Abstract
Objective:
Renal transplantation recipients are at increased risk of Mycobacterium tuberculosis infection because of immunosuppression. The aim of this study was to assess high-resolution CT (HRCT) findings in renal transplantation recipients diagnosed with pulmonary tuberculosis (TB).
Methods:
We reviewed HRCT findings from patients diagnosed with pulmonary TB, established by M. tuberculosis detection in bronchoalveolar lavage, sputum or biopsy sample. Two observers independently reviewed HRCT images and reached consensus decisions on the presence and distribution of: (i) miliary nodules, (ii) cavitation and centrilobular tree-in-bud nodules, (iii) ground-glass attenuation and consolidation, (iv) mediastinal lymph node enlargement and (v) pleural effusion.
Results:
The sample comprised 40 patients [26 males, 14 females; median age, 45 years (range, 12–69 years)]. The main HRCT pattern was miliary nodules (40%), followed by cavitation and centrilobular tree-in-bud nodules (22.5%), ground-glass attenuation and consolidation (15%), mediastinal lymph node enlargement (12.5%) and pleural effusion (10%). The distribution of findings in patients with miliary nodules was random. In patients with cavitation and centrilobular tree-in-bud nodules, 66.6% of abnormalities were found in the upper lobes. Pleural effusion was unilateral in 75% of cases. The overall mortality rate was 27.5%. This rate was 50% in patients with miliary nodules, and 72.6% of all deaths occurred in this group. Thus, mortality was increased significantly in patients with miliary nodules (p < 0.05).
Conclusion:
The main HRCT finding in renal transplantation recipients with pulmonary TB was miliary nodules, followed by cavitation and centrilobular tree-in-bud nodules. Miliary nodules were associated with a worse prognosis in these patients.
Advances in knowledge:
We report the first series on HRCT findings of microbiologically confirmed pulmonary TB exclusively in renal transplantation recipients. The main HRCT finding was miliary nodules, and mortality was increased significantly in these patients.
INTRODUCTION
Kidney transplantation is the treatment of choice for the majority of patients with end-stage renal disease.1 However, post-transplantation tuberculosis (TB) is a life-threatening infection affecting long-term outcomes in kidney transplantation recipients. The incidence of TB is 20–74 times greater in kidney recipients than in the general population.2 Immunosuppression in transplantation recipients accounts for progressive impairment in cellular immune function, allowing infection by Mycobacterium tuberculosis, an intracellular pathogen.3
With the emergence of new and potent immunosuppressive regimens, the incidence of TB among transplantation recipients may increase. Most transplantation patients with TB manifest clinical pulmonary infection. Fever, night sweats and constitutional symptoms occur frequently, but the immunocompromised state can alter the clinical presentation and delay diagnosis.
Radiologic findings described in transplantation recipients include miliary nodules, pleural effusion, parenchymal cavitation, nodules, pulmonary infiltrate, and hilar or mediastinal adenopathy.4,5 However, no study has described the high-resolution CT (HRCT) findings of TB developing exclusively after renal transplantation. The aim of this study was to assess HRCT patterns at presentation in renal transplantation recipients diagnosed with pulmonary TB.
METHODS AND MATERIALS
Our Institutional Review Board approved this study and waived the requirement for informed patient consent. Data from all renal transplantation recipients with pulmonary TB treated in two Brazilian hospitals between 1995 and 2015 were reviewed retrospectively. All data used in this study were anonymized. Inclusion criteria were: positive M. tuberculosis culture from sputum, bronchoalveolar lavage or lung biopsy sample, and availability of HRCT images obtained at the diagnosis. All patients diagnosed with TB were followed for a minimum of 2 years, and only deaths related to infection or its treatment were considered in this analysis. Patients diagnosed with coexistent pulmonary infections due to other pathogens were excluded from our sample based on a review of clinical and laboratory data.
HRCT scans were carried out with a 64-multidetector CT scanner (LightSpeed® VCT; GE Healthcare, Waukesha, WI). The parameters used were: 250 mA; 120 kVp; pitch, 1.375; time, 0.8 s; and inspiratory volumetric acquisition with 1-mm collimation at 1-mm increments. We performed a high-spatial frequency reconstruction algorithm, and images were obtained with parenchymal (width: 1200–1600 HU; level: −500 to −700 HU) and mediastinal (width: 350–450 HU; level: 20–40 HU) window settings.
The Fleischner Society's Glossary of Terms6 was used to assess the HRCT images. Findings were classified as: (i) miliary nodules, (ii) cavitation and centrilobular tree-in-bud nodules, (iii) ground-glass attenuation and consolidation, (iv) mediastinal lymph node enlargement and (v) pleural effusion. A nodule was defined as a rounded or irregular opacity that was well or poorly defined and ≤3 cm in diameter. Nodules were classified as small (diameter ≤10 mm) or large (diameter >10 mm). The measure of 1.2-cm short-axis diameter of mediastinal lymph nodes was used as a threshold to define pathological lymph node enlargement. A cavity was defined as a gas-filled space, seen as a lucency or low-attenuation area, within a pulmonary consolidation, mass or nodule. The tree-in-bud pattern describes the appearance of multiple centrilobular nodules with a linear branching pattern. Ground-glass opacities refer to a hazy area of increased attenuation in the lung with preserved vascular markings. Consolidation is defined as increased attenuation of the parenchyma causing obscuration of pulmonary vessels. The distribution of CT findings was classified by site using the categories of upper, middle and lower lobes, and further categorized as focal (unilobar) or diffuse (more than one lobe).
Two chest radiologists with more than 12 years' of experience independently assessed HRCT scans. The HRCT images were then reviewed together with a third chest radiologist to reach final consensus decisions. All radiologists were blinded to the patients' clinical data, except M. tuberculosis infection. Mortality within 2 years was also evaluated and correlated with imaging findings. Data were entered into Excel® (2010; Microsoft® Corp., Redmond, WA) and then exported to SPSS® v. 15.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) for statistical analysis. Quantitative variables were described using means, standard deviations, medians and variance. Qualitative variables were described using absolute and relative frequencies. The χ2 test and Student's t-test were used to compare proportions, mean and median values. A p-value <0.05 was considered statistically significant.
RESULTS
Data from 4128 patients who underwent kidney transplantation between 1995 and 2015 were reviewed, and 47 patients diagnosed with pulmonary TB were initially included in the analysis. However, the final sample consisted of 40 patients because 5 patients were excluded because of pneumonia caused by other aetiological agents, including bacteria (n = 2 patients), cytomegalovirus (n = 2) and fungus (n = 1); and 2 patients were excluded because they did not perform CT because of unavailability of a CT scanner. Of these, 26 patients were male and 14 were female (median age, 45 years; range, 12–69 years). The mean interval between transplantation and infection diagnosis was 8.6 months (standard deviation, 5.1 months); this interval was 3.3 months (standard deviation, 2.3 months) among patients presenting the miliary pattern.
Imaging findings are summarized in Table 1. The main HRCT pattern was miliary nodules (40% of patients; Figure 1), followed by cavitation and centrilobular tree-in-bud nodules (22.5%; Figure 2), ground-glass attenuation and consolidation (15%; Figure 3), mediastinal lymph node enlargement (12.5%; Figure 4) and pleural effusion (10%). The distribution of findings in patients with miliary nodules was random. In patients with cavitation and centrilobular tree-in-bud nodules, 66.6% of abnormalities were found in the upper lobes. Pleural effusion was unilateral in 75% of cases.
Table 1.
Features of renal transplantation recipients with pulmonary tuberculosis (n = 40)
| Feature | Dataa |
|---|---|
| Clinical characteristics | |
| Sex (male) | 26 (65%) |
| Median age, years (range) | 45 (12–69) |
| Mean interval (months) between transplantation and infection diagnosis (SD) | 8.6 (5.1) |
| HRCT findings | |
| Miliary nodules | 16 (40%) |
| Cavitation/tree-in-bud nodules | 9 (22.5%) |
| Ground-glass/consolidation | 6 (15%) |
| Mediastinal lymph node enlargement | 5 (12.5%) |
| Pleural effusion | 4 (10%) |
HRCT, high-resolution CT; SD, standard deviation.
Data are presented as the number (%) of patients unless otherwise indicated.
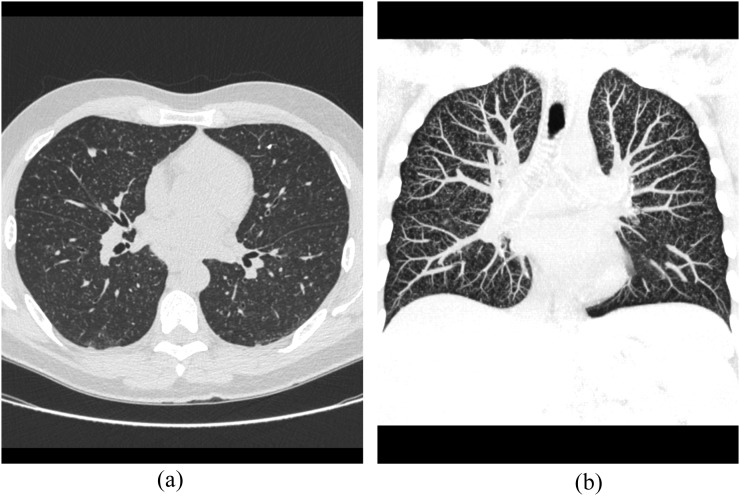
Figure 1.
A 48-year-old renal transplantation recipient with asthenia and cough. (a) Axial CT image shows bilateral miliary nodules. (b) CT image with coronal reconstruction demonstrates the same findings.
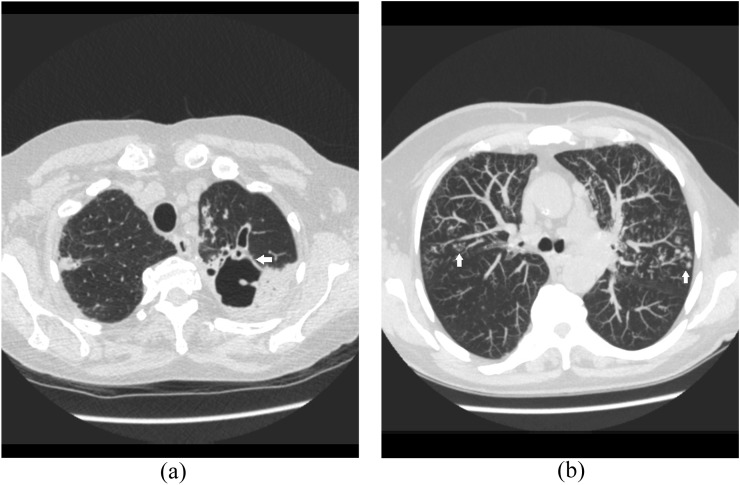
Figure 2.
A 52-year-old renal transplantation recipient with asthenia, cough and fever. Axial CT images show a thick-walled cavity in the left upper lobe (a, arrow) and bilateral centrilobular tree-in-bud nodules (b, arrows).
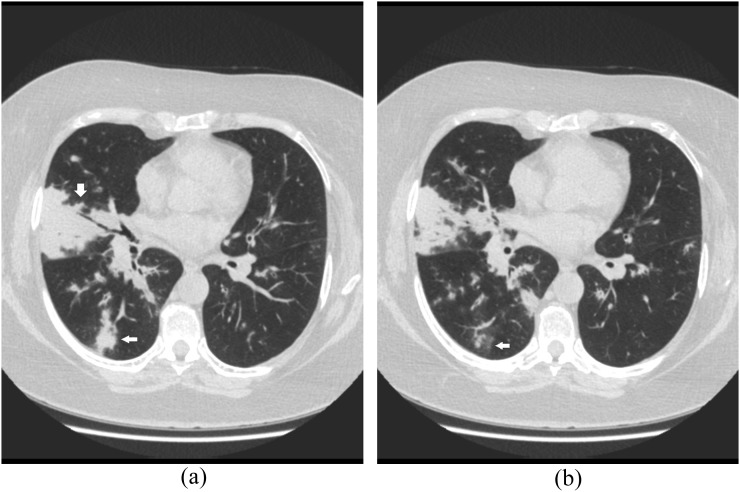
Figure 3.
A 47-year-old renal transplantation recipient with dyspnoea and cough. Axial CT images shows consolidation (a, arrows) and ground-glass opacities (b, arrow) in the right inferior and middle lobes.
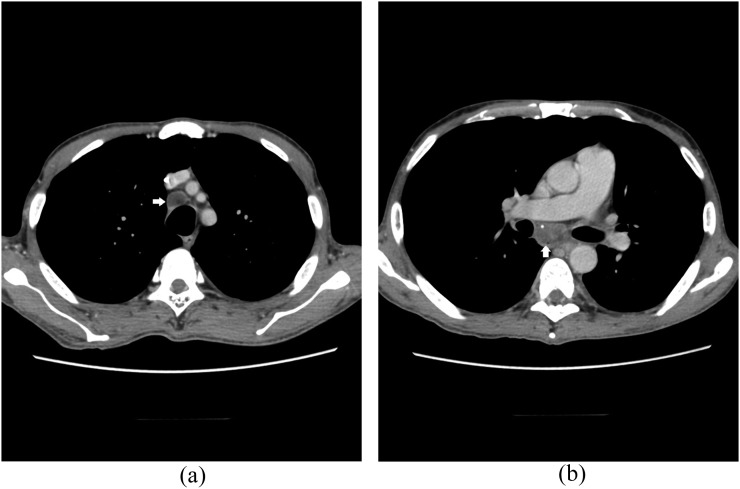
Figure 4.
A 39-year-old renal transplantation recipient with asthenia and cough. Axial CT images obtained in the mediastinal window show lymphadenopathy in the right inferior paratracheal (a, arrow) and subcarinal chains (b, arrow).
The overall mortality rate was 27.5% (n = 11). The mortality rate was 50% (n = 8) among patients with miliary nodules, and 72.6% of all deaths occurred in this group. Thus, mortality was increased significantly in patients with miliary nodules (p < 0.05). In our sample of 40 patients, there were 4 (10%) other deaths unrelated to TB because of graft dysfunction (n = 2 patients), stroke (n = 1) and renal cell carcinoma in the native kidney (n = 1).
DISCUSSION
Renal transplantation recipients are at increased risk of contracting TB because of immunosuppression, particularly in countries where the disease is endemic. The reported prevalence of TB in renal transplantation recipients ranges from 0.5% to 15%.3,7 Differences in these values may be attributed largely to epidemiological factors in developed and developing regions. TB also presents particular challenges because of delayed diagnosis and side effects of therapy. The clinical features of TB can be unusual, and they may be masked by blunted response to infection. Common clinical abnormalities include pyrexia, pulmonary infiltrates, exudative pleural effusion and exudative ascites.3,7,8
The resurgence of TB since the 1980s due to the acquired immunodeficiency syndrome epidemic and increasing numbers of other immunocompromised individuals, including organ transplantation recipients, makes this disease a topic of universal concern.9 Pulmonary TB can demonstrate a variety of CT findings, including consolidation, cavities, features of endobronchial spread, pleural effusion, miliary nodules and adenopathy. Tubercular adenopathy typically shows low-density centres with prominent rim enhancement.9–13 TB was classically divided into primary and post-primary (reactivated) disease. Primary TB typically manifests on CT as air-space consolidation, adenopathy, pleural effusion, miliary nodules and/or atelectasis. Post-primary disease results from the reactivation of a previously dormant infection in most cases. Classical CT findings in post-primary TB are patchy, ill-defined consolidations in the upper lobes, associated with cavitation. Cavities are often multiple and demonstrate thick and irregular walls. Endobronchial spread is a common complication that represents granulomatous infection in the airways, which manifests on HRCT as branching centrilobular nodules with the “tree-in-bud” appearance. Other findings indicating reactivated TB include bronchovascular structure distortion, fibrotic strands and bronchiectasis.9,12,13 However, the advent of molecular epidemiology in the 1990s has led to the discovery that the radiographic appearance of TB depends on immune status and is independent of the time since infection. Upper-lobe cavitary disease is usually seen in infected immunocompetent hosts, whereas immunocompromised patients usually present with lower-lung disease, adenopathy and effusions, among other findings.13 This revised classification correlates well with the findings of the present study. Renal transplantation recipients are immunosuppressed and thus might be expected to present with features of primary disease, regardless of whether they have had previous TB exposure. The miliary pattern can be an indication of the severe immunocompromised state and is thus associated with a poorer prognosis.
Miliary nodules have been reported in 2.4–28.5% of TB cases after kidney transplantation.3,14–16 Other radiographic findings in these patients include infiltrates (27.6–37.5%), cavities (6.2–20.7%), nodules (12.5%), lymph node enlargement (12.5%) and pleural effusion (31.2–41.4%).3,8,14–20 However, most previous studies describing imaging findings in these patients have used conventional radiography. The sensitivity and specificity of chest radiography are limited in patients with diffuse lung disease, and chest radiography findings are normal in up to approximately 10% of immunocompromised patients with acute lung disease.21 HRCT is more sensitive than chest radiography for the evaluation of acute and chronic lung disease.12 Our analysis, which to our knowledge is the first study of HRCT findings exclusively in renal transplantation recipients with TB, revealed a greater prevalence of the miliary pattern.
Gulati et al12 reported on HRCT findings in renal transplantation recipients with pulmonary infection. However, only 11 pulmonary TB cases were included in their sample, and TB diagnoses were based on the detection of thin-walled cavities and bronchiectasis (n = 4 patients), lobular consolidation in the upper lobes (n = 3), tree-in-bud nodules (n = 2) and right paratracheal adenopathy (n = 1). HRCT findings in the remaining patients were non-specific and included bilateral patchy areas of ground-glass attenuation, nodular opacities and lobar consolidation in the lower lobes. Sputum, blood culture and bronchoalveolar lavage were negative in 4 of these 11 patients, who were placed on an antitubercular regimen based on chest radiography and HRCT findings; all 4 patients responded favourably. The authors concluded that HRCT provided additional information in comparison with conventional radiography in 64.7% of renal transplantation recipients with pulmonary infection, particularly in TB cases with findings such as cavitation, bronchiectasis and endobronchial spread.12
Jiang et al22 evaluated CT findings of pulmonary infection after kidney transplantation. TB infection was confirmed in only 7 of 89 (7.9%) cases for which HRCT images were available, and the miliary pattern was found in only 1 of these cases. The tree-in-bud sign, seen in 57% of TB cases, was a significant marker differentiating bacterial infection from TB in this series.22
Both of these studies were limited because they included few patients with TB. Thus, our evaluation only of cases of TB infection after renal transplantation complements the results of previous reports. The incidence of disseminated disease is higher in renal transplantation recipients than in the general population. Sustained depressed immunity offers a logical explanation for this phenomenon.23 The increased frequency of the miliary pattern observed in this study underscores the importance of immune status in attempts to control this infectious disease.
Immunosuppressive therapy is the most important factor predisposing a patient to TB development after transplantation, and this therapy has changed substantially in the past two decades.18,19 For many years, basic immunosuppression consisted of the combined use of three types of drugs: a glucocorticosteroid (prednisone), a purine antagonist (azathioprine or mycophenolate mofetil) and a calcineurin inhibitor (cyclosporine or tacrolimus). Because of numerous cases of potential glucocorticoid toxicity and calcineurin inhibitor toxicity, many new regimens that incorporate rapid glucocorticoid elimination or calcineurin inhibitor dose reduction or elimination have been developed. Sirolimus has been used as a substitute for calcineurin inhibitors. The choice of agents is often protocol driven but is usually adapted to each recipient's risk profile.24–26 The immunosuppressive actions of these drugs are mediated through several pathways and particularly affect T-cells and phagocytes. Thus, transplantation recipients show progressive impairment of cellular immune function. T-cell-mediated immunity is crucial for TB control, and immunosuppressant agents may increase the risk of TB reactivation and dissemination. Neutropenia is not a major immunological defect in recipients of solid organ transplantations, as in other immunocompromised patients due to hematological malignancies, and those who have undergone haematopoietic stem-cell transplantation.15,19,24
The mortality rate observed in this study falls within the previously reported range of 12.5–32% among kidney transplantation recipients with post-transplantation TB.3,19 Mortality is higher when TB develops in the first year after kidney transplantation among poorly nourished patients and hypoxic patients treated with steroids.3 In our sample, most deaths occurred in the group of patients with miliary nodules, which showed a significant increase in mortality.
The limitations of this study include its retrospective design. In addition, we could not precisely exclude coexistent self-limited infections because of other organisms at the time of diagnosis, as in other immunocompromised individuals. However, we identified and excluded five patients diagnosed with concomitant infections potentially affecting the lungs. Despite these limitations, we report on HRCT findings in the largest published series of microbiologically confirmed pulmonary TB in renal transplantation recipients.
CONCLUSION
In conclusion, predominant HRCT findings in renal transplantation recipients with TB infection were miliary nodules, followed by cavitation and centrilobular tree-in-bud nodules. Mortality was increased significantly in patients with miliary nodules. HRCT is an important examination tool for the early diagnosis of post-transplantation pulmonary TB, and it should be included in investigative protocols for renal transplantation recipients suspected of having pulmonary infection.
Contributor Information
Marisa Pereira, Email: lisapereira@terra.com.br.
Fernando F Gazzoni, Email: gazzoni4@gmail.com.
Edson Marchiori, Email: edmarchiori@gmail.com.
Klaus Irion, Email: Klaus.Irion@lhch.nhs.uk.
Jose Moreira, Email: jmmoreirapn@gmail.com.
Irai L Giacomelli, Email: iraigiacomelli@gmail.com.
Alessandro Pasqualotto, Email: acpasqualotto@hotmail.com.
Bruno Hochhegger, Email: brunohochhegger@gmail.com.
REFERENCES
- 1.Akbar SA, Jafri SZ, Amendola MA, Madrazo BL, Salem R, Bis KG. Complications of renal transplantation. Radiographics 2005; 25: 1335–56. doi: 10.1148/rg.255045133 [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Goez JF, Linares L, Benito N, Cervera C, Cofán F, Ricart MJ, et al. Tuberculosis in solid organ transplant recipients at a tertiary hospital in the last 20 years in Barcelona, Spain. Transplant Proc 2009; 41: 2268–70. doi: 10.1016/j.transproceed.2009.06.080 [DOI] [PubMed] [Google Scholar]
- 3.Boubaker K, Gargah T, Abderrahim E, Abdallah TB, Kheder A. Mycobacterium tuberculosis infection following kidney transplantation. Biomed Res Int 2013; 2013: 347103. doi: 10.1155/2013/347103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis 1998; 27: 1266–77. doi: 10.1086/514993 [DOI] [PubMed] [Google Scholar]
- 5.Torre-Cisneros J, Doblas A, Aguado JM, San Juan R, Blanes M, Montejo M, et al. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis 2009; 48: 1657–65. doi: 10.1086/599035 [DOI] [PubMed] [Google Scholar]
- 6.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 7.Zhang XF, Lv Y, Xue WJ, Wang B, Liu C, Tian PX, et al. Mycobacterium tuberculosis infection in solid organ transplant recipients: experience from a single center in China. Transplant Proc 2008; 40: 1382–5. doi: 10.1016/j.transproceed.2008.01.075 [DOI] [PubMed] [Google Scholar]
- 8.Basiri A, Hosseini-Moghaddam SM, Simforoosh N, Einollahi B, Housseini M, Foirouzan A, et al. The risk factors and laboratory diagnostics for post renal transplant tuberculosis: a case-control, country-wide study on definitive cases. Transpl Infect Dis 2008; 10: 231–5. doi: 10.1111/j.1399-3062.2007.00271.x [DOI] [PubMed] [Google Scholar]
- 9.Harisinghani MG, McLoud TC, Shepard JA, Ko JP, Shroff MM, Mueller PR. Tuberculosis from head to toe. Radiographics 2000; 20: 449–70. doi: 10.1148/radiographics.20.2.g00mc12449 [DOI] [PubMed] [Google Scholar]
- 10.Padley SP, Adler B, Muller NL. High-resolution computed tomography of the chest: current indications. J Thorac Imaging 1993; 8: 189–99. doi: 10.1097/00005382-199322000-00004 [DOI] [PubMed] [Google Scholar]
- 11.Padley SP, Hansell DM, Flower CD, Jennings P. Comparative accuracy of high-resolution computed tomography and chest radiography in the diagnosis of chronic diffuse infiltrative lung disease. Clin Radiol 1991; 44: 222–6. doi: 10.1016/S0009-9260(05)80183-7 [DOI] [PubMed] [Google Scholar]
- 12.Gulati M, Kaur R, Jha V, Venkataramu NK, Gupta D, Suri S. High resolution CT in renal transplant patients with suspected pulmonary infections. Acta Radiol 2000; 41: 237–41. doi: 10.1080/028418500127345415 [DOI] [PubMed] [Google Scholar]
- 13.Rozenshtein A, Hao F, Starc MT, Pearson GD. Radiographic appearance of pulmonary tuberculosis: dogma disproved. AJR Am J Roentgenol 2015; 204: 974–8. doi: 10.2214/AJR.14.13483 [DOI] [PubMed] [Google Scholar]
- 14.Arslan H, Ergin F, Oner-Eyuboglu F, Akcay S, Karakayali H, Haberal M. Tuberculosis in renal transplant recipients. Transplant Proc 2003; 35: 2680–1. doi: 10.1016/j.transproceed.2003.09.091 [DOI] [PubMed] [Google Scholar]
- 15.Chen SY, Wang CX, Chen LZ, Fei JG, Deng SX, Qiu J, et al. Tuberculosis in southern Chinese renal-transplant recipients. Clin Transplant 2008; 22: 780–4. doi: 10.1111/j.1399-0012.2008.00878.x [DOI] [PubMed] [Google Scholar]
- 16.Sayiner A, Ece T, Duman S, Yildiz A, Ozkahya M, Kiliçaslan Z, et al. Tuberculosis in renal transplant recipients. Transplantation 1999; 68: 1268–71. doi: 10.1097/00007890-199911150-00009 [DOI] [PubMed] [Google Scholar]
- 17.Canet E, Dantal J, Blancho G, Hourmant M, Coupel S. Tuberculosis following kidney transplantation: clinical features and outcome. A French multicenter experience in the last 20 years. Nephrol Dial Transplant 2011; 26: 3773–8. doi: 10.1093/ndt/gfr156 [DOI] [PubMed] [Google Scholar]
- 18.Ghafari A, Makhdoomi K, Ahmadpoor P, Afshari AT, Fallah MM, Rezaee K. Tuberculosis in Iranian kidney transplant recipients: a single-center experience. Transplant Proc 2007; 39: 1008–11. doi: 10.1016/j.transproceed.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 19.Ou SM, Liu CJ, Teng CJ, Lin YT, Chang YS, Chiang SC, et al. Impact of pulmonary and extrapulmonary tuberculosis infection in kidney transplantation: a nationwide population-bases study in Taiwan. Transpl Infect Dis 2012; 14: 502–9. doi: 10.1111/j.1399-3062.2012.00737.x [DOI] [PubMed] [Google Scholar]
- 20.Ram R, Swarnalatha G, Prasad N, Dakshinamurty KV. Tuberculosis in renal transplant recipients. Transpl Infect Dis 2007; 9: 97–101. doi: 10.1111/j.1399-3062.2006.00182.x [DOI] [PubMed] [Google Scholar]
- 21.Barloon TJ, Galvin JR, Mori M, Stanford W, Gingrich RD. High-resolution ultrafast chest CT in the clinical management of febrile bone marrow transplant patients with normal or nonspecific chest roentgenograms. Chest 1999; 99: 928–33. doi: 10.1378/chest.99.4.928 [DOI] [PubMed] [Google Scholar]
- 22.Jiang T, Xue F, Zheng X, Yu H, Tao X, Xiao X, et al. Clinical data and CT findings of pulmonary infection caused by different pathogens after kidney transplantation. Eur J Radiol 2012; 81: 1347–52. doi: 10.1016/j.ejrad.2011.03.070 [DOI] [PubMed] [Google Scholar]
- 23.Lopez de Castilha D, Schluger NW. Tuberculosis following solid organ transplantation. Transpl Infect Dis 2010; 12: 106–12. doi: 10.1111/j.1399-3062.2009.00475.x [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Lian JD, Cheng CH, Wu MJ, Lee WC, Shu KH. Mycobacterium tuberculosis infection following renal transplantation in Taiwan. Transpl Infect Dis 2006; 8: 148–56. doi: 10.1111/j.1399-3062.2006.00147.x [DOI] [PubMed] [Google Scholar]
- 25.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004; 351: 2715–29. doi: 10.1056/NEJMra033540 [DOI] [PubMed] [Google Scholar]
- 26.Ahsan N, Hricik D, Matas A, Rose S, Tomlanovich S, Wilkinson A, et al. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil—a prospective randomized study. Steroid Withdrawal Study Group. Transplantation 1999; 68: 1865–74. [DOI] [PubMed] [Google Scholar]