Abstract
Objective:
Risk of nodal involvement in patients with sinonasal small-cell carcinoma and sinonasal undifferentiated carcinoma (SNUC) has not been well defined because of their rarity. We describe a population-based assessment of specific nodal level involvement in this group of rare neuroectodermal tumours.
Methods:
The Surveillance, Epidemiology and End Results (SEER) database from 2004 to 2011 identified patients with SNUC and sinonasal small-cell carcinoma. Overall neck involvement and individual nodal level involvement at presentation were assessed, and comparison was made with a contemporaneous cohort of patients with a borderline clinically significant risk of nodal involvement and recurrence.
Results:
Of 141 patients, 31 (22%) had gross nodal involvement at presentation (range 14–33% by site and histology). Non-nasal, non-ethmoid site with SNUC histology has the highest rates of initial nodal involvement, whereas higher stage and size do not predict for higher nodal involvement rates. Bilateral Levels 2–3 for all sinonasal small cell; Levels 2–3 for nasal or ethmoid SNUC; and bilateral Levels 1–3 in non-nasal/non-ethmoid SNUC have the highest rates of involvement compared with a clinical reference standard.
Conclusion:
We found high rates of initial nodal involvement in all SNUC and sinonasal small-cell carcinoma. We found higher initial involvement of Levels 2 and 3 and in certain cases to the Level 1 nodal levels, hypothesizing benefit for elective treatment to those levels.
Advances in knowledge:
With small single-institution series reporting conflicting nodal involvement rates, our data support high rates of nodal presentation at diagnosis, hypothesizing benefit for elective nodal treatment in this cohort.
INTRODUCTION
Sinonasal undifferentiated carcinoma (SNUC) and sinonasal small-cell carcinoma represent entities in the spectrum of neuroectodermal and neuroendocrine neoplasms of the head and neck.1 A population-based analysis shows SNUC representing <2% of tumours of the nasal cavity2 and 3% of all sinonasal tumours,3 with sinonasal small-cell carcinomas representing an even rarer disease. SNUC was first described in the 1980s and is known to be particularly aggressive,4,5 while the sparse information on sinonasal small-cell carcinomas indicates that this is an aggressive disease.6 Little is known about optimal treatment for sinonasal small-cell carcinoma. For SNUC, there are indications that multimodality treatment combining chemotherapy, radiation and resection may offer prolonged survival.7
Lymphatic drainage from the paranasal sinuses and nasal cavity is heterogeneous, with drainage including connections to the facial/buccal, submandibular, parotid and parapharyngeal nodal regions.8 Owing to the rarity of these tumours and lack of clear guidance in the literature, the treating physician has been faced with the quandary of whether or not to electively treat the neck, and if so, which nodal levels to treat in this group of patients. We therefore undertook a population-based analysis to help address this issue.
METHODS AND MATERIALS
The study population was extracted from the Surveillance, Epidemiology and End Results (SEER) Program v. 8.1.5 (National Cancer Institute, Bethesda, MD), encompassing patients treated from 2004 to 2011.9
Patients with small-cell carcinoma and SNUC of any paranasal sinus site were included. The American Joint Commitee on Cancer 7th edition was utilized for staging.10 The primary size was recorded for many cases. The grade was not analysed, as these tumours are uniformly considered to be high grade. Nodal region involvement, including facial, retropharyngeal (RP), each of neck Levels 1–5, parotid and contralateral/bilateral nodes, was recorded. Patients with incomplete information on staging or nodal involvement were excluded, as were patients with prior cancers.
A combined rate of initial nodal involvement and subsequent nodal recurrence of 15% has typically been used as a threshold indicating the need for elective nodal treatment. A limitation of the SEER database is that since it limits evaluation of nodal involvement to a period of 4 months around the patient's initial diagnosis,11 it is not possible to obtain a combined rate of initial nodal involvement and subsequent nodal recurrence. In cases in which the rate of initial nodal involvement alone approaches or is >15% in a population-based analysis, this crude incidence alone may support elective nodal treatment.
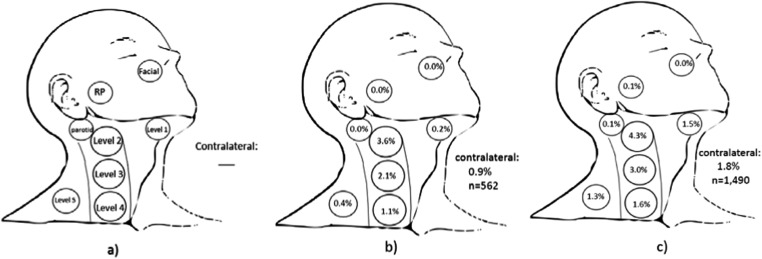
Although a 15% rate for initial and subsequent nodal involvement is generally accepted as indicating a need for elective nodal treatment, there is no accepted numerical threshold for elective neck treatment to specific nodal levels. In order to determine the significance of involvement at presentation of individual nodal levels, comparison to a reference standard of patients with head and neck cancer was used to make clinically meaningful hypotheses regarding the role of elective treatment. The reference standard used for statistical purposes consisted of T2 glottic larynx squamous cell carcinomas (SqCCs) with hypomobility only and without supraglottic/subglottic invasion in the SEER database also diagnosed from 2004 to 2011. Such patients are typically treated to the primary site at the larynx only and, based on the largest series from the University of Florida (Gainesville, FL), are considered to have a 13% risk of overall neck involvement or neck recurrence.12 By contrast, patients with supraglottic and/or subglottic carcinoma and without clinically evident nodal involvement at presentation commonly undergo elective treatment to Levels 2–4 as the standard of care because of a significantly higher risk of nodal involvement at those levels.13 Owing to the well-described natural history of and standard treatment paradigm for T2 glottic laryngeal SqCC with cord hypomobility and without supra/subglottic involvement, any difference in nodal risk compared with this subset of T2 glottic larynx patients should meet or exceed the 15% threshold used for elective neck treatment. For T2 glottic SqCCs, owing to impaired vocal cord movement only (reference group), there was a 7.12% (40/562) rate of nodal involvement, whereas the T2 glottic patients with supra/subglottic involvement had a 9.73% (145/1490) rate of nodal involvement. Of all patients, 95.4% had either no neck dissection or had biopsy of the neck node(s) only. We note that the rate of nodal involvement at presentation for T2 glottic SqCC with supraglottic involvement (9.7%) in the SEER is lower than the standard 15% threshold for the combination of initial nodal involvement and nodal recurrence, which is expected because the SEER database contains no data on nodal recurrence after the 4-month period.
To compare involvement of Levels 2–4, involvement of these nodal levels in SNUC and sinonasal small-cell carcinoma were compared with the same nodal levels in the reference T2 glottic larynx with vocal cord hypomobility only (Figure 1b), tabulated from the contemporaneous SEER data set. Stata® v. 13 (StataCorp., College Station, TX) for Fisher exact test and logistic regression analysis was used to determine factors at the primary site, histology and size associated with increased risk of nodal involvement. Fisher-Freeman-Halton or χ2 analysis was used in the statistical comparison between these groups. For Level 1, Level 5, parotid, RP and facial nodes, comparison was made to Level 4 involvement of the reference group. Owing to small sample sizes, borderline statistical significance was ascribed when a two-side p-value <0.15 was achieved. Statistical significance was ascribed when a two-sided p-value <0.05 was obtained.
Figure 1.
Nodal metastases at presentation: (a) depiction of each of Levels 1–5, retropharyngeal (RP), intraparotid and facial nodes. Reference standard pictorial representation of risk of nodal involvement in patients with T2 glottic squamous cell carcinoma (b) with impaired mobility only (7.1% nodal involvement, n = 562) used for statistical comparison and (c) with subglottic or supraglottic extension (9.7% nodal involvement, n = 1490).
RESULTS
There were a total of 167 patients with sinonasal small-cell carcinoma and SNUC in the SEER database. Staging information was available for all patients. Excluding patients with prior cancers (26), a total of 141 patients with sinonasal mucosal small cell carcinomas (29) and SNUCs (112) were analysed (Table 1). Of these, 136 (96.5%) had no or selective neck dissection. In the entire cohort, 31 (22.0%) had nodal involvement. Skip nodal metastases to secondary echelon nodal levels were negligible (0.7%, 0.7%, 0.7%, 0%, and 0% for Levels 3–5, parotid and RP nodes, respectively). Neither tumour size nor T-stage was significant on univariate analysis for risk of nodal involvement with either small cell or SNUC (Table 2). Non-nasal/ethmoid sites SNUC had a tendency towards higher rates of initial nodal involvement compared with nasal/ethmoid SNUC (32.7% vs 16.7%, p = 0.07), both of which were above the typical 15% threshold for initial or recurrent presentation commonly used for elective treatment. There was no difference by primary site in the incidence of nodal disease in patients with small-cell carcinoma (15% nasal/ethmoid vs 11% non-nasal/non-ethmoid small cell, p = 1.00).
Table 1.
Patient characteristics
| Small cell, n (%) | SNUC, n (%) | |
|---|---|---|
| Gender | ||
| Male | 16 (55) | 68 (61) |
| Female | 13 (45) | 44 (39) |
| Age (years) | ||
| <40 | 6 (21) | 18 (16) |
| 40–60 | 11 (38) | 53 (47) |
| >60 | 12 (41) | 41 (37) |
| Site | ||
| Nasal | 12 (41) | 42 (38) |
| Ethmoid | 8 (28) | 18 (16) |
| Maxillary | 5 (17) | 23 (21) |
| Sphenoid | 2 (7) | 4 (4) |
| Accessory | 1 (3) | 8 (7) |
| Overlapping | 1 (3) | 13 (12) |
| Frontal | 0 (0) | 4 (4) |
| Local stage | ||
| T1 | 3 (10) | 8 (8) |
| T2 | 3 (10) | 3 (3) |
| T3 | 8 (28) | 22 (20) |
| T4a | 11 (38) | 41 (37) |
| T4b | 4 (14) | 38 (34) |
| Neck dissection | ||
| None | 27 (93) | 104 (93) |
| Selective | 0 | 4 (4) |
| Extensive | 2 (7) | 3 (3) |
| Unknown | 0 | 1 (1) |
SNUC, sinonasal undifferentiated carcinoma.
Table 2.
Nodal involvement by quartile of size and T-stage
| All small cell (nodal involvement) | Nasal/ethmoid SNUC (nodal involvement) | Non-nasal/non-ethmoid SNUC (nodal involve) | |
|---|---|---|---|
| Local stage (p-value) | (p = 0.10 between T1–3 and T4, n = 29) | (p = 0.70 between T1–3 and T4, n = 60) | (p = 0.76 between T1–3 and T4, n = 52) |
| T1–T3 | 0/14 (0%) | 3/15 (20%) | 5/18 (28%) |
| T4 | 4/15 (27%) | 7/45 (16%) | 12/34 (35%) |
| Local tumour size (p-value) | (p = 1.00 between <median and >median, n = 19) | (p = 1.00 between <median and >median, n = 33) | (p = 0.69 between <median and >median, n = 34) |
| <median | 2/10 (20%) | 3/17 (18%) | 4/20 (20%) |
| >median | 1/9 (11%) | 2/16 (13%) | 4/14 (29%) |
SNUC, sinonasal undifferentiated carcinoma.
Not all patients had information on local tumour size available, with size of each relevant group defined in the headings. There was no difference by size or T-stage for nodal involvement in any group (p > 0.05 in all groups).
Clinically evident involvement for each of Levels 1–5, intraparotid, RP, facial nodes and contralateral and/or bilateral neck was analysed separately (Figure 1a). Patients with SNUC had a high rate of overall neck involvement of 24.1%. For sinonasal small-cell carcinoma, the initial nodal involvement rate approached the threshold of 15% for combined initial nodal involvement and recurrence used for justifying elective nodal treatment (13.8%, Table 3). By way of example, the absolute value was higher than that for a reference standard of T2 glottic larynx patients with supra/subglottic involvement, in whom elective nodal treatment is the standard of care (9.7%; Figure 1c and Table 3). For SNUC of the nasal/ethmoid sinuses, the overall rate of nodal involvement exceeded the 15% threshold (16.7%, Table 3) as did the rate for SNUC of other paranasal sinuses (32.7%, Table 3); both rates were significantly higher than those for the group of patients with T2 glottic larynx cancers by way of example. Our data therefore support a high nodal involvement rate in all patients with SNUC as well as sinonasal small-cell carcinoma.
Table 3.
Nodal involvement at presentation by site and histology
| Site/histology | % nodal involvement (n) | p-value (compared with the reference standard) | Levels at highest risk |
|---|---|---|---|
| All small cell | 13.8 (29) | 0.26b | IIa, IIIb, contralaterala |
| Nasal/ethmoid SNUC | 16.7 (60) | 0.02a | IIa |
| Non-nasal/non-ethmoid SNUC | 32.7 (52) | <0.001a | Ia, IIa, IIIa, contralateralb, parotidb |
| T2 glottic larynx without supraglottic extension (reference standard) | 7.1 (562) | N/A | N/A |
| T2 glottic larynx with supraglottic extension | 9.7 (1490) | N/A | N/A |
Reference standard (T2 glottic without supra/subglottic involvement = 7.1%, n = 562).
p < 0.05.
p > 0.05, but absolute incidence higher than T2 glottic larynx with subglottic or supraglottic extension patients receive elective nodal treatment as standard of care.
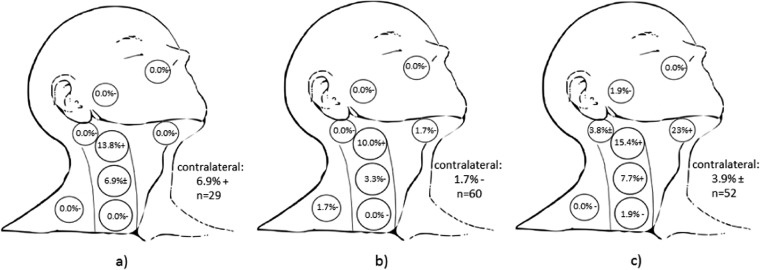
For patients with sinonasal small-cell carcinoma (n = 29; Figure 2a), the highest rate of nodal involvement for all sinonasal small-cell carcinomas were in Levels 2 and 3 (13.8% and 6.9% respectively), with a relatively high rate of contralateral neck involvement at presentation (6.9%). Nodal involvement of Level 2 was significantly higher (p = 0.025) than that of the reference standard. Although there was not a statistically significant difference in involvement of Level 3 compared with the reference standard (6.9%, p = 0.15), this was numerically greater than the 3% rate of involvement of Level 3 in T2 glottic cancers with sub/supraglottic involvement for whom the standard of care entails elective nodal treatment. The 6.9% contralateral/bilateral neck involvement was statistically significant compared with the reference standard in patients with sinonasal small-cell carcinoma (p = 0.04).
Figure 2.
Nodal levels at clinically significant risk. (a) All sinonasal small cell carcinoma (13.8% nodal involvement, n = 29, p = 0.26), (b) nasal and ethmoid SNUC (16.7% nodal involvement, n = 60, p = 0.02 compared with the reference standard in Figure 1b) and (c) non-nasal and non-ethmoid SNUC (32.7% nodal involvement, n = 52, p < 0.001). (+) denotes p < 0.05 compared with the reference standard representing nodal levels hypothesized to be included in treatment fields. (−) denotes p > 0.05 and therefore nodal levels that may be excluded from elective fields. (±) denotes p ≥ 0.05 and p < 0.15 compared with the reference standard of T2 glottic larynx with cord immobility only (Figure 1b), and also with absolute incidence higher than T2 glottic larynx with subglottic or supraglottic extension who ordinarily receive elective nodal treatment as the standard of care.
For patients with SNUC of the nasal cavity and ethmoid sinus (n = 60; Figure 2b), the highest rate of nodal involvement was in Level 2, which was statistically significant compared with the reference standard (10%, p = 0.031). There were insufficient numbers of patients coded as having bilateral/contralateral disease to demonstrate clinically significant bilateral nodal risk, although both the nasal cavity and ethmoid sinus are midline structures. For SNUC of sinonasal sites outside the nasal cavity and ethmoid sinus (n = 52; Figure 2c), the highest rates of nodal involvement were in Level 1 (23%, p < 0.001 compared with the reference standard), Level 2 (15%, p < 0.001) and Level 3 (8%, p = 0.04). The numerically higher rate of contralateral nodal involvement in non-nasal/non-ethmoid SNUC, which is of borderline statistical significance (3.9%, p = 0.11) compared with the reference standard as well as higher than the 1.8% rate of contralateral involvement in T2 glottic laryngeal cancers with supraglottic involvement (Figure 1c), suggests that elective treatment of the bilateral neck may be considered in this combination of site and histology (Table 3).
DISCUSSION
In this population-based series of patients with sinonasal small-cell carcinomas and SNUC diagnosed from 2004 to 2011, we find that the extent of nodal involvement is solely dependent on site (nasal/ethmoid vs other sinus) and histology (SNUC vs small cell) and not on factors relating to local tumour extension such as T stage and primary tumour size. The highest rates of nodal involvement are in patients with SNUC centered in sites outside the nasal cavity and ethmoid, and exceed the 15% risk of initial neck involvement or any subsequent neck recurrence that has generally been accepted as a threshold meriting elective neck dissection or radiation. In our series, which is limited by the SEER database to just initial rates of nodal involvement, we find that SNUC and sinonasal small-cell carcinomas have rates of nodal involvement that are higher than or approximating this commonly accepted threshold. In our data, the extent of elective neck treatment is solely dependent on histology (SNUC vs small cell) and site for SNUC (nasal/ethmoid vs other sinus) and not on factors relating to local tumour extension such as T stage and primary tumour size. There are insufficient patient numbers in the small-cell cohort to assess for effect of primary site.
In order to determine the clinical significance of individual nodal level involvement at presentation, a reference standard of T2 glottic larynx cancers that undergo observation or elective treatment of the neck based on involvement of the sub- or supraglottic larynx as standard of care provides a comparison, however imperfect, regarding which nodal levels should be included in an elective neck dissection or radiation field. In particular, we find that Levels 2 and 3 and the contralateral/bilateral neck are at a higher risk of involvement in small-cell carcinomas of the paranasal sinus. For nasal and ethmoid SNUC, we find that Levels 2 and 3 have higher rates of nodal involvement. For SNUC outside the nasal cavity and ethmoid sinus, we find that Levels 1–3 have the highest rates of nodal involvement while the parotid and contralateral/bilateral neck also have borderline significant rates of nodal involvement.
Most series for sinonasal small-cell carcinoma have included between one and seven patients. The experience from MD Anderson (Houston, TX) demonstrated three of seven sinonasal small-cell patients with nodal disease at presentation, with three of seven patients (44%) experiencing regional failure.14 Whether the four of seven patients with no gross nodal disease at presentation received elective nodal treatment was not described. The Memorial Sloan-Kettering (New York, NY) experience of six patients was mainly characterized by local failure; there were few details concerning initial staging and whether the neck underwent elective treatment, although no regional recurrences were reported.15 In a series of 21 patients from 8 French hospitals in which 2 patients underwent neck dissection and 4 patients with N0 disease received elective nodal irradiation, 3 of 21 patients experienced nodal recurrence. Two of the three patients with nodal recurrences had initially presented with N0 disease and did not undergo elective neck treatment.16 In a series of four patients from Tokyo, there was nodal involvement at presentation in one patient, with neck recurrences in two of the three patients with sinonasal small-cell carcinoma who did not have nodal involvement at presentation.17 In the available literature (Table 4), there was an 11% rate of initial nodal involvement in patients with sinonasal small cell carcinoma, with a 25% nodal recurrence rate in patients without elective nodal treatment (or no information on elective neck treatment). Of the six patients in the literature with initial N0 disease known to have received elective neck treatment, one patient suffered nodal recurrence.
Table 4.
Prior literature on nodal presentation at diagnosis or subsequent nodal recurrence in patients with sinonasal undifferentiated carcinoma (SNUC) or sinonasal small-cell carcinoma in those who initially presented with N0 disease
| Author/institution | Site/histology | (n, evaluable) | Initial nodal involvement | Subsequent nodal recurrence (no elective treatment or no information), initial N0 | Subsequent nodal recurrence (initial elective treatment), initial N0 |
|---|---|---|---|---|---|
| Rosenthal et al,14 MD Anderson | Small cell | 7 | 3/7 (43%) | 3/4 (75%) | N/R |
| Perez-Ordonez et al,15 Memorial Sloan-Kettering | Small cell | 6 | 0/6 | 0/6 | N/R |
| Babin et al,16 France | Small cell | 21 | 0/21 | 2/15 (13%) | 1/6 (17%) |
| Kameya et al,17 Tokyo | Small cell | 4 | 1/4 (25%) | 2/3 (67%) | N/A |
| Total evaluable (sinonasal small cell carcinoma) | 38 | 4/38 (11%) | 7/28 (25%) | 1/6 (17%) | |
| Total sinonasal small cell carcinoma (including current study) | 67 | 8/67 (12%) | |||
| Cerilli et al,18 UVA | SNUC | 25 | 1/25 (4%) | 8/15 (53%) | N/A |
| Jeng et al,19 Taiwan | SNUC | 36 | 6/36 (17%) | N/R | N/R |
| Rischin et al,20 Peter MacCallum | SNUC | 10 | 3/10 (30%) | N/R | N/R |
| Al-Mamgani et al,21 Erasmus | SNUC | 21 | 2/21 (9.5%) | 2/11 (18%) | 0/8 |
| Chen et al,22 UCSF | SNUC | 21 | 2/21 (9.5%) | 1/4 (25%) | 1/15 (6.7%) |
| Kim et al,23 UCLA | SNUC | 8 | 0/8 | 2/5 (40%) | 0/3 |
| Tanzler et al,24 University of Florida | SNUC | 15 | 2/15 (13%) | 2/6 (33%) | 0/7 |
| Total evaluable (SNUC) | 127 | 16/136 (12%) | 15/41 (37%) | 1/33 (3.0%) | |
| Total SNUC (including current study) | 239 | 34/239 (14%) |
N/A, not applicable; N/R, not reported; UCLA, the University of California at Los Angeles; UCSF, the University of California at San Francisco; UVA, the University of Virginia.
The relatively higher prevalence of SNUC has allowed for studies with larger sample sizes. In a series from the University of Virginia (Charlottesville, VA) with 25 patients treated with induction chemotherapy followed by radiation and craniofacial resection when possible, information on 16 patients was available. There was a 56% (9/16) rate of local and 50% (8/16) rate of neck recurrence. Of the seven patients who developed distant metastases, three had concurrent neck recurrence.18 The largest series of 36 patients from Taiwan did not report patterns of recurrence, but 6 patients (17%) presented with nodal involvement.19 A series from the Peter MacCallum Institute (Melbourne, Australia) of 10 patients describing an approach similar to the University of Virginia did not report on nodal recurrences, although 3/10 had nodal involvement at presentation.20 In the retrospective experience of the Erasmus Cancer Center with 21 patients, 2 patients had nodal disease at presentation. Eight patients without initially evident nodal disease but with skin, intratemporal fossa, pterygoid or cribiform involvement underwent elective nodal radiation to ipsilateral Levels I–III. None of these patients developed regional failure, whereas 2 of the remaining 13 patients experienced nodal recurrence.21 In a University of California San Francisco (San Francisco, CA) case series of 21 patients, 15 of 19 patients with clinically N0 necks received elective nodal irradiation. Although there was no description of the nodal levels covered in the elective field, there were no isolated regional failures. Two patients developed regional failure in the context of prior distant recurrence; one of the patients with regional and distant recurrence had received elective nodal irradiation.22 A case series of eight patients from the University of California Los Angeles (Los Angeles, CA) demonstrated that of the five patients who did not receive nodal treatment two recurred in the neck, whereas none of the three patients receiving elective neck treatment developed a regional recurrence.23 In a case series of 15 patients from the University of Florida, 13 patients had N0 disease at presentation; 7 of these patients received elective nodal treatment, whereas 6 did not. Patients receiving elective nodal treatment had 100% regional control while two of six patients who did not receive elective nodal treatment developed regional recurrence (regional control of 67%). One of these patients died with neck disease alone.24 Of the 127 patients reported in the available literature (Table 4), there was a 12% rate of initial nodal involvement; a 37% rate of regional recurrence in patients with N0 neck disease at presentation who did not undergo elective neck treatment or in whom no information on elective neck treatment was specified; and a 3% rate of regional recurrence in initially N0 patient who underwent elective neck treatment. In a review of the literature, only three of six (50%) patients with SNUC who did not receive elective nodal treatment and who experienced isolated regional recurrence could be salvaged. Of the 20 patients with N0 disease at presentation without elective neck treatment who subsequently developed distant metastases, seven (35%) also developed neck recurrence, suggesting that the cohort of N0 patients with SNUC may be at high risk for both regional and distant recurrence.
The extent of elective nodal treatment for the patients with N0 sinonasal small-cell carcinoma or SNUC is not well described in the literature. Although comprehensive treatment of the bilateral RP and Levels 1–5 nodal levels would likely lead to the lowest rates of nodal recurrence, this would be accompanied by significant toxicities. Avoiding radiation treatment or neck dissection to nodal levels with low rates of involvement for patients with SNUC and sinonasal small-cell carcinomas with N0 disease at presentation may help prevent long-term quality of life issues and allow for lower rates of radiotherapy treatment interruptions.25
Suggestions for when and which nodal level(s) to treat electively using initial rates of nodal disease is based on historical precedent. The benefit for elective nodal treatment for SqCC of the nasopharynx, oropharynx, oral cavity and larynx was established with pioneering work from MD Anderson in 1972 (Lindberg). In this work, patients who presented to their head and neck clinic had nodal level involvement tabulated at initial presentation, based on clinical examination only, and without data on nodal recurrence patterns. Rates of initial presentation only were used to make suggestions for elective nodal treatment and which nodal levels were to undergo elective treatment. It must be noted that Lindberg's study of clinical nodal involvement, which predates modern imaging techniques for staging, does not provide information on an absolute threshold for elective treatment to a specific nodal level. For example, the rate of contralateral Level 3 nodal involvement for hypopharyngeal carcinomas at initial presentation is only 1.5% in the study by Lindberg,26 yet this nodal level is always included in elective nodal fields for hypopharyngeal carcinomas and is considered a primary drainage level. For sinonasal small-cell carcinoma and SNUC, in which single-institution studies provide insufficient numbers to make suggestions regarding which nodal levels should be electively treated, we make similar hypotheses based on the larger numbers possible using a population-based study.
An advantage of our series is that it encompasses a modern cohort of patients from 2004 onwards, antedating confusion on how to pathologically distinguish sinonasal small-cell carcinoma and SNUC from other neuroectodermal tumours of the head and neck. This series of patients is also from an era in which modern radiographic tools for diagnosis and staging are available, as opposed to single-institution studies that may span several decades.
It is unknown whether prophylactic nodal treatment for patients with sinonasal small-cell carcinoma or SNUC provides a survival advantage, and this is beyond the scope of our work. All patients with sinonasal small-cell carcinoma or SNUC should undergo proper staging work-up of both the neck and potential sites of distant metastases. In head and neck cancers in general, regional neck recurrence confers a higher risk of concurrent or subsequent distant metastatic disease.27 As we have outlined, the retrospective literature suggests that elective nodal treatment may decrease the incidence of nodal recurrences. Whether or not elective neck treatment confers a distant metastases-free or overall survival advantage is unclear, yet we hypothesize that the deleterious effect of neck recurrence on quality of life may merit elective neck treatment to at least the high-risk nodal levels identified in the different subgroups of patients. This study cannot address whether there is inherent value in decreasing the risk of isolated nodal recurrence, as salvage may be feasible if a patient undergoes isolated recurrence in the unirradiated or undissected neck although the rates of salvage reported to date (50%) are not high, and in accordance with data for salvage of neck recurrence (4/7, 43%) in patients with paranasal esthesioneuroblastoma at the University of Michigan.28
A disadvantage of this work is that observational studies such as the SEER suffer from bias from unmeasured confounding as a limitation. This includes information on how patients were staged. Additionally, akin to the experience from the 1960s and 1970s noted above for Lindberg's study of head and neck mucosal SqCC, a significant potential flaw in our methodology is the assumption that recurrence risks are proportional to rates of initial nodal involvement and our further assumption that the ratio of initial nodal presentation to subsequent nodal recurrence is similar between our sinonasal population and that of the T2 glottic reference group.
Of importance is the rarity of these tumours; in the SEER database encompassing almost 25% of the US population in our study, there were <3 cases a year of sinonasal small-cell carcinoma and 14 cases a year of SNUC. Given this rarity, single-institution retrospective series are unlikely to ever have sufficient numbers to clarify this question and may themselves be subject to confounding secondary to referral patterns, varying practices regarding elective nodal treatment, as well as the commonly decades-long time span required to obtain these small numbers. Given the limitations of current retrospective series, comparisons utilizing population-based databases are therefore a useful tool to obtain higher patient numbers to develop data-driven hypotheses regarding optimal treatment and which nodal levels may be electively treated. Using statistical comparisons with nodal risk in a better-understood cancer to generate hypotheses regarding elective neck treatment may be useful in controlling for some of these unmeasured confounders inherent to the database itself. These may include the possibility of any underreporting of nodal involvement intrinsic to the SEER database and the different diagnostic modalities used to determine the extent of nodal involvement. We would reiterate that the lack of information on follow-up nodal recurrences is a serious limitation inherent to the SEER database and therefore of this work.
Although our study is population-based and the largest study of which we are aware for both SNUC and sinonasal small-cell carcinoma, subcategories of site, stage and size have very small patient numbers which may limit the analysis. Furthermore, our finding that rates of Level 1 nodal involvement are highest with non-nasal and non-ethmoid SNUC may be less applicable if there are high levels of involvement of tumours with epicentre inside the nasal cavity/ethmoid extending into the lymphatic draining subsites outside the nasal cavity or ethmoid sinus. Although we have generated hypotheses concerning whether and to which nodal levels elective treatment of the neck may be performed in patients with clinical N0 disease, patients with N+ clinical nodal involvement at presentation would likely warrant dissection or nodal irradiation to a comprehensive neck field.
CONCLUSION
There have been no consistent data presented on overall neck and specific nodal risk in sinonasal small-cell carcinomas and SNUCs because of their extreme rarity. Since population-based studies such as the SEER have limitations secondary to the lack of patterns of failure data, only hypotheses regarding the role and elective fields for treatment or observation of the clinically uninvolved neck can be generated. Our findings from the SEER demonstrate high rates of initial nodal involvement, generating a hypothesis that all patients with SNUC or sinonasal small cell carcinoma have a high risk of clinical or subclinical nodal involvement. We find that the particular nodal levels at highest risk may be dependent on histology and on the involved sinonasal site, but generally do not involve the entire neck. However, we would note that both this work and associated review of the literature do not provide a definitive level of proof that elective neck treatment is warranted in patients with SNUC or sinonasal small cell carcinoma, nor do they provide a definitive level of proof about the specific nodal levels at clinically significant risk of involvement.
CONFLICT OF INTEREST STATEMENT
Dr Ahn reports a pending patent on a “Patient positioning system and methods for diagnostic radiology and radiotherapy”.
Contributor Information
Peter H Ahn, Email: peter.ahn@uphs.upenn.edu.
Nandita Mitra, Email: nanditam@mail.med.upenn.edu.
Michelle Alonso-Basanta, Email: Michelle.Alonso-Basanta@uphs.upenn.edu.
Nithin D Adappa, Email: Nithin.Adappa@uphs.upenn.edu.
James N Palmer, Email: James.Palmer@uphs.upenn.edu.
Bert W O'Malley, Jr, Email: Bert.O'Malley@uphs.upenn.edu.
Christopher H Rassekh, Email: Christopher.Rassekh@uphs.upenn.edu.
Ara Chalian, Email: ChalianA@uphs.upenn.edu.
Roger B Cohen, Email: Roger.Cohen@uphs.upenn.edu.
Alexander Lin, Email: Alexander.Lin2@uphs.upenn.edu.
REFERENCES
- 1.Mills SE. Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Mod Pathol 2002; 15: 264–78. doi: 10.1038/modpathol.3880522 [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N. Cancer of the nasal cavity: survival and factors influencing prognosis. Arch Otolaryngol Head Neck Surg 2002; 128: 1079–83. doi: 10.1001/archotol.128.9.1079 [DOI] [PubMed] [Google Scholar]
- 3.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck 2012; 34: 877–85. doi: 10.1002/hed.21830 [DOI] [PubMed] [Google Scholar]
- 4.Frierson HF, Jr, Mills SE, Fechner RE, Taxy JB, Levine PA. Sinonasal undifferentiated carcinoma. An aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol 1986; 10: 771–9. doi: 10.1097/00000478-198611000-00004 [DOI] [PubMed] [Google Scholar]
- 5.Levine PA, Frierson HF, Jr, Stewart FM, Mills SE, Fechner RE, Cantrell RW. Sinonasal undifferentiated carcinoma: a distinctive and highly aggressive neoplasm. Laryngoscope 1987; 97: 905–8. [PubMed] [Google Scholar]
- 6.Renner G. Small cell carcinoma of the head and neck: a review. Semin Oncol 2007; 34: 3–14. doi: 10.1053/j.seminoncol.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 7.Musy PY, Reibel JF, Levine PA. Sinonasal undifferentiated carcinoma: the search for a better outcome. Laryngoscope 2002; 112: 1450–5. doi: 10.1097/00005537-200208000-00023 [DOI] [PubMed] [Google Scholar]
- 8.Lang J. Clinical anatomy of the nose, nasal cavity and paranasal sinuses. Stuttgart, Germany: Thieme; 1989. [Google Scholar]
- 9.Available from: http://seer.cancer.gov
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–4. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 11.Available from: http://training.seer.cancer.gov/staging/time.html
- 12.Mendenhall WM, Amdur RJ, Morris CG, Hinerman RW. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol 2001; 19: 4029–36. [DOI] [PubMed] [Google Scholar]
- 13.Gregoire V, Coche E, Cosnard G, Hamoir M, Reychler H. Selection and delineation of lymph node target volumes in head and neck conformal radiotherapy. Proposal for standardizing terminology and procedure based on the surgical experience. Radiother Oncol 2000; 56: 135–50. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal DI, Barker JL, El-Naggar AK, Glisson BS, Kies MS, Diaz EM, et al. Sinonasal malignancies with neuroendocrine differentiation. Cancer 2004; 101: 2567–73. doi: 10.1002/cncr.20693 [DOI] [PubMed] [Google Scholar]
- 15.Perez-Ordonez B, Caruana SM, Huvos AG, Shah JP. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. Hum Pathol 1998; 29: 826–32. doi: 10.1016/S0046-8177(98)90452-X [DOI] [PubMed] [Google Scholar]
- 16.Babin E, Rouleau V, Vedrine PO, Toussaint B, de Raucourt D, Malard O, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Laryngol Otol 2006; 120: 289–97. doi: 10.1017/S0022215106000594 [DOI] [PubMed] [Google Scholar]
- 17.Kameya T, Shimosato Y, Adachi I, Abe K, Ebihara S, Ono I. Neuroendocrine carcinoma of the paranasal sinus a morphological and endocrinological study. Cancer 1980; 45: 330–9. doi: [DOI] [PubMed] [Google Scholar]
- 18.Cerilli LA, Holst VA, Brandwein MS, Stoler MH, Mills SE. Sinonasal undifferentiated carcinoma: immunohistochemical profile and lack of EBV association. Am J Surg Pathol 2001; 25: 156–63. doi: 10.1097/00000478-200102000-00003 [DOI] [PubMed] [Google Scholar]
- 19.Jeng YM, Sung MT, Fang CL, Huang HY, Mao TL, Cheng W, et al. Sinonasal undifferentiated carcinoma and nasopharyngeal-type undifferentiated carcinoma: two clinically, biologically, and histopathologically distinct entities. Am J Surg Pathol 2002; 26: 371–6. doi: 10.1097/00000478-200203000-00012 [DOI] [PubMed] [Google Scholar]
- 20.Rischin D, Porceddu S, Peters L, Martin J, Corry J, Weih L. Promising results with chemoradiation in patients with sinonasal undifferentiated carcinoma. Head Neck 2004; 26: 435–41. doi: 10.1002/hed.10396 [DOI] [PubMed] [Google Scholar]
- 21.Al-Mamgani A, van Rooij P, Mehilal R, Tans L, Levendag PC. Combined-modality treatment improved outcome in sinonasal undifferentiated carcinoma: single-institutional experience of 21 patients and review of the literature. Eur Arch Otorhinolaryngol 2013; 270: 293–9. doi: 10.1007/s00405-012-2008-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen AM, Daly ME, El-Sayed I, Garcia J, Lee NY, Bucci MK, et al. Patterns of failure after combined-modality approaches incorporating radiotherapy for sinonasal undifferentiated carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2008; 70: 338–43. doi: 10.1016/j.ijrobp.2007.06.057 [DOI] [PubMed] [Google Scholar]
- 23.Kim BS, Vongtama R, Juillard G. Sinonasal undifferentiated carcinoma: case series and literature review. Am J Otolaryngol 2004; 25: 162–6. doi: 10.1016/j.amjoto.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 24.Tanzler ED, Morris CG, Orlando CA, Werning JW, Mendenhall WM. Management of sinonasal undifferentiated carcinoma. Head Neck 2008; 30: 595–9. doi: 10.1002/hed.20748 [DOI] [PubMed] [Google Scholar]
- 25.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003; 66: 253–62. doi: 10.1016/S0167-8140(02)00404-8 [DOI] [PubMed] [Google Scholar]
- 26.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer 1972; 29: 1446–9. doi: [DOI] [PubMed] [Google Scholar]
- 27.Leibel SA, Scott CB, Mohiuddin M, Marcial VA, Coia LR, Davis LW, et al. The effect of local-regional control on distant metastatic dissemination in carcinoma of the head and neck: results of an analysis from the RTOG head and neck database. Int J Radiat Oncol Biol Phys 1991; 21: 549–56. doi: 10.1016/0360-3016(91)90669-U [DOI] [PubMed] [Google Scholar]
- 28.Demiroz C, Gutfeld O, Aboziada M, Brown D, Marentette LJ, Eisbruch A. Esthesioneuroblastoma: is there a need for elective neck treatment? Int J Radiat Oncol Biol Phys 2011; 81: e255–61. doi: 10.1016/j.ijrobp.2011.03.036 [DOI] [PubMed] [Google Scholar]