Abstract
Community sexual bridging may influence the socio-geographic distribution of heterosexually transmitted HIV. In a cross-sectional study, heterosexual adults at high-risk of HIV were recruited in New York City (NYC) in 2010 for the Centers for Disease Control and Prevention-sponsored National HIV Behavioral Surveillance system. Eligible participants were interviewed about their HIV risk behaviors and sexual partnerships and tested for HIV. Social network analysis of the geographic location of participants’ recent sexual partnerships was used to calculate three sexual bridging measures (non-redundant ties, flow-betweenness and walk-betweenness) for NYC communities (defined as United Hospital Fund neighborhoods), which were plotted against HIV prevalence in each community. The analysis sample comprised 494 participants and 1534 sexual partnerships. Participants were 60.1 % male, 79.6 % non-Hispanic black and 19.6 % Hispanic race/ethnicity; the median age was 40 years (IQR 24–50); 37.7 % had ever been homeless (past 12 months); 16.6 % had ever injected drugs; in the past 12 months 76.7 % used non-injection drugs and 90.1 % engaged in condomless vaginal or anal sex; 9.6 % tested HIV positive (of 481 with positive/negative results). Sexual partnerships were located in 33 (78.6 %) of 42 NYC communities, including 13 “high HIV-spread communities”, 7 “hidden bridging communities”, 0 “contained high HIV prevalence communities”, and 13 “latent HIV bridging communities”. Compared with latent HIV bridging communities, the population racial/ethnic composition was more likely (p < 0.0001) to be black or Hispanic in high HIV-spread communities and to be black in hidden bridging communities. High HIV-spread and hidden bridging communities may facilitate the maintenance and spread of heterosexually transmitted HIV in black and Hispanic populations in NYC.
Keywords: HIV, Sexual networks, Bridging, Heterosexual, New York City, Communities
Introduction
Heterosexual transmission is the second largest risk category among persons living with HIV/AIDS (PLWHA) and of those newly diagnosed. In 2012, of 908,071 adult and adolescent (13 years of age or older) PLWHA in the United States (US), 18.9 %, were infected through heterosexual transmission, as were 19.8 %, of 115,871 adult and adolescent PLWHA in New York City (NYC) [1, 2]. Among adults and adolescents newly diagnosed with HIV in 2012, heterosexual transmission accounted for 15.0 % of 41,643 new HIV diagnoses in the US, and 20.1 %, of 2985 new HIV diagnoses in NYC [1, 2].
Heterosexually transmitted HIV is often concentrated in low-income geographic areas with majority black or Hispanic populations [3, 4]. Although public health resources to address heterosexually transmitted HIV are typically directed to those areas with high HIV prevalence in heterosexuals [5–7], identifying areas in which future HIV outbreaks or epidemics among heterosexuals may occur could help to inform a more comprehensive approach. Such an approach may be developed by conceptualizing at-risk geographic areas as part of a geographic HIV transmission network.
An HIV transmission network comprises nodes and the links between nodes, such as high-risk sexual relationships, through which HIV is spread. A geographic HIV transmission network describes the spread of HIV between geographic areas, especially from higher prevalence to lower prevalence areas. The sexual spread of HIV from a higher prevalence to a lower prevalence area requires sexual “bridging” whereby people or places may be bridges for the spread of sexually transmitted HIV between different geographic areas [8–12]. When people are bridges, HIV infected people from higher prevalence areas may travel to lower prevalence areas where they sexually transmit HIV to their sexual partners in these areas, who in turn may transmit HIV to their sexual partners in these same areas (a similar process can occur for uninfected people from lower prevalence areas who travel to and acquire sexually transmitted HIV in higher prevalence areas). When a place is a sexual bridge, infected and uninfected people from higher and lower prevalence areas may sexually mix in these places, such as bars or neighborhood “cruising” locations, which may be in areas where neither infected nor uninfected people are from, e.g., in “red light” districts in urban areas [13–19].
In the following, social network analysis is used to identify urban geographic communities (the term “communities” is used interchangeably with “areas” and “neighborhoods, as appropriate) in NYC by their bridging potential for facilitating the geographic spread of heterosexually transmitted HIV. This analysis uses self-reported data on the geographic location of recent sexual partnerships obtained from a 2010 study of heterosexuals at high-risk of HIV acquisition in NYC. Building on an approach used by Youm et al. to measure community sexual bridging in Chicago, three bridging measures were calculated (non-redundant ties, flow-betweenness and walk-betweenness) [20, 21]. A hierarchical spatial bridging measure was derived from these measures to provide a comprehensive assessment of community sexual bridging that may influence the geographic distribution of heterosexually transmitted HIV in NYC.
Methods
Sampling, Eligibility and Protocol
In 2010, heterosexuals at high-risk of HIV, defined below, were recruited in NYC to participate in the second heterosexual cycle (HET2) of the National HIV Behavioral Surveillance (NHBS) system, which is sponsored by the Centers for Disease Control and Prevention (CDC) and is conducted in 20 cities throughout the United States and in Puerto Rico. NHBS is a cross-sectional study that monitors HIV risk behaviors, testing history, the use of HIV prevention services, and HIV prevalence among men who have sex with men, injection drug users, and heterosexuals at high-risk in 3-year cycles [22, 23]. Data for the analysis described herein are from the NYC site of NHBS.
Heterosexuals at high-risk were defined as persons who both engaged in sex with an opposite gender partner in the past 12 months and who resided in a high-HIV-risk area (HRA) or were recruited through a social network recruitment chain that originated in a HRA. HRAs were geographic areas where heterosexuals were at greater risk of heterosexually acquired HIV compared with other areas in the Metropolitan Statistical Area. In HET2, the target HRAs in NYC were developed from the overlap of high poverty census tracts (based on U.S. Census data) with zip codes that were in the top 50 % of the heterosexual HIV case rate for new diagnoses between 2005 and 2008 (based on HIV surveillance data from the NYC Department of Health and Mental Hygiene (DOHMH)). Three main HRA clusters were identified, in Central and Northern Brooklyn, Northern Manhattan, and South and Central Bronx.
The sample was accrued using respondent-driven sampling (RDS) [24, 25]. Ethnographers selected eight initial recruits (seeds) from the target HRAs. Eligible seeds had to reside in a HRA, be eligible for study participation, be socially gregarious (i.e., be sociable and outgoing), have large social networks comprised of their existing social contacts, (i.e., people they know who they had seen recently), and had to have never injected drugs. After completing the study, seeds were asked to recruit up to three peers in their social networks (friends, relatives or people they were close to, aged 18–60 years, who resided in NYC, and who they had seen in the past 30 days). Study participants were in turn asked to recruit their peers, and so on, until the target sample size was met. Participants could not recruit others if they (the participants) had a household income that was above the poverty guidelines and they had greater than a high school education, or if they had injected drugs in the past 12 months.
Other participant eligibility criteria included self-identifying as male or female, having had vaginal or anal sex with opposite gender partners in the past 12 months, aged 18–60 years, residing in NYC, being able to complete the interview in English or Spanish (which was determined by interviewing staff during the eligibility screening process), and not previously participating in HET2. Heterosexuals at high-risk were not excluded if in addition to opposite-gender partners they also had same gender partners.
After giving their informed consent, participants were administered a computer-assisted, structured interview in private by trained interviewers, with interviewers entering participants’ responses into the computer. Interviews were conducted at two study research offices, one of which was in Crown Heights in Brooklyn, and the other in Central Harlem in Manhattan. After the interview was completed, a trained phlebotomist used venipuncture to obtain blood specimens from consenting participants and asked them to return for their HIV test results in 2 weeks after the specimens had been tested by the Public Health Laboratory of the NYC DOHMH. Those testing positive and those testing indeterminate were referred to HIV testing services. Participants were paid $20 for the interview, $10 for the HIV test, and $10 for each eligible participant they recruited. Participation in the study was anonymous.
Ethics
All procedures involving human subjects were reviewed and approved by the Institutional Review Boards of the NYC DOHMH, National Development and Research Institutes, and CDC.
Data Sources
The questionnaire included core questions for the national study and local questions to address local research interests. Core questions asked participants about their sociodemographic characteristics, drug and alcohol use, sexual risk behaviors and partnerships, and other HIV-related information. Risk behaviors are for the past 12 months unless specified otherwise. Blood specimens were screened for HIV antibody (Genetic Systems HIV-1/ HIV-2 PLUS “O” EIA, Bio-Rad Laboratories, Hercules, CA, USA) and positive specimens were confirmed using HIV1 Western blot platforms (Genetic Systems HIV-1 Western blot, Bio-Rad Laboratories, Hercules, CA, USA).
Communities
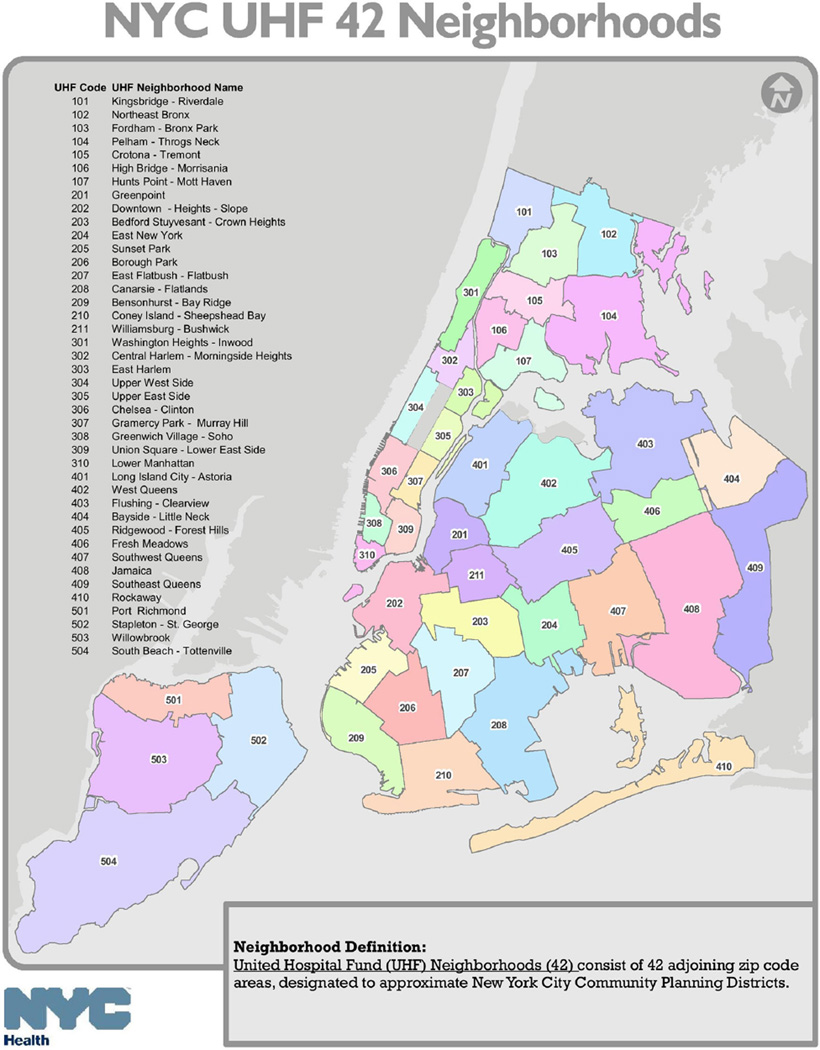
In the local NYC questionnaire, participants were asked to identify the closest street intersection of the place where they last had sex with up to 6 of their most recent partners in the past 12 months. The street names for the intersection were entered into “Geocoder”, a geocoding program that was developed and is maintained by the NYC DOHMH, to generate zip codes from geographic coordinates. The zip codes were recoded into United Hospital Fund neighborhoods for NYC. United Hospital Fund neighborhoods consist of 42 adjoining zip code areas, designated to approximate NYC Community Planning Districts [26] (see Fig. 1).
Fig. 1.
New York City United Hospital Fund Communities
Bridging Measures
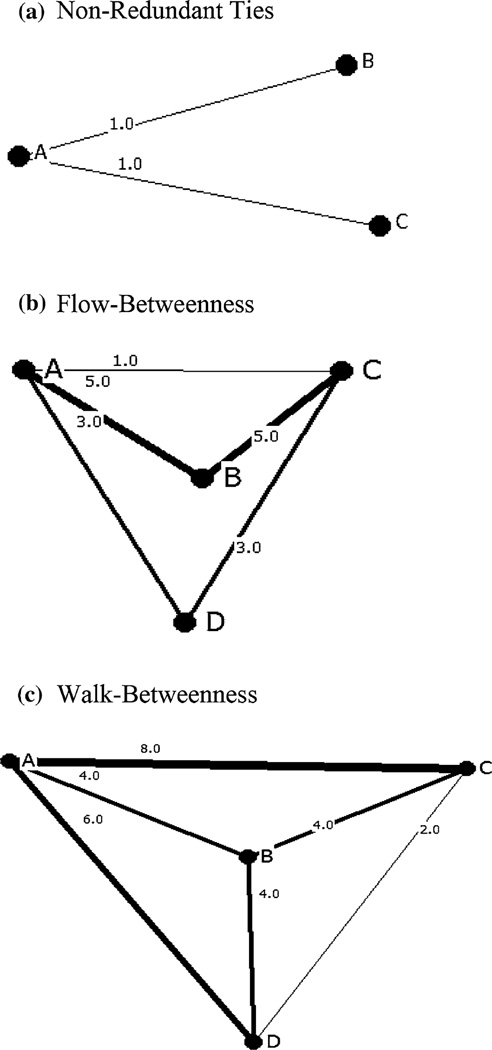
Bridging measures were calculated using relational data obtained in the NYC local questionnaire, with communities as the network nodes and the sexual ties between these communities as the links between the nodes. For example, if a participant had sex with a partner in community A and with another partner in community B, then a sexual tie would link community A with community B (A, B) and community B with community A (B, A). Additional sexual ties would be created for the index participant in community A (A, A) and community B (B, B) if the participant had sex with a partner in each community. UCINET© version 6.0 was used to create a symmetrical square data matrix in which both the rows and the columns were the communities and the cell elements were the number of sexual ties between communities or within the same community (the matrix diagonal). Three bridging measures were calculated, including “non-redundant ties” [27], “flow-betweenness” [28], and “walk-betweenness” [21, 29]. For each measure, the greater the bridging score of a node, then the greater the node’s bridging potential. The non-redundant ties and flow-betweenness measures were calculated using, respectively, the “structural holes” and “flow-betweenness” procedures in UCINET, and walk-betweenness using a customized MATLAB program [21]. Network diagrams using hypothetical data to illustrate the network structure of the bridging measures were constructed using NetDraw © version 2.099 and are shown in Fig. 2a–c.
Fig. 2.
Network structures of community sexual ties illustrating three bridging measures. a Non-Redundant Ties. (In the UCINET © version 6.0 “structural holes” procedure, the number of non-redundant ties for a given node is the node’s “effective network size”, which is the number of other nodes to which that node is directly linked minus the average number of the other nodes which are directly linked to each other; in the example, A is directly linked to 2 other nodes and B and C are directly linked to an average of 0 other nodes, excluding A, so that A’s effective network size is 2.0.) b Flow-betweenness. c Walk-betweenness. A, B, C, and D are the network nodes; “_n_” is the number of sexual ties between network nodes; Lines are scaled according to “n”, the number of sexual ties between network nodes
The non-redundant ties measure utilizes the direct ties of a node with other nodes and the ties between these other nodes. If the nodes in a network are not all directly connected to one another then there are “structural holes”, i.e., some nodes can only be connected by intermediary nodes. Non-redundant ties are those ties which are necessary to link nodes that otherwise would not be linked. For example, if in a local network comprising three nodes (A, B, and C) B is only connected to C through A, and C is only connected to B through A, then A’s ties with B and C are non-redundant, i.e., A has two non-redundant ties, while B and C each have one non-redundant tie (with A) (Fig. 2a).
The flow-betweenness measure utilizes both direct and indirect paths between nodes. Direct paths directly link nodes without any intermediary nodes, whereas indirect paths between nodes go through intermediary nodes in order to link other nodes in a network. The flow-betweenness score for any intermediary node represents the number of ties between all other nodes in the network which must go through the intermediary node. For example, in a community sexual network with four community nodes (A, B, C and D), with B as an intermediary node of interest (e.g., B is a community where many motels are located which charge room rates by the hour for commercial sex encounters between partners from different communities), A to C is linked by nine direct or indirect sexual ties, A to D by six, and D to C by six (Fig. 2b). The maximum number (or “maximum flow”) of sexual ties carried by A, C and D totals 21 sexual ties. For example, of the nine sexual ties linking A to C, one is a direct tie between A and C, three are indirect ties through D, and five are indirect ties through B. With B as the intermediary node of interest, in addition to the five ties that pass through B on the A to C paths, there are two on the A to D paths, and two on the C to D paths. Of the 21 sexual ties linking communities A, C and D, nine must go through B, so that the normalized flow-betweenness score for B, expressed as a proportion of maximum flow, is 0.429 (9/ 21).
The walk-betweenness measure developed by Youm [21], utilizes both direct and indirect ties between nodes, which are assumed to be linked through walks, i.e., an alternating sequence of nodes and links between nodes with each link connected to the node that immediately precedes and follows it. In contrast to the flow-betweenness measure, the walk-betweenness measure allows for multiple and repeated ties between nodes, e.g., multiple HIV infected individuals may repeatedly travel to certain communities where they transmit HIV to their sex partners in these communities. The walk-betweenness score for an intermediary node is the proportion of the total ties between all other nodes in a network which go through the intermediary node. For example, if in a community sexual network with four nodes (A, B, C and D) there are 14 sexual ties that connect nodes A and C, four of which pass through an intermediary node, B, then B’s walk-betweenness score for the A to C tie is 0.29 (4/14). If B’s walk-betweenness score is 0.33 (4/12) for the A to D tie, and 0.33 (4/12) for the C to D tie, then B’s walk-betweenness score for the network of A, B, C, and D is 0.95 (the sum of 0.29, 0.33, and 0.33) (Fig. 2c).
NYC Community HIV Prevalence for Non-MSM
HIV prevalence by NYC communities (United Hospital Fund neighborhoods) and in NYC overall used 2010 NYC HIV/AIDS case surveillance data, which geocodes cases using a case’s last known address [30]. The HIV prevalence rate was calculated as the number of PLWHA per 100,000 people for NYC overall and for each NYC community. PLWHA whose transmission risk was “men who have sex with men” were excluded from the numerator because the focus of the analysis was on heterosexuals.
Analysis
For individual participants, categorical data were analyzed using frequencies, percentages, and the prevalence ratio, normally distributed continuous data were analyzed using means and standard deviations, and non-normally distributed continuous data using medians and interquartile ranges (IQR).
The unit of analysis for analyzing community sexual bridging was the NYC community. Each bridging score (the vertical axis), was plotted against community HIV prevalence rates for non-MSM per 100,000 residents (the horizontal axis). For each bridging measure, the median bridging score and the overall NYC HIV prevalence rate for non-MSM per 100,000 residents were used, respectively, as horizontal and vertical plot references to determine each community’s bridging potential. Bridging scores above the horizontal plot reference line indicate higher bridging and bridging scores below the horizontal plot reference line indicate lower bridging. Community HIV prevalence to the right of the vertical reference line indicates higher community HIV prevalence, and community HIV prevalence to the left of the vertical reference line indicates lower community HIV prevalence. Based on the combination of bridging position and community HIV prevalence position, communities were categorized as: higher bridging and higher HIV prevalence; higher bridging and lower HIV prevalence; lower bridging and higher HIV prevalence; and lower bridging and lower HIV prevalence [20]. All three bridging measures were used to derive a 4-category hierarchical community sexual bridging measure: (1) any higher bridging and higher community HIV prevalence (“high HIV-spread communities”); (2) any higher bridging and lower community HIV prevalence (“hidden bridging communities”); (3) only lower bridging and higher community HIV prevalence (“contained high HIV prevalence communities”); and (4) only lower bridging and lower community HIV prevalence (“latent HIV bridging communities”). The association of the community bridging categories with the population racial/ethnic composition of NYC communities was examined using US Census 2010 population data aggregated at the United Hospital Fund neighborhood level.
RDS weights were not used for the statistical analysis. RDS weights are often used to estimate the population parameters of individual participant attributes (e.g., race/ ethnicity) from a RDS sample in order to adjust for differences in participants’ social network size and homophily (i.e., preferential in-group recruitment), which can bias the probability of participant recruitment. However, the focus in this analysis was on sexual networks between communities in NYC rather than on individual participant attributes.
Results
Sample Analyzed
The eight seeds generated 625 non-seed recruits as potential participants. The seeds resided in three HRA clusters, including Central Brooklyn (three seeds), Central Harlem (three seeds), and the South Bronx (two seeds). They included four females and four males, seven blacks and one Hispanic, and were between 20 and 54 years of age (median 33 years). Of the 625 recruited, 523 were eligible participants who had 1896 sexual partnerships. Of the 523 eligible participants, 29 (5.5 %) provided no geographic data on their sexual partnerships. Of the 1896 sexual partnerships, 88 (4.6 %) contained no geographic data and 274 (14.5 %) had invalid geographic data, including geographic data with missing street names, streets that did not intersect, or unknown street names. The analytic data set comprised 494 participants and 1534 sexual partnerships.
Compared with participants who provided valid geographic data for all of their sexual partners (n = 347), those who reported no sexual partner geographic data (n = 29) or did not report valid geographic data for all of their sexual partners (n = 147) were significantly (p < 0.05) less likely to be married or cohabiting and, in the past 12 months, were more likely to have ever been homeless, to have engaged in condomless anal sex, and to have had sex exchange partners (data not shown).
Participant Characteristics
The 494 participants analyzed resided in Brooklyn (53.2 %), Manhattan (31.4 %), Bronx (13.6 %), and Queens (1.8 %) (Table 1). Participants were 60.1 % male and 39.9 % female, 79.6 % were non-Hispanic black race/ ethnicity and 19.6 % Hispanic, the median age was 40 years (IQR 24–50), and 23.9 % reported a non-heterosexual identity. Ever being homeless in the past 12 months was reported by 37.7 %. Other sociodemographic characteristics are shown in Table 1.
Table 1.
Characteristics of participants analyzed, heterosexuals at high-risk, National HIV Behavioral Surveillance system, New York City 2010
| Characteristics | Total |
|
|---|---|---|
| N | ||
| Total | 494 | 100 |
| Borough of residence | ||
| Manhattan | 155 | 31.4 |
| Queens | 9 | 1.8 |
| Brooklyn | 263 | 53.2 |
| Bronx | 67 | 13.6 |
| Staten Island | 0 | 0 |
| Gender | ||
| Male | 297 | 60.1 |
| Female | 197 | 39.9 |
| Race/ethnicity | ||
| Black | 393 | 79.6 |
| Hispanic | 97 | 19.6 |
| White | 2 | 0.4 |
| Other | 2 | 0.4 |
| Age | ||
| 18–29 | 173 | 35.0 |
| ≥30 years | 321 | 65.0 |
| Median age (IQR) | 40 (24–50) | |
| Non-heterosexual identity | 118 | 23.9 |
| Not married/not living as married | 421 | 85.2 |
| Less than high school graduation/GED | 212 | 42.9 |
| Income <10 k (past 12 months) | 276 | 56.2 |
| Homeless (past 12 months) | 186 | 37.7 |
| Arrested (past 12 months) | 157 | 31.8 |
| Drug and alcohol use | ||
| Drug injection (lifetime) | 82 | 16.6 |
| Drug injection (past 12 months) | 21 | 4.3 |
| NI drug use (any NI drugs) | 379 | 76.7 |
| NI crack use | 112 | 22.7 |
| NI cocaine use | 114 | 23.1 |
| NI heroin use | 110 | 22.3 |
| NI methamphetamine | 6 | 1.2 |
| NI marijuana | 308 | 62.4 |
| Binge alcohol usea (past 12 months) | 285 | 57.7 |
| Sexual risks (past 12 months) | ||
| Condomless vaginal sex | 443 | 89.8 |
| Condomless anal sex | 218 | 44.1 |
| >3 heterosexual sexual partners | 251 | 50.8 |
| Median number of sex partners (IQR) | 4 (2–7) | |
| Sex exchange | 147 | 29.8 |
| UHF community location of sexual partnerships | ||
| Own community only | 286 | 57.9 |
| Outside communities only | 59 | 11.9 |
| Both own and outside communities | 149 | 30.2 |
| Self-reported HIV infection status | ||
| Positive | 15 | 3.0 |
| Negative | 391 | 79.2 |
| Unknown | 88 | 17.8 |
| HIV infection status | ||
| Positive | 46 | 9.3 |
| Negative | 435 | 88.1 |
| Unknown (indeterminate or test not done) | 13 | 2.6 |
NI non-injection
5 drinks at one sitting for men and 4 drinks at one sitting for women
In the past 12 months, 4.3 % injected drugs and 16.6 % had ever during their lifetime injected drugs (which includes both current and former injectors). Many used non-injection drugs (for specific non-injection drugs see Table 1) and engaged in binge alcohol use (respectively, 76.7 and 57.7 %). In the past 12 months, most (89.8 %) engaged in condomless vaginal sex and many (44.1 %) in condomless anal sex, with 90.1 % engaging in condomless vaginal or anal sex. The median number of sexual partners was 4 (IQR 2–7); 29.8 % engaged in exchange sex.
The majority (57.9 %) reported sexual partnerships in their own NYC community only, 11.9 % in outside communities only, and 30.2 % both in their own and outside communities. Participants reported sexual partnerships in 33 (78.6 %) of 42 NYC communities. A network diagram, created using NetDraw, of sexual partner ties (dichotomized) between NYC communities is shown in Fig. 3.
Fig. 3.
NYC United Hospital Fund Communities with sexual network ties (The community nodes are scaled according to the number of other communities to which they are directly connected [i.e., their community degree, e.g., Bedford Stuyvesant—Crown Heights is directly connected to 24 other communities and Canarsie—Flatlands to 5])
Few (3.0 %) reported being HIV positive, 79.2 % reported being HIV negative, and 17.8 % did not know their HIV status. In the study, 9.3 % tested positive, 88.1 % negative and 2.6 % were unknown (indeterminate or test not done). Of 481 with HIV positive or negative test results, 46 (9.6 %) tested positive and 435 (90.4 %) tested negative. Of those testing positive, 43.5 % reported a history of ever injecting drugs during their lifetime, compared with 13.4 % of those testing negative (prevalence ratio 3.2; 95 % CI 2.1, 4.7; p < 0.0001).
Community Sexual Bridging
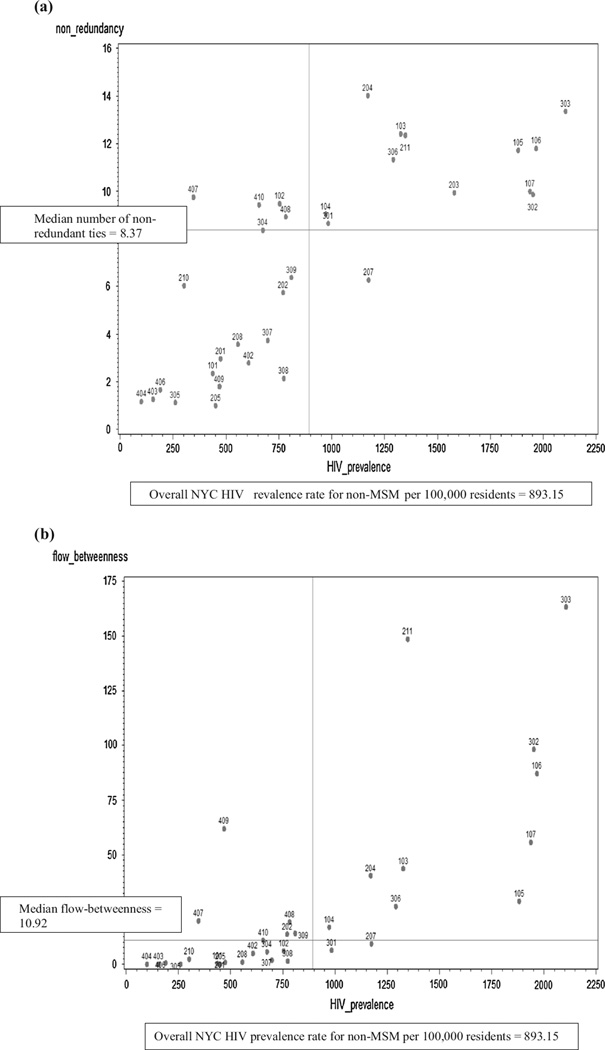
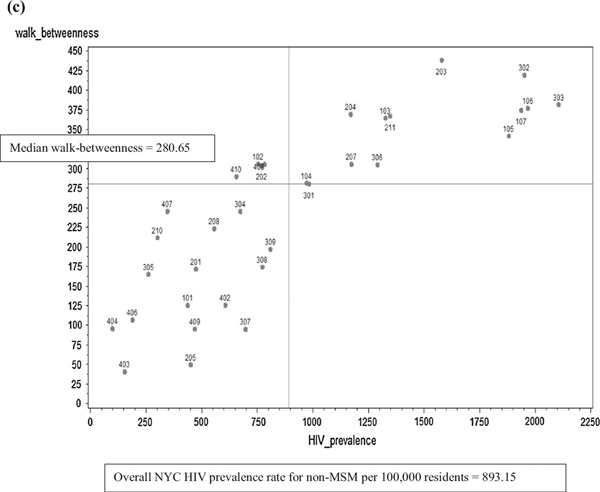
Plots of community sexual bridging are shown in Fig. 4a – c for each network bridging measure. The median score for each bridging measure, described below, is represented on the bridging measure plots as the horizontal reference line. The overall NYC HIV prevalence rate for non-MSM per 100,000 residents was 893.15 and is represented on the bridging measure plots as the vertical line; it was calculated from the number of non-MSM PLWHA in NYC divided by the NYC population in 2010 multiplied by 100,000, or (73,016/8,175,133) * 100,000.
Fig. 4.
Bridging scores. a Non-redundancy by United Hospital Fund Community HIV prevalence rate (per 100,000). b Flow-between-ness by United Hospital Fund Community HIV prevalence rate (per 100,000). (The flow-betweenness scores are the absolute, unnormalized values, and are calculated in UCINET© version 6.0 using an approach that was introduced in version 5.2.0.0 and later. Both versions yield the same flow-betweenness rank order for United Hospital Fund Communities relative to the median flow-betweenness score. The United Hospital Fund Community with the highest flow-betweenness score, United Hospital Fund Community 203, Bedford-Stuyvesant—Crown Heights [flow-betweenness score = 241.58, HIV prevalence rate = 1579.82/100,000], was removed from the plot to improve readability). c Walk-betweenness by United Hospital Fund Community HIV prevalence rate (per 100,000)
The median number of non-redundant ties was 8.37 (IQR 2.79–9.94) (Fig. 4a). Many higher HIV prevalence communities also had higher non-redundant ties. As shown in the upper left quadrant of Fig. 4a, four communities (102, 407, 408, and 410) had lower HIV prevalence but a higher number of non-redundant ties.
The median flow-betweenness score was 10.92 (IQR 1.45–40.52) (Fig. 4b). Community 203, Bedford-Stuyvesant—Crown Heights (not shown on the plot), had the highest flow-betweenness score (241.58) and higher HIV prevalence (1579.82/100,000). Five communities (202, 309, 407, 408, and 409) had lower HIV prevalence but higher flow-betweenness scores. Of these, three (202, 309, and 409) were not identified as higher bridging using non-redundant ties.
The median walk-betweenness score was 280.65 (IQR 165.30–341.86) (Fig. 4c). Community 203, Bedford-Stuyvesant—Crown Heights, had the highest walk-betweenness score (438.70). Four communities (102, 202, 408, and 410) had lower HIV prevalence but higher walk-betweenness scores. Community 207, a higher-HIV-prevalence community, which was categorized as lower bridging using the other measures, was higher bridging using the walk-betweenness measure.
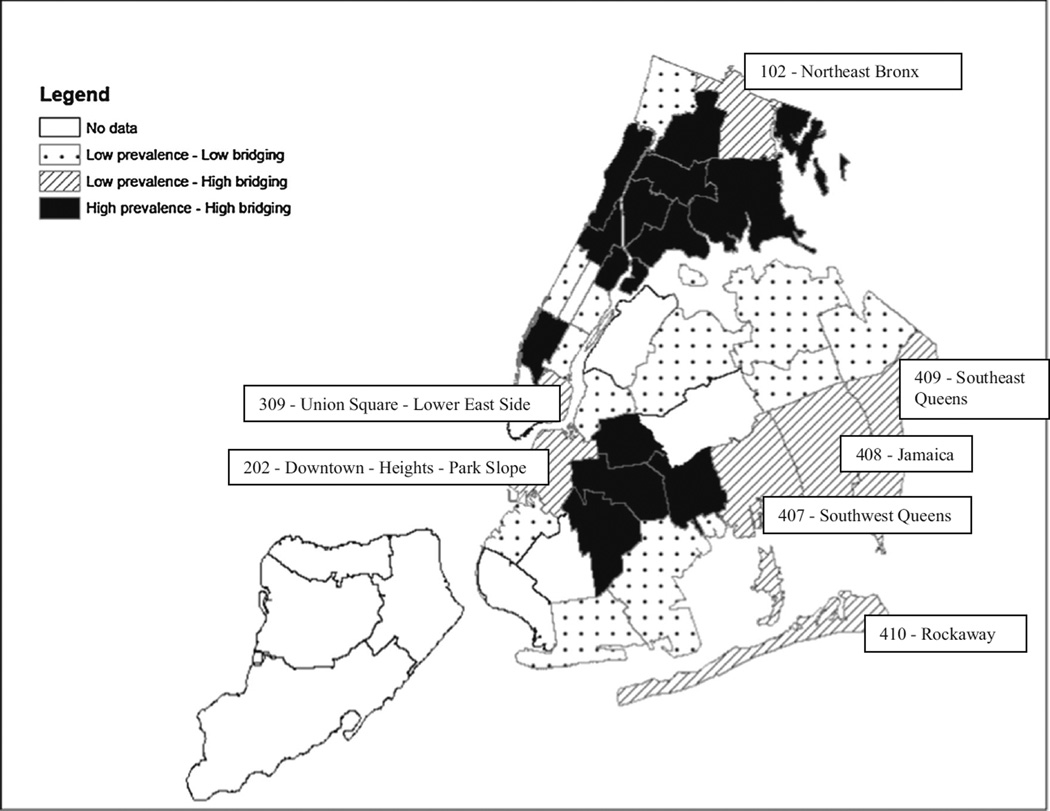
Using the hierarchical bridging measure (Table 2), of the 33 communities in the sample, 20 (60.6 %) overall were higher bridging, including 13 (39.4 %) high HIV-spread communities and 7 (21.2 %) hidden bridging communities. There were 13 (39.4 %) latent HIV bridging communities. None of the higher HIV prevalence communities were contained.
Table 2.
Hierarchical community sexual bridging by high and low United Hospital Fund Community HIV prevalence rates
| Hierarchical community sexual bridging | United Hospital Fund Community HIV prevalence rate | |
|---|---|---|
| High | Low | |
| Any high bridging | “High HIV-spread communities” | “Hidden bridging communities” |
| 103—Fordham—Bronx Park | 102—Northeast Bronx | |
| 104—Pelham—Throgs Neck | 202—Downtown—Heights—Park Slope | |
| 105—Crotona—Tremont | 309—Union Square—Lower East Side | |
| 106—High Bridge—Morrisania | 407—Southwest Queens | |
| 107—Hunts Point—Mott Haven | 408—Jamaica | |
| 203—Bedford Stuyvesant—Crown Heights | 409—Southeast Queens | |
| 204—East New York | 410—Rockaway | |
| 207—East Flatbush—Flatbush | ||
| 211—Williamsburg—Bushwick | ||
| 301—Washington Heights Inwood | ||
| 302—Central Harlem—Morningside Heights | ||
| 303—East Harlem | ||
| 306—Chelsea-Clinton | ||
| Only lower bridging | “Contained high HIV prevalence communities” | “Latent HIV bridging communities” |
| NA | 101—Riverdale | |
| 201—Greenpoint | ||
| 205—Sunset Park | ||
| 208—Canarsie—Flatlands | ||
| 210—Coney Island Sheepshead Bay | ||
| 304—Upper West Side | ||
| 305—Upper East Side | ||
| 307—Gramercy Park—Murray Hill | ||
| 308—Greenwich Village—SoHo | ||
| 402—West Queens | ||
| 403—Flushing—Clearview | ||
| 404—Bayside—Littleneck | ||
| 406—Fresh Meadows | ||
The high HIV-spread communities were geographically clustered (Fig. 5). The two main clusters were in Central and Northern Brooklyn, and in Northern Manhattan combined with South, Central and Eastern Bronx. Chelsea-Clinton, in South-Western Manhattan, was also a high HIV-spread community. Four of the seven hidden bridging communities were adjacent to high HIV-spread communities while three, in Eastern Queens, were adjacent to other hidden bridging communities or to latent HIV bridging communities.
Fig. 5.
Hierarchical community sexual bridging by United Hospital Fund Communities (hidden bridging communities identified)
The community sexual bridging categories were associated with the population racial/ethnic composition of the NYC communities. Compared with latent HIV bridging communities, the population in high HIV-spread communities had a significantly (p < 0.0001, χ2 = 1,393,700) greater percentage of black or Hispanic residents (37.8 vs. 9.5 % for blacks, and 43.0 vs. 22.8 % for Hispanics) than residents who were white or other race/ethnicity (13.0 vs. 45.4 % for whites, and 6.1 vs. 22.3 % for other). Hidden bridging communities compared with latent HIV bridging communities contained a significantly (p < 0.0001, χ2 = 1,393,700) greater percentage of black residents (33.8 vs. 9.5 %) and a significantly lower percentage of white residents (25.9 vs. 45.4 %).
Discussion
Community sexual bridging was common in this NYC sample of heterosexuals at high-risk of HIV. Moreover, HIV prevalence was high, with 9.6 % testing positive of those with positive or negative results compared with the overall 2010 NYC prevalence of PLWHA of 1.4 % [30], and many engaged in HIV risk behaviors. Most participants engaged in condomless sex and three-quarters used non-injection drugs. A substantial proportion of participants reported a lifetime history of ever injecting drugs, and of those testing HIV positive close to half had ever injected drugs. This suggests that the risk of acquiring heterosexually transmitted HIV from condomless sex with current or former drug injectors is considerable in NYC [31, 32].
A majority of NYC communities in the sample were higher bridging communities, and a majority of these were high HIV-spread communities. Most high HIV-spread communities overlapped with or were geographically proximate to the HRAs where sample recruitment was initiated. A majority of the hidden bridging communities were adjacent to high HIV-spread communities. The geographic distribution of higher bridging communities in NYC suggests that heterosexual sexual networks in these communities cover multiple, often geographically proximate communities which, along with risk behaviors and relevant social factors, facilitate the maintenance of endemic levels of heterosexually transmitted HIV [4, 11, 33]. In addition, hidden bridging communities with inadequate HIV prevention resources may continue to propagate the HIV epidemic among heterosexuals since they maintain social and sexual ties with higher prevalence communities.
Community sexual bridging was associated with the population racial/ethnic composition of NYC communities. Higher bridging communities had a greater percentage of black or Hispanic residents compared with latent bridging communities. This association parallels the population distribution of heterosexually transmitted HIV in NYC, which is predominantly and disproportionately found among black women followed by Hispanic women [30, 34]. Hidden bridging communities were more likely to include black residents, which may facilitate the spread of heterosexually transmitted HIV among black women in lower-HIV-prevalence communities.
A possible explanation for the association of the racial/ ethnic composition of NYC communities with community sexual bridging is that sexual partner selection between different communities may be influenced by racial/ethnic homophily, i.e., preferential in-group association by race/ ethnicity [35–37]. In particular, racial/ethnic sexual homophily has been found to be greater among blacks compared with other race/ethnic groups [36]. An analysis, using data from the 494 participants in the analysis data set, was conducted of the race/ethnicity of participants’ sex partnerships in the past 12 months dichotomized by the race/ethnicity of their sex partners, e.g., having “any black sex partners” yes/no, (N = 700 dichotomized sex partnerships). Black participants were significantly (p < 0.0001, χ2 = 46.46) more likely to have any black sex partners (69.6 % of 549), than were Hispanics to have any Hispanic sex partners (50.7 % of 144), whites to have any white sex partners (0.0 % of 3), and “other” to have any “other” sex partners (0.0 % of 4). Racial/ethnic homophily in the preferential selection of sex partners may also reflect residential location and segregation by race/ ethnicity. Sexual partners may be selected from those who are geographically proximate, particularly if there is also a high level of residential segregation by race/ethnicity in proximate communities. This may create a “small world phenomenon”, with clusters of sex partners in geographically proximate communities that are linked to each other through relatively short paths involving sexual network bridging [38], a network structure which is consistent with the network of NYC community heterosexual sexual ties shown in Fig. 3.
Community sexual bridging in NYC may also be associated with homelessness and subsequent geographic mobility. Many participants experienced homelessness in the past 12 months. A high prevalence of homelessness and geographic mobility, especially in high HIV-spread communities, may facilitate the spread of heterosexually transmitted HIV between NYC communities. A structural process that may have contributed to high levels of homelessness in HRAs in NYC is “urban desertification”, whereby mass housing abandonment and arson, such as occurred in the South Bronx during the 1980s, contributes to the fragmentation of social networks, high levels of geographic mobility by individuals at risk of or infected with HIV, and the spread of HIV across urban neighborhoods [39].
Describing and understanding patterns of community sexual bridging can enhance geographically targeted HIV interventions in urban areas. If public health resources are only allocated to high HIV prevalence areas, the role of hidden bridging communities in the spread of HIV may be overlooked. Similarly, high HIV prevalence localities imbedded within communities with lower HIV prevalence may be missed [7]. Using social network analysis to identify communities where community sexual bridging by heterosexuals occurs and implementing appropriate interventions in these communities may have a multiplier effect by interrupting the spread of heterosexually transmitted HIV across urban communities. Such an approach may be useful in NYC as well as in other cities with similar epidemics of heterosexually transmitted HIV.
Limitations
The study has several limitations. A third of participants did not provide adequate geographic data on their sexual partnerships, which may have led to underestimating the extent of community sexual bridging. Intra-NYC community differences in HIV prevalence and bridging may not have been measured for heterogeneous United Hospital Fund neighborhoods which cover a large geographic area or with a large population [7]. Although study participants who recruited their peers may have had a financial incentive to increase the likelihood that their peers would be eligible, this source of recruitment bias may have been minimized because of the broadness of the eligibility criteria. It is possible that men in black and Hispanic majority communities may be more likely than men in other communities to underreport having sex with men because of higher levels of stigma against male homosexuality in these communities. This reporting bias could lead to an underreporting of men who have sex with men in the case surveillance data and in the study, although men who engaged in sex with men and women were eligible for study participation. The data are cross-sectional and consequently the dynamics of community sexual bridging could not be measured, e.g., if participants’ sex partners moved after the reported sexual encounter. Recall bias may be more likely for sexual encounters which occurred earlier in the 12 month recall period. Other factors that may influence community HIV risk and sexual bridging among heterosexuals, including the socioeconomic dynamics between communities, community viral load, community social cohesion, and whether some people engage in more bridging between communities than do others were not analyzed but may be areas for future research. RDS sampling tends to recruit participants from well-connected social networks who, in this study, may have had more sexual partnerships than those who were less socially connected [40]. Caution is therefore necessary in generalizing the results of this study to heterosexuals in NYC who may be less socially connected.
Conclusions
Social network analysis revealed patterns of sexual bridging between NYC communities. While many higher HIV prevalence communities were also higher bridging communities that may sustain endemic heterosexually transmitted HIV, other communities were lower in HIV prevalence but had higher community sexual bridging with the potential to spark outbreaks in lower prevalence communities. Together, high HIV-spread and hidden bridging communities may facilitate the maintenance and spread of heterosexually transmitted HIV in black and Hispanic populations in NYC. In order to interrupt the chain of heterosexual HIV transmission across urban communities with populations at high-risk, interventions can be enhanced by targeting both high HIV-spread and hidden bridging communities.
Acknowledgments
The authors would like to acknowledge Sarah Braunstein, PhD, Blayne Cutler, MD, PhD, and James Hadler, MD, of the New York City Department of Health and Mental Hygiene, and Kent Sepkowitz, MD, for their review of earlier drafts of the paper. We would like to thank Elizabeth DiNenno, Amy Drake, Amy Lansky, and Isa Miles of the CDC for contributing to the NHBS study design locally and nationally, and the New York City NHBS field staff for their efforts in data collection, as well as the study participants who consented to be in the study. This research was funded by a cooperative agreement between the New York City Department of Health and Mental Hygiene (NYC DOHMH) and the Centers for Disease Control and Prevention (CDC) (Grant# 5U62PS000964-03).
References
- 1.Centers for Disease Control and Prevention. [Accessed 02 Jul 2015];HIV surveillance report. 2013 Report No.: 25. http://www.cdc.gov/hiv/pdf/g-1/hiv_surveillance_report_vol_25.pdf.
- 2.New York City Department of Health and Mental Hygiene. [Accessed 02 Jul 2015];New York City HIV/AIDS Annual Surveillance Statistics. 2012 http://www.nyc.gov/html/doh/downloads/pdf/ah/surveillance2012-table-all.pdf.
- 3.Centers for Disease Control and Prevention. [Accessed 20 Aug 2013];HIV and AIDS in the United States by Geographic Distribution. 2013 http://www.cdc.gov/hiv/pdf/statistics_geographic_distribution.pdf.
- 4.Rothenberg R, Muth SQ, Malone S, Potterat JJ, Woodhouse DE. Social and geographic distance in HIV risk. Sex Transm Dis. 2005;32(8):506–512. doi: 10.1097/01.olq.0000161191.12026.ca. [DOI] [PubMed] [Google Scholar]
- 5.New York City Department of Health and Mental Hygiene. [Accessed 08 Oct 2013];Health Department launches Bronx-wide HIV testing initiative [press release] 2008 http://www.nyc.gov/html/pr2008/pr045-08.shtml.
- 6.Renaud TC, Bocour A, Irvine MK, Bernstein KT, Begier EM, Sepkowitz KA, et al. The free condom initiative: promoting condom availability and use in New York City. Public Health Rep. 2009;124(4):481–489. doi: 10.1177/003335490912400404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepard CW, Gortakowski HW, Nasrallah H, Cutler BH, Begier EM. Using GIS-based density maps of HIV surveillance data to identify previously unrecognized geographic foci of HIV burden in an urban epidemic. Public Health Rep. 2011;126(5):741–749. doi: 10.1177/003335491112600517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris M, Podhisita C, Wawer MJ, Handcock MS. Bridge populations in the spread of HIV/AIDS in Thailand. AIDS. 1996;10(11):1265–1271. doi: 10.1097/00002030-199609000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Helleringer S, Kohler HP. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS. 2007;21(17):2323–2332. doi: 10.1097/QAD.0b013e328285df98. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg R. HIV transmission networks. Curr Opin HIV AIDS. 2009;4(4):260. doi: 10.1097/COH.0b013e32832c7cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aral SO. Behavioral aspects of sexually transmitted diseases: core groups and bridge populations. Sex Transm Dis. 2000;27(6):327–328. doi: 10.1097/00007435-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rothenberg R, Jenkins R, Lambert E. Special issue: Sexual Acquisition and Transmission of HIV Cooperative Agreement Program (SATHCAP), July 2009: commentary. J Urban Health. 2009;86(Suppl 1):144–148. doi: 10.1007/s11524-009-9374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auerbach DM, Darrow WW, Jaffe HW, Curran JW. Cluster of cases of the acquired immune deficiency syndrome. Patients linked by sexual contact. Am J Med. 1984;76(3):487–492. doi: 10.1016/0002-9343(84)90668-5. [DOI] [PubMed] [Google Scholar]
- 14.Potterat JJ, Rothenberg RB, Woodhouse DE, Muth JB, Pratts CI, Fogle JS. Gonorrhea as a social disease. Sex Transm Dis. 1985;12(1):25–32. doi: 10.1097/00007435-198501000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Wylie JL, Jolly A. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex Transm Dis. 2001;28(1):14–24. doi: 10.1097/00007435-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Klovdahl AS, Graviss EA, Yaganehdoost A, Ross MW, Wanger A, Adams GJ, et al. Networks and tuberculosis: an undetected community outbreak involving public places. Soc Sci Med. 2001;52(5):681–694. doi: 10.1016/s0277-9536(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 17.De P, Singh AE, Wong T, Yacoub W, Jolly AM. Sexual network analysis of a gonorrhoea outbreak. Sex Transm Infect. 2004;80(4):280–285. doi: 10.1136/sti.2003.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams ML, Atkinson J, Klovdahl A, Ross MW, Timpson S. Spatial bridging in a network of drug-using male sex workers. J Urban Health. 2005;82(1 Suppl 1) doi: 10.1093/jurban/jti022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wylie JL, Shah L, Jolly A. Incorporating geographic settings into a social network analysis of injection drug use and bloodborne pathogen prevalence. Health Place. 2007;13(3):617–628. doi: 10.1016/j.healthplace.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Youm Y, Mackesy-Amiti ME, Williams CT, Ouellet LJ. Identifying hidden sexual bridging communities in Chicago. J Urban Health. 2009;86(Suppl 1):107–120. doi: 10.1007/s11524-009-9371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youm YA. sociological interpretation of emerging properties in STI transmission dynamics: walk-betweenness of sexual networks. Sex Transm Infect. 2010;86(Suppl 3):ii24–ii28. doi: 10.1136/sti.2010.044008. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher KM, Sullivan PS, Lansky A, Onorato IM. Behavioral surveillance among people at risk for HIV infection in the U.S.: the National HIV Behavioral Surveillance System. Public Health Rep. 2007;122(Suppl):1. doi: 10.1177/00333549071220S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lansky A, Sullivan PS, Gallagher KM, Fleming PL. HIV behavioral surveillance in the U.S.: a conceptual framework. Public Health Rep. 2007;122(Suppl 1):16. doi: 10.1177/00333549071220S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–199. [Google Scholar]
- 25.Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc Probl. 2002;49(1):11–34. [Google Scholar]
- 26.New York City Department of Health and Mental Hygiene. [Accessed 07 Oct 2013];New York City United Hospital Fund neighborhoods and NYC ZIP code areas. http://home2.nyc.gov/html/doh/downloads/pdf/survey/uhf_map_100604.pdf.
- 27.Burt RS. Structural holes: the social structure of competition. Cambridge: Harvard University Press; 1992. [Google Scholar]
- 28.Freeman LC, Borgatti SP, White DR. Centrality in valued graphs: a measure of betweenness based on network flow. Soc Netw. 1991;13(2):141–154. [Google Scholar]
- 29.Newman MEJ. A measure of betweenness centrality based on random walks. Soc Netw. 2005;27:39–54. [Google Scholar]
- 30.New York City Department of Health and Mental Hygiene. [Accessed 26 Mar 2012];New York City HIV/AIDS Annual Surveillance Statistics. 2010 http://www.nyc.gov/html/doh/downloads/pdf/ah/surveillance2010-tables-all-pdf.
- 31.Jenness SM, Neaigus A, Hagan H, Murrill CS, Wendel T. Heterosexual HIV and sexual partnerships between injection drug users and noninjection drug users. AIDS Patient Care STDS. 2010;24(3):175–181. doi: 10.1089/apc.2009.0227. [DOI] [PubMed] [Google Scholar]
- 32.Neaigus A, Miller M, Gyarmathy VA, Friedman SR. HIV heterosexual sexual risk from injecting drug users among HIV-seronegative noninjecting heroin users. Subst Use Misuse. 2011;46(2–3):208–217. doi: 10.3109/10826084.2011.521473. [DOI] [PubMed] [Google Scholar]
- 33.Wasserheit JN, Aral O. The dynamic topology of sexually transmitted disease epidemics: implications for prevention strategies. J Infect Dis. 1996;174(suppl 2):S201–S213. doi: 10.1093/infdis/174.supplement_2.s201. [DOI] [PubMed] [Google Scholar]
- 34.United States Census Bureau. American FactFinder fact sheet. New York City; 2010. [Accessed 14 Jun 2014]. http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF. [Google Scholar]
- 35.Ford K, Norris A. Sexual networks of African-American and Hispanic youth. Sex Transm Dis. 1997;24(6):327–333. doi: 10.1097/00007435-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26(5):250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Bingham TA, Harawa NT, Johnson DF, Secura GM, MacKellar DA, Valleroy LA. The effect of partner characteristics on HIV infection among African American men who have sex with men in the Young Men’s Survey, Los Angeles, 1999–2000. AIDS Educ Prev. 2003;15(1 Suppl A):39–52. doi: 10.1521/aeap.15.1.5.39.23613. [DOI] [PubMed] [Google Scholar]
- 38.Watts DJ. Networks, dynamics, and the small world phenomenon. Am J Sociol. 1999;105:493–527. [Google Scholar]
- 39.Wallace R. Urban desertification, public health and public order: ‘Planned shrinkage’, violent death, substance abuse and AIDS in the Bronx. Soc Sci Med. 1990;31(7):801–813. doi: 10.1016/0277-9536(90)90175-r. [DOI] [PubMed] [Google Scholar]
- 40.Jenness SM, Neaigus A, Wendel T, Gelpi-Acosta C, Hagan H. Spatial recruitment bias in respondent-driven sampling: implications for HIV prevalence estimation in urban heterosexuals. AIDS Behav. 2014;18(12):2366–2373. doi: 10.1007/s10461-013-0640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]