Summary
The introduction of intravenous immunoglobulin (IVIG) for modulation of inflammation in acute Kawasaki disease (KD) was a great therapeutic triumph. However, three decades later, the mechanisms underlying immune regulation by IVIG are only beginning to be revealed. Stimulation of an immature myeloid population of dendritic cells (DC) that secretes IL-10 and the elucidation of Fc-specific, HLA-restricted natural regulatory T cells (Treg) provide insights into mechanisms of IVIG. Other potential mechanisms include provision of agent-specific neutralizing antibody, anti-idiotype and anti-cytokine antibodies, blockade of activating Fcγ receptors, and stimulation of the inhibitory FcγRIIb receptor. New initiatives must seek to understand the mechanisms of IVIG in order to one day replace it with more affordable and more targeted therapies.
Keywords: pediatric vasculitis, coronary artery aneurysms, immune regulation, Kawasaki disease, acquired heart disease, myocardial infarction
Kawasaki disease (KD) is a self-limited vasculitis that is the most common cause of acquired heart disease in infants and children. The condition is currently diagnosed according to clinical criteria that rely on a constellation of clinical signs in conjunction with laboratory data revealing high levels of systemic inflammation [1]. Although KD was described by Tomisaku Kawasaki in the 1960s in Japan, awareness of the disease in Western countries began in 1974 with the English language publication of the original series of 50 patients[2]. If untreated, 25% of children will develop aneurysms of the coronary arteries and the extent to which KD may contribute to the burden of adult cardiovascular disease is of growing concern [3].
Historical perspective
In 1981, the landmark paper by Imbach and colleagues described the use of IVIG to treat idiopathic thrombocytopenic purpura (ITP) in children, and the paper was widely read in Japan[4]. Reasoning that KD was an acute inflammatory process and that IVIG appeared to modulate the immune system, Furusho in Japan used IVIG to treat 14 acute KD patients within the first 10 days after fever onset[5]. Patients were studied by coronary angiography in the subacute phase and 7 of 40 historical control patients developed coronary artery aneurysms compared to none of the 14 IVIG-treated patients. On the basis of these suggestive data, the U.S. Multicenter KD Study Group launched a randomized, open label trial of IVIG plus aspirin compared to aspirin alone[6]. The trial was stopped prematurely by the Data Safety Monitoring Board when 18 of 78 children (23%) in the aspirin group, as compared with 6 of 75 (8%) in the gamma globulin group developed coronary artery aneurysms at the two week time point (p = 0.01).
The efficacy of IVIG administered in the acute phase of KD is now well-established. A variety of dose regimens were initially used in Japan and the United States. A meta-analysis by Durongpisitkul demonstrated that the prevalence of coronary artery abnormalities was lower among patients who received the high-dose regimen of 2 gm/kg as a single dose as compared to those receiving lower-dose regimens[7,8]. It remains unknown whether the presumed higher peak level or its earlier attainment contribute more to the superior efficacy of the single high-dose regimen.
Mechanisms of action of IVIG in KD
There has been much speculation, but little data, on the mechanism of action of IVIG in acute KD. It is certainly plausible that IVIG interacts with many different arms of the immune and vascular systems to achieve the down-regulation of inflammation. The major goal of IVIG treatment of KD is to prevent coronary artery damage and to reduce tissue levels of inflammation. However, IVIG also mediates the rapid disappearance of fever, rash, conjunctival injection, and systemic malaise. Thus, the majority of KD patients, even those who go on to become IVIG-resistant or develop aneurysms, experience a dramatic clinical improvement within hours of initiating the IVIG infusion. The effect has been likened to the disappearance of the rash and clinical signs in scarlet fever patients during administration of horse anti-streptococcal antiserum in the pre-antibiotic era, raising the possibility that one mechanism of IVIG action is the neutralization of a toxin. Neutralization of conventional antigens or superantigens are also potential mechanisms of action, but elucidation of the causative agent or environmental trigger for KD will likely be necessary before these mechanisms can be fully understood. While these rapid anti-inflammatory actions of IVIG certainly benefit the patients, it is important to remember that the most important goal of therapy is to protect the vascular system and myocardium from immune-mediated damage.
Studies of IVIG action, largely from animal models, have demonstrated effects on pathways involving Fc receptors, complement, cytokines, and auto-antibodies. Cellular targets include endothelial cells and cells participating in both innate and adaptive immunity [9,10]. Shortcomings of the body of research on IVIG are that the majority of the work has been done in animal models and much of it delineates the phenomenology of IVIG treatment but fails to provide mechanism. The interactions of IVIG with components of the immune system are considered below and aspects relevant for KD treatment are noted.
Interaction with macrophage/monocytes: Blockade of Fc receptors on the reticuloendothelial cell system, most notably the spleen, appears to be a major mode of action for reducing antibody-mediated platelet destruction in ITP. Recent studies demonstrate that in a human model, sialylation is not necessary and that IgG dimers were most effective in blocking the FcgRs on monocyte-derived macrophages[11]. Stimulation of the inhibitory FcgRIIb by sialylated IVIG was demonstrated in the collagen-induced arthritis model in mice[12]. Although polymorphisms in both the activating (FcgRIIa and FcgRIIIb-NA1) and inhibitory (FcgRIIb) receptors have been variously reported to influence disease susceptibility, response to IVIG, and coronary artery outcome in KD, no direct link between IVIG and either receptor blockade or stimulation has been demonstrated in KD patients[13–18]. Down-regulation of gene expression of two FCGRs (FCGRIA, and FCGR3A) on activated monocytes from KD patients following IVIG infusion has been demonstrated but no mechanistic studies have expanded on this observation[19].
Interaction with dendritic cells (DC): DC are professional antigen presenting cells (APC) that prime naive T cells in vivo. IVIG dose-dependent DC-mediated expansion of peripherally-induced regulatory T cells (iTreg) has been reported in human cells and linked to prostaglandin E2 production[20]. This effect required only the Fab’ portion of the IgG molecule and required sialylation. It has been shown that the Fab’ activates follicular T helper cells, T helper 17 and T helper 1 cells that later become iTreg under appropriate antigen presentation and homing conditions. Our group recently demonstrated that in acute KD patients, an immature myeloid DC population is stimulated by the Fc to secrete IL-10 and influence the differentiation of T cells to Treg[21]. These tolerogenic DC do not decrease after IVIG in the subacute phase of KD as for canonical mature myeloid DC and further expand in patients treated with infliximab.
Interaction with antibodies: IVIG can neutralize auto-antibodies by providing anti-idiotypic IgG and can delay maturation of the germinal centers[9]. While these mechanisms have been postulated in KD, there is currently no evidence for a direct pathogenic role for autoantibodies in the pathogenesis of KD.
Interaction with T cells: Reported effects include modulation of T cell differentiation and cytokine release, increased T cell regulation, decreased proliferation of Th17 cells, and reduced cytokine release[22,23]. Both Fab’ and Fc effects have been described. The suppression of Th17 differentiation from naïve human T cells and the blocking of Th17 cell proliferation and pro-inflammatory cytokine release were mediated by the Fab’ through an inhibition of STAT3 phosphorylation. In contrast, effects on expansion of Treg are mediated by the Fc. In KD patients, direct evidence for an Fc-mediated mechanism of expansion of natural regulatory T cells (nTreg) and increased IL-10 production has recently been described by our group and is further discussed below[24].
Interaction with neutrophils: IVIG contains antibodies against siglecs expressed on the surface of neutrophils and binding to these lectins can result in cell death[25].
Interaction with NK cells: In KD patients, enumeration of circulating NK cells pre- and post-IVIG demonstrated a rapid increase in the number of circulating NK cells that presumably represented the influx of demarginated cells bound to endothelium[26].
Role of regulatory T cells in KD
The self-limited nature of KD and our T cell clonal studies in the acute phase suggest that Treg play an important role in mitigating the pro-inflammatory effect of pathogenic effector T cells that participate in the destruction of the media and internal elastic lamina of the coronary artery [27–30]. Two main Treg lineages have been described [31,32]: 1) natural (n)Treg are derived from the thymus during fetal life and recognize self peptides [33,34]; 2) peripherally-induced (i)Treg recognize not-self, arise from naïve T cells under appropriate conditions (i.e. TGFb) [35,36] and repeated antigenic stimulation [37–39]. Treg cells limit inflammation and restore immune tolerance to self and allo-antigens in humans, thus playing a critical role in allergy, autoimmunity, and transplantation [40–42]. Their most relevant feature is the secretion of IL-10, a suppressive cytokine that down-regulates the effector functions of cells of the innate and adaptive immune system [43,44]. During acute inflammation, the IL-10-mediated regulation of the innate and adaptive immune response can determine the balance between health and chronic immune-mediated diseases [45].
Mechanisms of IVIG action in KD
A lengthy catalogue of changes following IVIG administration in KD patients is largely descriptive and fails to get at mechanisms of action. Reduction in cytokine and chemokine levels, changes in cell populations including decreased numbers of circulating CD14+ monocyte/macrophages, neutrophils, activated T cells, increased numbers of circulating NK cells, and changes in lymphocyte subsets have all been noted following administration of IVIG in KD patients. However, the precise manner in which IVIG brings about these changes remains unknown.
Clinical trials using an Fc-enriched IVIG preparation showed similar efficacy to intact IVIG while a pepsin-treated IVIG enriched for Fab’ fragments was not effective in preventing coronary artery abnormalities [39, 40]. These results suggest that at least some of the beneficial effects of IVIG are mediated through the Fc. Our laboratory demonstrated that the function of the Fc following IVIG administration is related to the induction of immune-regulation in KD via two mechanisms: 1) stimulation of an immature myeloid population of dendritic cells (DC) that secretes IL-10 [41], which, in turn, leads to the expansion of iTreg [41]; and 2) stimulation in an antigen-specific, HLA-restricted nTreg population that recognize the Fc of IgG [42]. We found an association between the development of coronary artery abnormalities (CAA+) in KD patients and failure to expand Fc-specific Treg after IVIG [42].
Minimal Fc epitopes for Treg recognition
Short Fc-derived peptides (15 amino acids) tailored to fit the T cell receptor (TcR) and to bind the HLA without antigen processing are recognized by Treg in KD patients following IVIG treatment (Figure 1). We defined discrete immunodominant regions within the Fc protein that rapidly expand nTreg in vitro[46]. Epitope-specific Treg responses were also documented in KD patients who failed to respond to the whole Fc protein after IVIG. The same Fc sequences were recognized by healthy controls suggesting that IVIG stimulates the expansion of a population of nTreg important for preserving vascular homeostasis. These Fc-specific nTreg were detectable in pediatric patients with a variety of acute infections associated with fever and inflammation, but were not detectable in acute KD patients prior to IVIG treatment.
Figure 1.
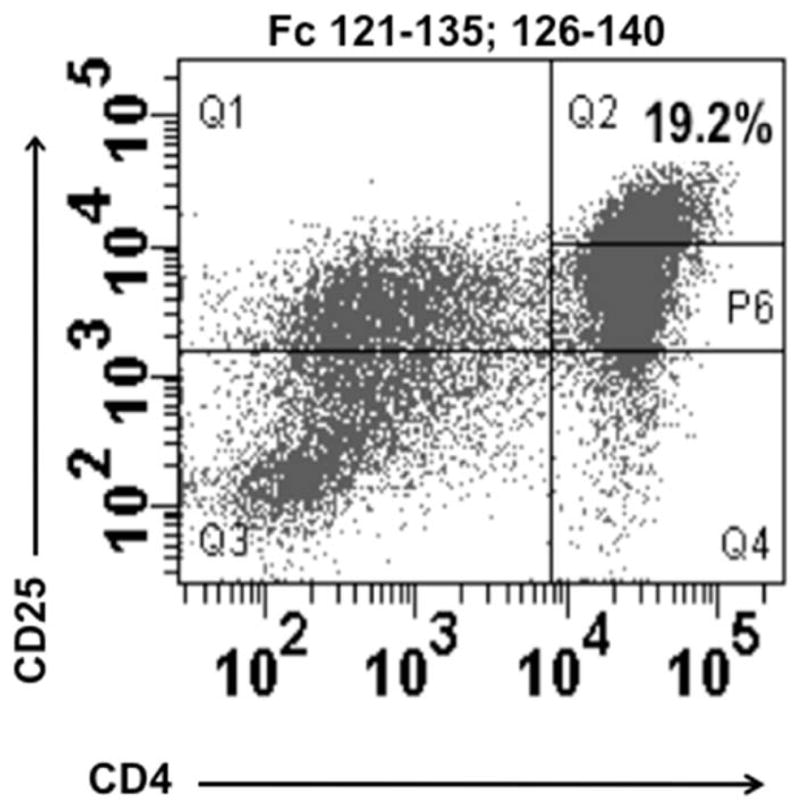
CD4+ CD25high nTreg rapidly expand when peripheral blood mononuclear cells derived from a sub-acute KD patient after IVIG are stimulated in vitro for four days with Fc peptide sequences 121–135 and 126–140. Reproduced with permission from Informa Healthcare [46].
These results suggest that the identification of immunodominant Fc epitopes capable of binding multiple HLA alleles could lead to the development of a valuable alternative to IVIG for KD patients. Recently, in a large genome-wide association study, KD susceptibility in Japanese children was associated with a polymorphism near the HLA-DQA2 locus on 6p21.3 [47]. The association of polymorphisms in HLA/DQB2 and HLD/DOB were recently validated in a study of KD trios of European descent, thus broadening the suspected importance of HLA in KD susceptibility [17]. The role of the HLA in the Fc-specific Treg response is currently under study by our laboratory.
IVIG Resistance
Although the majority of patients respond to single dose of IVIG with cessation of fever and improvement in clinical signs and laboratory markers of inflammation, a minority will have so- called IVIG resistance defined as persistent or recrudescent fever at 36 hours after the completion of the initial IVIG infusion. The immunologic basis for IVIG resistance is unknown and researchers have tried to glean clues from the success or failure of alternative therapies.
Based on the apparent dose response to IVIG, administration of a second dose of IVIG to resistant patients became first-line therapy for these patients and remains so today[9]. Alternative treatments include infliximab (5–10 mg/kg over 2 hours), steroids (prednisone 2 mg/kg/day for extended period), cyclosporine, anakinra, and plasmapheresis[10–14]. In a two-center, retrospective study of either second IVIG infusion or infliximab as the first re-treatment, patients with IVIG resistance who were treated with infliximab had more rapid resolution of fever and inflammatory markers, fewer days in hospital, and lower costs of care[15]. There was no difference in coronary artery outcomes between groups.
A different strategy pursued by several investigators has been to select agents that could be added to the initial IVIG regimen to prevent recrudescent fever. The RAISE study in Japan used a scoring system that works well in Japanese patients to select those who were likely to be refractory to initial therapy[16]. These patients were then randomized to receive either 2 mg/kg of prednisolone for 3–5 weeks (based on normalization of C-reactive protein levels) or placebo in addition to standard therapy. There were significantly fewer patients with coronary artery abnormalities in the group receiving prednisolone plus IVIG (3% vs. 23%, CI 0.12–0.28, p<0.001). Caveats to the adoption of this protocol for other countries is that the scoring system has been shown to have poor predictive value in mixed ethnic populations and patients with coronary artery abnormalities on their first echocardiogram at diagnosis were excluded[17, 18]. In a U.S. population, of the patients who go on to develop aneurysms, 81% had an abnormal first echocardiogram and would have been excluded from the RAISE study[19]. Two randomized, controlled trials of intensification of initial therapy with either high-dose pulse methylprednisolone or infliximab were not successful in preventing IVIG resistance [20, 21]. In the infliximab trial, 13% of KD patients treated with either infliximab (5 mg/kg) or placebo followed by standard 2 gm/kg IVIG plus aspirin within the first 10 days after fever onset were IVIG-resistant [21]. The group receiving infliximab did have more rapid resolution of inflammatory markers, fewer fever days, and more rapid improvement in Z score of the left anterior descending coronary artery.
Selection of alternative treatments in the setting of IVIG resistance remains confounded by an incomplete understanding of the mechanisms of action IVIG in acute KD. In a study of the role of sialylation of both infused and native IVIG in KD patients, no relationship was seen between IVIG resistance and level of sialylation of the infused IVIG[22]. However, patients with IVIG resistance had lower levels of sialylation of their endogenous IgG and lower transcript and protein levels of the enzyme, ST6GAL1 that adds sialic acid to the terminal galactose on the Fc of IgG. These findings were stable over time suggesting a genetic basis to this difference in glycosylation patterns. The hypothesis is that patients who are IVIG-resistant have a genetic difference in the enzyme system (ST6GAL1 and others) that adds terminal sialic acid to the GalNac structure on the Fc and other proteins. However, thus far, the small, unreplicated genetic association studies of IVIG resistance in KD patients have not uncovered determinants affecting sialylation[23]. The largest genetic case:control study to date of IVIG responsive versus resistant subjects of European descent identified loci in intergenic regions of several genes that have also been associated with immune pathways and autoimmune diseases[17]. These results will need to be validated in additional studies, although small sample size will always be a limiting factor.
Expert commentary
Administration of IVIG in conjunction with aspirin should be the mainstay of therapy for all KD patients. Few other therapies in pediatrics have accumulated the same wealth of supporting clinical trial evidence. While the data supporting the clinical use are compelling, understanding the mechanisms through which IVIG works its magic are sadly lacking. Investigators have carefully catalogued the changes in cytokines, cell populations, and gene expression before and after IVIG administration, but few are working with patient samples and cells to get at the specific mechanisms that are operative in KD. Given the current body of information, it seems likely that both the Fc and Fab’ portions of the IgG molecule play a role. The fact that patients who develop coronary artery abnormalities fail to expand the Fc-specific nTreg population argues for an important role of nTregs in modulating tissue-level inflammation. The rapid reduction of clinical signs of inflammation is more likely due to Fab’-mediated effects by anti-cytokine, anti-idiotype, and possibly anti-toxin or anti-causative agent antibodies, but the exact mechanisms have not been elucidated.
Five-year view
Important steps forward over the next five years will hopefully include epitope mapping of the Fc to determine the critical peptide sequences for nTreg recognition and the role of HLA on antigen presentation to the Fc-specific nTregs. The importance of sialylation and IgG aggregates must be further studied to understand both host effects and the role of sialic acid in the binding of IVIG to APCs. We must not lose sight of the fact that KD is a vasculitis and further studies of the effects of IVIG on the endothelium must be pursued. The genetics of IVIG-resistance must be understood through large cooperative studies to gather sufficient study subjects for robust genotyping and validation cohorts. The genetics of host sialylation and its effect on response to therapy and disease outcome should also be a research priority. Finally, we must remember that the majority of the children in the world who suffer from KD live in resource-challenged settings where IVIG is neither available nor affordable. Thus, the motivating factor behind the study of mechanisms of action of IVIG should be its eventual replacement by more affordable recombinant small molecules, peptides, or antibodies.
Key Issues.
IVIG likely acts through many different pathways in KD to achieve the downregulation of systemic and tissue-level inflammation but little is known about the underlying mechanisms
Mechanisms involving the Fab’ region may include neutralization of the inciting agent, toxin, or superantigen and neutralization of pro-inflammatory molecules including cytokines, but there is no specific experimental data that addresses these possibilities.
T cell regulation appears to be important at the tissue level for the resolution of inflammation in KD and evidence suggests that a) the Fc stimulate immature myelogenic DC to secrete IL-10 and lead to the expansion of Treg and b) Fc peptides are processed and presented by APCs to a specialized subset of Fc-specific nTregs that proliferate and secrete IL-10. It is proposed that these mechanisms modulate vascular inflammation as Fc-specific nTreg from patients who develop aneurysms fail to expand in vitro when incubated with purified Fc fragments but do expand when incubated with recombinant peptides from specific regions of the Fc.
Preliminary data suggests that enzymes involved in sialylation of native IgG molecules may play a role in IVIG resistance.
Polymorphisms associated with IVIG resistance have been described but await validation. These findings suggest that IVIG resistance may influenced by host genetics.
Footnotes
Financial and competing interest’s disclosure
The authors were supported by grants from the National Institutes of Health (grant numbers: NIH R01 HL103536 to A Franco and JC Burns and U54 HL108460 to JC Burns) and from the University of California (UCSD – UL1 RR031980 to A Franco). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment and long term management of Kawaski disease: a statement for helath professionals from the Committee for Rheumatic Fever, Endocarditis and Kawaski Disease, Council on Cardiovascular Diseases in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki T, Kosaki F, Okawa S, et al. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54(3):271–276. [PubMed] [Google Scholar]
- 3.Burns JC, Shike H, Gordon JB, et al. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253–257. doi: 10.1016/0735-1097(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 4.Imbach P, Barandun S, d’Apuzzo V. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 5.Furusho K, Sato K, Soeda T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1983;2(8363):1359. doi: 10.1016/s0140-6736(83)91109-1. [DOI] [PubMed] [Google Scholar]
- 6*.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. The New England journal of medicine. 1986;315(6):341–347. doi: 10.1056/NEJM198608073150601. Randomized, open-label study of IVIG plus aspirin versus aspirin alone that established IVIG as effective therapy for KD. At 2 weeks after enrollment, coronary artery abnormalities were present in 18 of 78 children (23%) in the aspirin alone group, as compared with 6 of 75 (8%) in the IVIG group (P = 0.01) [DOI] [PubMed] [Google Scholar]
- 7*.Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96(6):1057–1061. Important meta-analysis that helped to settle the debate about optimal dose of IVIG in KD and suggested a dose-response threshold. [PubMed] [Google Scholar]
- 8.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. The New England journal of medicine. 1991;324(23):1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 9.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. The New England journal of medicine. 2001;345(10):747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 10.Tha-In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29(12):608–615. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Nagelkerke S, Dekkers G, Kustiawan I, et al. Inhibition of FcgR-mediated phagocytosis by IVIG is independent of IgG-Fc sialylation and FcgRIIb in human macrophages. Blood. 2014;124:3709–3718. doi: 10.1182/blood-2014-05-576835. [DOI] [PubMed] [Google Scholar]
- 12.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khor CC, Davila S, Breunis WB, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nature genetics. 2011;43(12):1241–1246. doi: 10.1038/ng.981. [DOI] [PubMed] [Google Scholar]
- 14.Duan J, Lou J, Zhang Q, et al. A Genetic Variant rs1801274 in FCGR2A as a Potential Risk Marker for Kawasaki Disease: A Case-Control Study and Meta-Analysis. PLoS ONE. 2014;9(8):e103329. doi: 10.1371/journal.pone.0103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrestha S, Wiener H, Shendre A, et al. Role of activating FcgammaR gene polymorphisms in Kawasaki disease susceptibility and intravenous immunoglobulin response. Circ Cardiovasc Genet. 2012;5(3):309–316. doi: 10.1161/CIRCGENETICS.111.962464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha S, Wiener HW, Olson AK, et al. Functional FCGR2B gene variants influence intravenous immunoglobulin response in patients with Kawasaki disease. The Journal of allergy and clinical immunology. 2011;128(3):677–680. doi: 10.1016/j.jaci.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shendre A, Wiener HW, Zhi D, et al. High-density genotyping of immune loci in Kawasaki disease and IVIG treatment response in European-American case-parent trio study. Genes and immunity. 2014 doi: 10.1038/gene.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onouchi Y, Ozaki K, Burns JC, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nature genetics. 2012;44(5):517–521. doi: 10.1038/ng.2220. [DOI] [PubMed] [Google Scholar]
- 19.Abe J, Jibiki T, Noma S, Nakajima T, Saito H, Terai M. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. J Immunol. 2005;174(9):5837–5845. doi: 10.4049/jimmunol.174.9.5837. [DOI] [PubMed] [Google Scholar]
- 20.Trinath J, Hegde P, Sharma M, et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood. 2013;122(8):1419–1427. doi: 10.1182/blood-2012-11-468264. [DOI] [PubMed] [Google Scholar]
- 21.Burns JC, Song Y, Bujold M, et al. Immune-monitoring in Kawasaki disease patients treated with infliximab and intravenous immunoglobulin. Clin Exp Immunol. 2013;174(3):337–344. doi: 10.1111/cei.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Ephrem A, Chamat S, Miquel C, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111(2):715–722. doi: 10.1182/blood-2007-03-079947. First attempt at understanding the role of sialylation in response to IVIG. Study showed that levels of α2,6-linked sialic acid on native IgG from patients with IVIG-resistance was lower compared to IVIG-responsive patients and controls. Sialic acid levels on infused IVIG product was not different between the groups. [DOI] [PubMed] [Google Scholar]
- 23.Maddur MS, Vani J, Hegde P, et al. Inhibition of differentiation, amplification, and function of human TH17 cells by intravenous immunoglobulin. The Journal of allergy and clinical immunology. 2011;127(3):823–830. e821–827. doi: 10.1016/j.jaci.2010.12.1102. [DOI] [PubMed] [Google Scholar]
- 24**.Franco A, Touma R, Song Y, et al. Specificity of regulatory T cells that modulate vascular inflammation. Autoimmunity. 2014;47:95–104. doi: 10.3109/08916934.2013.860524. Describes one mechanism by which IVIG might modulate inflammation in acute KD through expansion of Fc-specific nTreg that expand and secrete IL-10 following IVIG infusion. Experiments have implications beyond KD and describe a new story in T cell/B cell cooperation. [DOI] [PubMed] [Google Scholar]
- 25.von Gunten S, Schaub A, Vogel M, et al. Immunologic and functional evidence for anti-Siglec-9 autoantibodies in intravenous immunoglobulin preparations. Blood. 2006;108(13):4255–4259. doi: 10.1182/blood-2006-05-021568. [DOI] [PubMed] [Google Scholar]
- 26.Finberg RW, Newburger JW, Mikati MA, et al. Effect of high doses of intravenously administered immune globulin on natural killer cell activity in peripheral blood. J Pediatr. 1992;120(3):376–380. doi: 10.1016/s0022-3476(05)80900-x. [DOI] [PubMed] [Google Scholar]
- 27.Franco A, Shimizu C, Tremoulet, et al. Memory T cells and characterization of peripheral T cell clones in acute Kawasaki disease. Autoimmunity. 2010;43:317–324. doi: 10.3109/08916930903405891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco A, Almanza G, Burns JC, et al. Endoplasmic reticulum stress drives a regulatory phenotype in human T cell clones. Cell Immunol. 2010;266:1–6. doi: 10.1016/j.cellimm.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu C, Oharaseiki T, Takahashi K, Kottek A, Franco A, Burns JC. The role of TGF-b and myofibroblasts in the arteritis of Kawasaki disease. Human pathology. 2012;44:189–198. doi: 10.1016/j.humpath.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franco A, Touma R, Song Y, et al. Specificity of regulatory T cells that modulates vascular inflammation. Autoimmunity. 2014;47:95–104. doi: 10.3109/08916934.2013.860524. [DOI] [PubMed] [Google Scholar]
- 31.Makoto M, Yoshioka Y, Kitoh A, et al. Functional delination and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 32*.Feuerer M, Hill JA, Mathis D, Benoit C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nature Immunology. 2010;10:698–695. doi: 10.1038/ni.1760. Microarray paper that outlines changes in transcript abundance patterns in response to IVIG in KD patients. Provides a good catalogue of gene expression changes over time in response to IVIG. [DOI] [PubMed] [Google Scholar]
- 33.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 34.Miyara M, Yoshioka Y, Kito A, et al. Functional delineation and differentiation dinamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Chen WJ, Jin W, Hardegen N, et al. Conversion of Peripheral CD4+CD25- Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kretschmer K, Apostolou I, Hawiger D, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nature Immunol. 2005;6:1–9. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 37.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 38.Apostolou I, von Boemer H. In vivo instruction od suppressor committment in naive T cells. Journal of Experimental Medicine. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivino L, Gruarin RL, Steinfelder, et al. CCR6 is expressed on IL-10 producing, autoreactive memory T cell population with context-dependent regulatory function. J Exp Med. 2010;207:565–577. doi: 10.1084/jem.20091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roncarolo MG, Battaglia M. Regulatory T cell immunotherapy for tolerance to self antigens and to alloantigens in humans. Nature Reviews. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 41.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature Immunology. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 42.von Boehmer H, Melchers F. Checkpoints in lymphocytes development and autoimmune disease. Nature Immunology. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 43.Kassiotis G, O’Garra A. Immunology. Immunity benefits from a little suppression. Science. 2008;320:1168–1169. doi: 10.1126/science.1159090. [DOI] [PubMed] [Google Scholar]
- 44.O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 46**.Burns JC, Touma R, Song Y, et al. Fine specificities of natural regulatory T cells after IVIG therapy in patients with Kawasaki disease. Autoimmunity. 2015:1–8. doi: 10.3109/08916934.2015.1027817. In vitro experiments with patient cells defines discrete immunodominant regions within the Fc protein that expand a subset of nTreg. Epitope-specific Treg responses were documented in KD patients who failed to respond to the whole Fc protein after IVIG. Suggests that antigen processing or presentation may define KD patients who develop aneurysms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onouchi Y, Ozaki K, Burns JC, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nature Genetics. 2012;44:517–521. doi: 10.1038/ng.2220. [DOI] [PubMed] [Google Scholar]


