Abstract
To measure the toxic potential of asbestos fibers—a known cause of asbestosis, lung cancer, and malignant mesothelioma—asbestos minerals are generally first ground down to small fibers, but it is unknown whether the grinding condition itself changes the fiber toxicity. To evaluate this, we ground chrysotile ore with or without water for 5–30 min and quantified asbestos-induced reactive oxygen species generation in elicited murine peritoneal macrophages as an indicator of fiber toxicity. The toxicity of dry-ground fibers was higher than the toxicity of wet-ground fibers. Grinding with or without water did not materially alter the mineralogical properties. However, dry-ground fibers contained at least 7 times more iron than wet-ground fibers. These results indicate that grinding methods significantly affect the surface concentration of iron, resulting in changes in fiber-induced reactive oxygen species generation or toxicity. Therefore, fiber preparation conditions should be accounted for when comparing the toxicity of asbestos fibers between reported studies.
Graphical abstract
INTRODUCTION
Exposure to asbestos, a group of naturally occurring fibrous silicate materials, can lead to serious health effects including asbestosis, malignant mesothelioma, pleural disorders, and both lung and stomach cancers.1–3 Despite the known toxicity resulting from asbestos exposure, nearly 2 million metric tons of asbestos are mined globally per year.4 Both the mining of asbestos minerals and the production of asbestos fibers from asbestos-containing materials continue to pose serious health hazards to vulnerable populations. To assess the toxic potential of asbestos fibers, asbestos minerals or asbestos-containing materials are broken into small fibers by mechanical grinding or ultrasonic treatment for extended periods of time.5,6 However, it is unknown whether the grinding method impacts the measured asbestos toxicity, despite evidence that the grinding method can change the fiber shape, size, and structure.5–8
The nature of active surface sites plays a critical role in determining the carcinogenic potential of the fibers.9 The surface chemistry of the fiber may change based on the grinding condition and the characteristics of the particular liquid used during grinding.7 In particular, grinding in water may dissolve iron, which can be present as an impurity (e.g., chrysotile) or as a structural component (e.g., amosite, crocidolite, actinolite). Recent studies have shown that an increase in iron concentration in fibers correlates with an increase in toxicity,10,11 partly due to enhanced production of reactive oxygen species (ROS) from surface reactive iron, causing oxidative stress and DNA damage to surrounding cells.12–14 Because iron may be present in the crystal lattice structure of asbestos fibers,11,15 any method that breaks or exposes the crystal lattice may potentially increase fiber toxicity. For instance, Pollastri et al.16 show that iron is typically present in octahedral sites in the fiber that can be exposed during dissolution in water. However, previous studies that examined the effect of grinding methods on fiber properties did not measure iron concentrations of the ground fiber or fiber toxicity.5–8 Thus, the extent to which the grinding method changes fiber surface properties and toxicity is unknown.
The purpose of this study was to examine whether and how grinding conditions affect the cytotoxicity of ground asbestos fibers—a necessary pretreatment method to lower the fiber size for toxicity measurement. The scope of the current study is not to examine the effect of possible asbestos fragmentation that may occur in the workplace environment. We hypothesized that grinding in the presence of water would remove a fraction of total iron from fibers, in turn decreasing cytotoxicity, whereas dry grinding, through pulverizing fibers, would either preserve or expose more iron, thereby increasing toxicity. To test these hypotheses, we ground chrysotile—the most commonly used asbestos mineral—with or without water for 5–30 min and measured the fiber toxicity based on the generation of asbestos-induced ROS in elicited murine peritoneal macrophages as a model of tissue phagocytic response to the presence of asbestos in the pleural space.17 Macrophages are immune cells that play a critical role in tumor development. When exposed to foreign material such as bacteria or asbestos, macrophages generate ROS. However, excessive ROS release can cause inflammation and DNA damage, which may lead to tumor development. Therefore, ROS generation in macrophages has been used as a proxy to differentiate tumor-associated macrophages from alternatively activated macrophages.18
EXPERIMENTAL METHODS
Asbestos Grinding
Chrysotile ore (Glove, Arizona) was first broken using a hammer to separate fibrous bundles from other rock impurities. The handpicked fiber bundles were ground for 5, 15, or 30 min in a high-energy vibratory ball mill (Model 8000, SPEX Industries, Inc.) with or without deionized water. The wet samples were oven-dried for 24 h at 70 °C. Asbestos fibers were prepared and handled inside the fume hood to minimize asbestos exposure. On the basis of the guideline recommended by the Office of Environmental Health and Radiation Safety at the University of Pennsylvania, we used appropriate personal protective equipment and cleaned the workplace following the use of asbestos fibers.
Characterization of Asbestos Fibers
To assess changes in mineral properties of asbestos fibers due to the presence of water during grinding, we compared mineral phase, morphology, and surface element concentrations of fibers ground for 15 min under dry and wet conditions. Mineralogy was determined using X-ray diffraction analysis (X’Pert Powder Diffractometer with X’Celerator Detector, PANalytical B.V., Almelo, The Netherlands). Samples were back-packed into 26 mm diameter holders and exposed to Cu Kα radiation over a range of 5–70° 2θ at 2 s per 0.02° step. The XRD data were analyzed qualitatively for mineral phases present using HighScore Plus (Version 4.3, Panalytical). The size and morphology of ground fibers were determined via scanning electron microscopy (SEM) and energy-dispersive X-ray spectrographic (EDS) analysis (Quanta 600 FEG Mark II low vacuum, FEI Company, Hillsboro, OR, U.S.A.). Fiber samples were homogeneously suspended in deionized water, and a 10 µL drop of the suspension was air-dried on a support grid (holey carbon on 200 mesh Cu, SPI supplies). The grid with asbestos fibers was then mounted on double-sided carbon tape and analyzed for size, morphology, and concentrations of iron and other elements found in asbestos fibers. Suspension of fiber in DI water prior to SEM-EDS analysis may displace some iron from asbestos fibers. However, this displacement is assumed to have a minimal effect on the comparison of iron concentration between dry- and wet-ground fibers because both types of fibers were prepared using the same SEM protocol.
Quantification of Asbestos-Induced ROS in Elicited Murine Peritoneal Macrophages
To examine the effect of grinding time, we compared the asbestos-induced ROS generation in fibers ground in the presence of water for 5, 15, and 30 min. To examine the effect of different grinding methods, we compared the asbestos-induced ROS generation of fibers ground for 15 min with (wet) or without water (dry). We measured asbestos-induced ROS in peritoneal macrophages (MF) as an indicator of the fiber toxicity as described previously.17 Macrophages from mice were harvested from the peritoneum following elicitation using thioglycollate broth (see the Supporting Information for method details). We utilized a fluorogenic probe (CellROX Green Reagent, Molecular Probes by Life Technologies, Eugene, OR, U.S.A.) to determine levels of oxidative stress in live murine peritoneal macrophages. CellROX Green Reagent (CGR) is a cellpermeant dye that produces a green photostable fluorescent signal upon oxidation in the presence of ROS. Plated MF cells were treated with vehicle (PBS) with or without the selected ground fibers at a concentration of 20 µg/cm2. The fiber concentration was chosen based on a fiber dose–response relationship tested in our previous study.17 At 6 h post-asbestos exposure, cells were stained with 5 µM CGR (and DAPI for fluorescent imaging) by adding the probe(s) to complete media and incubating at 37 °C for 30 min. Cells were washed three times with PBS and the fluorescence intensity was then measured using a SpectraMax i3Multi-Mode Microplate Detection Platform (Molecular Devices, Sunnyvale, CA, U.S.A.) using an excitation wavelength of 485 nm, with fluorescence emission detection at 520 nm. Data are presented as mean ± standard error of the mean. Fluorescence microscopy was also performed on stained cells, and images were captured on a Eclipse TE2000-U microscope (Nikon, Japan) equipped with a digital camera (Retiga 2000R, QImaging, Surrey, BC, Canada) using 20× magnification.
Statistical Analysis
Statistically significant differences in ROS levels between vehicle and wet/dry conditions were determined using unpaired t tests (GraphPad Prism v6, La Jolla CA, U.S.A.). To identify statistically significant differences between results of dry- and wet-grinding treatments, one-way analysis of variance (ANOVA) was performed with Tukey’s post hoc test using R. Statistically significant differences were determined at a p-value of 0.05. Asterisks shown in figures indicate significant differences between groups (* = p < 0.05).
RESULTS AND DISCUSSION
Effect of Grinding Conditions on the Fiber Properties
Dry grinding of chrysotile ore produced typical white fibers, whereas wet grinding produced gray fibers (Figure 1). Increasing the grinding duration produced darker gray fibers. X-ray diffraction analysis of dry- and wet-ground fibers did not reveal any significant change in the mineralogy of chrysotile fibers (Figure 2). With an increase in dry-grinding duration from 5 to 30 min, the characteristic peak height of chrysotile fibers became smaller. This result indicates an increase in amorphous powder or a decrease in crystallite size during dry-grinding treatment, implying a net shortening of the fibers. This result is expected based on observations from previous studies,5,8 which show that dry grinding can reduce the size of fibers and alter their structure. Suquet5 shows that the basal spacing of the peaks from ground chrysotile become less intense due to fragmentation of fibers, explaining the observed decrease in peak height of the principal diffraction angles with an increase in grinding duration. In contrast, increasing the wet-grinding duration from 15 to 30 min increased the peak height, suggesting that the wet-grinding method did not destroy the fibers. Water is known to adsorb on the surface of fibers and protect the fiber from amorphization in water.7 Thus, the increase in grinding duration may only enhance the separation of individual fibrils from the associated bundles, thus increasing the apparent crystallite numbers and increasing peak intensities. Compared to dry-ground fibers, wet-ground fibers produced a peak at 48.25°. The identity of the peak could not be verified with certainty, although it is likely a weathering product of the chrysotile, such as talc, which features a noticeable peak near this angle. Other typical weathering products (e.g., vermiculite, smectite) could not be ruled out, as there was not enough of this phase after 30 min of grinding. Expanding the scan angle to 90°, in order to see the talc peak ca. 80°, could help address this issue, but the exact nature of the phase was not important to the study.
Figure 1.
Grinding of chrysotile fiber bundles (A) with or without water affected the color of fibers produced. Dry grinding produced typical white fibers (B), whereas wet grinding produced gray fibers (C). The dry-ground fibers were soaked in water before the picture was taken.
Figure 2.
X-ray diffraction result for chrysotile fibers exposed to wetand dry-grinding treatments for 5, 15, and 30 min. Fibers produced by 5 min of wet grinding were too large for XRD analysis. The vertical dashed lines indicate the characteristic peaks of chrysotile fibers.
Using SEM/EDS-EDX, we compared the morphology and elemental properties of asbestos fibers from wet- and dry-grinding treatment for 15 min. The result shows that wet grinding (or the presence of water) preserved fiber integrity and created individual fibrils with a high aspect ratio, whereas dry grinding broke fibers along the axis, primarily creating fiber bundles with a smaller aspect ratio (Figure 3). The dry-ground fiber length was less than 20 µm; conversely, fiber length after wet grinding exceeded 100 µm (Figure 3). The result provides further evidence that water protects the fiber during grinding.
Figure 3.
Chrysotile fibers produced by 15 min of grinding in dry (left) and wet (right) conditions. Wet-grinding method created fibers with high aspect ratios and disassociated fiber bundles, whereas dry-grinding method produced fiber bundles with a shorter aspect ratio.
Comparing the EDS spectrum (data not shown) of dry- and wet-ground fibers, we found that the iron content of dry-ground fibers (2.3% by weight) was nearly 7 times higher than the iron content of wet-ground fibers (0.3%). We attributed this result to two factors: First, dry grinding broke the asbestos bundle along the axis,5 which potentially exposes more structural iron. Second, wet grinding could dissolve brucite from the chrysotile crystal,19,20 which in turn would permit dissolution of iron from the fiber surface. The change in surface iron concentration during wet grinding in our study demonstrates that the grinding condition affects not only fiber morphology but also its elemental composition.
Effect of Grinding Conditions on the Fiber-Induced ROS Generation
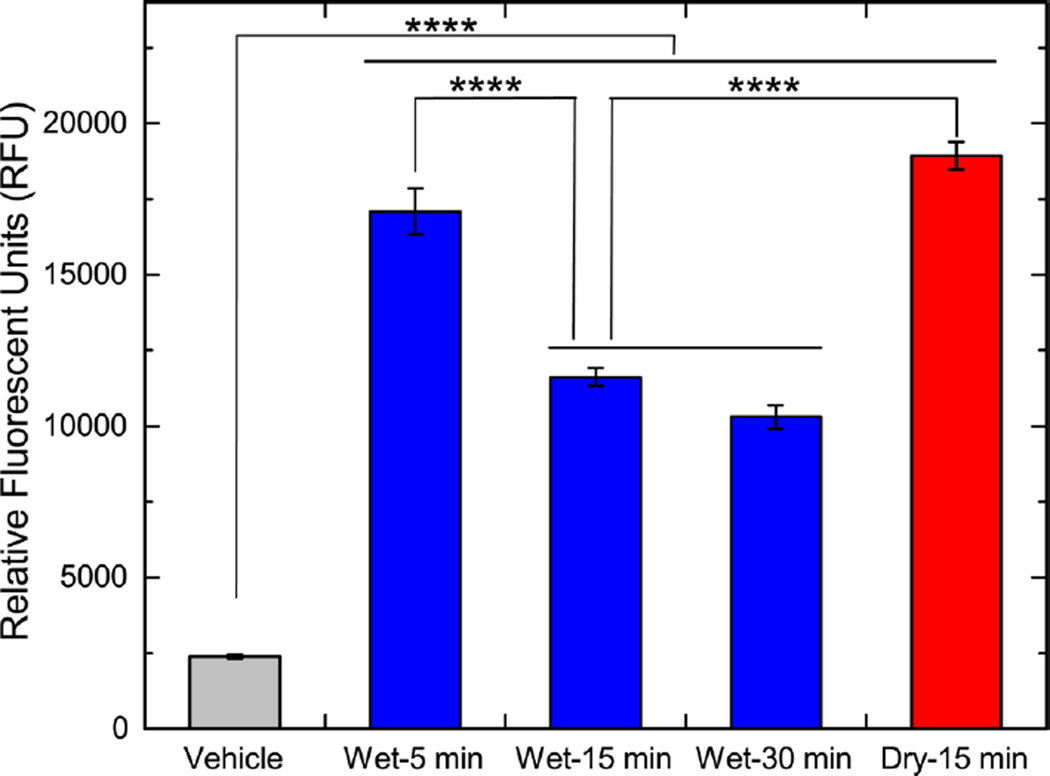
To assess the toxicity of fibers created by dry- and wet-grinding methods, we determined levels of asbestos-induced ROS in elicited murine peritoneal macrophages at 6 h after asbestos exposure. Compared to vehicle (PBS)-treated macrophages, exposure to asbestos fibers led to a significant (p < 0.0001) increase in ROS (Figure 4). On the basis of the one-way ANOVA test, 15 min dry-ground fibers generated significantly higher (p < 0.0001) ROS than 15 min wet-ground fibers, which indicates that the presence of water during grinding lowered the amount of ROS generated by the ground fibers. Wet grinding of asbestos fibers from 5 to 15 min caused a significant decrease in the generation of ROS (p < 0.0001), whereas grinding beyond 15 min did not significantly (p = 0.504) decrease ROS generation. Unlike in the wet-ground group, in the dry-ground group we did not examine the effect of various grinding times on ROS generation. This is because longer periods of dry grinding, such as for 30 min, significantly damaged the fibers by lowering the fiber size and creating amorphous chrysotile dust, as explained earlier. This change in fiber properties, in addition to the change in surface iron concentration, would have a confounding effect on ROS generation. Asbestos fibers have been shown to participate in redox reactions generating reactive oxygen species through multiple mechanisms, including hydroxyl radicals generated either through a redox reaction or by catalyzing a Fenton-like reaction in exposed cells.21 In this experiment, asbestos fiber internalization generated a significant increase in intracellular ROS as determined by a fluorescent dye. Compared to wet-ground fibers (15 min), dry-ground fibers (15 min) generated significantly (p < 0.0001, Figure 4) more ROS, likely due to higher iron content. On the basis of SEM-EDS analysis and asbestos-induced ROS production by macrophages, we conclude that wet grinding causes a net reduction in fiber iron content. A decrease in asbestos-induced ROS with an increase in grinding duration in the presence of water further confirm this idea.
Figure 4.
Effect of grinding method for chrysotile asbestos on the levels of asbestos-induced ROS in murine peritoneal macrophages, as assessed via the fluorescent probe, CellROX Green Reagent. Data are presented as mean ± standard deviation of the mean and **** indicates p < 0.0001.
In summary, we show that fiber preparation conditions can affect the fiber toxicity. Cytotoxicity (as determined by the generation of asbestos-induced ROS) of fibers produced by the dry-grinding method was higher than cytotoxicity of fibers resulting from wet grinding. Estimating the iron concentration in chrysotile after dry and wet grinding, we showed that differences in the surface iron concentration directly relate to the cytotoxicity of the fibers. Thus, it is important to consider the fiber preparation method and resulting changes in surface chemical properties when conducting future research examining toxicity of asbestos fibers or comparing asbestos toxicity between reported studies.
Supplementary Material
Acknowledgments
This work was funded in part by NIH-R03 CA180548 (M.C.-S.) by pilot project support from 1P30 ES013508-02 awarded to M.C.-S., and the National Institute Environmental Health Sciences of the National Institutes of Health under award number P42 ES023720 (J. K. W. and M.C.-S). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH. We thank the reviewers for their comments.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Method for isolation of elicited murine peritoneal macrophages; fluorescent images of asbestos-induced ROS activity in murine peritoneal macrophages (Figure S1). (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.Cunningham HM, Pontefract R. Asbestos Fibres in Beverages and Drinking Water. Nature. 1971;232:332–333. doi: 10.1038/232332a0. [DOI] [PubMed] [Google Scholar]
- 2.Fortunato L, Rushton L. Stomach cancer and occupational exposure to asbestos: a meta-analysis of occupational cohort studies. Br. J. Cancer. 2015;112:1805–1815. doi: 10.1038/bjc.2014.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Research Council. Asbestos: Selected Cancers. Washington, DC: National Academies Press; 2006. p. xi. [PubMed] [Google Scholar]
- 4.Frank AL, Joshi TK. The Global Spread of Asbestos. Ann. Glob. Health. 2014;80:257–262. doi: 10.1016/j.aogh.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Suquet H. Effects of dry grinding and leaching on the crystal structure of chrysotile. Clays Clay Miner. 1989;37:439–445. [Google Scholar]
- 6.Assuncao J, Corn M. The effects of milling on diameters and lengths of fibrous glass and chrysotile asbestos fibers. Am. Ind. Hyg. Assoc. J. 1975;36:811–819. doi: 10.1080/0002889758507347. [DOI] [PubMed] [Google Scholar]
- 7.Papirer E. Grinding of chrysotile in hydrocarbons, alcohol, and water. Clays Clay Miner. 1981;29:161–170. [Google Scholar]
- 8.Spurny KR, Stöber W, Opiela H, Weiss G. On the problem of milling and ultrasonic treatment of asbestos and glass fibers in biological and analytical applications. Am. Ind. Hyg. Assoc. J. 1980;41:198–203. doi: 10.1080/15298668091424609. [DOI] [PubMed] [Google Scholar]
- 9.Bonneau L, Malard C, Pezerat H. Studies on surface properties of asbestos: II. Role of dimensional characteristics and surface properties of mineral fibers in the induction of pleural tumors. Environ. Res. 1986;41:268–275. doi: 10.1016/s0013-9351(86)80188-8. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L, Akatsuka S, Nagai H, Chew SH, Ohara H, Okazaki Y, Yamashita Y, Yoshikawa Y, Yasui H, Ikuta K, Sasaki K, Kohgo Y, Hirano S, Shinohara Y, Kohyama N, Takahashi T, Toyokuni S. Iron overload signature in chrysotile-induced malignant mesothelioma. J. Pathol. 2012;228:366–377. doi: 10.1002/path.4075. [DOI] [PubMed] [Google Scholar]
- 11.Foresti E, Fornero E, Lesci IG, Rinaudo C, Zuccheri T, Roveri N. Asbestos health hazard: A spectroscopic study of synthetic geoinspired Fe-doped chrysotile. J. Hazard. Mater. 2009;167:1070–1079. doi: 10.1016/j.jhazmat.2009.01.103. [DOI] [PubMed] [Google Scholar]
- 12.Aust A, Lund L. The role of iron in asbestos-catalyzed damage to lipids and DNA. Biol. Oxid. Syst. 1990;2:597–605. [Google Scholar]
- 13.Aust EA, Lund LG, Chao C-C, Park S-H, Fang R. Role of iron in the cellular effects of asbestos. Inhalation Toxicol. 2000;12:75–80. doi: 10.1080/08958378.2000.11463232. [DOI] [PubMed] [Google Scholar]
- 14.Fubini B, Mollo L. Role of iron in the reactivity of mineral fibers. Toxicol. Lett. 1995;82–83:951–960. doi: 10.1016/0378-4274(95)03531-1. [DOI] [PubMed] [Google Scholar]
- 15.Pascolo L, Gianoncelli A, Schneider G, Salome M, Schneider M, Calligaro C, Kiskinova M, Melato M, Rizzardi C. The interaction of asbestos and iron in lung tissue revealed by synchrotron-based scanning X-ray microscopy. Sci. Rep. 2013;3:1123. doi: 10.1038/srep01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollastri S, D’Acapito F, Trapananti A, Colantoni I, Andreozzi GB, Gualtieri AF. The chemical environment of iron in mineral fibres. A combined X-ray absorption and Mössbauer spectroscopic study. J. Hazard. Mater. 2015;298:282–293. doi: 10.1016/j.jhazmat.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Pietrofesa R, Velalopoulou A, Albelda S, Christofidou- Solomidou M. Asbestos Induces Oxidative Stress and Activation of Nrf2 Signaling in Murine Macrophages: Chemopreventive Role of the Synthetic Lignan Secoisolariciresinol Diglucoside (LGM2605) Int. J. Mol. Sci. 2016;17:322. doi: 10.3390/ijms17030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, Liu Z-G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campopiano A, Cannizzaro A, Angelosanto F, Astolfi ML, Ramires D, Olori A, Canepari S, Iavicoli S. Dissolution of glass wool, rock wool and alkaline earth silicate wool: Morphological and chemical changes in fibers. Regul. Toxicol. Pharmacol. 2014;70:393–406. doi: 10.1016/j.yrtph.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Gronow JR. The Dissolution of Asbestos Fibers in Water. Clay Miner. 1987;22:21–35. [Google Scholar]
- 21.Blake DJ, Bolin CM, Cox DP, Cardozo-Pelaez F, Pfau JC. Internalization of Libby amphibole asbestos and induction of oxidative stress in murine macrophages. Toxicol. Sci. 2007;99:277–288. doi: 10.1093/toxsci/kfm166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.