. The ethics committee approved this consent procedure.
Patients
From January 2008 to November 2008, ten consecutive post-gastrectomy patients were enrolled in the study. All of the patients were aged from 19 to 63 years (median 48 years) and pathologically staged as T3-4/N + (IB-IV) according to the 2002 American Joint Committee on Cancer TNM staging system.
Treatment
Surgery
Surgery was performed through a median laparotomy with either total or subtotal gastrectomy, and the operating surgeon recorded the extent of lymphadenectomy. All of the patients underwent a spleen-conserving D2 dissection (removal of all involved N2 lymph nodes).
Chemoradiotherapy
The radiation target volume included the tumour bed, anastomotic stoma, gastric remnant (T3, T4) and regional draining lymph nodes, which were determined from pre- and post-operative CT, surgical clips and barium examination. The barium swallow was required to define the intestine and gastric remnants. Perigastric, celiac, local para-aortic, splenic, hepatoduodenal or hepatic-portal and pancreaticoduodenal lymph nodes were included in the radiation target volume. For patients with tumours of the gastro-oesophageal junction, the paracardial and lower para-oesophageal lymph nodes were included in the radiation target volume, but pancreaticoduodenal radiation was not required. Exclusion of the splenic nodes was allowed in patients with antral lesions if it was necessary to spare the left kidney.
Each patient was fixed to an individual vacuum pad (Med-Tec Corporation, Orange City, 1A) for both simulation and treatment. The prescribed dose was 45 Gy in 25 fractions, and the treatment was delivered 5 days per week. The ABC technique (Elekta Oncology System, Crawley, UK) was used to reduce the respiratory uncertainty.30 The treatments were delivered with intensity-modulated RT using a 6-MV X-ray (Elekta Oncology System). Doses were limited as follows: (1) <30% of the hepatic volume received 30 Gy, and the mean dose was ≤23 Gy; (2) <50% of each kidney received 15 Gy, and the mean dose was ≤16 Gy; and (3) <30% of the cardiac volume received >40 Gy.
According to the guidelines of our institute, all patients were recommended to receive radiotherapy with concurrent chemotherapy (5-fluorouracil or capecitabine) and four to six cycles of epirubicin-based triplet adjuvant chemotherapy both before (1–2 cycle) and after (4–5 cycles) chemoradiation.
Image registration
Three CT data sets were acquired for each patient with an empty stomach or five at least 3 hours after having meal in the CT simulator (Philips Medical Systems, Madison, WI) with a 5-mm slice thickness in different consecutive 3 days. The ABC technique was performed in the imaging process for all the patients. The three data sets were marked CT1, CT2 and CT3 according to the imaging sequences. All images were transferred to a commercial treatment planning system (Pinnacle v. 8.0 m; Philips Radiation Oncology Systems, Milpitas, CA) for registration and contouring. CT2 and CT3 were rigidly registered to CT1 using a CT-to-CT automatic registration algorithm, and then a fine adjustment was manually performed to provide a better alignment in the vertebral bodies. Four ROs marked as A, B, C and D (two attending doctors and two professors) contoured the CTV and normal tissues (liver and two kidneys) in the treatment planning system, referring to the institutional target contouring guideline, based on the consensus statement of Smalley et al2 and the Japanese Classification of Gastric Carcinoma lymph node stations.31 Each physician contoured the CTV and normal organs in all of the image data sets. During the contouring, the ROs were required to turn off all of the other contours to diminish their influence. For each contoured volume, a point of interest was automatically placed in the centre of the mass (CM).
Variation analysis
Size variation
The maximum dimensions of the anteroposterior (AP) and left-lateral RT fields were recorded. We also registered the volume of the CTV and normal organs, and we compared the interobserver and inter-CT difference of the maximal dimensions and volume of contours from different CTs and different physicians.
CTV variation
The interobserver and inter-CT CTV variations for each contour were investigated using the distance between the CMs and the conformity index (CI). The CI indicates the overlapping ratio between two volumes of interest and is defined as: CI = (A∩B)/(A∩B), where A and B are the two volumes of interest. A perfect match gives a CI equal to 1. Furthermore, a slice-by-slice CT comparison was performed to calculate the exact area of the difference in CTV determination. The interobserver and inter-CT variations were analysed.
Dosimetric effects
Finally, for the treatment target volume and normal organs, we analysed the variation of the dose distribution. We created the planning target volume (PTV) from the CTV plus the set-up error margin, the margin of cranial–foot direction was 1.2 cm and in other directions was 0.7 cm. We took the PTV which was actually clinically used to formulate the treatment plan, for CTV and PTV by using the parameters D99, D95 and D1 in addition to the mean dose (Dm) of PTV. Each patient had one treatment plan. Dx was the dose that only encompassed x% of the CTV or PTV. For the liver, we used V30 and Dm, and for the kidneys, we used V15, V18 and Dm, where Vx is the percentage of the volume that received x Gy irradiation.
All statistical analyses were performed using the SPSS® v. 13.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). A one-way analysis of variance (ANOVA) was used to compare the interobserver and inter-CT variability, and p < 0.05 (two-sided) was considered statistically significant.
RESULTS
Size variation
Maximal dimension
The maximum dimensions from different observers and different CT scans were obtained. X and Y indicated the field width and length of the AP portal and Z indicated the anterior–posterior field extent of the left lateral portal. The average variations of the maximum dimension were 32.5, 22 and 16.24 mm, respectively. ANOVA showed no significant difference (interobserver or inter-CT) in the field dimensions.
Volume study
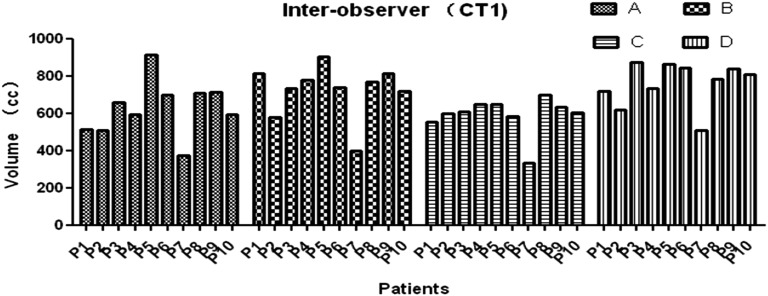
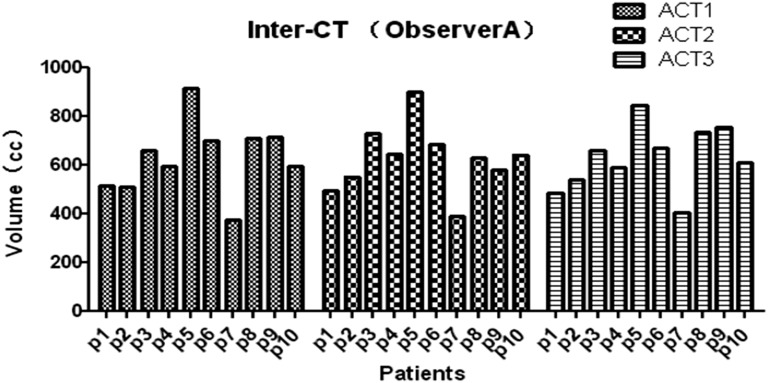
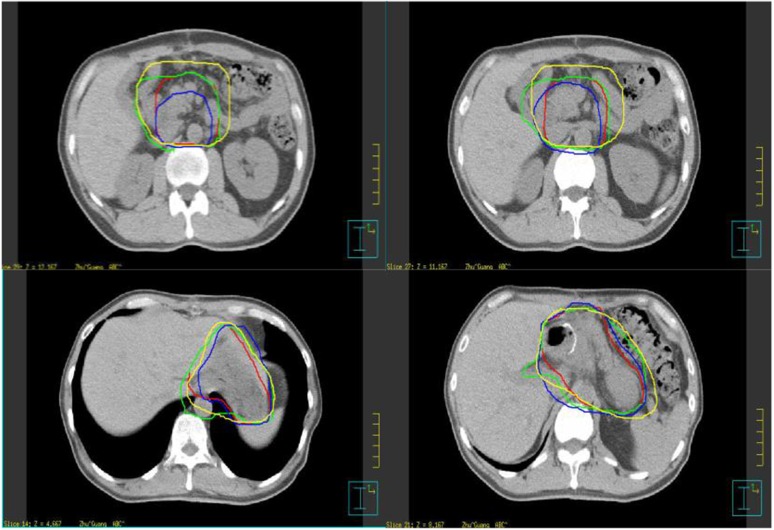
The average volume [standard deviation (SD)] of the CTV, liver, left kidney and right kidney of all 3 CTs for all patients and all observers (120 volumes) was 674 (138) cm3 (range, 332–969 cm3), 1000 (138) cm3 (range, 714–1320 cm3), 149 (13) cm3 (range, 104–183 cm3) and 141 (21) cm3 (range, 110–186 cm3), respectively. There were significant interobserver differences among the three CT data sets (CT1: p = 0.0182; CT2: p = 0.0136; and CT3: p = 0.0356), whereas there was no significant difference among different CT scans (Observer A: p = 0.9964; Observer B: p = 0.8985; Observer C: p = 0.8648; and Observer D: p = 0.9964) (Figures 1 and 2).
Figure 1.
Clinical tumour volume (CTV) determination for different observers for each patient in CT1. (The CTV determination for different observers for each patient in CT1, not all the CT series of CT1, CT2 and CT3, p = 0.0182.)
Figure 2.
Clinical tumour volume (CTV) determination of different CTs for each patient by Observer A. (The CTV determination of different CTs for each patient by Observer A, p = 0.9964.) ACT, adjuvant chemotheraphy.
Target location difference
Distance between centres of the mass
The CM distances between different contours for different observers and different CTs are shown in Tables 1 and 2. The average interobserver discrepancy in the CM distance of the CTV contours was larger than that of the liver and kidney contours, whereas the average inter-CT discrepancy in the CM distances of the CTV contours was smaller than that of the liver and kidney contours.
Table 1.
Centre of mass distances between contours for different observers
| CT | Difference between observers (cm) | CTV | Liver | L-kidney | R-kidney |
|---|---|---|---|---|---|
| CT1 | A–B | 0.92 | 0.20 | 0.04 | 0.06 |
| A–C | 1.19 | 0.61 | 0.18 | 0.12 | |
| A–D | 1.02 | 0.55 | 0.19 | 0.17 | |
| B–C | 0.86 | 0.51 | 0.19 | 0.14 | |
| B–D | 0.76 | 0.62 | 0.19 | 0.18 | |
| C–D | 0.85 | 0.86 | 0.13 | 0.21 | |
| Mean | 0.93 | 0.56 | 0.15 | 0.15 | |
| CT2 | A–B | 1.00 | 0.18 | 0.07 | 0.05 |
| A–C | 1.20 | 0.49 | 0.13 | 0.10 | |
| A–D | 1.13 | 0.63 | 0.16 | 0.17 | |
| B–C | 0.93 | 0.54 | 0.17 | 0.07 | |
| B–D | 0.81 | 0.58 | 0.19 | 0.15 | |
| C–D | 1.07 | 0.62 | 0.18 | 0.17 | |
| Mean | 1.02 | 0.51 | 0.15 | 0.12 | |
| CT3 | A–B | 0.95 | 0.21 | 0.10 | 0.05 |
| A–C | 1.29 | 0.71 | 0.14 | 0.17 | |
| A–D | 1.07 | 0.50 | 0.23 | 0.21 | |
| B–C | 0.87 | 0.56 | 0.22 | 0.18 | |
| B–D | 0.75 | 0.50 | 0.21 | 0.23 | |
| C–D | 0.96 | 0.73 | 0.23 | 0.18 | |
| Mean | 0.98 | 0.54 | 0.19 | 0.17 |
CTV, clinical tumour volume; L, left; R, right.
Table 2.
Centre of mass distances between contours for different CT scans
| Observer | Difference between CTs (cm) | CTV | Liver | L-kidney | R-kidney |
|---|---|---|---|---|---|
| A | 1–2 | 0.51 | 0.59 | 0.60 | 0.66 |
| 1–3 | 0.28 | 0.50 | 0.63 | 0.50 | |
| 2–3 | 0.40 | 0.43 | 0.68 | 0.60 | |
| Mean | 0.40 | 0.51 | 0.64 | 0.59 | |
| B | 1–2 | 0.28 | 0.54 | 0.62 | 0.66 |
| 1–3 | 0.31 | 0.40 | 0.57 | 0.46 | |
| 2–3 | 0.26 | 0.36 | 0.71 | 0.62 | |
| Mean | 0.28 | 0.43 | 0.63 | 0.58 | |
| C | 1–2 | 0.49 | 0.69 | 0.57 | 0.66 |
| 1–3 | 0.43 | 0.75 | 0.66 | 0.57 | |
| 2–3 | 0.52 | 0.68 | 0.67 | 0.60 | |
| Mean | 0.48 | 0.71 | 0.63 | 0.61 | |
| D | 1–2 | 0.46 | 0.72 | 0.63 | 0.72 |
| 1–3 | 0.42 | 0.47 | 0.68 | 0.63 | |
| 2–3 | 0.43 | 0.58 | 0.73 | 0.64 | |
| Mean | 0.44 | 0.59 | 0.68 | 0.66 |
CTV, clinical tumour volume; L, left; R, right.
The conformity index
The average CTV CIs for different observers and CTs are shown in Tables 3 and 4. A lower average CTV CI was observed for different observers than for different CT scans, indicating that the uncertainty was more apparent between observers than between CT scans.
Table 3.
Average inter-observer conformity index (CI)
| Item | Mean CI of 10 patients (%) |
|---|---|
| 1CTV_A^B/1CTV_A&B | 80.86 |
| 1CTV_A^C/1CTV_A&C | 69.72 |
| 1CTV_A^D/1CTV_A&D | 68.82 |
| 1CTV_B^C/1CTV_B&C | 68.95 |
| 1CTV_B^D/1CTV_B&D | 72.39 |
| 1CTV_C^D/1CTV_C&D | 63.89 |
| 2CTV_A^B/1CTV_A&B | 81.78 |
| 2CTV_A^C/1CTV_A&C | 68.48 |
| 2CTV_A^D/1CTV_A&D | 67.40 |
| 2CTV_B^C/1CTV_B&C | 67.52 |
| 2CTV_B^D/1CTV_B&D | 69.95 |
| 2CTV_C^D/1CTV_C&D | 63.25 |
| 3CTV_A^B/1CTV_A&B | 83.21 |
| 3CTV_A^C/1CTV_A&C | 69.76 |
| 3CTV_A^D/1CTV_A&D | 68.22 |
| 3CTV_B^C/1CTV_B&C | 70.50 |
| 3CTV_B^D/1CTV_B&D | 70.73 |
| 3CTV_C^D/1CTV_C&D | 66.11 |
| Average CTV CI | 70.64 |
CTV, clinical tumour volume.
Table 4.
Average inter-CT conformity index (CI)
| Item | Mean CI of 10 patients (%) |
|---|---|
| ACTV_1^2/ACTV_1&2 | 81.58 |
| ACTV_1^3/ACTV_1&3 | 84.59 |
| ACTV_2^3/ACTV_2&3 | 81.97 |
| BCTV_1^2/BCTV_1&2 | 84.86 |
| BCTV_1^3/BCTV_1&3 | 85.24 |
| BCTV_2^3/BCTV_2&3 | 85.97 |
| CCTV_1^2/CCTV_1&2 | 76.01 |
| CCTV_1^3/CCTV_1&3 | 79.05 |
| CCTV_2^3/CCTV_2&3 | 76.91 |
| DCTV_1^2/CCTV_1&2 | 82.33 |
| DCTV_1^3/CCTV_1&3 | 81.11 |
| DCTV_2^3/CCTV_2&3 | 81.92 |
| Average CTV CI | 81.79 |
CTV, clinical tumour volume.
CT slice-by-slice comparison
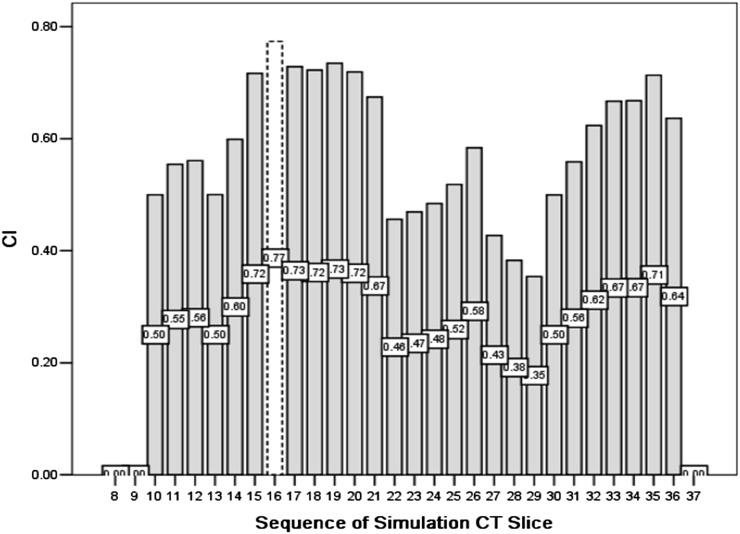
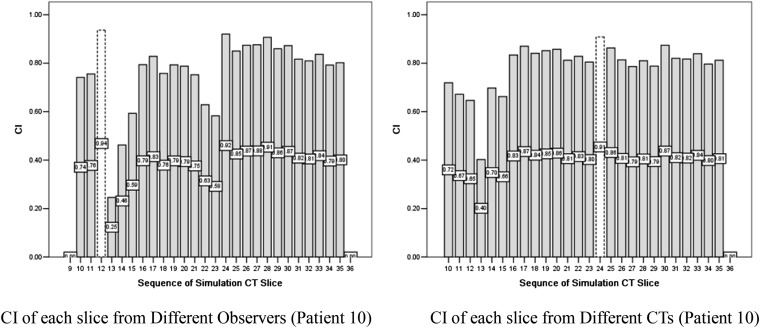
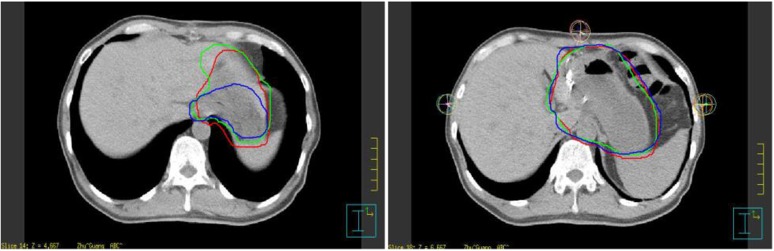
We also calculated the interobserver and inter-CT CI of each slice of the CTV to determine the slices in which uncertainty was more likely to occur. The CI histogram of Patient 10 is shown in Figures 3 and 4, and the corresponding contour examples are shown in Figures 5 and 6. The slices in which variations more likely occur were the slices of the lower verge of the hilum of the spleen and porta hepatis, then the para-oesophageal lymph nodes region and abdominal aorta, and the inferior vena cava, and the variation in the craniocaudal orientation from the interobserver was more predominant compared to that from inter-CT.
Figure 3.
Conformity index (CI) of different observers for each slice of Patient 10.
Figure 4.
Inter-observer and inter-CT conformity index (CI) of clinical tumour volume from Patient 10.
Figure 5.
Contour examples of classic slices of inter-observers (Patient 10).
Figure 6.
Contour examples of classic slices of inter-CT (Patient 10).
Dose distribution
There are significant differences in D99 and Dmean for PTV of different observer (p = 0.004 and p = 0.036), whereas no significant difference was found for CTV, liver or kidneys of different observer (p > 0.05) (Table 5).
Table 5.
Dose distribution statistical analysis
| Dose | F | Significance |
|---|---|---|
| PTV–D99 | 5.270 | 0.004 |
| PTV–D95 | 2.664 | 0.063 |
| PTV–D1 | 0.035 | 0.991 |
| PTV–Dmean | 3.176 | 0.036 |
| L-Kidney–V15 | 0.116 | 0.950 |
| L-Kidney–V18 | 0.149 | 0.929 |
| L-Kidney–Dmean | 0.064 | 0.978 |
| R-Kidney–V15 | 0.013 | 0.998 |
| R-Kidney–V18 | 0.021 | 0.996 |
| R-Kidney–Dmean | 0.021 | 0.996 |
| Liver–V30 | 0.482 | 0.697 |
| Liver–Dmean | 0.539 | 0.658 |
| CTV–D99 | 0.861 | 0.470 |
| CTV–D95 | 0.886 | 0.458 |
| CTV–D1 | 0.064 | 0.979 |
| CTV–Dmean | 0.578 | 0.633 |
CTV, clinical tumour volume; L, left; PTV, planning target volume; R, right.
DISCUSSION
In the volume size of CTV for post-operative gastric cancer, there were significant variations among multiple observers while there was no variation between different CTs, and there was no significant interobserver or inter-CT variation in the volume of OARs. The variation of OARs between different CTs may come from spatial location. The slices in which variations more likely occur were the slices of the lower verge of the hilum of the spleen and porta hepatis, then the para-oesophageal lymph nodes region and abdominal aorta, and the inferior vena cava, and the variation in the craniocaudal orientation from the interobserver was more predominant compared with that from inter-CT. Most of the variations occurred on the border of the target and where the target range changed sharply, especially in the local lymph node region. Moreover, a significant interobserver influence on the dose distribution was found, despite the use of institutional delineation guidelines. We primarily found the slices in which variation most likely occurred during the course of target determination. The slices in which variations more likely occur were the slices of the lower verge of the hilum of the spleen and porta hepatis, then the para-oesophageal lymph nodes region and abdominal aorta, and the inferior vena cava, and the variation in craniocaudal orientation from the interobserver was more predominant compared with that from inter-CT.
Evaluation of the treatment plans of the INT0116 study, which used a two-dimensional (2D) technique, showed that 35% of treatment plans had major or minor variations. Leong et al19 reported a similar result at the 47th American Society for Radiation Oncology (ASTRO) annual meeting. These two studies indicate that uncertainties exist in the definition of target volume in gastric cancer and that further study is required to describe the reason for and effect of the variation. Chung et al4 evaluated the inter- and intraclinician variability in RT field delineation using conventional 2D and 3D techniques and showed that despite the use of guidelines and a departmental protocol, significant variations in the RT field areas were observed among ROs for both 2D and 3D planning. The CRITICS study was the first exploratory study that evaluated the interobserver variation of the CTV in gastric cancer,20 and it indicated that the variability of the CTV in post-operative chemoradiotherapy for gastric cancer is large and that strict and clear delineation guidelines should be provided, especially in Phase III multicentre studies. The adaptations of these guidelines should be evaluated in clinical studies.
In the present study, we used the ABC technique to reduce the influence of breathing movements to enable precise, simultaneous evaluation of interobserver and inter-CT variation. We analysed the size difference and the geometry or spatial uncertainty, which allowed us to describe the contour variation comprehensively. In our study, the average volume and SD of the CTV were 674 and 138 cm3, respectively. The mean CTV was greater than that of the CRITICS study, and we also had a smaller SD. These differences may be explained by our use of an institutional target delineation guideline, which may have influenced the definition of targets, in addition to the single-centre nature of our study, as ROs from the same institution may have similar ideas regarding target volume. ANOVA showed that the interobserver difference in the volume of the CTV was significant, whereas there was no significant inter-CT difference in the target volume. We also calculated the CI and the distance between the CMs of the CTVs to evaluate the geometry of the spatial variation. The interobserver and inter-CT maximum, minimum and average CI were 83.21%, 63.89% and 70.64%, and 85.97%, 76.01% and 81.79%, respectively. The average inter-CT differences in the distance between the CMs of the CTV, liver, left kidney and right kidney were 0.40, 0.56, 0.65 and 0.6 cm, respectively; the differences between the observers were 0.98, 0.53, 0.16 and 0.15 cm, respectively. This finding indicates that the interobserver variation (approximately 1.0 cm) of the CTV includes volume size and spatial differences and that the inter-CT variation of the CTV may be mainly derived from spatial and volumetric differences. For the same CT scan, the variation in the kidney (0.2 cm) was lower than that between different CT scans (0.6 cm), whereas the variation in liver delineation was stable for different CTs and different ROs. This finding may be attributed to the clearer border of the kidney and easier delineation of the kidney relative to the liver or CTV. An interfraction CT variation of 0.6 cm was still observed when we used ABC to obtain the simulation data, and this variation was similar to the thickness (0.5 cm) of the CT slice. In the slice-by-slice analysis of CI, we also found that the interobserver craniocaudal difference reached 1.5 cm (three CT slices), which may indicate that the mean the interobserver distance of CMs for CTV is approximately 1.0 cm.
In the slice-by-slice analysis of CI, we analysed the region where variations were more likely to occur and found that the CIs were significantly lower in slices of the lower border of the hilum of the spleen and porta hepatis, the para-oesophageal lymph node region and abdominal aorta, and the inferior vena cava region (anterior and lateral border); these slices were all in the local lymph node region. The potential reasons for the interobserver variation in these regions are as follows. First, regarding oncological factors, because the target mainly included subclinical regions, there was no clearly defined border; therefore, the comprehension of the target differed among the ROs, such as in the tumour bed and regional lymph node region. Additionally, iconographic factors, including a partial volume effect, the resolution and different image parameters (window level and window width), were present. The inter-CT variation may be attributed to geometric factors, the different extents of control of breathing movements, volumetric factors and the set-up error between different CT scans. To define the target and normal organs more precisely, multiple CT simulation images may therefore represent the treatment region more comprehensively, even when the ABC technique is used during simulation and treatment. Therefore, when the CTV is contoured, the post-operative CTV of gastric cancer should be defined according to a combination of factors and more attention should be paid to these regions. More precise guidelines are needed to help define post-operative gastric cancer targets, especially the guidelines regarding lymph node delineation.
We also investigated the influence of interobserver variation on dose distribution. Because no “gold-standard” PTV is available, we used the PTV that was actually used to design the treatment plan rather than the median PTV, which was applied in the CRITICS study. ANOVA showed that the PTV only differed significantly from D99 and Dmean. For different CTVs and normal organs contoured by different ROs, there was no significant difference in the parameters that we compared. We applied a conformal treatment technique in our study rather than conventional treatment technique as in the CRITICS study, and this was reflected in the influence that the interobserver variation in target delineation had on dose distribution.
Inevitably, there were some issues that we did not resolve. First, the inter-CT variation also included image fusion and intraobserver uncertainty. We used bony structures as references by using the CT–CT fusion option and cross-correlation algorithm to perform image fusion. In this manner, the fusion error was decreased and could be considered negligible. We contoured the CTVs of different CT images at one time to reduce the influence of intraobserver uncertainty as much as possible. Second, we did not analyse the influence of inter-CT variation because no standard CT scans were available. Therefore, the influence of inter-CT variation is not conclusive, and we will continuously study the target delineation and update the delineation guidelines to provide references for the uniform standard.
FUNDING
Our present project is supported by the National Natural Science Foundation of China (Grant No. 81302097).
Contributor Information
Gui-Chao Li, Email: guichaoli11@163.com.
Zhen Zhang, Email: zhenzhang6@163.com.
Xue-Jun Ma, Email: maxuejun123456789@163.com.
Xiao-Li Yu, Email: stephanieyxl@hotmail.com.
Wei-Gang Hu, Email: Jackhu88@yeah.net.
Jia-Zhou Wang, Email: wangjiazhou88@yeah.net.
Qi-Wen Li, Email: 0556245@fudan.edu.cn.
Li-Ping Liang, Email: llp8521@gmail.com.
Li-Jun Shen, Email: yeahslj@163.com.
Hui Zhang, Email: zhanghui_19860605@163.com.
Ming Fan, Email: 07301010259@fudan.edu.cn.
REFERENCES
- 1.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001; 345: 725–30. doi: 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- 2.Smalley SR, Gunderson L, Tepper J, Martenson JA, Jr, Minsky B, Willett C, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys 2002; 52: 283–93. doi: 10.1016/S0360-3016(01)02646-3 [DOI] [PubMed] [Google Scholar]
- 3.Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol 2002; 12: 187–95. doi: 10.1053/srao.2002.30827 [DOI] [PubMed] [Google Scholar]
- 4.Chung HT, Shakespeare TP, Wynne CJ, Lu JJ, Mukherjee RK, Back MF. Evaluation of a radiotherapy protocol based on INT0116 for completely resected gastric adenocarcinoma. Int J Radiat Oncol Biol Phys 2004; 59: 1446–53. doi: 10.1016/j.ijrobp.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 5.Leong T, Willis D, Joon DL, Condron S, Hui A, Ngan SY. 3D conformal radiotherapy for gastric cancer–results of a comparative planning study. Radiother Oncol 2005; 74: 301–6. doi: 10.1016/j.radonc.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 6.Urie MM, Goitein M, Doppke K, Kutcher JG, LoSasso T, Mohan R, et al. The role of uncertainty analysis in treatment planning. Int J Radiat Oncol Biol Phys 1991; 21: 91–107. doi: 10.1016/0360-3016(91)90170-9 [DOI] [PubMed] [Google Scholar]
- 7.Hurkmans CW, Borger JH, Pieters BR, Russell NS, Jansen EP, Mijnheer BJ. Variability in target volume delineation on CT scans of the breast. Int J Radiat Oncol Biol Phys 2001; 50: 1366–72. doi: 10.1016/S0360-3016(01)01635-2 [DOI] [PubMed] [Google Scholar]
- 8.Senan S, van Sörnsen de Koste J, Samson M, Tankink H, Jansen P, Nowak PJ, et al. Evaluation of a target contouring protocol for 3D conformal radiotherapy in non-small cell lung cancer. Radiother Oncol 1999; 53: 247–55. doi: 10.1016/S0167-8140(99)00143-7 [DOI] [PubMed] [Google Scholar]
- 9.Van De Steene J, Linthout N, de Mey J, Vinh-Hung V, Claassens C, Noppen M, et al. Definition of gross tumor volume in lung cancer: inter-observer variability. Radiother Oncol 2002; 62: 37–49. doi: 10.1016/S0167-8140(01)00453-4 [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Nagata Y, Okajima K, Ishigaki T, Murata R, Mizowaki T, et al. Differences in target outline delineation from CT scans of brain tumours using different methods and different observers. Radiother Oncol 1999; 50: 151–6. doi: 10.1016/S0167-8140(99)00015-8 [DOI] [PubMed] [Google Scholar]
- 11.Weltens C, Menten J, Feron M, Bellon E, Demaerel P, Maes F, et al. Interobserver variations in gross tumor volume delineation of brain tumors on computed tomography and impact of magnetic resonance imaging. Radiother Oncol 2001; 60: 49–59. doi: 10.1016/S0167-8140(01)00371-1 [DOI] [PubMed] [Google Scholar]
- 12.Logue JP, Sharrock CL, Cowan RA, Read G, Marrs J, Mott D. Clinical variability of target volume description in conformal radiotherapy planning. Int J Radiat Oncol Biol Phys 1998; 41: 929–31. doi: 10.1016/S0360-3016(98)00148-5 [DOI] [PubMed] [Google Scholar]
- 13.Fiorino C, Reni M, Bolognesi A, Cattaneo GM, Calandrino R. Intra- and inter-observer variability in contouring prostate and seminal vesicles: implications for conformal treatment planning. Radiother Oncol 1998; 47: 285–92. doi: 10.1016/S0167-8140(98)00021-8 [DOI] [PubMed] [Google Scholar]
- 14.Rasch C, Barillot I, Remeijer P, Touw A, Van Herk M, Lebesque JV. Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys 1999; 43: 57–66. doi: 10.1016/S0360-3016(98)00351-4 [DOI] [PubMed] [Google Scholar]
- 15.Seddon B, Bidmead M, Wilson J, Khoo V, Dearnaley D. Target volume definition in conformal radiotherapy for prostate cancer: quality assurance in the MRC RT-01 trial. Radiother Oncol 2000; 56: 73–83. doi: 10.1016/S0167-8140(00)00191-2 [DOI] [PubMed] [Google Scholar]
- 16.Valicenti RK, Sweet JW, Hauck WW, Hudes RS, Lee T, Dicker AP, et al. Variation of clinical target volume definition in three-dimensional conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 1999; 44: 931–5. doi: 10.1016/S0360-3016(99)00090-5 [DOI] [PubMed] [Google Scholar]
- 17.Weiss E, Richter S, Krauss T, Metzelthin SI, Hille A, Pradier O, et al. Conformal radiotherapy planning of cervix carcinoma: differences in the delineation of the clinical target volume. A comparison between gynaecologic and radiation oncologists. Radiother Oncol 2003; 67: 87–95. doi: 10.1016/S0167-8140(02)00373-0 [DOI] [PubMed] [Google Scholar]
- 18.Tai P, Van Dyk J, Yu E, Battista J, Stitt L, Coad T. Variability of target volume delineation in cervical esophageal cancer. Int J Radiat Oncol Biol Phys 1998; 42: 277–88. doi: 10.1016/S0360-3016(98)00216-8 [DOI] [PubMed] [Google Scholar]
- 19.Leong T, Michael M, Lim Joon D, Jayamoham J, Spry N, Harvey J, et al. Adjuvant chemoradiation for gastric cancer using epirubicin, cisplatin, and 5-FU (ECF) before and after 3D-conformal radiotherapy with continuous infusional 5-FU: a multicentre study of the Trans-tasman Radiation Oncology Group (TROG). Int J Radiat Oncol Biol Phys 2007; 69: S107. doi: 10.1016/j.ijrobp.2007.07.197 [DOI] [PubMed] [Google Scholar]
- 20.Jansen E, Nijkamp J, Gubanski M, Lind P, Verheij M. Interobserver variation of clinical target volume delineation in gastric cancer Int J Radiat Oncol Biol Phys 2010; 77: 1166–70. doi: 10.1016/j.ijrobp.2009.06.023 [DOI] [PubMed] [Google Scholar]
- 21.Somigliana A, Zonca G, Loi G, Sichirollo AE. How thick should CT/MR slices be to plan conformal radiotherapy? A study on the accuracy of three-dimensional volume reconstruction. Tumori 1996; 82: 470–2. [DOI] [PubMed] [Google Scholar]
- 22.Melian E, Mageras GS, Fuks Z, Leibel SA, Niehaus A, Lorant H, et al. Variation in prostate position quantitation and implications for three-dimensional conformal treatment planning. Int J Radiat Oncol Biol Phys 1997; 38: 73–81. doi: 10.1016/S0360-3016(97)00221-6 [DOI] [PubMed] [Google Scholar]
- 23.Nehmeh S, Erdi Y, Ling C, Rosenzweig KE, Squire OD, Braban LE, et al. Effect of respiratory gating on reducing lung motion artifacts in PET imaging of lung cancer. Med Phys 2002; 29: 366–71. doi: 10.1118/1.1448824 [DOI] [PubMed] [Google Scholar]
- 24.Caldwell C, Mah K, Skinner M, Danjoux C. Can PET provide the 3D extent of tumor motion for individualized internal target volumes? A phantom study of the limitation of CT and promise of PET. Int J Radiat Oncol Biol Phys 2003; 55: 1381–93. doi: 10.1016/S0360-3016(02)04609-6 [DOI] [PubMed] [Google Scholar]
- 25.Dubois D, Prestidge B, Hotchkiss L, Prete J, Bice WJ. Intraobserver and interobserver variability of MR imaging and CT derived prostate volumes after transperineal interstitial permanent prostate brachytherapy. Radiology 1998; 207: 785–9. doi: 10.1148/radiology.207.3.9609905 [DOI] [PubMed] [Google Scholar]
- 26.Rasch C, Remeijer P, Barillot I, Van Herk M, Lebesque JV. Observer imaging modality (ct, Mri) related definition prostate. Int J Radiat Oncol Biol Phys 1997; 39: 228. [DOI] [PubMed] [Google Scholar]
- 27.Remouchamps VM, Letts N, Vicini FA, Sharpe MB, Kestin LL, Chen PY, et al. Initial clinical experience with moderate deep-inspiration breath hold using an active breathing control device in the treatment of patients with left-sided breast cancer using external beam radiation therapy. Int J Radiat Oncol Biol Phys 2003; 56: 704–15. doi: 10.1016/S0360-3016(03)00010-5 [DOI] [PubMed] [Google Scholar]
- 28.Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys 2003; 55: 392–406. doi: 10.1016/S0360-3016(02)04143-3 [DOI] [PubMed] [Google Scholar]
- 29.Muralidhar KR, Murthy PN, Mahadev DS, Subramanyam K, Sudarshan G, Raju AK. Magnitude of shift of tumor position as a function of moderated deep inspiration breath-hold: an analysis of pooled data of lung patients with active breath control in image-guided radiotherapy. J Med Phys 2008; 33: 147–53. doi: 10.4103/0971-6203.44475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong JW, Sharpe MB, Jaffray DA, Kini VR, Robertson JM, Stromberg JS, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys 1999; 44: 911–9. doi: 10.1016/S0360-3016(99)00056-5 [DOI] [PubMed] [Google Scholar]
- 31.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 2nd English Edition. Gastric Cancer 1998; 1: 10–24. doi: 10.1007/PL00011681 [DOI] [PubMed] [Google Scholar]