Abstract
Objective:
The purpose of this study was to investigate the safety, tolerability and preliminary effectiveness of topical epigallocatechin-3-gallate (EGCG) for radiation dermatitis in patients with breast cancer receiving adjuvant radiotherapy.
Methods:
Patients with breast cancer who received radiotherapy to the chest wall after mastectomy were enrolled. EGCG solution was sprayed to the radiation field from the initiation of Grade 1 radiation dermatitis until 2 weeks after completion of radiotherapy. EGCG concentration escalated from 40 to 660 μmol l−1 in 7 levels with 3–6 patients in each level. EGCG toxicity was graded using the NCI (National Cancer Institute Common Terminology Criteria for Adverse Events) v. 3.0. Any adverse event >Grade 1 attributed to EGCG was considered dose-limiting toxicity. The maximum tolerated dose was defined as the dose level that induced dose-limiting toxicity in more than one-third of patients at a given cohort. Radiation dermatitis was recorded weekly by the Radiation Therapy Oncology Group scoring and patient-reported symptoms.
Results:
From March 2012 to August 2013, 24 patients were enrolled. Acute skin redness was observed in 1 patient and considered to be associated with the EGCG treatment at 140 μmol l−1 level. Three more patients were enrolled at this level and did not experience toxicity to EGCG. The dose escalation stopped at 660 μmol l−1. No other reported acute toxicity was associated with EGCG. Grade 2 radiation dermatitis was observed in eight patients during or after radiotherapy, but all decreased to Grade 1 after EGCG treatments. Patient-reported symptom scores were significantly decreased at 2 weeks after the end of radiotherapy in pain, burning, itching and tenderness, p < 0.05.
Conclusion:
The topical administration of EGCG was well tolerated and the maximum tolerated dose was not found. EGCG may be effective in treating radiation dermatitis with preliminary investigation.
Advances in knowledge:
EGCG solution seemed to be feasible for treating radiation dermatitis in patients with breast cancer after mastectomy. It should be tested as a way to reduce radiation-induced normal tissue toxicity and complications in future years.
EGCG; BREAST NEOPLASMS; DERMATITIS, RADIATION-INDUCED
Skin toxicity (radiation dermatitis) is the most common acute side effect of radiotherapy to the breast, varying from mild erythema to moist desquamation and occasionally ulceration.1 Even with modern techniques such as intensity-modulated radiation therapy, 31.2% of patients experience moist desquamation during or up to 6 weeks after the radiation treatment.2 Radiation dermatitis can significantly impose discomfort and interfere with patients' daily living activities and quality of life.3 Severe toxicity may compromise treatment efficacy if the treatment is interrupted while the injury heals.4 Hence, it is important to identify approaches aimed at preventing or treating radiation dermatitis in patients with breast cancer.
No evidence-based standard of care has been established for reducing radiation dermatitis.5 The Phase III trial [Radiation Therapy Oncology Group (RTOG) 97-13] showed that trolamine did not reduce skin toxicity compared with best supportive care during adjuvant radiotherapy for breast cancer.6 In another randomized Phase III trial, no benefit was found from the use of the topical hyaluronic acid-based gel for reducing the development of ≥Grade 2 dermatitis after adjuvant radiotherapy for breast cancer.7 There remains a need to continue investigating new products and novel approaches for minimizing radiation dermatitis.
An expanding body of pre-clinical evidence suggested that epigallocatechin-3-gallate (EGCG), the major catechin found in green tea, had potential in inhibiting radiation-induced damage in vitro and in vivo.8–10 It was found that EGCG was most efficient at inhibiting erythema response evoked by ultraviolet radiation in human health volunteers.11 At the same time, the toxicity test of green tea extract also did not show any sign of irritation in the skin in patients with allergic contact dermatitis.12 Therefore, we conducted this Phase I trial of topical EGCG in patients with breast cancer receiving post-operative radiotherapy. The primary purpose was to define the safety and maximum tolerated dose (MTD) of topical EGCG. The second purpose was to investigate preliminarily the effectiveness of EGCG in treating radiation dermatitis.
METHODS AND MATERIALS
Patients
Eligible patients had to have a pathologically proven breast cancer with a planned course of radiotherapy to the chest wall after modified radical mastectomy. Other inclusion criteria were age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, normal haematological function (granulocyte count ≥1.5 × 109 cells per litre, platelet count ≥100 × 109 cells per litre and haemoglobin ≥100 g l−1) and organ function (creatinine clearance >50 ml min−1 and aspartate aminotransferase/alanine aminotransferase ≤2.5 of upper normal limit). Exclusion criteria included the presence of rash or unhealed wound in the radiation field, known allergy or hypersensitivity to green tea or EGCG, pregnancy or lactation, history of/current connective tissue disorder and prior radiation to the thorax. Informed consent was obtained from all patients. The study was approved by the Institutional Review and Ethical Committees at the Shandong Cancer Hospital and Institute and registered at ClinicalTrials.gov (NCT01481818).
Radiotherapy
Radiation treatment was delivered to the chest wall, including the surgical scar and regional lymph nodes, i.e. supraclavicular and infraclavicular nodes. All patients underwent simulation for verification of the irradiated fields and determination of chest wall thicknesses. Additional boluses were added according to chest wall thickness variation. The electron energy was determined by the chest wall thickness in the midplane (range, 6–12 MeV). The dose given was 50 Gy in 25 fractions over 5 weeks. The field arrangement involved an anterior photon field against the supraclavicular and infraclavicular regions and an anterior electron field against the chest wall.13 Patients with sharp surface irregularities, large planning target volumes (i.e. very obese patients or positive deep margins) or in whom the tumour bed was located under an irregular contour (i.e. axillary folds or inframammary folds), which can produce localized hot spots and cold spots in the underlying tissue, were suggested to receive three-dimensional conformal radiotherapy/intensity-modulated radiation therapy in our hospital. Therefore, these patients would not be included initially.
Epigallocatechin-3-gallate administration and maximum tolerated dose definition
EGCG (purity ≥95% by high performance liquid chromatography) was purchased from HEP Biotech Co., Ltd (Ningbo, Zhejiang, China) and freshly dissolved in 0.9% saline solution. The EGCG concentration escalated from 40 μmol l−1, 80 μmol l−1, 140 μmol l−1, 210 μmol l−1, 300 μmol l−1 and 440 μmol l−1 to 660 μmol l−1. EGCG administration was initiated once Grade 1 dermatitis occurred. The solution was sprayed three times a day at 0.05 ml cm−2 to 2 cm beyond the whole radiation field until 2 weeks after radiation completion. No other prophylactic agent was allowed in the radiation field. Patients were instructed to cleanse the skin regularly with warm water and mild soap. If Grade 3 dermatitis occurred, EGCG administration was discontinued and additional treatments were given at the physician's discretion.
Toxicity of EGCG was graded using the NCI Common Terminology Criteria for Adverse Events v. 3.0. Any adverse event >Grade 1 attributed to EGCG was considered dose-limiting toxicity (DLT). Three patients were assigned to each dose level. If no DLT was observed, the next level was opened. If the DLT was observed in one of the three patients, three additional patients were accrued at this level. If no more DLT was observed, then the dose was escalated to the next level. If two or more patients at any dose level experienced DLT, there was no further dose escalation. MTD was defined as the dose level that induced DLT in more than one-third of patients in a given cohort. The recommend dose level of EGCG for the Phase II study was defined as that below the level of MTD or the highest concentration if MTD was not observed.
Skin toxicity evaluation
Skin toxicity of radiotherapy was evaluated every day, once radiation began. EGCG administration was given immediately when Grade 1 dermatitis occurred, and then dermatitis was recorded weekly. The score at the end of radiotherapy was the one of the last week of radiotherapy. The evaluation continued until 2 weeks after the end of radiotherapy with two approaches. One was the RTOG score defined by the observers.14 The other was patient-reported symptom scores adapted from the Skin Toxicity Assessment Tool as pain, burning, itching, pulling and tenderness in the treatment area.15
Statistical analysis
SPSS® (v. 17.0; IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) was used for statistical analysis. The differences in the score before, during and after treatment were analysed using paired t-test. A value of p < 0.05 was defined as statistical significance.
RESULTS
34 patients were screened from March 2012 to August 2013. No radiation dermatitis of >Grade 1 was found in nine patients. One patient withdrew informed consent during the treatment. 24 patients completed the course of therapy and were finally analysed. The patients and disease characteristics are listed in Table 1.
Table 1.
Patient demographics and disease characteristics
| Variable | Number of patients (N = 24) | % |
|---|---|---|
| Age (years) | ||
| Median | 44 | |
| Range | 22–63 | |
| Smoking status | ||
| Yes | 6 | 25.0 |
| No | 18 | 75.0 |
| Performance status (ECOG) | ||
| 0 | 10 | 41.7 |
| 1 | 14 | 58.3 |
| Comorbidities | ||
| None | 15 | 62.5 |
| Diabetes | 2 | 8.3 |
| Hypertension | 2 | 8.3 |
| Hyperlipaemia | 3 | 12.5 |
| Coronary heart disease | 1 | 4.2 |
| Arrhythmia | 1 | 4.2 |
| T stage | ||
| T1 | 4 | 16.7 |
| T2 | 14 | 58.3 |
| T3 | 6 | 25.0 |
| N stage | ||
| N1 | 3 | 12.5 |
| N2 | 21 | 87.5 |
| AJCC stage | ||
| IIB | 1 | 4.2 |
| IIIA | 23 | 95.8 |
| Surgery to | ||
| Right breast | 11 | 45.8 |
| Left breast | 13 | 54.2 |
| Histology | ||
| Invasive ductal carcinoma | 22 | 91.7 |
| Invasive lobular carcinoma | 2 | 8.3 |
AJCC, American Joint Commitee on Cancer; ECOG, Eastern Cooperative Oncology Group.
The EGCG dose, treatment period and RTOG skin toxicity scores for each patient are shown in Table 2. The median duration of the EGCG treatment was 4 weeks. The EGCG solution was well tolerated. Acute skin redness extending outside the radiation field was observed immediately after EGCG administration in one patient (140 μmol l−1) at the 4th day, which was considered to be associated with the EGCG. Three more patients were enrolled at this level and no more patient-experienced toxicities of EGCG. The dose escalation stopped at 660 μmol l−1. No other reported acute toxicity was considered to be associated with EGCG. No patient needed dose reduction or delay in radiotherapy because of skin toxicity. MTD was not found, and the highest dose of this Phase I trial (660 μmol l−1) was defined as a recommended dose for the Phase II trial.
Table 2.
Epigallocatechin-3-gallate (EGCG) treatment and radiation dermatitis scoring
| Patient number | BMI | Radiation dose as EGCG treatment | EGCG dose (μmol l−1) | RTOG score |
EGCG treatment time (weeks) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EGCG treatment start | 1 week | 2 weeks | 3 weeks | 4 weeks | 1 week after radiation | 2 weeks after radiation | |||||
| 1 | 24.8 | 30 Gy/15f | 40 | 1 | 1 | 1 | 1 | 1 | 4 | ||
| 2 | 24.7 | 16 Gy/8f | 40 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 6 |
| 3 | 23.8 | 26 Gy/13f | 40 | 1 | 1 | 1 | 1 | 2 | 1 | 5 | |
| 4 | 23.7 | 20 Gy/10f | 80 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 6 |
| 5 | 22.5 | 34 Gy/17f | 80 | 1 | 1 | 1 | 1 | 0 | 4 | ||
| 6 | 18.6 | 40 Gy/20f | 80 | 1 | 1 | 1 | 0 | 3 | |||
| 7 | 23.1 | 28 Gy/14f | 140 | 1 | 0 | 1 | 2 | 2 | 1 | 5 | |
| 8 | 25.6 | 16 Gy/8f | 140 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 6 |
| 9 | 26.4 | 36 Gy/18f | 140 | 1 | 1 | 1 | 1 | 0 | 4 | ||
| 10 | 25.3 | 40 Gy/20f | 140 | 1 | 1 | 2 | 1 | 3 | |||
| 11 | 25.6 | 30 Gy/15f | 140 | 1 | 1 | 1 | 1 | 0 | 4 | ||
| 12 | 32.4 | 40 Gy/20f | 140 | 1 | 1 | 2 | 1 | 3 | |||
| 13 | 22.7 | 36 Gy/18f | 210 | 1 | 1 | 1 | 1 | 3 | |||
| 14 | 29.7 | 34 Gy/17f | 210 | 1 | 1 | 1 | 1 | 0 | 4 | ||
| 15 | 23.8 | 30 Gy/15f | 210 | 1 | 1 | 1 | 1 | 0 | 4 | ||
| 16 | 20.7 | 40 Gy/20f | 300 | 1 | 2 | 2 | 1 | 3 | |||
| 17 | 24.9 | 34 Gy/17f | 300 | 1 | 1 | 1 | 1 | 0 | 4 | ||
| 18 | 21.5 | 30 Gy/15f | 300 | 1 | 1 | 1 | 1 | 0 | 4 | ||
| 19 | 25.4 | 32 Gy/16f | 440 | 1 | 1 | 1 | 1 | 1 | 4 | ||
| 20 | 18.8 | 36 Gy/18f | 440 | 1 | 1 | 1 | 1 | 1 | 4 | ||
| 21 | 24.5 | 32 Gy/16f | 440 | 1 | 1 | 1 | 1 | 1 | 4 | ||
| 22 | 26.7 | 28 Gy/14f | 660 | 1 | 1 | 2 | 2 | 1 | 4 | ||
| 23 | 18.7 | 32 Gy/16f | 660 | 1 | 1 | 1 | 1 | 0 | 4 | ||
| 24 | 30.3 | 22 Gy/11f | 660 | 1 | 1 | 1 | 1 | 1 | 0 | 5 | |
BMI, body mass index; F, fractions; RTOG, Radiation Theraphy Oncology Group.
Grade 1 radiation dermatitis was observed in all the patients, which appeared in four patients at the 2nd week, seven patients at the 3rd week and others at the 4th week. Radiation dermatitis developed into Grade 2 in four patients at the end of radiotherapy (Patient number 4, 7, 16 and 22). Four more patients with Grade 2 dermatitis were found at 1 week after the radiotherapy (Patient number 2, 3, 10 and 12). As the EGCG treatment was performed continuously, all these Grade 2 reactions were decreased to Grade 1 at 2 weeks after the end of radiotherapy. For patients with Grade 1 dermatitis at the end of the radiotherapy, 62.5% (10/16) of patients were scored as Grade 0 and others as Grade 1 at 2 weeks afterwards.
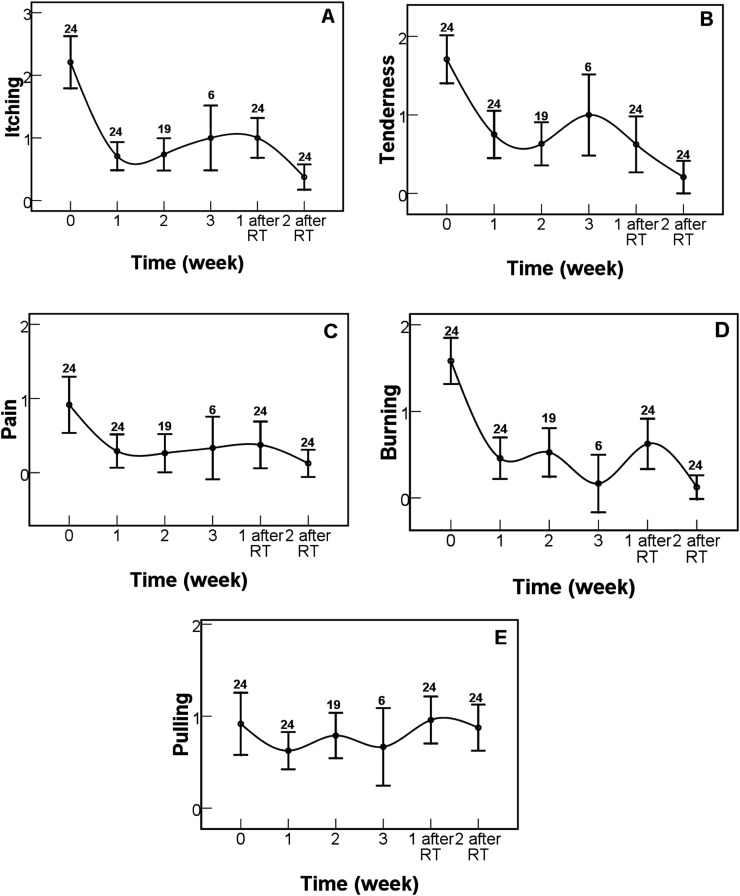
The patient-reported symptoms assessed by the Skin Toxicity Assessment Tool were compared before, during and after the EGCG treatment (Figure 1). Rapid relief after EGCG treatment of 1 week was observed in itching (p < 0.001, Figure 1a), tenderness (p < 0.001, Figure 1b), pain (p < 0.001, Figure 1c), burning (p < 0.001, Figure 1d) and pulling (p = 0.032, Figure 1e). Patient-reported symptom scores were significantly decreased at 2 weeks after the end of radiotherapy as comparison with that at the beginning of the EGCG treatment in itching (p < 0.001), tenderness (p < 0.001), pain (p < 0.001) and burning (p < 0.001). There was no statistically significant difference in pulling symptom scoring (p = 0.840). Since only three patients received the EGCG treatment for 6 weeks, their scores are not shown and compared. The regression of patient-reported symptoms related to acute skin reactions did not seem to correlate with the onset time and the dose of EGCG.
Figure 1.
Changes in patient-reported symptom scores during and after the treatments. (a) Itching, (b) tenderness, (c) pain, (d) burning and (e) pulling. Error bars represent standard error of the mean. Numbers indicate how many patients were analysed. RT, radiotherapy.
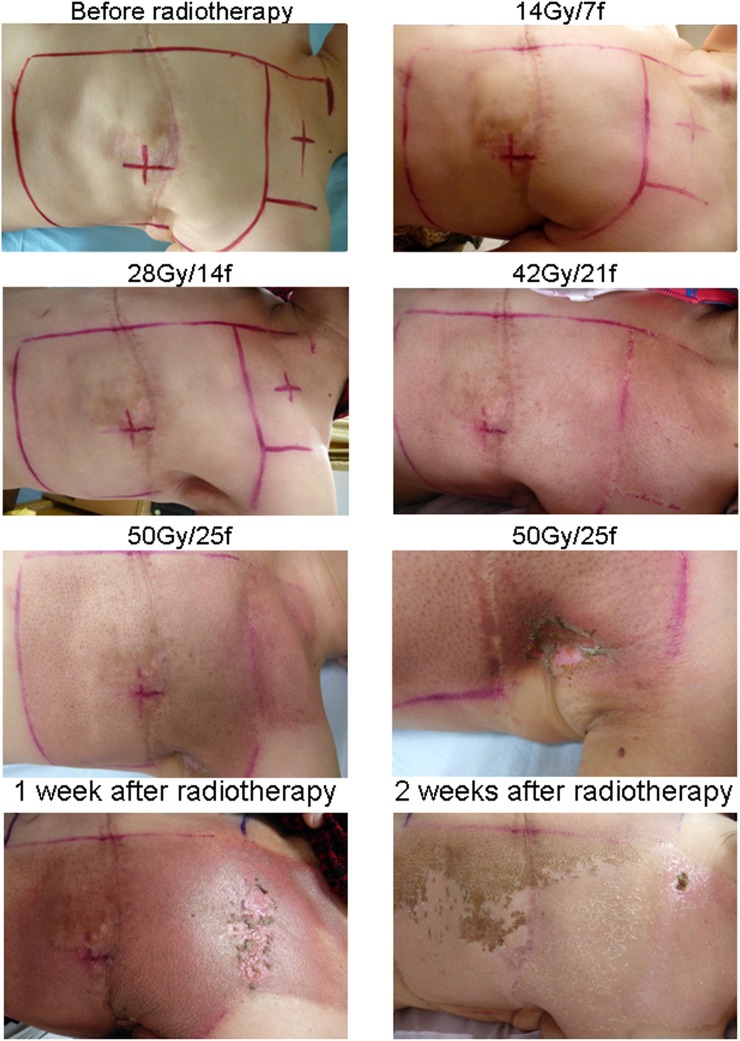
One representative case is shown in Figure 2. The patient (Patient number 22, pT2N2M0) developed Grade 1 radiation dermatitis at 28 Gy/14 fractions of radiotherapy and started receiving the EGCG treatment at 660 μmol l−1 dose level. The dermatitis was scored as Grade 2 at the end of radiotherapy (50 Gy/25 fractions) and was decreased to Grade 1 at 2 weeks after the end of radiotherapy. Total EGCG treating time was 4 weeks.
Figure 2.
A representative case receiving epigallocatechin-3-gallate treatment at 660 μmol l−1 (patient number 22).
DISCUSSION
In this Phase I trial, we found that topical EGCG for patients with breast cancer during adjuvant radiotherapy was well tolerated. No DLT was observed, and no other obvious adverse effect was observed to be related to topical EGCG treatments. Therefore, the MTD was not defined, and the highest dose tested (660 μmol l−1) was defined as a recommended dose in further Phase II study. In terms of efficacy, we chose to begin the application of EGCG only once Grade 1 dermatitis occurred in order to find its therapeutic effect easily.16 Most patients only suffered Grade 1 toxicity according to the RTOG criteria and all Grade 2 dermatitis were decreased to Grade 1 with EGCG treatments at 2 weeks after the end of radiotherapy. More importantly, relief of symptoms of radiation dermatitis was observed in most of the patients after EGCG treatments. This study may provide first-hand clinical evidence for topical EGCG treatment to minimize radiation dermatitis.
Table 3 lists the randomized studies on the prevention of acute radiation skin reactions in patients with breast cancer receiving radiotherapy in last 5 years.4,17–28 Randomized controlled studies have generated mostly negative results for use of skin care products in preventing or treating radiation dermatitis. Remarkably, patients receiving topical corticosteroids (mometasone furoate and betamethasone) during radiotherapy might experience reduced acute skin toxicity compared with placebo or moisturizing creams according to the result of three double-blind studies with a larger population. The symptoms of radiodermatitis were also alleviated sometimes owing to the effect of corticosteroids.18,22,27 However, the side effects of corticosteroids such as periorificial dermatitis, skin atrophy and mycotic infection had to be taken into account in clinical practice. Therefore, studies investigating new and more efficient treatments to prevent or treat radiation skin toxicity are increasing. A retrospective study showed that a skin care programme containing topically applied tea extracts for radiation-induced Grade ≥2 skin lesions helped to restore skin integrity in the head and neck and pelvic regions.29 It suggested that the higher content of EGCG accounted for the shorter duration of Grade ≥2 toxicity in patients treated with green tea extracts.29 However, black/green tea bag in water following filtering was used in that trial.29 Therefore, the safety and efficacy of EGCG in treating radiation dermatitis warranted systematic prospective studies.
Table 3.
Study descriptions and outcomes of trials on the prevention of acute radiation skin reactions in patients with breast cancer receiving radiotherapy
| Study | Number | Treatment arms | Study design | End point and scale name | Outcomes | |
|---|---|---|---|---|---|---|
| Rollmann et al17 | 42 | Ultra emu oil vs placebo | 2 : 1, randomized, double blind | The AUC of Skindex-16 scale scores |
No differences | |
| Maximum grade of radiation dermatitis using CTCAE v. 3.0; QL assessed with a questionnaire |
||||||
| Hindley et al18 | 112 | Mometasone furoate vs diprobase | Randomized, double blind | Mean RTOG score and DLQI |
Significant difference (p=0.046) | |
| Lewis et al19 | 333 | Aluminium-containing deodorant vs non-aluminium containing deodorant vs control | 3 arm | Objective measurements: axilla sweating (Hyperhidrosis Disease Severity Scale), skin toxicity (RTOG), pain, itching and burning using VAS |
No differences | |
| Randomized, controlled | QL assessed with QLQ-C30 |
|||||
| Chan et al20 | 174 | Natural oil-based emulsion containing allantoin vs aqueous cream | Randomized | Skin toxicity using CTCAE v. 3.0 |
No differences | |
| Skin-related QL scores |
||||||
| Herst et al21 | 78 | Mepitel film vs aqueous cream | Intrapatient, randomized | Skin reaction severity was assessed using RISRAS and RTOG scales |
Significant difference (p < 0.001) | |
| Ulff et al22 | 104 | Betamethasone vs Essex®vs Canoderm® | 2 : 1 : 1 | Dermatitis measured with RTOG score and by colorimeter |
Significant difference (p=0.05) in RTOG score | |
| Randomized, double blind | Symptoms using VAS |
No differences in symptoms | ||||
| Sharp et al23 | 411 | Calendula Weleda cream vs Essex® cream | Randomized, double blind | The difference in proportion of patients with ARSR, assessed with RTOG score |
No differences | |
| Patient-reported outcome measure: QLQ-C30, sleep disturbances (MOS sleep questionnaire) and symptoms (VAS) |
||||||
| Pinnix et al4 | 74 | Topical hyaluronic acid vs standard skin care | Single blind, randomized, Phase III | ≥Grade 2 radiation dermatitis according to CTCAE v. 3.0 |
No differences | |
| Hemati et al24 | 102 | Topical silver sulfadiazine vs standard skin care | Randomized, double blind, controlled |
The severity of radiation dermatitis according to RTOG score | Significant difference (p < 0.001) | |
| Kirova et al25 | 190 | Hyaluronic acid vs placebo | Randomized |
The clinical evaluation of erythema according to RTOG score | No differences | |
| Skin colourimetry performed with a chromameter (Minolta CR300), pain with VAS and QL measured by QLQ-C30 | ||||||
| Jensen et al26 | 66 | Oil-in-water emulsion vs untreated | Randomized, controlled |
Clinical scoring and pruritus (ONS radiation skin reaction scoring) | Significant difference (p < 0.05) in pruritus | |
| Biophysical measurements (Corneometer® and the Tewameter® TM210) | No difference in ONS scores and biophysical measurements | |||||
| Miller et al27 | 176 | Mometasone furoate vs placebo | Phase III, randomized, double blind, controlled |
Provider-assessed maximal grade of radiation dermatitis (CTCAE v. 3.0) | No difference in the mean maximum grade of radiation dermatitis | |
| Provider-assessed ≥Grade 3 radiation dermatitis and adverse event monitoring; the patient-reported outcome measures included the Skindex-16, Skin Toxicity Assessment Tool, Symptom Experience Diary and QL self-assessment. | Significant difference in ≥Grade 3 (p = 0.04) and patient-reported outcome measures (p < 0.05) | |||||
| Gosselin et al28 | 208 | Aquaphor®vs Biafine® RE vs RadiaCare™ vs placebo | Randomized, double blind, controlled | ≥Grade 2 radiation dermatitis according to RTOG scales participant self-assessment | No differences |
ARSR, acute radiation skin reaction; AUC, area under the curve; CTCAE, Common Terminology Criteria for Adverse Events; DLQI, the dermatology life quality index score; MOS, Medical Outcomes Study; QL, quality of life; QLQ-C30, Quality of Life Questionnaire; RISRAS, radiation-induced skin reaction assessment scale; RTOG, Radiation Therapy Oncology Group; VAS, visual analogue scale.
Green tea as a beverage is generally regarded as safe, and the oral administration of EGCG is available commercially as a dietary supplement. The reported complications associated with oral EGCG in health cohort were mild, such as in Grade 1, excess gas, upset stomach, nausea, heartburn, stomach ache, abdominal pain, dizziness, headache and muscle pain.30 Recently, our clinical study showed that oral administration of EGCG was feasible and safe in treating oesophagitis during concurrent chemoradiotherapy in patients with unresectable Stage III non-small-cell lung cancer.31 MTD was not found in that Phase I trial, and the highest dose was defined as 440 μmol l−1 escalated from 40 μmol l−1. In the present study, the daily total dose is up to 18 mg, which is much lower than that in previous studies.30 The optimal dose/concentration of EGCG solution in clinical setting may be at relatively wide range and warrants further study.
EGCG has a scavenging activity for superoxide anion, hydroxyl radical and hydrogen peroxide.32,33 It can defend the DNA against radiation injury by intercalating into the DNA, binding to the free radicals or repairing the damage due to free radicals. Inhibition of the proteasome, a key regulator of inflammation, by EGCG has also been reported earlier.34 Green extracts inhibited cleavage activities of the proteasome in vitro and caused a significant decrease in the release of the proinflammatory cytokines interleukin-1β, interleukin-6, interleukin-8, tumour necrosis factor-α and prostaglandin E in vivo. In addition, tea polyphenols have been shown to modulate nuclear factor-κ gene binding activity through the p38 mitogen-activated protein kinase pathway and direct inhibition of inhibitory κ B alpha kinases.29,35,36 However, the EGCG molecular mechanisms underlying the beneficial effects in acute radiation-induced skin toxicity are complex and involve antibacterial and anti-inflammatory processes.29 Molecular mechanism study is being incorporated in a further Phase II trial.
Susceptibility to degradation in water solution might hinder the routine use of EGCG in treating radiation dermatitis. Therefore, in the present study, the EGCG solution was prepared freshly before each administration. It was reported that epimerization processes of EGCG, the main reaction occurring in the EGCG degradation, did not significantly alter the antioxidant activity, absorption and metabolism of the original catechins.37 In addition, it was proven that the catechin product released from the EGCG degradation, namely gallocatechin gallate, was more effective in reducing plasma cholesterol and triglyceride concentrations than EGCG.38,39 Some previous studies also suggested that lower storage temperature, lower pH and using a reducing agent could provide a better storage and stability for EGCG.40 All these information were helpful in developing oral/topical agents for clinical usage and biopharmaceutical studies.
There were some limitations in the study. Firstly, the radiation dosimetry of the chest wall could not be confirmed without three-dimensional planning radiotherapy. Therefore, the Phase II/III study aimed at efficacy assessment would use some techniques to provide improved target homogeneity and conformality. Secondly, self-resolution of radiation dermatitis might also attribute to the result of promising activity. The prospective randomized, placebo-controlled design is warranted in the further study.
CONCLUSION
Based on clinical data from this trial, topical administration of EGCG solution seems to be feasible for treating radiation dermatitis in patients with breast cancer after mastectomy. Phase II studies are under way to assess the efficacy of EGCG solution in the treatment of radiation dermatitis for patients after mastectomy or breast-conservative surgery. Randomized controlled trials will establish how applicable our findings are to other populations of patients with cancer receiving radiotherapy.
Acknowledgments
ACKNOWLEDGMENTS
A part of this work was presented at the 2014 Annual Meeting of the American Society of Radiation Oncology (ASTRO), San Francisco, CA, USA. This study was supported by the Shandong Provincial key scientific and technological project of China No.2015GSF118132.
Contributor Information
Hanxi Zhao, Email: Zhaoxg2011@163.com.
Wanqi Zhu, Email: wanqi5145@126.com.
Li Jia, Email: 34577559@qq.com.
Xiaorong Sun, Email: yujmwin@126.com.
Guanxuan Chen, Email: 22253780@qq.com.
Xianguang Zhao, Email: Zhaoxg2011@163.com.
Xiaolin Li, Email: daisylinjinan@163.com.
Xiangjiao Meng, Email: mengxiangjiao@126.com.
Lingling Kong, Email: cloverkll@126.com.
Ligang Xing, Email: wanqi5145@163.com.
Jinming Yu, Email: sdyujinming@126.com.
REFERENCES
- 1.Lilla C, Ambrosone CB, Kropp S, Helmbold I, Schmezer P, von Fournier D, et al. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res Treat 2007; 106: 143–50. doi: 10.1007/s10549-006-9480-9 [DOI] [PubMed] [Google Scholar]
- 2.Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 2008; 26: 2085–92. doi: 10.1200/JCO.2007.15.2488 [DOI] [PubMed] [Google Scholar]
- 3.Azria D, Belkacemi Y, Romieu G, Gourgou S, Gutowski M, Zaman K, et al. Concurrent or sequential adjuvant letrozole and radiotherapy after conservative surgery for early-stage breast cancer (CO-HO-RT): a phase 2 randomised trial. Lancet Oncol 2010; 11: 258–65. doi: 10.1016/S1470-2045(10)70013-9 [DOI] [PubMed] [Google Scholar]
- 4.Pinnix C, Perkins GH, Strom EA, Tereffe W, Woodward W, Oh JL, et al. Topical hyaluronic acid vs. standard of care for the prevention of radiation dermatitis after adjuvant radiotherapy for breast cancer: single-blind randomized phase III clinical trial. Int J Radiat Oncol Biol Phys 2012; 83: 1089–94. doi: 10.1016/j.ijrobp.2011.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan RJ, Larsen E, Chan P. Re-examining the evidence in radiation dermatitis management literature: an overview and a critical appraisal of systematic reviews. Int J Radiat Oncol Biol Phys 2012; 84: e357–62. doi: 10.1016/j.ijrobp.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 6.Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys 2000; 48: 1307–10. doi: 10.1016/S0360-3016(00)00782-3 [DOI] [PubMed] [Google Scholar]
- 7.Graham PH, Plant N, Graham JL, Browne L, Borg M, Capp A, et al. A paired, double-blind, randomized comparison of a moisturizing durable barrier cream to 10% glycerine cream in the prophylactic management of postmastectomy irradiation skin care: trans Tasman Radiation Oncology Group (TROG) 04.01. Int J Radiat Oncol Biol Phys 2013; 86: 45–50. doi: 10.1016/j.ijrobp.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka H, Kurosaki H, Yoshinaga K, Saito K, Yoshioka H. Beta ray-induced scission of DNA in tritiated water and protection by a green tea percolate and (-)-epigallocatechin gallate. Biosci Biotechnol Biochem 1997; 61: 1560–3. doi: 10.1271/bbb.61.1560 [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Cao JJ, Liu P, Guo DH, Wang YP, Yin J, et al. Protective role of tea polyphenols in combination against radiation-induced haematopoietic and biochemical alterations in mice. Phytother Res 2011; 25: 1761–9. doi: 10.1002/ptr.3483 [DOI] [PubMed] [Google Scholar]
- 10.Peng Z, Xu ZW, Wen WS, Wang RS. Tea polyphenols protect against irradiation-induced injury in submandibular glands’ cells: a preliminary study. Arch Oral Biol 2011; 56: 738–43. doi: 10.1016/j.archoralbio.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 11.Elmets CA, Singh D, Tubesing K, Matsui M, Katiyar S, Mukhtar H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol 2001; 44: 425–32. doi: 10.1067/mjd.2001.112919 [DOI] [PubMed] [Google Scholar]
- 12.Kim HK, Choi SY, Chang HK, Baek SY, Chung JO, Rha CS, et al. Human skin safety test of green tea cell extracts in condition of allergic contact dermatitis. Toxicol Res 2012; 28: 113–16. doi: 10.5487/TR.2012.28.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gez E, Assaf N, Bar-Deroma R, Rosenblatt E, Kuten A. Postmastectomy electron-beam chest-wall irradiation in women with breast cancer. Int J Radiat Oncol Biol Phys 2004; 60: 1190–4. doi: 10.1016/j.ijrobp.2004.05.036 [DOI] [PubMed] [Google Scholar]
- 14.López E, Núñez MI, Guerrero MR, del Moral R, de Dios Luna J, del Mar Rodríguez M, et al. Breast cancer acute radiotherapy morbidity evaluated by different scoring systems. Breast Cancer Res Treat 2002; 73: 127–34. doi: 10.1023/A:1015296607061 [DOI] [PubMed] [Google Scholar]
- 15.Neben-Wittich MA, Atherton PJ, Schwartz DJ, Sloan JA, Griffin PC, Deming RL, et al. Comparison of provider-assessed and patient-reported outcome measures of acute skin toxicity during a Phase III trial of mometasone cream versus placebo during breast radiotherapy: the North Central Cancer Treatment Group (N06C4). Int J Radiat Oncol Biol Phys 2011; 81: 397–402. doi: 10.1016/j.ijrobp.2010.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott EA, Wright JR, Swann RS, Nguyen-Tân F, Takita C, Bucci MK, et al. ; Radiation Therapy Oncology Group Trial 99-13. Phase III Trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Trial 99-13. J Clin Oncol 2006; 24: 2092–7. doi: 10.1200/JCO.2005.04.9148 [DOI] [PubMed] [Google Scholar]
- 17.Rollmann DC, Novotny PJ, Petersen IA, Garces YI, Bauer HJ, Yan ES, et al. Double-blind, placebo-controlled pilot study of processed ultra emu oil versus placebo in the prevention of radiation dermatitis. Int J Radiat Oncol Biol Phys 2015; 92: 650–8. doi: 10.1016/j.ijrobp.2015.02.028 [DOI] [PubMed] [Google Scholar]
- 18.Hindley A, Zain Z, Wood L, Whitehead A, Sanneh A, Barber D, et al. Mometasone furoate cream reduces acute radiation dermatitis in patients receiving breast radiation therapy: results of a randomized trial. Int J Radiat Oncol Biol Phys 2014; 90: 748–55. doi: 10.1016/j.ijrobp.2014.06.033 [DOI] [PubMed] [Google Scholar]
- 19.Lewis L, Carson S, Bydder S, Athifa M, Williams AM, Bremner A. Evaluating the effects of aluminum-containing and non-aluminum containing deodorants on axillary skin toxicity during radiation therapy for breast cancer: a 3-armed randomized controlled trial. Int J Radiat Oncol Biol Phys 2014; 90: 765–71. doi: 10.1016/j.ijrobp.2014.06.054 [DOI] [PubMed] [Google Scholar]
- 20.Chan RJ, Mann J, Tripcony L, Keller J, Cheuk R, Blades R, et al. Natural oil-based emulsion containing allantoin versus aqueous cream for managing radiation-induced skin reactions in patients with cancer: a phase 3, double-blind, randomized, controlled trial. Int J Radiat Oncol Biol Phys 2014; 90: 756–64. doi: 10.1016/j.ijrobp.2014.06.034 [DOI] [PubMed] [Google Scholar]
- 21.Herst PM, Bennett NC, Sutherland AE, Peszynski RI, Paterson DB, Jasperse ML. Prophylactic use of Mepitel Film prevents radiation-induced moist desquamation in an intra-patient randomised controlled clinical trial of 78 breast cancer patients. Radiother Oncol 2014; 110: 137–43. doi: 10.1016/j.radonc.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 22.Ulff E, Maroti M, Serup J, Falkmer U. A potent steroid cream is superior to emollients in reducing acute radiation dermatitis in breast cancer patients treated with adjuvant radiotherapy. A randomised study of betamethasone versus two moisturizing creams. Radiother Oncol 2013; 108: 287–92. doi: 10.1016/j.radonc.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 23.Sharp L, Finnilä K, Johansson H, Abrahamsson M, Hatschek T, Bergenmar M. No differences between Calendula cream and aqueous cream in the prevention of acute radiation skin reactions–results from a randomised blinded trial. Eur J Oncol Nurs 2013; 17: 429–35. doi: 10.1016/j.ejon.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 24.Hemati S, Asnaashari O, Sarvizadeh M, Motlagh BN, Akbari M, Tajvidi M, et al. Topical silver sulfadiazine for the prevention of acute dermatitis during irradiation for breast cancer. Support Care Cancer 2012; 20: 1613–18. doi: 10.1007/s00520-011-1250-5 [DOI] [PubMed] [Google Scholar]
- 25.Kirova YM, Fromantin I, De Rycke Y, Fourquet A, Morvan E, Padiglione S, et al. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy? Results of phase III randomised trial. Radiother Oncol 2011; 100: 205–9. doi: 10.1016/j.radonc.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 26.Jensen JM, Gau T, Schultze J, Lemmnitz G, Fölster-Holst R, May T, et al. Treatment of acute radiodermatitis with an oil-in-water emulsion following radiation therapy for breast cancer: a controlled, randomized trial. Strahlenther Onkol 2011; 187: 378–84. doi: 10.1007/s00066-011-2224-8 [DOI] [PubMed] [Google Scholar]
- 27.Miller RC, Schwartz DJ, Sloan JA, Griffin PC, Deming RL, Anders JC, et al. Mometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4. Int J Radiat Oncol Biol Phys 2011; 79: 1460–6. doi: 10.1016/j.ijrobp.2010.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosselin TK, Schneider SM, Plambeck MA, Rowe K. A prospective randomized, placebo-controlled skin care study in women diagnosed with breast cancer undergoing radiation therapy. Oncol Nurs Forum 2010; 37: 619–26. doi: 10.1188/10.ONF.619-626 [DOI] [PubMed] [Google Scholar]
- 29.Pajonk F, Riedisser A, Henke M, McBride WH, Fiebich B. The effects of tea extracts on proinflammatory signaling. BMC Med 2006; 4: 28. doi: 10.1186/1741-7015-4-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks JA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res 2003; 9: 3312–19. [PubMed] [Google Scholar]
- 31.Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun X, et al. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiother Oncol 2014; 110: 132–6. doi: 10.1016/j.radonc.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 32.Richi B, Kale RK, Tiku AB. Radio-modulatory effects of green tea catechin EGCG on pBR322 plasmid DNA and murine splenocytes against gamma-radiation induced damage. Mutat Res 2012; 747: 62–70. doi: 10.1016/j.mrgentox.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 33.Mitrica R, Dumitru I, Ruta LL, Ofiteru AM, Farcasanu IC. The dual action of epigallocatechin gallate (EGCG), the main constituent of green tea, against the deleterious effects of visible light and singlet oxygen-generating conditions as seen in yeast cells. Molecules 2012; 17: 10355–69. doi: 10.3390/molecules170910355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem 2001; 276: 13322–30. doi: 10.1074/jbc.M004209200 [DOI] [PubMed] [Google Scholar]
- 35.Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol 2001; 60: 528–33. [PubMed] [Google Scholar]
- 36.Pan MH, Lin-Shiau SY, Ho CT, Lin JH, Lin JK. Suppression of lipopolysaccharide-induced nuclear factor-kappaB activity by theaflavin-3,3'-digallate from black tea and other polyphenols through down-regulation of IkappaB kinase activity in macrophages. Biochem Pharmacol 2000; 59: 357–67. doi: 10.1016/S0006-2952(99)00335-4 [DOI] [PubMed] [Google Scholar]
- 37.Xu JZ, Yeung SY, Chang Q, Huang Y, Chen ZY. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br J Nutr 2004; 91: 873–81. doi: 10.1079/BJN20041132 [DOI] [PubMed] [Google Scholar]
- 38.Ikeda I, Kobayashi M, Hamada T, Tsuda K, Goto H, Imaizumi K, et al. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate. J Agric Food Chem 2003; 51: 7303–7. doi: 10.1021/jf034728l [DOI] [PubMed] [Google Scholar]
- 39.Lee SM, Kim CW, Kim JK, Shin HJ, Baik JH. GCG-rich tea catechins are effective in lowering cholesterol and triglyceride concentrations in hyperlipidemic rats. Lipids 2008; 43: 419–29. doi: 10.1007/s11745-008-3167-4 [DOI] [PubMed] [Google Scholar]
- 40.Fangueiro JF, Parra A, Silva AM, Egea MA, Souto EB, Garcia ML, et al. Validation of a high performance liquid chromatography method for the stabilization of epigallocatechin gallate. Int J Pharm 2014; 475: 181–90. doi: 10.1016/j.ijpharm.2014.08.053 [DOI] [PubMed] [Google Scholar]