Abstract
Objective:
We aimed to investigate whether the standardized uptake values, volumetric parameters and intratumoral heterogeneity of fluorine-18-fludeoxyglucose (18F-FDG) uptake could predict regional lymph node (rLN) metastasis in oesophageal cancer.
Methods:
51 patients with surgically resected oesophageal cancer were included in the present study. The 18F-FDG positron emission tomography (PET)/CT findings and rLN metastasis were compared with the histopathological results. The intratumoral metabolic heterogeneity was represented by the heterogeneity factor (HF), which was determined for each patient. Univariate and multivariate analyses were used to analyse the associations between the rLN metastasis and clinical findings, standardized uptake values, metabolic tumour volume (MTV), total lesion glycolysis (TLG) and HF.
Results:
The rLN(+) group showed statistically significant higher values of MTV (median, 13.59 vs 6.6; p = 0.0085), TLG (median, 119.18 vs 35.96; p = 0.0072) and HF (median, 3.07 vs 2.384; p = 0.0002) than the rLN(−) group. Univariate analysis showed that maximum standardized uptake value, mean standardized uptake value, MTV, TLG and HF were significantly associated with pathologic rLN involvement. However, in multivariate analysis, the HF was a potent associated factor for the prediction of pathologic rLN metastasis in oesophageal cancer.
Conclusion:
In conclusion, 18F-FDG PET/CT parameters such as maximum standardized uptake value, mean standardized uptake value, MTV, TLG and HF were useful for the prediction of pathologic rLN status in patients with oesophageal cancer. However, HF might be the most powerful predictor of rLN metastasis of patients with oesophageal cancer.
Advances in knowledge:
Assessment of intratumoral heterogeneity of 18F-FDG PET/CT may be a useful adjunct for rLN staging of oesophageal cancer.
INTRODUCTION
Oesophageal cancer is a high-grade malignancy with a poor prognosis. In the USA, approximately 16,980 new cases of oesophageal cancer are diagnosed each year; the estimated deaths were 15,590 in 2015.1 The 5-year survival rate is <10% for patients suffering from oesophageal cancer, and showed poor prognosis.
Accurate stage and evaluation of the disease extent is fundamental to determine resectability and overall prognosis. Also, determination of regional lymph node (rLN) metastasis is necessary to determine operability of disease.2 Locoregional recurrence after resection is attributed to lymph node (LN) involvement in approximately 40% of patients.3
Accurate assessment of rLN in oesophageal cancer is more complex but essential for selecting appropriate treatments and forecasting disease progression. Current imaging modalities for pre-operative characterization are CT, endoscopic ultrasonography and fluorine-18-fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT.4 Although PET using 2-deoxy-2-18F-FDG has been reported to be a promising functional imaging technique for cancer detection, previous studies showed that 18F-FDG PET/CT has a limited role in the identification of early rLN metastasis but is highly useful for detecting distant metastasis.5 Also, diagnosing rLN metastasis is often difficult from its size and maximum standardized uptake value (SUVmax).6
Recently, several new 18F-FDG PET/CT parameters including metabolic tumour volume (MTV), total lesion glycolysis (TLG) and intratumoural heterogeneity of FDG uptake represented by heterogeneity factor (HF) were developed to predict prognosis in various cancers.7–12 Although these newly described 18F-FDG PET/CT parameters seemed to be effective for predicting prognosis of various patients with cancer, no study has attempted to adapt and to compare these parameters for the evaluation of rLN status of patients with oesophageal cancer.
The aim of the present study was to investigate whether these various 18F-FDG PET/CT parameters could predict malignant rLN status and to compare the diagnostic accuracies in patients with oesophageal cancer.
METHODS AND MATERIALS
Patients
The present retrospective study included 51 patients with oesophageal cancer (49 males and 2 females; age range, 51–80 years; median, 69 years old) who underwent surgical treatment. All patients were checked for 18F-FDG PET/CT for initial staging work-up. For staging, physical examination, routine laboratory tests, oesophagogastroduodenoscopy, bonchoscopy and CT from the neck to pelvis were performed in all patients. All patients underwent CT within 1 week of 18F-FDG PET/CT. The exclusion criteria were patients who: (1) received previous neoadjuvant chemoradiotherapy, (2) had cancer of oesophagogastric junction, (3) had previous history of other cancer, (4) who refused operation and (5) had histology other than squamous cell carcinoma.
The 18F-FDG PET/CT findings of oesophageal cancer and rLN status were compared with the pathologic findings within 6 weeks after surgical treatment. The study protocol was approved by the institutional review board and written informed consent was waived for retrospective character of the present study.
Fluorine-18-fludeoxyglucose positron emission tomography/CT imaging
18F-FDG PET/CT images were obtained using a Biograph40 (Siemens Healthcare, Knoxville, TN). Standard patient preparation included a fasting period of at least 8 h and a serum glucose level <6.7 mmol l−1 (120 mg dl−1) before 18F-FDG injection. PET/CT imaging was performed 60 min after injection of 18F-FDG (5 MBq kg−1 of body weight). The emission scan time per bed position was 2 min 30 s, and six bed positions were acquired. The average total PET/CT examination was 20 min.
Fluorine-18-fludeoxyglucose positron emission tomography/CT image analysis
The 18F-FDG PET/CT images were reviewed by two experienced nuclear medicine physicians, and any disagreement was resolved by consensus. To calculate SUVmax, manually defined circular region of interest were drawn on tumour. The MTV was determined as the total number of voxels with the threshold standardized uptake value (SUV) of ≥40% of the SUVmax in the volume of interest. The TLG was calculated as the MTV multiplied by its mean standardized uptake value (SUVmean).
Measurement of heterogeneity factor
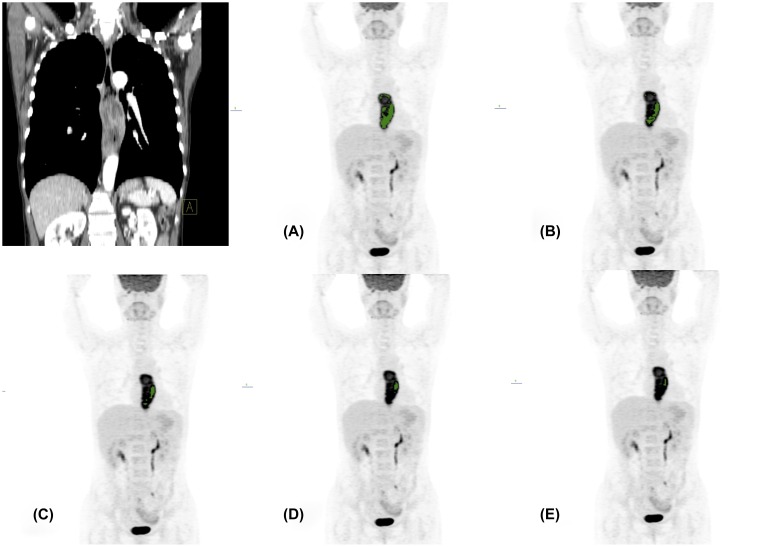
A region of interest was placed to include the primary tumour and a surrounding region of normal tissue to determine the HF.13 The MTVs were calculated with several SUV thresholds. The SUV thresholds of <40% and >80% were not included in the HF calculation.8,14 Linear regression was performed, and the HF was calculated by finding the derivative (dV dT−1). Because the HF values pose negative values as shown in Figure 1, the calculated HF values were modified into absolute values.
Figure 1.
Representative case of a 56-year-old male patient without regional lymph node metastasis. The metabolic tumour volumes (MTVs) are decreasing from 40% to 80% (a–e) threshold of maximum standardized uptake value (SUVmax). The MTVs were 11.08, 4.76, 2.43, 1.12 and 0.65 cm3 from 40% to 80% thresholds of SUVmax. The calculated slope was −0.245 and heterogeneity factor was 0.245.
Statistical analysis
Statistical analyses were performed using commercially available software v. 14.2 (MedCalc, Mariakerke, Belgium). Receiver-operating characteristic (ROC) curves of 18F-FDG PET/CT parameters were calculated and evaluated by comparing the areas under the curves. The sensitivity and specificity of each parameter were determined at the optimal cut-off values. The χ2 test, Fisher's exact test and Mann–Whitney U test were used to analyse statistical differences in categorical data, 18F-FDG PET/CT parameters between rLN as appropriate. Univariate analysis was used to analyse the associations between the pathologic rLN status and clinical characteristics and tumoral features, and 18F-FDG PET/CT parameters. Multivariate analysis was performed with logistic multivariate analysis to assess the joint effects and interactions of the variables on rLN involvement. Variables with p < 0.1 in univariate analysis were included in a multivariate analysis. Statistical significance was defined as p < 0.05.
RESULTS
Patient characteristics
Table 1 shows the characteristics of the present cohort. rLN metastases were present in 23 of 51 (45.1%) patients. The rLN metastasis was associated with American Joint Commitee on Cancer stage, MTV, TLG and HF. Figure 1 shows CT and 18F-FDG PET images for the calculation of HF (volume-threshold graph).
Table 1.
Characteristics of patients
| Variables | Lymph node status |
p-value | |
|---|---|---|---|
| Positive number (%) | Negative number (%) | ||
| Age (years) | 0.6217 | ||
| >69 | 9 (39.1) | 14 (60.9) | |
| ≤69 | 14 (50) | 14 (50) | |
| Sex | 1 | ||
| Male | 21 (45.7) | 25 (54.3) | |
| Female | 2 (40) | 3 (60) | |
| T stage | 0.3059 | ||
| T1 | 4 (36.4) | 7 (63.6) | |
| T2 | 2 (22.2) | 7 (77.8) | |
| T3 | 15 (53.6) | 13 (46.4) | |
| T4 | 2 (66.7) | 1 (33.3) | |
| AJCC stage | <0.0001 | ||
| I | 0 (0) | 7 (100) | |
| II | 6 (23.1) | 20 (76.9) | |
| III | 17 (94.4) | 1 (5.6) | |
| Location | 0.7022 | ||
| Upper | 5 (50) | 5 (50) | |
| Middle | 10 (50) | 10 (50) | |
| Lower | 8 (38.1) | 13 (61.9) | |
| Differentiation | 0.2755 | ||
| Well | 12 (52.2) | 11 (47.8) | |
| Moderate | 8 (50) | 8 (50) | |
| Poor | 3 (25) | 9 (75) | |
| SUVmax | 0.0694 | ||
| >12.2 | 15 (60) | 10 (40) | |
| ≤12.2 | 8 (30.8) | 18 (69.2) | |
| SUVmean | 0.2103 | ||
| >7.15 | 14 (56) | 11 (44) | |
| ≤7.15 | 9 (34.6) | 17 (65.4) | |
| MTV (cm3) | 0.0174 | ||
| >7.3 | 16 (64) | 9 (36) | |
| ≤7.3 | 7 (26.9) | 19 (73.1) | |
| TLG | 0.0174 | ||
| >60.17 | 16 (64) | 9 (36) | |
| ≤60.17 | 7 (26.9) | 19 (73.1) | |
| HF | 0.0033 | ||
| >2.705 | 17 (68) | 8 (32) | |
| ≤2.705 | 6 (23.1) | 20 (76.9) | |
AJCC, American Joint Commitee on Cancer; HF, heterogeneity factor; MTV, metabolic tumour volume; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TLG, total lesion glycolysis.
Comparison of 18F-FDG positron emission tomography/CT parameters
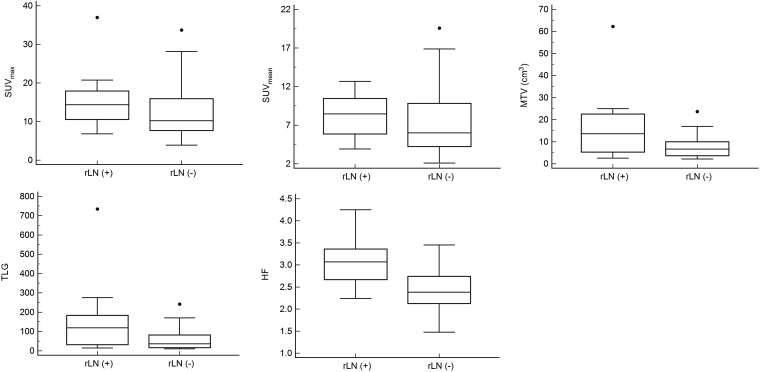
Figure 2 demonstrates the differences of 18F-FDG PET/CT parameters according to pathologic rLN status. In the rLN(+) group, the MTV (median, 13.59 vs 6.6; p = 0.0085), TLG (median, 119.18 vs 35.96; p = 0.0072) and HF (median, 3.07 vs 2.384; p = 0.0002) were significantly higher than the rLN(−) group. However, the SUVmax (median, 14.36 vs 10.19; p = 0.0584) and SUVmean (median, 8.46 vs 6; p = 0.1161) showed no statistical differences.
Figure 2.
The differences of fluorine-18-fludeoxyglucose positron emission tomography/CT parameters according to pathologic regional lymph node (rLN) status. Dots indicate outsider values. HF, heterogeneity factor; MTV, metabolic tumour volume; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TLG, total lesion glycolysis.
Prediction of regional lymph node metastasis
Table 2 summarizes the results of ROC analyses. The SUVmax [area under curve (AUC), 0.655; 95% confidence interval (CI), 0.509–0.783; p = 0.0484], MTV (AUC, 0.716; 95% CI, 0.572–0.833; p = 0.0039), TLG (AUC, 0.720; 95% CI, 0.577–0.837; p = 0.0023) and HF (AUC, 0.811; 95% CI, 0.676–0.907; p < 0.0001) were associated with rLN metastasis.
Table 2.
Receiver-operating characteristic curve analyses for determination of pathologic regional lymph node status
| Variables | Sensitivity (%) | Specificity (%) | PLR | NLR | AUC | SE | p-value |
|---|---|---|---|---|---|---|---|
| SUVmax > 10.28 | 82.6 | 53.5 | 1.78 | 0.32 | 0.655 | 0.0787 | 0.0484 |
| SUVmean > 4.68 | 91.3 | 42.8 | 1.6 | 0.2 | 0.629 | 0.0799 | 0.1068 |
| MTV > 11.14 cm3 | 56.5 | 85.7 | 3.96 | 0.51 | 0.716 | 0.0747 | 0.0039 |
| TLG > 94.46 | 60.8 | 82.1 | 3.41 | 0.48 | 0.720 | 0.0724 | 0.0023 |
| HF > 2.762 | 73.9 | 82.1 | 4.14 | 0.32 | 0.811 | 0.0604 | <0.0001 |
AUC, area under curve; HF, heterogeneity factor; MTV, metabolic tumour volume; NLR, negative likelihood ratio; PLR, positive likelihood ratio; SE, standard error; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TLG, total lesion glycolysis.
Comparison of receiver-operating characteristic curves
Table 3 shows pairwise comparison of ROC analyses of 18F-FDG PET/CT parameters for prediction of pathologic rLN involvement. The statistical differences were not found between 18F-FDG PET/CT parameters for prediction of pathologic rLN status.
Table 3.
Pairwise comparison of 18F-FDG positron emission tomography/CT parameters for prediction of pathologic regional lymph node status
| Variables | SUVmax | SUVmean | MTV | TLG |
|---|---|---|---|---|
| SUVmean | ||||
| DBA | 0.0264 | |||
| SE | 0.0161 | |||
| 95% CI | −0.00516 to 0.058 | |||
| p-value | 0.1011 | |||
| MTV | ||||
| DBA | 0.0606 | 0.087 | ||
| SE | 0.0889 | 0.0865 | ||
| 95% CI | −0.114 to 0.235 | −0.0825 to 0.256 | ||
| p-value | 0.4955 | 0.3146 | ||
| TLG | ||||
| DBA | 0.0652 | 0.0916 | 0.00466 | |
| SE | 0.0629 | 0.0596 | 0.0326 | |
| 95% CI | −0.0581 to 0.189 | −0.0252 to 0.208 | −0.0592 to 0.0685 | |
| p-value | 0.2999 | 0.1241 | 0.8863 | |
| HF | ||||
| DBA | 0.155 | 0.182 | 0.0947 | 0.0901 |
| SE | 0.0945 | 0.0977 | 0.103 | 0.102 |
| 95% CI | −0.0299 to 0.34 | −0.00983 to 0.373 | −0.108 to 0.297 | −0.109 to 0.289 |
| p-value | 0.1003 | 0.063 | 0.3588 | 0.3754 |
CI, confidence interval; DBA, difference between areas; HF, heterogeneity factor; MTV, metabolic tumour volume; SE, standard error; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TLG, total lesion glycolysis.
Univariate and multivariate analyses
In univariate analysis, SUVmax, SUVmean, MTV, TLG and HF were significantly associated with pathologic rLN involvement (Table 4). However, in multivariate analysis, the HF was associated with pathologic rLN involvement in oesophageal cancer.
Table 4.
Univariate and multivariate analysis of factors associated with pathologic regional lymph node status
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years) | ||||
| >69 | 0.73 (0.23–2.33) | 0.6038 | ||
| Sex | ||||
| Male | 1.26 (0.19–8.26) | 0.8097 | ||
| T stage | ||||
| >I | 1.58 (0.39–6.27) | 0.5082 | ||
| Location | ||||
| Upper | 1.27 (0.32–5.1) | 0.7286 | ||
| Differentiation | ||||
| Poor | 1.68 (0.55–5.14) | 0.3588 | ||
| SUVmax | ||||
| >12.2 | 5.48 (1.47–20.29) | 0.0109 | 0.59 (0.02–16.13) | 0.7552 |
| SUVmean | ||||
| >7.15 | 7.87 (1.53–40.28) | 0.0132 | 4.59 (0.12–162.8) | 0.4018 |
| MTV | ||||
| >7.3 cm3 | 7.8 (2.03–29.8) | 0.0027 | 2.56 (0.20–32.5) | 0.4666 |
| TLG | ||||
| >60.17 | 7.15 (1.99–25.71) | 0.0026 | 8.9 (0.32–241.1) | 0.1941 |
| HF | ||||
| >2.705 | 10.3 (2.84–37.98) | 0.0004 | 29.8 (3.04–293.4) | 0.0036 |
CI, confidence interval; HF, heterogeneity factor; MTV, metabolic tumour volume; OR, odd ratio; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; TLG, total lesion lycolysis.
DISCUSSION
The present study demonstrates that 18F-FDG PET/CT parameters such as SUVmax, SUVmean, MTV, TLG and HF were independent risk factors associated with rLN metastasis in patients with oesophageal cancer. Also, our study further suggests that intratumoral heterogeneity of 18F-FDG uptake might be the most potent predictor of rLN involvement of patients with oesophageal cancer.
Exact pre-operative staging is important for determining the most appropriate therapeutic procedure for curative surgery of oesophageal cancer. Previous studies showed that the 5-year survival rate of patients with oesophageal cancer with metastatic LNs was <15%, compared with that of >40% in patients without LN metastasis.15,16
18F-FDG PET and PET/CT characterize cellular characteristic on the basis of altered tissue glucose metabolism.17 Several previous studies demonstrated that 18F-FDG PET and PET/CT are useful for staging of oesophageal cancer, especially with regard to the tumour depth invasion definition and rLN status.18–21 However, 18F-FDG PET/CT has a limited role in the identification of early rLN metastasis but is highly useful for detecting remote organ metastasis.5 Furthermore, determination of rLN metastasis is often difficult from its size and SUVmax.6 To overcome this limited diagnostic value of SUVmax for the detection of rLN involvement, metabolic parameters of 18F-FDG PET/CT were described. Previously, we reported that MTVs were potent factors associated with pathologic rLN involvement, and MTV is a more accurate predictor than SUVmax with regard to rLN staging in oesophageal cancer.22
Intratumoural heterogeneity is a well-documented character of malignant tumours and is associated with many tumour phenotypes such as cellular morphology, gene expression, metabolism and metastatic potential.23 Malignant cancer cells are composed of heterogenous components, not only biologic constituents but also gene expression, metabolic and behavioural characteristics.24–26 Heterogeneity varies in the same cancer and has a wide spectrum even in the same stage because there are differences in properties such as the growth rate, vascularity and necrosis within the same tumour cell population.27 Recently, there has been increasing interest in the assessment of intratumoural heterogeneity of 18F-FDG uptake demonstrating an association of prognosis of patients.11,12,28,29
Some previous studies have investigated the prognostic value of HF in patients with oral cavity cancer and breast cancer.11,12 In patients with breast cancer, intratumoral metabolic heterogeneity significantly affected the overall survival in patients with invasive ductal carcinoma. Therefore, they concluded the HF may act as a robust surrogate marker for the prediction of overall survival in patients with invasive ductal carcinoma.11 Kwon et al12 concluded that the intratumoural heterogeneity of 18F-FDG uptake may be a significant prognostic factor for overall survival in addition to cervical lymph node metastasis in oral cavity cancer. The present study adapted the HF for the prediction of rLN status in patients with oesophageal cancer and showed the most independent predictor for rLN involvement.
The present study has some limitations. First, it was a retrospective, single-centre study. Second, the intratumoral metabolic heterogeneity on 18F-FDG PET scans can be represented by various methods; for example, textural features, elliptic solid mathematical model with homogenous density and cumulative SUV volume histograms.28,30,31 Although the present study did not use the textural features, the textural features were used relatively widely. Also, a feasible and highly reproducible method for obtaining a heterogeneity parameter representing intratumoral metabolic heterogeneity is warranted. Finally, most patients of the present study are male. It could affect the results of the present study of the male predominance.
CONCLUSION
In conclusion, 18F-FDG PET/CT parameters such as SUVmax, SUVmean, MTV, TLG and HF were useful for the prediction of pathologic rLN status in patients with oesophageal cancer. However, HF of 18F-FDG uptake might be the most powerful predictor of rLN involvement of patients with oesophageal cancer. Further studies are needed to confirm these results and improve statistical accuracy.
Contributor Information
Seong-Jang Kim, Email: growthkim@daum.net.
Kyoungjune Pak, Email: ilikechopin@me.com.
Samuel Chang, Email: samuel.chang@ucdenver.edu.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. doi: 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Luketich JD. Resection of esophageal cancer: strategies for optimal management. Ann Thorac Surg 2008; 85: 751–6. [DOI] [PubMed] [Google Scholar]
- 3.Isono K, Onada S, Ishikawa T, Sato H, Nakayama K. Studies on the causes of death from esophageal carcinoma. Cancer 1982; 49: 2173–9. doi: [DOI] [PubMed] [Google Scholar]
- 4.Stahl M, Budach W, Meyer HJ, Cervantes A; ESMO Guidelines Working Group. Esophageal cancer: clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21: v46–9. doi: 10.1093/annonc/mdq163 [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, Faried A, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 2005; 103: 148–56. doi: 10.1002/cncr.20724 [DOI] [PubMed] [Google Scholar]
- 6.Tangoku A, Yamamoto Y, Furukita Y. The new era of staging as a key for an appropriate treatment for esophageal cancer. Ann Thorac Cardiovasc Surg 2012; 18: 190–9. doi: 10.5761/atcs.ra.12.01926 [DOI] [PubMed] [Google Scholar]
- 7.Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol 2010; 17: 115–22. doi: 10.1245/s10434-009-0719-7 [DOI] [PubMed] [Google Scholar]
- 8.Miller TR, Grigsby PW. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int J Radiat Oncol Biol Phys 2002; 53: 353–9. doi: 10.1016/S0360-3016(02)02705-0 [DOI] [PubMed] [Google Scholar]
- 9.Chen MK, Chen TH, Liu JP, Chang CC, Chie WC. Better prediction of prognosis for patients with nasopharyngeal carcinoma using primary tumor volume. Cancer 2004; 100: 2160–6. doi: 10.1002/cncr.20210 [DOI] [PubMed] [Google Scholar]
- 10.Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys 2007; 69: 328–33. doi: 10.1016/j.ijrobp.2007.04.036 [DOI] [PubMed] [Google Scholar]
- 11.Son SH, Kim DH, Hong CM, Kim CY, Jeong SY, Lee SW, et al. Prognostic implication of intratumoral metabolic heterogeneity in invasive ductal carcinoma of the breast. BMC Cancer 2014; 14: 585. doi: 10.1186/1471-2407-14-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon SH, Yoon JK, An YS, Shin YS, Kim CH, Lee DH, et al. Prognostic significance of the intratumoral heterogeneity of 18F-FDG uptake in oral cavity cancer. J Surg Oncol 2014; 110: 702–6. doi: 10.1002/jso.23703 [DOI] [PubMed] [Google Scholar]
- 13.Kidd EA, Grigsby PW. Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res 2008; 14: 5236–41. doi: 10.1158/1078-0432.CCR-07-5252 [DOI] [PubMed] [Google Scholar]
- 14.Soret M, Bachrach SL, Buvat I. Partial volume effect in PET tumor imaging. J Nucl Med 2007; 48: 932–45. doi: 10.2967/jnumed.106.035774 [DOI] [PubMed] [Google Scholar]
- 15.Aranda-Hernandez J, Cirocco M, Marcon N. Treatment of dysphagia in barrett esophagus. Clin Endosc 2014; 47: 55–64. doi: 10.5946/ce.2014.47.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka T, Matono S, Mori N, Shirouzu K, Fujita H. T1 squamous cell carcinoma of the esophagus: long-term outcomes and prognostic factors after esophagectomy. Ann Surg Oncol 2013; 21: 932–8. doi: 10.1245/s10434-013-3372-0 [DOI] [PubMed] [Google Scholar]
- 17.Bar-Shalom R, Valdivia AY, Blaufox MD. PET imaging in oncology. Semin Nucl Med 2000; 30: 150–85. doi: 10.1053/snuc.2000.7439 [DOI] [PubMed] [Google Scholar]
- 18.Kato H, Kuwano H, Nakajima M, Miyazaki T, Yoshikawa M, Ojima H, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 2002; 94: 921–8. doi: 10.1002/cncr.10330 [DOI] [PubMed] [Google Scholar]
- 19.Okada M, Murakami T, Kumano S, Kuwabara M, Shimono T, Hosono M, et al. Integrated FDG-PET/CT compared with intravenous contrast-enhanced CT for evaluation of metastatic regional lymph nodes in patients with resectable early stage esophageal cancer. Ann Nucl Med 2009; 23: 73–80. doi: 10.1007/s12149-008-0209-1 [DOI] [PubMed] [Google Scholar]
- 20.Gillies RS, Middleton MR, Maynard ND, Bradley KM, Gleeson FV. Additional benefit of (18)F-fluorodeoxyglucose integrated positron emission tomography/computed tomography in the staging of oesophageal cancer. Eur Radiol 2011; 21: 274–80. doi: 10.1007/s00330-010-1943-z [DOI] [PubMed] [Google Scholar]
- 21.Hsu WH, Hsu PK, Wang SJ, Lin KH, Huang CS, Hsieh CC, et al. Positron emission tomography-computed tomography in predicting locoregional invasion in esophageal squamous cell carcinoma. Ann Thorac Surg 2009; 87: 1564–8. doi: 10.1016/j.athoracsur.2009.02.065 [DOI] [PubMed] [Google Scholar]
- 22.I HS, Kim SJ, Kim IJ, Kim K. Predictive value of metabolic tumor volume measured by 18F-FDG PET for regional lymph node status in patients with esophageal cancer. Clin Nucl Med 2012; 37: 442–6. doi: 10.1097/RLU.0b013e318238f703 [DOI] [PubMed] [Google Scholar]
- 23.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta 2010; 1805: 105–17. doi: 10.1016/j.bbcan.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heppner GH. Tumor heterogeneity. Cancer Res 1984; 44: 2259–65. [PubMed] [Google Scholar]
- 25.Gillies RJ, Schornack PA, Secomb TW, Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia 1999; 1: 197–207. doi: 10.1038/sj.neo.7900037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lleonart ME, Martin-Duque P, Sanchez-Prieto R, Moreno A, Ramon y Cajal S. Tumor heterogeneity: morphological, molecular and clinical implications. Histol Histopathol 2000; 15: 881–9. [DOI] [PubMed] [Google Scholar]
- 27.Huang B, Chan T, Kwong DL, Chan WK, Khong PL. Nasopharyngeal carcinoma: investigation of intratumoral heterogeneity with FDG PET/CT. AJR Am J Roentgenol 2012; 199: 169–74. doi: 10.2214/AJR.11.7336 [DOI] [PubMed] [Google Scholar]
- 28.Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predict response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 2011; 52: 369–78. doi: 10.2967/jnumed.110.082404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 2013; 54: 19–26. doi: 10.2967/jnumed.112.107375 [DOI] [PubMed] [Google Scholar]
- 30.O'Sullivan F, Roy S, O'Sullivan J, Vernon C, Eary J. Incorporation of tumor shape into an assessment of spatial heterogeneity for human sarcomas imaged with FDG-PET. Biostatistics 2005; 6: 293–301. doi: 10.1093/biostatistics/kxi010 [DOI] [PubMed] [Google Scholar]
- 31.van Velden FH, Cheebsumon P, Yaqub M, Smit EF, Hoekstra OS, Lammertsma AA, et al. Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoral FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging 2011; 38: 1636–47. doi: 10.1007/s00259-011-1845-6 [DOI] [PMC free article] [PubMed] [Google Scholar]