Summary
A challenging component of vaccine development is the large serologic diversity of protective antigens. Remarkably, there is a conserved surface/capsular polysaccharide, one of the most effective vaccine targets, expressed by a large number of bacterial, fungal and eukaryotic pathogens: poly-N-acetyl glucosamine (PNAG). Natural antibodies to PNAG are poorly effective at mediating in vitro microbial killing or in vivo protection. Removing most of the acetate substituents to produce a deacetylated glycoform, or using synthetic oligosaccharides of poly-β-1-6-linked glucosamine conjugated to carrier proteins, results in vaccines that elicit high levels of broad-based immunity. A fully human monoclonal antibody is highly active in laboratory and preclinical studies and has been successfully tested in a phase-I setting. Both the synthetic oligosaccharide conjugate vaccine and MAb will be further tested in humans starting in 2016; but, even if effective against only a fraction of the PNAG-producing pathogens, a major advance in vaccine-preventable diseases will occur.
Keywords: Poly-N-acetyl glucosamine, conjugate vaccine, capsule, antibiotic-resistance, monoclonal antibody
Vaccine potential of PNAG
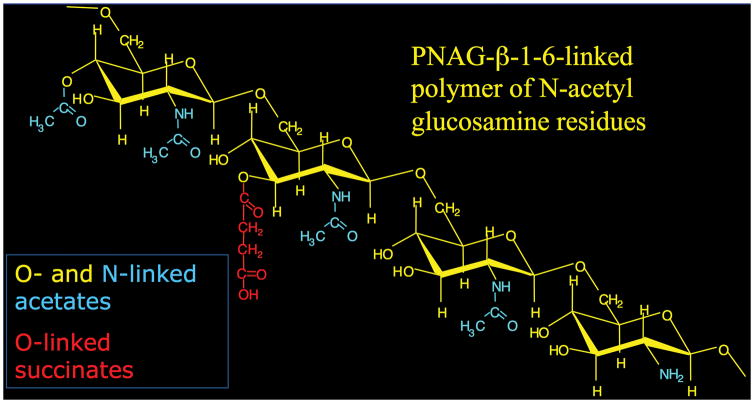
Vaccines are the most exciting and effective therapeutics for human and animal diseases. They were called by the Centers for Disease Control and Infection the most important medical advance of the 20th century [1], as they are exceedingly successful in preventing infections. Throughout the history of vaccine development, the largest challenge has been the serologic diversity of many, if not most, of the protective antigens, requiring either a large compendium of vaccine components for broad-based coverage (Streptococcus pneumoniae) [2], finding a means to deal with a continually evolving target antigen (influenza virus and human immunodeficiency virus) [3,4], or trying to address high in vivo antigenic shifts and drifts (Plasmodium spp.) [5]. Often vaccinologists must turn to finding conserved, but frequently suboptimal, antigens or epitopes as vaccines. Thus, a major paradigm underlying the success of many infectious agents is the utilization of antigenic variation to avoid host immunity and thus retain their virulence. Overall, it would seem that a conserved antigen, readily accessible to major cellular and humoral immune effectors, would be selected against by evolutionary forces that promote pathogen success. However, over the past 20 years, it has become apparent that such a vaccine target may exist for many non-viral pathogens and this vaccine might be developed for use in a single formulation to induce humoral immunity to the most common microbial pathogens of humans and animals, encompassing most Gram-negative and Gram-positive bacteria, including tuberculosis, as well as fungal pathogens such as Candida albicans, and even important protozoan pathogens such as those causing malaria [6]. The target is poly-N-acetyl glucosamine (PNAG), a polymer of β-(1-6)-linked N-acetylglucosamine residues with a relatively small amount of deacetylation occurring on 5–15% of the monosaccharide residues (Figure 1).
Figure 1.
To thwart host acquired immunity from being effective at preventing serious infection and disease against the collection of PNAG-producing pathogens, natural antibodies to this polysaccharide, found in most human and animal sera, are mostly ineffective at mediating microbial killing and protective immunity. Thus, these pathogens are unaffected by the native antibody response to PNAG. Fortuitously, it was discovered that by removing the acetates to produce a mostly polymeric β-1-6-linked N-glucosamine, or use of synthetic β-1-6-linked glucosamine (i.e., non-acetylated) oligosaccharides in conjugate vaccines [7–10], antibodies could be induced that not only bound to native, acetylated PNAG but also deposited complement onto microbial surfaces, mediated in vitro killing and showed protective efficacy against a large variety of PNAG-producing pathogens in pre-clinical studies [6,7,9]. We now stand on the brink of determining in humans and economically important commercial and companion animals whether immunity to PNAG will provide a massive increase in vaccine-preventable diseases, be efficacious against only some PNAG-producing microbes, or reveal un-expected consequences that could make safety considerations and side-effects outweigh the benefits of a PNAG vaccine. While animal studies to date, including some ongoing studies in farm animals, are highly encouraging that the benefits will be high from a PNAG vaccine, these tests are still in the early stages. Importantly, the evaluation of the vaccine has been initiated in farm animals, and will be tested in humans in 2016 along with a fully human IgG1 monoclonal antibody (MAb) to PNAG that has already undergone phase 1 testing [11], with phase II testing also scheduled for commencement in 2016. Those of us working on developing vaccines and MAbs to PNAG are cautiously optimistic about our chances to have a major impact on human and animal health, but also fully cognizant of the challenges, roadblocks and potential of unanticipated consequences that confront the clinical development of all drugs.
Biology of PNAG
From PS/A to PIA to SAA to PNAG
Some of the properties of what eventually turned out to be PNAG were initially described as the capsular polysaccharide/adhesin (PS/A) of S. epidermidis, although a definitive chemical composition and structure was not determined in these studies [12–14]. Mack et al. [13] first described the chemical proprieties of this surface polysaccharide and named it the polysaccharide intercellular adhesin (PIA) and attributed to PIA the property of mediating intercellular adherence of coagulase-negative staphylococci. McKenney et al. [14] found that PS/A and PIA were the same material and then reported in 1999 that the polymer was made by S. aureus, [15] which was also reported by Cramton et al. [16]. Another variant of the PNAG polymer containing more than 70% glucosamine residues was described as the slime-associated antigen (SAA) [17]. It is now clear that all 4 of these entities (PNAG, PS/A, PIA, SAA) are chemically related and nearly identical [18–20].
Functional properties of PNAG
PNAG plays a major role in biofilm formation by different microbial species (see ref [21] for a review). In 2004, experiments reported by Romeo and colleagues designed to identify components of the E. coli biofilm led to the discovery that many strains of this organism produced PNAG [22]. The locus in E. coli was named pga for polyglucosamine and bioinformatic investigations indicated a broad range of Gram-negative bacteria had the pga locus, implicating PNAG synthesis as a common factor among these organisms. Further investigations confirmed PNAG was a component of biofilms made by numerous Gram-negative bacterial species. In Acinetobacter baumannii, deletion of the pga locus led to loss of the biofilm phenotype, which was restored by complementation [23]. Similar findings were found with Burkholderia cepacia complex (BCC) [24], K. pneumoniae [25], and Bordetella pertussis [26]. Recently, PNAG’s role in biofilm formation was reported in Bacillus subtilis [27], the first Gram-positive bacillus found to produce PNAG.
PNAG has also been found to be an important virulence factor for several pathogens. In three different strains of S. aureus, Mn8, Newman and NCTC 10833 [28], loss of PNAG resulted in significantly reduced abilities to maintain bacterial levels in blood following intravenous or intraperitoneal (IP) injection in mice, or to spread systemically to the kidneys or induce a moribund/lethal state following IP infection. In E. coli, PNAG-deficient strains exhibited reduced fitness in the urinary bladder, kidneys and spleen compared to wild-type strains in murine models of ascending UTI and systemic infection, respectively [29]. In K. pneumoniae, PNAG-negative strains are less able to colonize the GI tract and to disseminate, and are lethal to significantly fewer mice after IP infection [25].
As a major component of the bacterial biofilm, PNAG has also been reported to help bacteria evade the immune system, and particularly opsonophagocytic killing [30], presumably by either preventing complement opsonins from binding to the bacterial cell surface or complement receptors on phagocytes from binding deposited opsonis, which are needed for efficient opsonic killing.
PNAG has also been reported to protect bacteria against environmental stresses. For instance, S. aureus, E. coli and K. pneumoniae producing PNAG were all better able to survive exposure to ethanol than isogenic PNAG-deficient mutants, suggesting PNAG serves as a type of microbial armor regulating interactions of planktonic cells with the external environment [6].
Genetics and biosynthesis of PNAG
The ica and pga loci
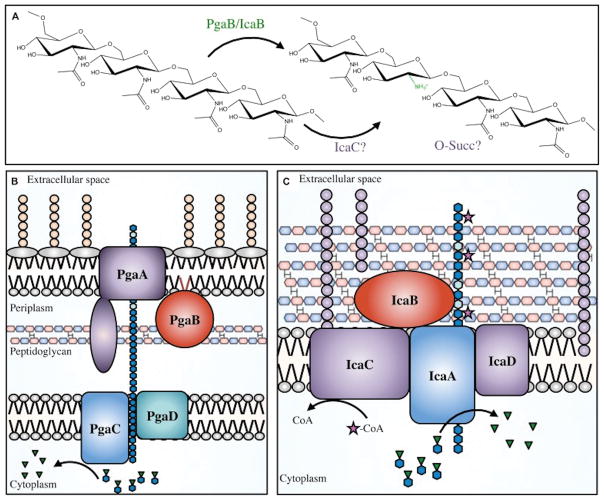
The molecular basis for PNAG production was initially discovered in S. epidermidis [31]. In 1996 Heilmann et al. [31] demonstrated that each one of three genes in a locus named icaABC were necessary for the production of PNAG. An ica operon mutant lost the ability to produce PNAG, which was restored by complementation with a plasmid carrying the ica locus. In addition, PNAG production was lost when any of the three genes were individually inactivated. In 1998, a fourth gene (icaD), encoded between icaA and icaB was identified by Gerke et al. [32] who also reported that the IcaA and IcaD proteins were located in the cytoplasmic membrane. Both exhibited N-acetylglucosaminyltransferase activity, in which IcaA represented a catalytic enzyme that required IcaD for full activity.
In 2004, Wang et al. [22] used random transposon (Tn) mutagenesis to identify novel genes required for biofilm development in E. coli. They found 17 Tn-insertions clustered in a small portion of the chromosome that resulted in E. coli mutants that were producing significantly less biofilm (~10-fold when compared to the parent strain). The locus, initially annotated as ycdSRQP, was renamed as pgaABCD. Each gene of the pga locus was necessary to produce a surface polysaccharide, whose chemical structure was determined by nuclear magnetic resonance analyses to be PNAG. From this discovery, homologs of the pga locus were found in numerous Gram-negative bacterial species [6,23–26,33]. The presence of pga genes is not enough, by itself, to firmly establish that the PNAG molecule is synthesized and expressed. Nonetheless, it is notable that, to date, essentially all the investigations finding pga genes in a microbe’s chromosome were able to confirm the presence of PNAG at the bacterial surface[6,22–24,26,33,34]. Interestingly, the introduction of the pga locus into a PNAG-negative bacteria can lead to the production of PNAG [27]. A schematic depiction of the biosynthesis of PNAG be proteins encoded by genes in the pga and ica loci is given in Figure 2, taken from reference [35].
Figure 2.
The hms locus of Y. pestis and eps locus of B. subtilis
PNAG has also been detected on the surface of bacterial species that have somewhat divergent genetic loci encoding PNAG biosynthetic enzymes and regulators. In Yesinia pestis, three operons, hmsHFRS, hmsT and hmsP, are responsible for the production of biofilms [33]. PNAG has been detected in biofilms of Y. pestis, synthesized by proteins encoded within the hmsHFRS locus, and production is controlled by the diguanylate cyclase HmsT and the EAL domain-containing phosphodiesterase, HmsP. These act as positive- and negative-regulators, respectively, of the transcription of the hmsHFRS locus. Amino acid sequence analysis showed that HmsR and HmsF are homologues of the S. epidermidis β-1,6-GlcNAc glycosyltransferase IcaA and deacetylase IcaB, respectively, and all four HmsHFRS proteins have 50–83% similarity to products encoded by pgaABCD.
PNAG was also detected in biofilms produced by Bacillus subtilis [27]. In these bacteria, 4 genes encoding the proteins for PNAG synthesis, epsHIJK, were found in a cluster within the 15 gene eps locus encpmassing epsA-epsO. Cloning each of the B. subtilis epsH-K genes into E. coli with individual in-frame deletions in each of the 4 PNAG biosynthetic genes, pgaA-D, respectively, restored PNAG production in E. coli. Cloning the entire B. subtilis epsHIJK locus into pga-deleted E. coli, K. pneumoniae, or alginate-negative P. aeruginosa also restored or conferred PNAG production [27]. In Y. pestis and B subtilis, and about 7 other bacterial species, when PNAG was extracted and analyzed, it was found to be a β-1-6-linked poly-N-acetylglucosamine molecule, with only small variations in the levels of amino-group acetylation (95 to 100%) and variation in the amount of O-linked acetates and succinates [22,36,37].
Detection of PNAG expression by a broad range of microbial pathogens
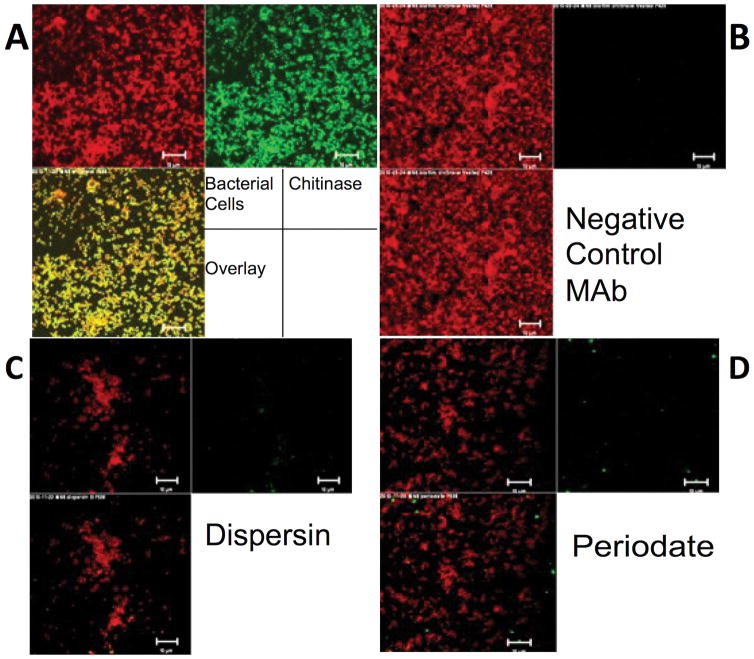
To detect the presence of PNAG on microbial cells, immunofluorescence microscopy using an antigen-specific MAb to PNAG, designated F598, is routinely used [38]. The specificity of the MAb has been confirmed in studies wherein binding is strongly detected using wild-type E. coli, S. aureus and A. baumannii cells, but not to these bacteria deleted for the pga or ica loci [6,23,39]. Binding is restored when an intact pga or ica locus is placed onto a plasmid or back into the chromosome. Another test uses the PNAG-degrading enzyme dispersin B that specifically hydrolyzes β-(1→6)–linked N-acetylglucosamine residues [40,41] to degrade the antigen and show concomitant loss of MAb F598 binding. Control treatment is with the β-1-4-linked N-acetylglucosamine hydrolyzing chitinase. Chitinase-resistant but dispersin B-sensitive binding of MAb F598 is highly specific to PNAG (Figure 3). This approach is particularly useful for identifying PNAG expression when encoding genes have not been fully identified. At the moment, PNAG production has been identified in over 30 microbial organisms (Table 1) including fungal (Candida albicans) and protozoan parasites (Plasmodium falciparum) [6], using chemical, genetic and/or immunologic analyses. For a number of these, the protective efficacy of antibody to PNAG in mouse models of infection has confirmed the antigen is expressed in vivo and a vaccine target [6]. Extensive work is ongoing to determine the precise genetic and molecular basis for PNAG production in all these species.
Figure 3.
Table 1.
Listing of organisms identified as making PNAG by chemical, genetic or immunologic criteria.
| A. Organisms verified to produce PNAG by chemical, genetic and immunologic criteria | |
| Actinobacillus pleuropneumoniae | Escherichia coli |
| Acinetobacter baumannii | Staphylococcus aureus |
| Aggregatibacter actinomycetemcomitans | Staphylococcus epidermidis |
| Bacillus subtilis | Vibrio parahemolyticus |
| Yersinia pestis | |
| B. Organisms verified to produce PNAG by genetic and immunologic criteria | |
| Bordetella pertussis | Klebsiella pneumoniae |
| B. parapertussis | Shigella spp. |
| B. bronchiseptica | Stenotrophomonas maltophilia |
| Burkholderia cepacia complex | Yersinia entercolitica |
| Enterobacter cloacae | Yersinia pseudotuberculosis |
| C. Organisms verified to produce PNAG by immunologic criteria | |
| Bacteroides fragilis | Neisseria meningitides |
| Candida albicans | Streptococcus pyogenes |
| Hemophilus ducreyi | Streptococcus agalactiae |
| Hemophilus influenzae | Streptococcus pneumoniae |
| Listeria monocytogenes | Plasmodium spp. |
| Mycobacterium tuberculosis | Trichmonas vaginalis |
| Neisseria gonorrhoeae | |
In vivo expression of PNAG
Previous studies have reported PNAG expression in the sputum and lung of CF patients infected with S. aureus [15], with PNAG detected by fluorescent microscopy at the surface of 80 to 90% of the bacterial cells. PNAG was also found on the surface of microbial cells in vivo in the lungs of patients infected by BCC [24] or M. tuberculosis [6]. In children, in vivo expression of PNAG was found in the middle ear effusions from S. pneumoniae or nontypable H. influenzae otitis media samples [6].
In animals, in vivo expression of PNAG was found in nasopharyngeal fluid of chinchillas experimentally infected by S. pneumoniae serogroup 19A, in the gastrointestinal tract of a mouse experimentally infected by C. rodentium, and in ocular tissues from mice with C. albicans keratitis [6]. PNAG is also expressed by major symbiont bacteria found in the human microbiota: the GI tract (E. coli among others), the skin (S. epidermidis) and the oral flora (A. actinomycetemcomitans). The extensive in vivo expression of PNAG raised the question of whether natural antibodies to PNAG were present in healthy individuals and whether these antibodies were protective.
Naturally-occurring antibodies to PNAG
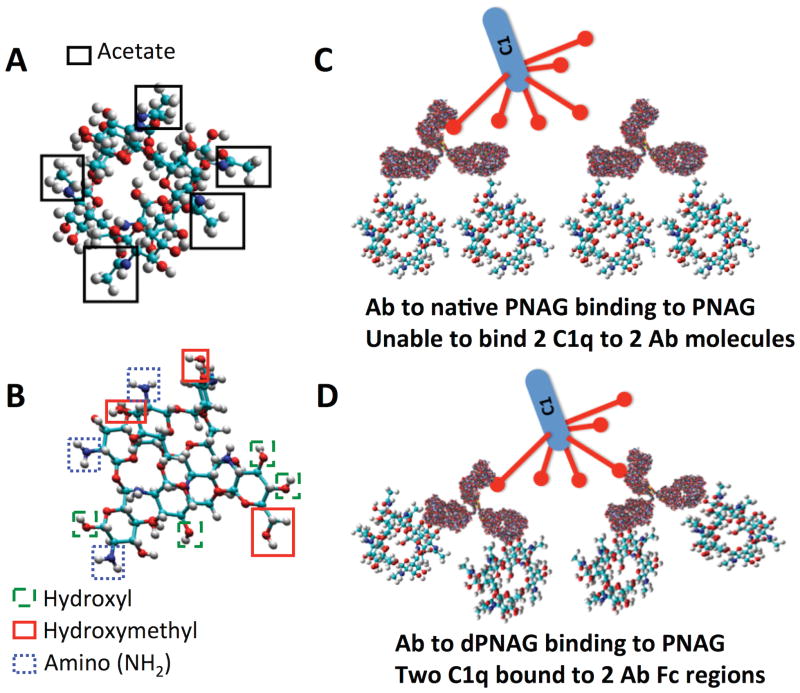
Interestingly, in several studies, high levels of antibodies to PNAG were detected in healthy humans [8,38,42,43] by enzyme-linked immunosorbent assay (ELISA). Notably, these antibodies do not appear to be functional or protective. Natural antibodies to PNAG are not able to mediate in vitro bactericidal or opsonophagocytic killing [8] and they do not protect animals against PNAG producing pathogenic bacteria such as S. aureus [42]. The natural antibodies predominantly require that the acetate substituents on PNAG be present in order to bind to the antigen, but the result is an inability to mediate complement activation and protective immunity [8,9,42]. Structural studies of PNAG indicate that in its preferred glycoform conformation the acetates are on the polymer’s surface, sticking out into the surrounding medium, and this forms the basis for the immunodominance of the acetate-dependent epitopes [44]. Apparently, then, the Fc regions of the antibodies bound to the acetate-containing epitopes are not sufficiently close together to effectively bind C1q and initiate complement activation [38,42].
Immunity to PNAG and capsular polysaccharides in S. aureus infections
In contrast to most normal human sera, in some infected patients functional antibodies to PNAG could be detected [8], especially opsonizing antibodies in patients with S. aureus bacteremia [43]. However, most patients with non-bloodstream, acute S. aureus infections, such as in the skin and lung, failed to make an opsonizing antibody response to PNAG. Thus, either a more intense, or longer-term, infection was required to elicit opsonic antibodies to PNAG in the context of S. aureus infection. However, the fact that humans could make such antibodies supported other data that altered glycoforms of PNAG could induce opsonic, protective antibody [9,39] indicating it would be feasible to develop an effective passive or active immunotherapy targeting PNAG.
With the discovery of extensive production of PNAG by clinical isolates of S. aureus it became very attractive to consider a multivalent vaccine incorporating this antigen with the S. aureus capsular polysaccharides (CP), based on analogies with successful capsular vaccines to other bacterial pathogens, including Streptococcus pneumoniae, Neisseria meningitidis, Hemophilus influenzae type b, and Salmonella enterica serovar Typhi [2]. S. aureus makes two CP antigens, types 5 and 8, expressed by 70–85% of clinical isolates, and are considered to be excellent candidate vaccine antigens [45]. Unfortunately, two clinical trials for preventing bacteremia in hemodialysis patients using CP antigens conjugated to a carrier protein as a vaccine both failed to meet their primary end points of reduced bacteremia [46,47]. Notably, in the first trial [46], there was 57% protective efficacy (P=0.02) after 40 weeks, as determined by a post hoc analysis, but at the predetermined 54-week endpoint efficacy was only 26% (P=0.23). Thus, a multivalent PNAG/CP5/CP8 vaccine seemed like a reasonable candidate to augment the limited efficacy of the bivalent CP vaccine.
However, when testing of antisera containing antibodies to these 3 components was started, instead of observing the expected additive or synergist effects, what was actually found were potent and mutually inhibitory effects on opsonic killing using in vitro assays and mouse models of bacteremia and skin infections [43]. Electron microscopy, isothermal calorimetry, and surface plasmon resonance (SPR) experiments indicated that antibodies to CP and PNAG bound together via an apparent charge-dependent, idiotype–anti-idiotype interaction. In the sera of patients recovering from S. aureus bacteremia antibody titer increases to both PNAG and CP antigens were found, but even in these sera there was a notable lack of in vitro and in vivo protective efficacy [42,43]. Consistent with the laboratory-based findings of potent inhibitory effects between immunization-induced antibodies to PNAG and to S. aureus CP antigens, infection-induced antibodies to both PNAG and the S. aureus capsular polysaccharides, which by themselves were able to mediate opsonic killing, did not do so when both antibodies were in the same serum sample. When either antibodies to PNAG or capsule were removed by absorption, the sera gained functional activity and this was again lost when the two antibodies were recombined [43]. In a follow-up study, it was reported that natural, non-opsonic antibodies to PNAG that are present in most NHS samples can also neutralize the functional activity of antibodies to S. aureus CP5 and CP8 antigens, but these natural antibodies to PNAG do not interfere with functional antibodies to PNAG [42]. These findings raised the possibility that a potential explanation for the failure of the CP-based vaccines in hemodialysis patients might have been loss of efficacy when the titers induced by CP immunization declined to the point where natural antibodies to PNAG neutralized their protective efficacy. Altogether, these findings show that the presence of natural antibodies to PNAG in both healthy and infected subjects could impact newer attempts to use the CP antigens as vaccines but also highlight the complexity of vaccine development using PNAG or CP antigens for S. aureus.
Discovery of the means to successfully induce functional immunity to PNAG
Before the full characterization of the chemical structure of PNAG, immunization-induced anti-bodies raised to what was most likely semi-purified PNAG that had undergone extraction processes for purification that likely altered its composition, were reported to be opsonic in vitro and active in vivo against S. epidermidis [48,49]. Once the composition of native PNAG was known, the basis for protective efficacy in comparison to the lack of functionality of natural antibodies to PNAG could be explained.
Analysis of purified PNAG that induced opsonic killing and animal protection showed a markedly reduced level of N-acetylation in this material [9,19]. Based on this finding, two conjugate vaccines using native, highly acetylated (~95%) PNAG and the deacetylated glycoform, termed dPNAG (~15% acetylation), conjugated to a carrier protein of mutant diphtheria toxoid were synthesized. Antibodies to dPNAG-DT were found to be much more effective than antibodies to native PNAG-DT at inducing opsonic killing and reductions in bacterial levels in the blood of bacteremic mice and at protecting mice in a model of lethal S. aureus infection[9].
An additional study examining the opsonic killing activity against S. aureus in serum samples from infected CF patients found that antibodies directed at the deacetylated (backbone) epitopes on PNAG (dPNAG) were superior at eliciting in vitro opsonic killing. More precisely, it was found that when opsonic killing activity was detected in the CF patient’s sera, antibodies directed to dPNAG were more functional in phagocyte-dependent killing than antibodies directed at the acetate-dependent epitope (PNAG). This result was obtained using both whole serum and affinity-purified antibody populations directed at the epitopes under examination [8]. Of note, the presence of such antibodies correlated with a healthier overall clinical condition in spite of the presence of S. aureus in sputum cultures. However, it is a hallmark of CF that patients with S. aureus are often healthier than those lacking this organism, although the most likely explanation is that lack of S. aureus is also associated with the presence of P. aeruginosa, the pathogen clearly most responsible for clinical deterioration in CF [50,51]. Nonetheless, the finding of functional antibodies to PNAG in S. aureus-infected CF patients could account for the relative lack of pathology associated with the presence of this pathogen in the lungs of these patients.
As both the animal-derived [9] and human-derived antibodies to dPNAG [8] were better able to mediate opsonophagocytic killing activity than antibodies to native PNAG, human monoclonal antibodies (MAbs) to PNAG and dPNAG were produced to further study this phenomena [38]. A MAb designated F598 that bound well to both native PNAG (expressed on microbial surfaces) and dPNAG (which binds the functional antibodies) had superior opsonophagocytic activity and protective activity in a mouse model of lethality induced by S. aureus than two MAbs that bound optimally to PNAG and minimally to dPNAG (MAbs F628 and F630). The better activity of antibodies to dPNAG was associated with deposition of osponically-active fragments of the first and third components of complement onto PNAG [38] (Figure 4).
Figure 4.
As the natural antibodies to PNAG are directed against its native, highly acetylated (>90% of N—acetyl groups) form, these results probably explain the lack of protection mediated by natural antibodies: they do not bind to dPNAG or deposit C3 opsonins onto bacterial surfaces [8,38]. In contrast, dPNAG elicits antibodies with excellent engagement of the complement system leading to robust in vitro killing activity and protection against infection in experimental animal models [8,38,42]. Thus, the dPNAG glycoform is the basis for the current vaccine approaches being evaluated against PNAG-producing microbes.
Production of synthetic oligosaccharide-protein conjugate vaccines
A further refinement to the development of a product for active immunization was achieved by conjugating synthetic oligosaccharides composed of either non-acetylated β-(1→6)-D-glucosamine (GlcNH2) or β-(1→6)-N-acetylglucosamine (GlcNAc) to carrier proteins [7,10]. Oligosaccharides of either 5 or 9 monosaccharides in length, with linkers on the reducing termini that could be activated to produce sulfhydryl groups for conjugation to bromoacetyl groups introduced onto carrier proteins, were synthesized [7]. Interestingly, synthetic 5-mer GlcNH2 (5GlcNH2) or 9GlcNH2 conjugated to tetanus toxoid (TT) elicited mouse antibodies that mediated opsonic killing, while the antibodies that were produced in response to the acetylated oligosaccharide conjugate vaccine, 5GlcNAc- or 9GlcNAc-TT, did not mediate opsonic killing [7]. Rabbit antibodies to 9GlcNH2-TT bound to PNAG and dPNAG antigens, mediated killing of S. aureus and E. coli, and protected against S. aureus skin abscesses and lethal E. coli peritonitis [7]. Subsequent studies showed other carrier proteins could induce protective antibodies against S. aureus and E. coli when conjugated to 5GlcNH2 or 9GlcNH2 [10,52]. Therefore, the synthetic 5GlcNH2 oligosaccharide now forms the basis for the clinical development of PNAG-targeting vaccines for humans and animals.
In vitro correlates of PNAG-mediated immunity
Eliciting antibodies to PNAG with the proper properties to mediate microbial killing and clearance is essential for achieving protective immunity, and predicting the level and functionality of antibodies that are indicative of protective immunity is key for evaluating vaccine doses and constructs for their potential to mediate protection. A number of in vitro assays have been developed as functional correlates of immunoprotection.
Antibody titer measured by ELISA
In order to distinguish the levels of naturally occurring, usually non-protective, antibodies to PNAG from functionally active, vaccine-elicited antibodies to dPNAG, ELISA-based assays using either purified PNAG- or dPNAG-coated plates are employed. The serum antibody titers are interpreted as the amount of PNAG or dPNAG bound antibody determined by optical density. Linear regression formulas are generated to calculate titers. While antibodies to both PNAG and dPNAG will bind native PNAG, only dPNAG-elicited antibodies will bind dPNAG, in contrast to most natural antibody to PNAG [7–9]. While a validated ELISA test or titer for either PNAG or dPNAG is not yet developed beyond those used in laboratory investigations, it is clear that protective antibodies must bind to both of these antigens, indicating that a high titer to dPNAG is a good measure of likely protective efficacy.
Complement mediated opsonic and bactericidal killing
For Gram-positive bacteria, the OPK assay can be used to demonstrate both antibody specificity for PNAG recognition, and functional activity, by use of neutrophil- and complement-mediated killing of opsonized pathogens. Similarly, for Gram-negative bacteria, the bactericidal assay using antibody to PNAG/dPNAG and complement demonstrates antibody specificity and functionality, requiring the complement membrane attack complex (MAC), consisting of complement components C5–C9, to be formed. These assays constitute core components of the in vitro evaluation of vaccine-induced antibodies to PNAG. Whether all strains of Gram-negative bacteria are killed in the absence of phagocytes is not known.
Complement deposition assays
Another means used to correlate the PNAG antibody titer with functional activity is use of a C3 deposition assay [38,42]. In the most recent iteration of this assay, ELISA plates are coated with purified PNAG and then varying concentrations of heat-inactivated (to remove endogenous complement activity) dilutions of pre- and post-immunization sera, and an active complement source, kept at a constant concentration, are added. Functionally active PNAG-specific antibodies bind to the purified PNAG on the ELISA plate and activate complement, resulting in C3 deposition. A C3-specific antibody is used to detect the amount of C3 deposited in each well by the PNAG-specific antibody. Thus far, the amount of C3 deposited has correlated well with PNAG-specific antibody titers and protection against experimental infection challenges, a finding applicable to both polyclonal and monoclonal antibodies [38].
More recently, it was found that protective antibodies to PNAG mediate their activity via activation of the classical complement pathway (Figure 4). PNAG itself can activate complement via the lectin pathway, as evidenced by antibody-independent C3 deposition occurring in C1-deficient serum [53]. However, this pathway is not optimal for mediating killing of many Gram-positive bacterial species [54], whereas antibody-mediated activation of the classical pathway is much more effective. Therefore, we have developed a C1q-deposition assay similar to that for C3 deposition, except an antibody specific to C1q is used to detect complement activation and binding of C1. Of note, C1q is highly conserved across species and a single antiserum to purified human C1q can be used to detect C1 deposition using sera obtained from a range of mammalian species as the complement source.
In vivo studies
Since the early 1990s, antibodies to PNAG have shown a broad-spectrum activity against numerous pathogens in laboratory animal models. Published results are summarized in Table 2 and Cywes-Bentley et al. [6] provides experimental results with S. pyogenes, L. monocytogenes, S. pneumoniae, and N. meningitidis serogroup B. In addition, the human MAb to PNAG protected T-bet/RAG2-deficient (TRUC) mice from infectious colitis [6,55].
Table 2.
Protective efficacy of antibody to the dPNAG glycoform against various microbial pathogens in mouse settings of infection.
| S. aureus: lethal peritonitis, bacteremia, skin infection, pneumonia, keratitis |
| E. coli: lethal peritonitis, GI infection by Stx+ strains, systemic infection by multidrug resistant strains |
| Burkholderia cenocepacia: lethal peritonitis |
| A. baumannii: bacteremia and pneumonia |
| N. meningitidis serogroup B: neonatal mouse meningitis infection |
| S. pyogenes, skin infection leading to lethal systemic infection |
| L. monocytogenes: systemic infection |
| S. pneumoniae: pneumonia |
| C. albicans: keratitis |
| Plasmodium berghei ANKA: mouse malaria |
In vivo protection against staphylococci infections
The first report of protective activity of antibodies to PNAG (called PS/A at the time) was in a rabbit model of bacteremia using catheters colonized by coagulase-negative staphylococci inserted into the right jugular vein and attached to a subcutaneous osmotic pump [48]. In this model, antibodies to PNAG were able to reduce the number of bacteremic days by approximately 60%. This approach was then extended to a rabbit model of endocarditis [49], and both passive and active immunization targeting PNAG protected against bacteremia and endocarditis cause by S. epidermidis. The antibodies to PNAG were next tested against S. aureus in numerous studies. In the first report [15], active and passive immunization significantly reduced the CFU recovered from the kidneys of mice after IV infection with different strains of S. aureus. Similarly, passive immunization significantly protected mice in a lethality model. In the ensuing sixteen years, antibodies to PNAG were repetitively shown to be protective against S. aureus infections, including mouse models of bacteremia and skin abscesses as well as sheep mastitis [7–9,19,28,38,42,43,56,57].
In vivo protection against infections caused by E. coli
E. coli represents a major challenge for vaccine development, mostly due to the large serologic diversity of protective antigens such as LPS O side chains and capsules [58–60]. After the report that the conserved PNAG was also present on the surface of E. coli [22], antibodies to this antigen were tested for protection against this organism. In several studies using a model of systemic dissemination after IP infection [7,34,39], both polyclonal and monoclonal antibodies to PNAG significantly protected mice against various strains of E. coli. For infections caused by E. coli colonizing the GI tract, the 9GlcNH2 synthetic oligosaccharide conjugated to Shiga toxin 1b subunit (9GlcNH2-Stx1b) was shown to induce rabbit antibodies that were protective against infection caused by different strains of Shiga-toxin producing E. coli [52]. Thus in multiple in vivo mouse settings, antibodies to PNAG were effective in reducing the consequences of E. coli infections.
In vivo protection against infections caused by multi-drug resistant (MDR) and pan-drug resistant (PDR) Gram-negative pathogens
Antibiotic resistance is a major concern impacting treatment of illnesses caused by Gram-negative bacteria, and a vaccine or immunotherapeutic alternative is highly desirous to participate in curing these infections. Among the main pathogenic Gram-negative bacteria that are naturally MDR as well as capable of acquiring additional resistances to become PDR are A. baumannii and stains within the BCC. Studies with A. baumannii, a major cause of nosocomial infections in immunocompromised patients, showed that rabbit antisera to 9GlcNH2-TT significantly reduced levels of A. baumannii in the lungs or blood of mice 2 or 24 h post-infection, respectively, compared to levels in control groups receiving normal rabbit sera (NRS) [36]. There are three major BCC species: B. cenocepacia, B. multivorans, and B. dolosa and they can all cause PDR infections, particularly in patients with cystic fibrosis. All three species were shown to produce PNAG both in vitro and in vivo. Antibodies to PNAG were able kill these bacteria in opsonophagocytic assays as well as protect mice from lethal peritonitis caused by BCC strains injected either alone or in combination with MRSA, chosen as another example of a PNAG-producing pathogenic and antibiotic-resistant bacterium [24]
Carbapenems are the main drug used to treat infections caused by MDR Gram-negative bacteria in general, and MDR enterobacteriacae in particular. Unfortunately, there are now more and more Carbapenem Resistant Enterobacteria (CRE), that have emerged mainly through the acquisition of genes encoding for production of carbapenemases such as the Klebsiella pneumoniae carbapenemase (KPC) or the New Delhi metallo β–lactamase (NDM) [61–63]. As with the other antibiotic resistant strains, antibodies to PNAG were able to kill CRE strains in vitro and protect mice in vivo against three clinically relevant species of enterobacteriacea (E. coli, Enterobacter cloacae and K. pneumoniae), whether or not the strains produced a carbapenemase [34]. Finally, while Pseudomonas aeruginosa is the only major drug-resistant pathogenic bacterium not producing PNAG naturally, antibodies to PNAG were shown to be able to protect mice infected by a PNAG producing, genetically engineered P. aeruginosa construct, suggesting that regardless of the Gram-negative bacterial species, PNAG expression is the sole determinant of the protective efficacy of antibodies to this antigen. The animal models used in these studies involved both IP and IV injections of the bacteria [34].
Killing and protection against pathogenic eukaryotes by antibodies to PNAG
PNAG has been detected at the surface of several eukaryotes, including human pathogens such as Candida albicans and Plasmodium falciparum [6]. Using a model of mouse keratitis, antibodies to PNAG significantly reduced cfu counts recovered from the cornea of C57BL/6 mice infected with C. albicans and treated with the human MAb F598 to PNAG either before or after the challenge by C. albicans [6]. These antibodies also killed C. albicans in vitro using an OPK. When Plasmodium berghei was used to infect mice, which is a relevant murine model for P. falciparum human infection, antibodies to PNAG significantly reduced the mortality of C57BL/6 mice from systemic PNAG-positive P. berghei ANKA infection initiated by IP injection [6]. Thus, the potential efficacy of PNAG as a vaccine extends to a very broad range of targets.
Route to a clinical testing
Although the first papers describing protective efficacy against S. epidermidis using injections of what is now known to be PNAG-containing semi-purified antigens were published 25 to almost 30 years ago [12,49], it has taken this long to bring this technology to the point of human testing. Two products are being evaluated. One is the fully human IgG1 molecule derived by cloning variable regions from a human B cell obtained from an individual recovering from a S. aureus infection into the TCAE 6.2 vector encoding the human gamma1 heavy chain constant regions and lambda light chain constant region [38]. The second is a synthetic pentamer of β-1-6-linked glucosamine conjugated to tetanus toxoid (5GlcNH2-TT) [7]. Notably, while designing phase II and III human trials of vaccines is challenging even when the target antigen is on a single species of pathogen, the plethora of pathogens making PNAG provides multiple opportunities for testing, which can, at times, make decisions difficult. Nonetheless, both the human MAb and vaccine are currently being manufactured under GMP conditions for further testing commencing in 2016.
Phase 1 clinical trial of MAb
The human IgG1 MAb was tested in 20 healthy humans in 2010 in a dose-escalating trial involving 4 volunteers per dose, 5 doses given IV in a 2-hour infusion starting at 0.85 mg/Kg and ending at 17.2 mg/Kg [11]. There were no significant adverse reactions, but two individuals given the highest dose had recordable events, one involving a rash that developed after infusion that was resolved by use of diphenhydramine and the other an infusion-reaction resolved by slowing the rate of infusion. The half-life of the MAb was ~28 days and high titers of functional, opsonic antibody to S. aureus were maintained throughout the 50-day observation and blood sampling periods. No reports of distress that might be related to mucosal surface reactions or disruption to the normal microbiota were recorded. Overall it appeared the MAb to PNAG is safe with an outstanding half-life and other properties, paving the way for phase II trials.
Phase 1 clinical trial of vaccine
The synthetic pentameric 5GlcNH2-TT has undergone about 2 years of development to scale up the synthesis of the oligosaccharide, which is produced as a dimer of two glucosamine pentamers bound together by a disulfide bond at the end of a linker, as described [7]. Commercially available, GMP-grade TT is used as the carrier protein. Trials of this vaccine, containing from 20–39 oligosaccharides per TT monomer, have commenced in economically important farm animals with some encouraging, albeit early, results indicating efficacy. As these trials are ongoing the full results are not yet available, but at least for some that are successful, it is anticipated that findings will be reported at appropriate meetings and potentially published in 2016.
Phase 2 clinical trials
Of note, with the broad range of pathogens producing PNAG, the challenge for phase II (and beyond) trials is finding a setting for testing both the MAb and vaccine where infection rates will be high. One obvious setting is human challenge models [64], and some that are currently available for PNAG producing pathogens include Neisseria gonorrhoeae [65], Plasmodium falciparum [66], Hemophilus ducreyi [67] and several GI pathogens [68,69]. Additional trials conducted in more traditional settings where infection rates with PNAG producing pathogens is high might include efficacy in intensive care units, GI tract surgery, mechanically-ventilated patients or similar high-risk locations within hospitals and health-care settings. Interventional studies with both the MAb and vaccine in either newly-diagnosed patients with long or difficult courses of antibiotic treatments, such as drug-resistant tuberculosis or MDR Gram-negative pathogens [24,34] or recurrent infections, such as methicillin-resistant S. aureus skin and soft tissue infections or E. coli urinary tract infections, are also being considered. Overall the final decisions will depend on numerous factors, taking into consideration concepts such medical need and potential impact on health, settings providing a proof of principle, economic feasibility of the intervention and any other related risk/benefit considerations.
Expert commentary
The potential for a single vaccine component to provide protective immunity to so many major pathogens is a clear paradigm shift in the field. In favor of realizing much of this potential is the broad-based expression of PNAG [6], indicative of an essential role in microbial biology inasmuch as so many divergent genetic and enzymatic methods to produce PNAG have evolved and survived in diverse microbial populations [22,23,27,31,33], detection of in vivo expression during human and animal infection [6,15], and preclinical data mostly in mice demonstrating protection based on use of the deacetylated glycoform of PNAG to induce immunity [7,9]. Furthermore, as a surface, essentially capsular, polysaccharide [6], PNAG is as available to immune effectors as are the more traditional capsules of S. pneumoniae, H. influenzae type B, and N. meningitides. Co-occurrence in the same physical space on the microbial surface with classic capsules, among our most successful vaccines, places PNAG in an excellent position to be an effective vaccine target. Many interesting questions remain related to the diversity of genes and proteins producing PNAG in so many different species, including their functions [32,70,71], how highly conserved the overall PNAG structure is, and how essential PNAG is to a given microbe’s fitness for survival. Nonetheless, the field is currently ready to see the results of the animal and human testing to ascertain if in settings of infections that are relevant to specific but different animals hosts immunity to PNAG can be effective.
On the down-side, use of the MAb in humans and the vaccine in animals is still quite limited, so overall safety considerations are still predicated on a small sample size. Long-term effects are not known, nor is the duration of immunity, and whether protective antibody isotypes will be universally induced cannot be predicted from the small sample sizes tested to date. Fortunately, there have been no indications that the natural antibodies to PNAG that most everyone possess, interfere with the vaccine-induced antibodies, but it would not be surprising if some situations arise wherein the efficacy of vaccine-induced antibodies is compromised by natural antibodies, including those to S. aureus CP antigens. Notably, several companies are pursuing S. aureus vaccines containing the CP5 and CP8 antigens [72,73] and if vaccines targeting both these antigens and PNAG are found to be efficacious, this could present a difficult problem if these vaccine-induced antibodies are cross-neutralizing, as has been reported using rabbit and mouse antibodies [42,43].
Five-year view
At this point, the vast majority of vaccine research is centered on finding individual vaccines for every important microbe. This is the basis for not only overall vaccine success to date but is also consistent with our modern understanding of immunity and host defense in that serologically variant and organism-specific antigens maximize their pathogenic potential. In the next five years extensive testing of the PNAG MAb and vaccine will ensue. During this period clear answers as to safety will be obtained, as will a reasonable amount of efficacy data, particularly in economically important animals. Success of either reagent in human challenge models, or a clearly medically important clinical setting, will likely propel the licensing of PNAG-directed immunotherapies. Given the exceptionally broad range of PNAG-targetable microbial species, it might be anticipated there will be some failures for specific organisms or in specific tissue settings. This could be due to lack of in vivo PNAG expression, difficulty in getting immune effectors (antibody, complement, phagocytes, Th17 cells, act.) to sites of infection (i.e., skin, urogenital tract, GI tract) or emergence of PNAG-negative variants that retain pathogenicity. Significant safety issues, mostly not anticipated at this time, could be a major barrier to development. Concerns about effects of antibody to PNAG on normal microbial flora has not been realized in any of the animal or short-term human studies conducted to date, but larger studies might find problems here. A difficult to predict consequence impacting the field might entail the success of immunity to PNAG in preventing infections with some pathogens, but possibly exacerbating infections by others, as antibody-mediated enhancement of infection is not unknown in infectious diseases, particularly with viral pathogens [74]. Overall, significant clinically relevant results in humans with the MAb and the 5GlcNH2-TT vaccine and in animals with the vaccine will be obtained in the next five years. These likely will determine the potential success or failure of targeting PNAG with a broad-spectrum antibody and vaccine, not unlike broad-spectrum antibiotics. Optimistically, the immunotherapies will have little downside and thus provide humanity with an important new tool to control infectious diseases.
Key issues.
PNAG is an exceptionally broadly expressed capsular./surface antigen on most major human and animal pathogens
Immunity to PNAG can be engendered by use of deacetylated/non-acetylated glycoforms, particularly as components of conjugate vaccines
PNAG likely has an essential role in microbial biology based on its broad expression and the diversity of genetic and enzymatic proteins involved in its synthesis among different microbial species
Natural immunity to PNAG is common but mostly ineffective due to an inability of natural antibodies to activate complement
Preclinical data in mice shows broad-protection against bacterial, fungal and protozoan pathogens
Phase I trials of a fully human IgG1 MAb to PNAG showed a long half life and overall excellent safety profile
Human clinical trials of the MAb and a conjugate vaccine consisting of 5GlcNH2-TT will commence, barring unforeseen difficulties, in 2016
Evaluation of the vaccine in economically important and companion animals has commenced with results likely available in 2016
Challenges remain in regard to long-term safety, efficacy against a range of pathogens, and unanticipated consequence.
Footnotes
Financial and competing interests disclosure
GB Pier is an inventor of intellectual properties [human monoclonal antibody to PNAG and PNAG vaccines] that are licensed by Brigham and Women’s Hospital to Alopexx Vaccine, LLC, and Alopexx Pharmaceuticals, LLC, entities in which GB Pier also holds equity. As an inventor of intellectual properties, GB Pier also has the right to receive a share of licensing-related in-come (royalties, fees) through Brigham and Women’s Hospital from Alopexx Pharmaceuticals, LLC, and Alopexx Vaccine, LLC. GB Pier’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners Healthcare in accordance with their conflict of interest policies. D Skurnik and C Cywes-Bentley are inventors of intellectual properties [use of human monoclonal antibody to PNAG and use of PNAG vaccines] that are licensed by Brigham and Women’s Hospital to Alopexx Vaccine, LLC, and Alopexx Pharmaceuticals, LLC. As inventors of intellectual properties, C Cywes-Bentley and D Skurnik also have the right to receive a share of licensing-related income (royalties, fees) through Brigham and Women’s Hospital from Alopexx Pharmaceuticals, LLC, and Alopexx Vaccine, LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Achievements in Public Health, 1900–1999: Control of Infectious Diseases. MMWR Morb Mort Wkly Rep. 1999;48(29):621–629. [PubMed] [Google Scholar]
- 2.Makela PH. Conjugate vaccines--a breakthrough in vaccine development. SE Asian J Trop Med Public Health. 2003;34(2):249–253. [PubMed] [Google Scholar]
- 3.Houser K, Subbarao K. Influenza vaccines: challenges and solutions. Cell Host Microbe. 2015;17(3):295–300. doi: 10.1016/j.chom.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubens M, Ramamoorthy V, Saxena A, Shehadeh N, Appunni S. HIV vaccine: Recent advances, current roadblocks, and future directions. J Immunol Res. 2015;2015:560347. doi: 10.1155/2015/560347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha S, Medhi B, Sehgal R. Challenges of drug-resistant malaria. Parasite. 2014;21:61. doi: 10.1051/parasite/2014059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Cywes-Bentley C, Skurnik D, Zaidi T, et al. Antibody to a conserved antigenic target is protective against diverse prokaryotic and eukaryotic pathogens. Proc Natl Acad Sci U S A. 2013;110(24):E2209–2218. doi: 10.1073/pnas.1303573110. This paper describes the broad based expression of PNAG among a range of microbes and documents the protective efficacy of polyclonal and MAb to PNAG against these pathogens. It also highlights te common in vivo expression of PNAG in infected tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Gening ML, Maira-Litran T, Kropec A, et al. Synthetic β-(1->6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect Immun. 2012;78(2):764–772. doi: 10.1128/IAI.01093-09. This paper documents the utility of synthetic oligosaccharides as a basis for a conjugate vaccine to PNAG. It also validates the need for non-acetylated glucosamines as the PNAG glycoform needed top induce protective immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly-Quintos C, Kropec A, Briggs S, Ordonez CL, Goldmann DA, Pier GB. The role of epitope specificity in the human opsonic antibody response to the staphylococcal surface polysaccharide poly N-acetyl glucosamine. J Infect Dis. 2005;192(11):2012–2019. doi: 10.1086/497604. [DOI] [PubMed] [Google Scholar]
- 9.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated Staphylococcal Poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2005;73(10):6752–6762. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PloS one. 2012;7(10):e46648. doi: 10.1371/journal.pone.0046648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlock D, Lee JC, Kropec-Huebner A, Pier GB. Pre-clinical and initial phase i evaluations of a fully human monoclonal antibody directed against the PNAG surface polysaccharide on Staphylococcus aureus. Abstracts of the 50th ICAAC 2010. 2010 Abstract G1-1654/329. [Google Scholar]
- 12.Tojo M, Yamashita N, Goldmann DA, Pier GB. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- 13.Mack D, Fischer W, Krokotsch A, et al. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178(1):175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenney D, Hubner J, Muller E, Wang Y, Goldmann DA, Pier GB. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66(10):4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenney D, Pouliot KL, Wang Y, et al. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284(5419):1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 16.Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67(10):5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldassarri L, Donnelli G, Gelosia A, Voglino MC, Simpson AW, Christensen GD. Purification and characterization of the staphylococcal slime-associated antigen and its occurrence among Staphylococcus epidermis clinical isolates. Infect Immun. 1996;64(8):3410–3415. doi: 10.1128/iai.64.8.3410-3415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller E, Hubner J, Gutierrez N, Takeda S, Goldmann DA, Pier GB. Isolation and characterization of transposon mutants of Staphylococcus epidermidis deficient in capsular polysaccharide/adhesin and slime. Infect Immun. 1993;61(2):551–558. doi: 10.1128/iai.61.2.551-558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maira-Litran T, Kropec A, Abeygunawardana C, et al. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect Immun. 2002;70(8):4433–4440. doi: 10.1128/IAI.70.8.4433-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyce JG, Abeygunawardana C, Xu Q, et al. Isolation, structural characterization, and immunological evaluation of a high-molecular-weight exopolysaccharide from Staphylococcus aureus. Carbohydr Res. 2003;338(9):903–922. doi: 10.1016/s0008-6215(03)00045-4. [DOI] [PubMed] [Google Scholar]
- 21.O’Gara JP. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270(2):179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 22**.Wang X, Preston JF, 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol. 2004;186(9):2724–2734. doi: 10.1128/JB.186.9.2724-2734.2004. In 2004 Romeo and collegaues found the genes encoding the PNAG biotsynthetic proteins in E. coli, leading to the identification of these genes in a large number of gram-negative pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi AHK, Slamti L, Avci FY, Pier GB, Maira-Litrán T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-β-1-6-N-acetyl glucosamine which is critical for biofilm formation. J Bacteriol. 2009;191(19):5953–5963. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skurnik D, Davis MR, Jr, Benedetti D, et al. Targeting pan-resistant bacteria with antibodies to a broadly conserved surface polysaccharide expressed during infection. J Infect Dis. 2012;205(11):1709–1718. doi: 10.1093/infdis/jis254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen KM, Chiang MK, Wang M, Ho HC, Lu MC, Lai YC. The role of pgaC in Klebsiella pneumoniae virulence and biofilm formation. Microb Pathog. 2014;77:89–99. doi: 10.1016/j.micpath.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Parise G, Mishra M, Itoh Y, Romeo T, Deora R. Role of a putative polysaccharide locus in Bordetella biofilm development. J Bacteriol. 2006;189(3):750–760. doi: 10.1128/JB.00953-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux D, Cywes-Bentley C, Zhang YF, et al. Identification of Poly-N-acetylglucosamine as a major polysaccharide component of the Bacillus subtilis biofilm matrix. J Biol Chem. 2015;290(31):19261–19272. doi: 10.1074/jbc.M115.648709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kropec A, Maira-Litran T, Jefferson KK, et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73(10):6868–6876. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subashchandrabose S, Smith SN, Spurbeck RR, Kole MM, Mobley HL. Genome-wide detection of fitness genes in uropathogenic Escherichia coli during systemic infection. PLoS Pathog. 2013;9(12):e1003788. doi: 10.1371/journal.ppat.1003788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74(8):4849–4855. doi: 10.1128/IAI.00230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20(5):1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. Ths publication provided the initial identification of the genetic locus encoding proteins invovled in PNAG synthesis and insight into the function of the these proteins. [DOI] [PubMed] [Google Scholar]
- 32.Gerke C, Kraft A, Sussmuth R, Schweitzer O, Gotz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273(29):18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 33.Bobrov AG, Kirillina O, Forman S, Mack D, Perry RD. Insights into Yersinia pestis biofilm development: topology and co-interaction of Hms inner membrane proteins involved in exopolysaccharide production. Environ Microbiol. 2008;10(6):1419–1432. doi: 10.1111/j.1462-2920.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 34.Skurnik D, Roux D, Pons S, Xi L, Cywes-Bentley C, Pier GB. Extended spectrum antibodies protective against carbapenemase producing Enterobacteriaceae. J Antimicrob Chemother. 2016 doi: 10.1093/jac/dkv448. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Whitfield GB, Marmont LS, Howell PL. Enzymatic modifications of exopolysaccharides enhance bacterial persistence. Front Microbiol. 2015;6471 doi: 10.3389/fmicb.2015.00471. This review article presents an outtanding overview of the biosynthesis of PNAG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentancor LV, O’Malley JM, Bozkurt-Guzel C, Pier GB, Maira-Litran T. Poly-N-acetyl-beta-(1-6)-glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect Immun. 2012;80(2):651–656. doi: 10.1128/IAI.05653-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoong P, Cywes-Bentley C, Pier GB. Poly-N-acetylglucosamine expression by wild-type Yersinia pestis is maximal at mammalian, not flea, temperatures. mBio. 2012;3(4):e00217–00212. doi: 10.1128/mBio.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Kelly-Quintos C, Cavacini LA, Posner MR, Goldmann D, Pier GB. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect Immun. 2006;74(5):2742–2750. doi: 10.1128/IAI.74.5.2742-2750.2006. This paper describes the production and characterization of MAb F598 to PNAG, n o in clinical trials in humans and also used as the reagent tod etect PNAG among microbial pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerca N, Maira-Litran T, Jefferson KK, Grout M, Goldmann DA, Pier GB. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proc Natl Acad Sci U S A. 2007;104(18):7528–7533. doi: 10.1073/pnas.0700630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan JB, Velliyagounder K, Ragunath C, et al. Genes involved in the synthesis and degradation of matrix polysaccharide in Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae biofilms. J Bacteriol. 2004;186(24):8213–8220. doi: 10.1128/JB.186.24.8213-8220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Ramasubbu N, Thomas LM, Ragunath C, Kaplan JB. Structural analysis of Dispersin B, a biofilm-releasing glycoside hydrolase from the periodontopathogen Actinobacillus actinomycetemcomitans. J Mol Biol. 2005;349(3):475–486. doi: 10.1016/j.jmb.2005.03.082. The use of Dispersin B as a tool in identifying and studying PNAG is exceptional. this paper reports on its properties and its specificty for the β-1-6-linkage between the glucosamines in PNAG. [DOI] [PubMed] [Google Scholar]
- 42.Skurnik D, Kropec A, Roux D, Theilacker C, Huebner J, Pier GB. Natural antibodies in normal human serum inhibit Staphylococcus aureus capsular polysaccharide vaccine efficacy. Clin Infect Dis. 2012;55(9):1188–1197. doi: 10.1093/cid/cis624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skurnik D, Merighi M, Grout M, et al. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J Clin Invest. 2010;120(9):3220–3233. doi: 10.1172/JCI42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grachev AA, Gerbst AG, Gening ML, et al. NMR and conformational studies of linear and cyclic oligo-(1-->6)-beta-D-glucosamines. Carbohydr Res. 2011;346(15):2499–2510. doi: 10.1016/j.carres.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verdier I, Durand G, Bes M, et al. Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J Clin Microbiol. 2007;45(3):725–729. doi: 10.1128/JCM.01572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346(7):491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 47.Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54(4):560–567. doi: 10.1093/cid/cir828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kojima Y, Tojo M, Goldmann DA, Tosteson TD, Pier GB. Antibody to the capsular polysaccharide/adhesin protects rabbits against catheter-related bacteremia due to coagulase-negative staphylococci. J Infect Dis. 1990;162(2):435–441. doi: 10.1093/infdis/162.2.435. [DOI] [PubMed] [Google Scholar]
- 49.Takeda S, Pier GB, Kojima Y, et al. Protection against endocarditis due to Staphylococcus epidermidis by immunization with capsular polysaccharide/adhesin. Circulation. 1991;84(6):2539–2546. doi: 10.1161/01.cir.84.6.2539. [DOI] [PubMed] [Google Scholar]
- 50.Farrell PM, Collins J, Broderick LS, et al. Association between mucoid Pseudomonas infection and bronchiectasis in children with cystic fibrosis. Radiology. 2009;252(2):534–543. doi: 10.1148/radiol.2522081882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293(5):581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 52.Lu X, Skurnik D, Pozzi C, et al. A poly-N-acetylglucosamine-Shiga toxin broad-spectrum conjugate vaccine for Shiga toxin-producing Escherichia coli. mBio. 2014;5(2):e00974-00914–e00974-00914. doi: 10.1128/mBio.00974-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallis R, Mitchell DA, Schmid R, Schwaeble WJ, Keeble AH. Paths reunited: Initiation of the classical and lectin pathways of complement activation. Immunobiol. 2010;215(1):1–11. doi: 10.1016/j.imbio.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brouwer N, Dolman KM, van Houdt M, Sta M, Roos D, Kuijpers TW. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J Immunol. 2008;180(6):4124–4132. doi: 10.4049/jimmunol.180.6.4124. [DOI] [PubMed] [Google Scholar]
- 55.Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131(1):33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez MM, Prenafeta A, Valle J, et al. Protection from Staphylococcus aureus mastitis associated with poly-N-acetyl beta-1,6 glucosamine specific antibody production using biofilm-embedded bacteria. Vaccine. 2009;27(17):2379–2386. doi: 10.1016/j.vaccine.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cerca N, Jefferson KK, Maira-Litran T, et al. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2007;75(7):3406–3413. doi: 10.1128/IAI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lukacova M, Barak I, Kazar J. Role of structural variations of polysaccharide antigens in the pathogenicity of Gram-negative bacteria. Clin Microbiol Infect. 2008;14(3):200–206. doi: 10.1111/j.1469-0691.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- 59.Samuel G, Reeves P. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr Res. 2003;338(23):2503–2519. doi: 10.1016/j.carres.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 60.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 61.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. The Lancet. 2009;9(4):228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 62.Poirel L, Pitout JD, Nordmann P. Carbapenemases: molecular diversity and clinical consequences. Future Microbiol. 2007;2(5):501–512. doi: 10.2217/17460913.2.5.501. [DOI] [PubMed] [Google Scholar]
- 63.Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-beta-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19(6):870–878. doi: 10.3201/eid1906.121515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Darton TC, Blohmke CJ, Moorthy VS, et al. Design, recruitment, and microbiological considerations in human challenge studies. The Lancet. 2015 doi: 10.1016/S1473-3099(15)00068-7. [DOI] [PubMed] [Google Scholar]
- 65.Hobbs MM, Sparling PF, Cohen MS, Shafer WM, Deal CD, Jerse AE. Experimental gonococcal infection in male volunteers: Cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front Microbiol. 2011;2:123. doi: 10.3389/fmicb.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laurens MB, Billingsley P, Richman A, et al. Successful human infection with P. falciparum using three aseptic Anopheles stephensi mosquitoes: A new model for controlled human malaria infection. PloS one. 2013;8(7):e68969. doi: 10.1371/journal.pone.0068969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Labandeira-Rey M, Dodd D, Fortney KR, et al. A Haemophilus ducreyi CpxR deletion mutant is virulent in human volunteers. J Infect Dis. 2011;203(12):1859–1865. doi: 10.1093/infdis/jir190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollard AJ, Savulescu J, Oxford J, et al. Human microbial challenge: the ultimate animal model. The Lancet. 2012;12(12):903–905. doi: 10.1016/S1473-3099(12)70292-X. [DOI] [PubMed] [Google Scholar]
- 69.Waddington CS, Darton TC, Jones C, et al. An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis. 2014;58(9):1230–1240. doi: 10.1093/cid/ciu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Little DJ, Bamford NC, Pokrovskaya V, Robinson H, Nitz M, Howell PL. Structural basis for the de-N-acetylation of Poly-beta-1,6-N-acetyl-D-glucosamine in Gram-positive bacteria. J Biol Chem. 2014;289(52):35907–35917. doi: 10.1074/jbc.M114.611400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Little DJ, Li G, Ing C, et al. Modification and periplasmic translocation of the biofilm exopolysaccharide poly-beta-1,6-N-acetyl-D-glucosamine. Proc Natl Acad Sci U S A. 2014;111(30):11013–11018. doi: 10.1073/pnas.1406388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nissen M, Marshall H, Richmond P, et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine. 2015;33(15):1846–1854. doi: 10.1016/j.vaccine.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 73.Lattar SM, Noto Llana M, Denoel P, et al. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun. 2014;82(1):83–91. doi: 10.1128/IAI.01050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor A, Foo SS, Bruzzone R, Vu Dinh L, King NJ, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev. 2015;268(1):340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]