Abstract
We investigated the risk factors for adenoma occurrence at surveillance colonoscopy, especially focusing on patient characteristics, including metabolic factors.
Surveillance colonoscopy intervals depend on baseline adenoma characteristics. However, patients’ characteristics may also influence the occurrence of adenomas.
Of 62,171 asymptomatic subjects who underwent colonoscopy for a health check-up between 2010 and 2011, 4869 subjects who underwent follow-up colonoscopy between 2012 and 2013 were included in this longitudinal study. The risk of adenoma occurrence was assessed using Cox proportional hazards modeling.
Of 4869 subjects, 2827 (58.1%), 1619 (33.3%), and 423 (8.7%) were assigned to the normal, low-risk, and high-risk groups, respectively, according to baseline adenoma characteristics. The mean interval between initial and follow-up colonoscopy was 2.2 ± 0.6 years. Certain patient factors, including older age (≥50 years; adjusted hazard ratio [aHR], 2.08; 95% CI, 1.73–2.49), male sex (aHR, 1.69; 95% CI, 1.30–2.19), metabolic syndrome (MetS) (aHR, 1.28; 95% CI, 1.09–1.51), obesity (aHR, 1.17; 95% CI, 1.02–1.34), elevated fasting blood glucose levels (aHR, 1.37; 95% CI, 1.19–1.58), and elevated triglyceride levels (aHR, 1.19; 95% CI, 1.03–1.37), as well as baseline adenoma characteristics, were associated with a higher risk of adenoma occurrence at follow-up colonoscopy. The cumulative incidence of adenoma occurrence in the high-risk group was higher than that in the low-risk group, whereas the incidence in the high-risk group without MetS was comparable with that in the low-risk group with MetS.
Patient characteristics, such as MetS, obesity, older age, and male sex, in addition to adenoma characteristics, were independent risk factors for adenoma occurrence at surveillance colonoscopy. These patient characteristics may be considered in surveillance colonoscopy intervals.
Keywords: colorectal adenoma, metabolic syndrome, surveillance
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in males and the second most common in females, making it the fourth most common leading cause of cancer-related deaths worldwide. [1] In Asian countries, the incidence of CRC has steeply increased in recent years.[ 2 3] Screening colonoscopy and polypectomy have the most significant effects on reducing the incidence and mortality of CRC.[ 4 5] However, patients who have adenomas are at increased risk for developing metachronous adenomas or cancer, compared with patients without adenomas. [6] Thus, patients with resected adenomas are recommended to have follow-up surveillance colonoscopy. [6 7 8]
The present guidelines on postpolypectomy surveillance have introduced the concept of “risk stratification” on the basis of baseline adenoma characteristics. [6 7 8] The guidelines stratify adenomas into 2 risk groups based on the likelihood of developing advanced colorectal neoplasm (ACRN) during surveillance and recommend repeat screening colonoscopy at 10 years for subjects with no adenoma, 5 to 10 years for patients with low-risk adenomas, and 3 years for those with high-risk adenomas. [6 7 8] However, patients’ characteristics, as well as adenoma characteristics, may influence the occurrence of colorectal adenomas. Numerous studies have investigated the features of adenomas associated with recurrent colorectal neoplasia (CRN), [9 10 11 12 13] whereas studies focusing on patient characteristics associated with recurrent CRN are few in number. [14 15 16]
Recently, many studies have reported that smoking, obesity, and metabolic syndrome (MetS), as well as older age and male sex, are risk factors for the development of colorectal adenoma. [17 18 19 20 21] However, the majority of these studies were cross-sectional investigations, which evaluate the association between the prevalence of adenoma and patients’ characteristics.[ 18 19 21 22] These patient characteristics may also be risk factors for developing metachronous adenomas. Several studies reported that older age and male sex were associated with a higher risk of adenoma recurrence.[ 9 12 23 24] However, data regarding the influence of modifiable risk factors, such as metabolic factors, on adenoma recurrence are extremely limited. Thus, we conducted this longitudinal study to identify patient-related risk factors associated with the occurrence of colorectal adenomas at time of follow-up surveillance colonoscopy, especially focusing on metabolic factors.
2. Methods
2.1. Study population
The study population consisted of asymptomatic subjects who had undergone a colonoscopy as part of a comprehensive health screening program at Kangbuk Samsung Hospital, Seoul and Suwon, Korea, between 2010 and 2011 (defined as “initial colonoscopy”) (n = 62,171). Of these participants, 7318 subjects underwent repeat colonoscopy as part of a health checkup between 2012 and 2013 (defined as “follow-up colonoscopy”). The exclusion criteria were as follows: poor bowel preparation (n = 1744), lack of an adequate biopsy (n = 43), missing anthropometric data (n = 47), a family history of CRC (n = 420), a history of CRC or colorectal surgery (n = 17), a history of inflammatory bowel disease (n = 70), detection of a colorectal carcinoid tumor during this study (n = 36), detection of a colorectal adenocarcinoma during this study (n = 3), and age <30 years old (n = 69). Finally, the total number of eligible subjects for the study was 4869 (Fig. 1). Poor bowel preparation was defined as “large amounts of solid fecal matter found, precluding a satisfactory study; unacceptable preparation; <90% of mucosa seen.” [25]
Figure 1.
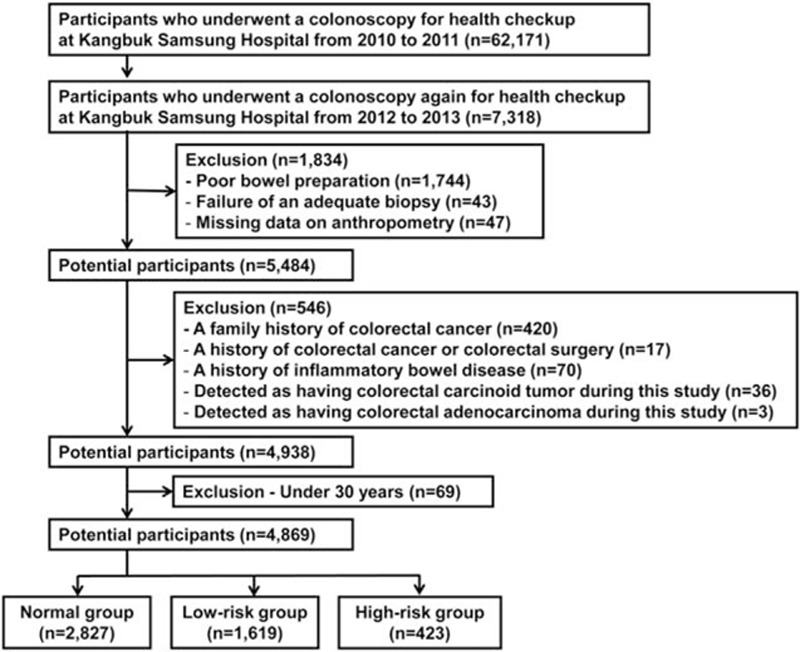
Flow diagram illustrating the selection of study subjects.
Before colonoscopy, general practitioners conducted interviews to ensure that the subjects had no gastrointestinal symptoms, such as visible rectal bleeding or abdominal pain. Subjects with these symptoms were urged to seek medical care and only asymptomatic participants continued this screening program.
In Korea, the Industrial Safety and Health Law requires employees to participate in annual or biennial health examinations. About 60% of the participants were employees of various companies and local governmental organizations and their spouses, with the remaining participants registering individually for the program. In most Korean companies, the mandatory retirement age is ∼55 years. As part of the welfare policy, companies often subsidize annual or biennial comprehensive health examinations including colonoscopies, regardless of age. Such programs are popular in Korea. [20] Therefore, our database had a relatively large group of subjects aged <50 years who underwent screening colonoscopy. In addition, although clinicians recommend that the frequency of undergoing surveillance colonoscopy should be as per the intervals mentioned in the present guideline (the Korean guideline recommends surveillance colonoscopy at 5 years for subjects with no adenoma or low-risk adenomas and at 3 years for those with high-risk adenomas), [7] some subjects opt to undergo a colonoscopy biennially in spite of having no adenomas or low-risk adenomas, regardless of the recommendation, because companies often subsidize annual or biennial health screening programs that include colonoscopies.
This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital, which exempted the requirement for informed consent, because we retrospectively accessed only de-identified data.
2.2. Measurements and definitions
Data on medical history and health-related behaviors were collected through a self-administered questionnaire, whereas the physical measurements and laboratory tests were performed by a trained staff. Self-reported use of nonsteroidal anti-inflammatory drugs (NSAIDs) (regular use over the previous month) was also assessed. Family history of CRC was defined as CRC in ≥1 first-degree relatives at any age.
The blood samples were collected from the antecubital vein after at least a 10-hour fast. The serum levels of the total cholesterol and triglycerides were determined using an enzymatic colorimetric assay. Fasting blood glucose (FBG) levels were measured using the hexokinase method. For the blood pressure (BP) measurements, the average of 3 measurements was used for the data analysis. Diabetes mellitus (DM) was defined as history of DM, use of glucose-lowering medications, FBG ≥126 mg/dL, or HbA1c ≥6.5%. MetS was diagnosed when ≥3 of the following criteria were satisfied: abdominal obesity, elevated FBG levels (≥100 mg/dL or use of glucose-lowering medications), elevated BP (≥130 mm Hg systolic, ≥85 mm Hg diastolic, or intake of antihypertensive drugs), elevated triglyceride levels (≥150 mg/dL), and reduced high-density lipoprotein cholesterol (HDL-C) levels (<40 and <50 mg/dL in men and women, respectively). [26] Because waist circumference measurements were not available for all participants, we substituted overall obesity for abdominal obesity. Obesity was defined as body mass index (BMI) ≥25 kg/m2, which is the proposed cut-off for the diagnosis of obesity in Asians. [27] BMI was calculated by dividing the measured weight (kg) by the square of the height (m2).
The presence or absence of fatty liver was examined through abdominal ultrasonography (US). A diagnosis of fatty liver was made on the basis of 4 known criteria: hepatorenal echogenic contrast, liver brightness, deep attenuation, and vascular blurring. [28] Abdominal US was performed using a 3.5-MHz transducer (Logiq 9; General Electric, Madison, WI) by 11 experienced radiologists, who were unaware of the study aims and blinded to clinical information. To assess intra-observer and inter-observer reliability of US diagnosis of fatty liver, random samples of 200 stored US images were re-read at least 2 weeks apart by the 11 radiologists. Inter-observer reliability was substantial (kappa statistic of 0.74), and intra-observer reliability was excellent (kappa statistic of 0.94). [29] However, fatty liver is just a factor in the steatohepatitis assessment tools and cannot be the sole factor in decision.
2.3. Colonoscopy and histologic examination
Colonoscopy was performed by 11, 12, 13, and 12 experienced gastroenterologists in 2010, 2011, 2012, and 2013, respectively, using an EVIS LUCERA CV-260 colonoscope (Olympus Medical Systems, Tokyo, Japan). The total number of unique gastroenterologists was 21. All the participants took 4 L of polyethylene glycol solution for bowel preparation.
All detected polypoid lesions were biopsied or removed and histologically assessed by experienced pathologists. Polyps were classified by number, size, and histologic characteristics (tubular, tubulovillous, or villous adenoma; hyperplastic polyp; inflammatory polyp; and sessile serrated adenoma or traditional serrated adenoma). Pathologic results of the hyperplastic polyps, inflammatory polyps, or lipomas were considered normal findings. The grade of dysplasia was classified as low or high. Advanced adenoma was defined as the presence of 1 of the following features: >10 mm diameter, tubulovillous or villous structure, and high-grade dysplasia (HGD). [6] Subjects simultaneously discovered to have nonadvanced and advanced adenomas were classified as having advanced adenoma. The study subjects were categorized into normal, low-risk, and high-risk groups according to initial colonoscopy findings. The normal group was defined as subjects with no adenoma, the low-risk group was defined as subjects with 1 to 2 tubular adenomas <10 mm, and the high-risk group was defined as subjects who have adenomas with villous histology, HGD, size ≥10 mm, or ≥3 adenomas at the initial colonoscopy. [6 7 8]
2.4. Statistical analysis
Data are expressed as mean ± standard deviation or frequency (%). In each normal, low-risk, and high-risk group, baseline characteristics between subjects with and without adenoma occurrence at follow-up colonoscopy were compared by χ2 analysis for categorical variables and by Student's t test for continuous variables.
The risks of the occurrence of any adenoma and advanced adenoma at follow-up colonoscopy according to baseline patient and adenoma characteristics were assessed using Cox proportional hazards modeling. Person-years were calculated as the sum of the intervals from initial to follow-up colonoscopy. We estimated the hazard ratios (HRs) with 95% confidence intervals (CIs) for the association of patient characteristics with the risks of adenoma occurrence using Cox proportional hazards models, adjusted for potentially confounding variables such as age, sex, smoking status, use of NSAIDs, fatty liver, and MetS. The analysis of DM, obesity, BP, FBG, HDL-C, and triglyceride levels was not adjusted for MetS.
In the subgroup analysis, the cumulative probabilities of adenoma occurrence were calculated using the Kaplan–Meier method, with differences determined using the log-rank test. All of the reported P-values were 2-tailed, and P-values <0.05 were considered to be statistically significant. SPSS Version 18 (IBM Corp., Armonk, NY) was used for statistical analyses.
3. Results
3.1. Baseline characteristics of study population
A total of 4869 participants were eligible for the analysis (Fig. 1). At baseline, the mean age of the 4869 subjects was 42.0 ± 6.8 years, and 87.1% of the subjects were men. The proportions of subjects aged 30 to 39 years, 40 to 49 years, and ≥50 years were 39.1%, 47.9%, and 13.0%, respectively. The mean interval between initial and follow-up colonoscopy was 2.2 ± 0.6 years, with a total of 10,618 person-years.
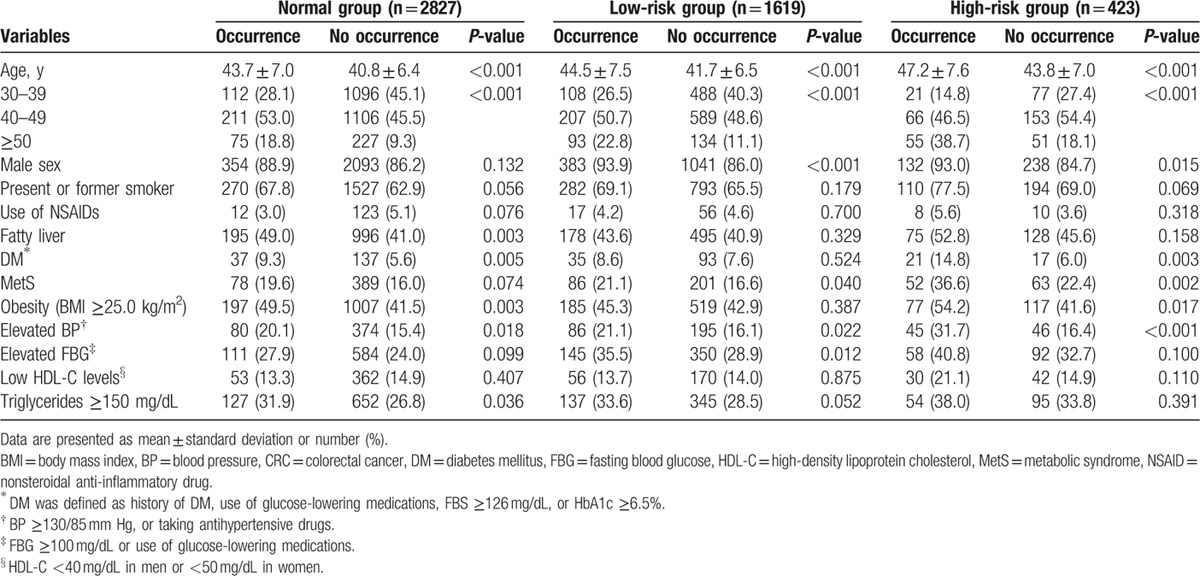
According to initial colonoscopy findings (baseline adenoma characteristics), 2827 (58.1%) subjects were assigned to the normal group, 1619 (33.3%) to the low-risk group, and 423 (8.7%) to the high-risk group. In each group, the baseline characteristics between subjects who experienced the occurrence of adenoma at follow-up surveillance colonoscopy and who did not are summarized in Table 1. In all groups, subjects with adenoma occurrence had a higher mean age compared with subjects without adenoma occurrence. In the normal group, subjects with adenoma occurrence had higher proportions of fatty liver, DM, obesity, elevated BP, and elevated triglyceride levels compared with subjects without adenoma occurrence. In the low-risk group, adenoma occurrence was seen in a higher proportion of males, MetS, elevated BP, and elevated FBG levels. Meanwhile, in the high-risk group, adenoma occurrence was more frequently seen in males, patients with DM, MetS, obesity, and elevated BP. In all groups, there was no significant difference in the proportion of subjects with a smoker, use of NSAIDs, and low HDL-C levels between subjects with and without adenoma occurrence.
Table 1.
Comparison of baseline characteristics between subjects with and without the occurrence of adenoma at follow-up colonoscopy.
3.2. Risks of adenoma occurrence according to baseline patient and adenoma characteristics
Table 2 shows the risk for the occurrence of any adenoma and advanced adenoma at the time of follow-up colonoscopy among the 4869 subjects, according to the patients’ clinical characteristics and adenoma characteristics at the time of initial colonoscopy. A Cox proportional hazard regression analysis was performed to determine the risk factors for the occurrence of any adenoma and advanced adenoma. In the univariate analyses, patients’ demographic factors including older age (≥50 years; HR, 2.07; 95% CI, 1.72–2.48), male sex (HR, 1.84; 95% CI, 1.46–2.32), present or former smoking (HR, 1.29; 95% CI, 1.13–1.49), fatty liver (HR, 1.27; 95% CI, 1.12–1.44), DM (HR, 1.47; 95% CI, 1.19–1.83), MetS (HR, 1.47; 95% CI, 1.27–1.72), obesity (HR, 1.28; 95% CI, 1.13–1.45), elevated BP (HR, 1.27; 95% CI, 1.09–1.48), elevated FBG levels (HR, 1.54; 95% CI, 1.34–1.76), elevated triglyceride levels (HR, 1.28; 95% CI, 1.12–1.47), and low-risk and high-risk groups (vs normal group; HR, 2.12; 95% CI, 1.84–2.43 and HR, 3.16; 95% CI, 2.61–3.82, respectively) were associated with a higher risk of any adenoma occurrence at follow-up surveillance colonoscopy. The risk for advanced adenoma occurrence increased in subjects with older age (≥50 years; HR, 6.35; 95% CI, 3.18–12.70), DM (HR, 2.40; 95% CI, 1.22–4.69), MetS (HR, 1.88; 95% CI, 1.09–3.23), elevated FBG levels (HR, 1.88; 95% CI, 1.14–3.09), and low-risk and high-risk groups (vs normal group; HR, 1.81; 95% CI, 1.02–3.22 and HR, 6.78; 95% CI, 3.73–12.32, respectively).
Table 2.
Risks of adenoma and advanced adenoma occurrence according to baseline patient and adenoma characteristics.
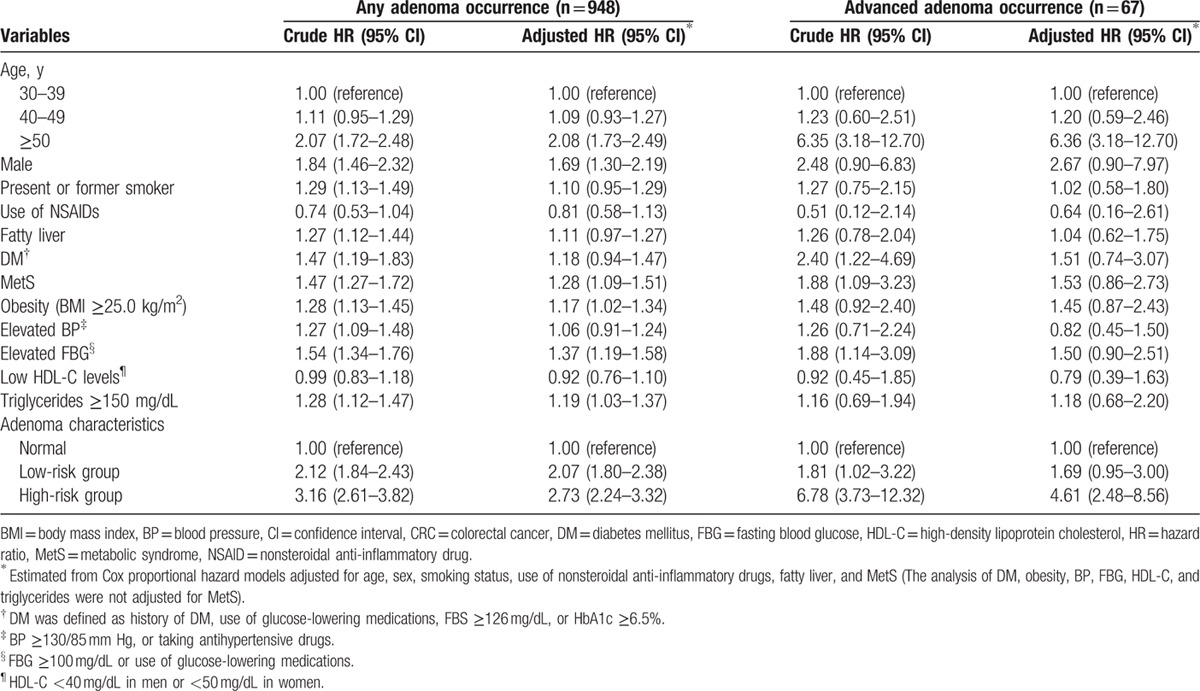
In the multivariate analyses, adjusted for age, sex, smoking status, use of NSAIDs, fatty liver, and MetS, the patients’ demographic factors, including older age [≥50 years; adjusted HR (aHR), 2.08; 95% CI, 1.73–2.49], male sex (aHR, 1.69; 95% CI, 1.30–2.19), MetS (aHR, 1.28; 95% CI, 1.09–1.51), obesity (aHR, 1.17; 95% CI, 1.02–1.34), elevated FBG levels (aHR, 1.37; 95% CI, 1.19–1.58), elevated triglyceride levels (aHR, 1.19; 95% CI, 1.03–1.37), and low-risk and high-risk grouping (vs normal group; aHR, 2.07; 95% CI, 1.80–2.38 and aHR, 2.73; 95% CI, 2.24–3.32, respectively), were associated with a higher risk of any adenoma occurrence at follow-up surveillance colonoscopy. In addition, older age (≥50 years; aHR, 6.36; 95% CI, 3.18–12.70) and high-risk classifications (vs normal group; aHR, 4.61; 95% CI, 2.48–8.56) were independent risk factors for advanced adenoma occurrence at follow-up surveillance colonoscopy.
3.3. Subgroup analysis by baseline adenoma characteristics
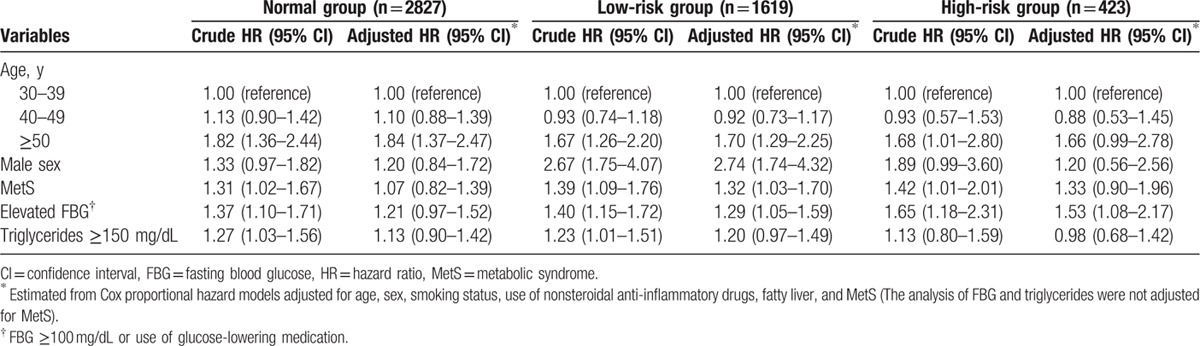
We performed subgroup analysis based on risk groups according to initial colonoscopy findings (Table 3). In the normal group, older age (≥50 years; aHR, 1.84; 95% CI, 1.37–2.47) was the only independent risk factor for adenoma occurrence at follow-up surveillance colonoscopy. In the low-risk group, older age (≥50 years; aHR, 1.70; 95% CI, 1.29–2.25), male sex (aHR, 2.74; 95% CI, 1.74–4.32), MetS (aHR, 1.32; 95% CI, 1.03–1.70), and elevated FBG levels (aHR, 1.29; 95% CI, 1.05–1.59) were independent risk factors for adenoma occurrence. In the high-risk group, elevated FBG levels (aHR, 1.53; 95% CI, 1.08–2.17) were independently associated with adenoma occurrence, whereas MetS was not an independent risk factor for adenoma occurrence (aHR, 1.33; 95% CI, 0.90–1.96).
Table 3.
Subgroup analysis for adenoma occurrence by baseline adenoma characteristics.
3.4. Influence of MetS on adenoma occurrence
We compared the cumulative incidence of adenoma occurrence at follow-up colonoscopy between low-risk and high-risk groups. The high-risk group showed a higher cumulative incidence of adenoma occurrence than the low-risk group (P <0.001, Fig. 2A). However, the cumulative incidences of adenoma occurrence in the high-risk group without MetS and the low-risk group with MetS were comparable (P = 0.948, Fig. 2B). In other words, the significant gap in adenoma occurrence according to baseline adenoma characteristics was offset by a patient-related risk factor that is MetS.
Figure 2.
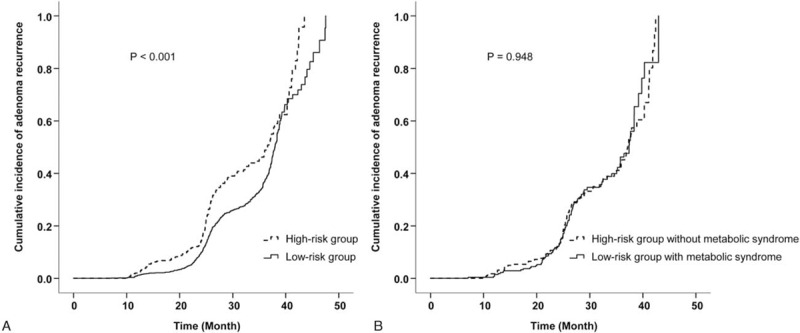
(A) Cumulative incidence of adenoma occurrence in the low-risk group vs high-risk group (P <0.001). (B) Cumulative incidence of adenoma occurrence in the low-risk group with MetS vs high-risk group without MetS (P = 0.948). MetS = metabolic syndrome.
4. Discussion
This large-scale, longitudinal study, which included 4869 asymptomatic participants, investigated the factors associated with the occurrence of colorectal adenomas at follow-up surveillance colonoscopy, focusing on patients’ clinical characteristics. We found that patients’ demographic factors, including older age, male sex, MetS, obesity, elevated FBG levels, and elevated triglyceride levels, as well as baseline adenoma characteristics (low-risk and high-risk adenomas), were associated with a higher risk of adenoma occurrence at surveillance colonoscopy. Moreover, the cumulative incidence of adenoma occurrence in the low-risk group with MetS was comparable with that in the high-risk group without MetS.
The present guidelines recommend intervals of surveillance colonoscopy according to adenoma characteristics, on the basis of the likelihood of developing ACRN during surveillance. [6 7 8] However, our study showed that patients’ characteristics, including MetS, obesity, older age, and male sex, also influence the occurrence of adenomas at surveillance colonoscopy. Our findings suggest that the surveillance colonoscopy interval may be individualized and optimized according to patient-related risk factors, in addition to adenoma characteristics. These tailored suggestions for surveillance colonoscopy intervals can improve the efficacy and cost effectiveness of surveillance colonoscopy and may reduce the risk of interval CRCs.[ 18 19 30 31 32]
To date, only a few studies have investigated the influence of these metabolic factors on adenoma occurrence at follow-up surveillance colonoscopy.[ 14 16 33 34 35] One Japanese study involving 1111 subjects who underwent removal of >5 mm adenomas at initial colonoscopy demonstrated that obesity and increased FBG were associated with an increased risk of adenoma recurrence. [33] Another 2 studies revealed that obesity was associated with an increased risk of adenoma recurrence and advanced adenoma recurrence.[ 14 34] Recently, a Korean study including 1792 subjects demonstrated that metabolic factors, especially high waist circumference, affect CRN occurrence at surveillance colonoscopy. [35] A Taiwan study including 4483 subjects also showed that MetS is a risk factor for the occurrence of an advanced adenoma at surveillance colonoscopy. [16] However, the Korean and Taiwan studies reported that high waist circumference and MetS had significant effects on the risk for CRN in the normal and low-risk groups, but not in the high-risk group.[ 16 35] On the contrary, in our study, MetS was associated with a higher risk of adenoma occurrence in the low-risk group and its component, elevated FBG, was associated with a higher risk of adenoma occurrence in both the high-risk and low-risk groups. Metabolic factors seem to accelerate adenoma occurrence in patients with a history of adenoma. Based on our results, we carefully suggest that patients with both high-risk adenomas and clinical risk factors such as MetS be categorized as “very high-risk patients,” and these patients may be recommended to undergo surveillance colonoscopy at <3 years. In addition, the low-risk group with clinical risk factors may be recommended to undergo surveillance colonoscopy earlier than 5 to 10 years, whereas the high-risk group without clinical risk factors may be recommended to undergo surveillance colonoscopy beyond 3 years. These suggestions are supported by our finding that the significant gap in adenoma recurrence according to baseline adenoma characteristics (low-risk vs high-risk group) was offset by patient-related risk factors such as MetS.
The main pathophysiological abnormality of MetS and obesity is visceral fat deposition. [36] Visceral fat is associated with insulin resistance and conditions which influence the carcinogenic process by increasing cell proliferation and reducing apoptosis. [37] In addition, visceral adipose tissues release inflammatory cytokines and adipocytokines, which increase the risk of development and growth of colorectal tumors. [38] Based on these biologic mechanisms, it is reasonable to conclude that MetS affects adenoma occurrence at surveillance colonoscopy, as well as adenoma prevalence.
The gut microbiome may be proposed as another mechanism linking MetS and CRN. Both animals and humans studies suggest that gut microbiota play a significant role in energy homeostasis, metabolic processes, modulation of inflammatory signaling pathways, and interference with the immune system, potentially contributing to MetS. [39] Recent studies also support a link between gut microbiome and CRN. [40] The gut microbiota can drive DNA damage via either specific proteins or metabolites and thus it may affect the risk of developing CRN.[ 40 25]
In the present study, obesity, MetS and its individual components, such as elevated FBG and triglyceride levels, showed a significant impact on the occurrence of adenomas. Especially, elevated FBG was an independent risk factor for adenoma occurrence in both low-risk and high-risk groups. Although the mechanisms linking elevated FBG and adenoma occurrence are not fully understood, there are several possible explanations. First, hyperinsulinemia has been reported to promote carcinogenesis through the effect of insulin-like growth factor 1. [41] Second, hyperglycemia can promote the formation of reactive oxygen species, which can damage DNA and trigger cancer progression. [42] Third, glucose may contribute to colorectal carcinogenesis through an increase in bile acids or energy supply to neoplastic cells. [43] Considering that components of MetS are modifiable and its incidence has dramatically increased worldwide, [44] interventions to alleviate MetS (such as exercise, diet control, or weight reduction) may play important roles in reducing adenoma occurrence. However, the evidence is lacking. Further long term, prospective, randomized, controlled studies are warranted to clarify whether the interventions to prevent MetS have merit in decreasing the development of metachronous adenoma.
On the contrary, we found that in the multivariate analysis, MetS was not associated with adenoma occurrence in the high-risk group (aHR, 1.33; 95% CI, 0.90–1.96). The reason may be that the number of patients in the high-risk group was insufficient to reach statistical significance. In addition, MetS itself does not have a significant impact on the risk for advanced adenoma. The most likely explanation is that the follow-up period (mean duration 2.2 years) might be too short to evaluate the risk for the occurrence of advanced adenoma. However, baseline adenoma characteristics were associated with a higher risk for advanced adenoma occurrence. Moreover, the most potent risk factor for adenoma occurrence (highest aHR) was baseline adenoma characteristics (high-risk adenoma). In other words, aHRs of patient-related risk factors were lower than the aHR of the high-risk group. Our findings indicate that although several metabolic factors have significant effects on the risk for adenoma occurrence, baseline adenoma characteristics remain to be the most important factors for risk stratification of adenoma occurrence during surveillance colonoscopy.
In the present study, older age and male sex were found to be independent risk factors for adenoma occurrence. Similar to our results, previous studies have also reported that older age and male sex are associated with a higher risk of adenoma occurrence.[ 13 15 16 33] In our study, older age was the most important patient-related risk factor (highest aHR among patient characteristics) for adenoma occurrence; it was also a risk factor for advanced adenoma occurrence. Based on the results of our study and previous studies, age and sex may also be considered in surveillance colonoscopy intervals.
The strength of our study was that it included a relatively larger number of subjects than other earlier related studies. However, there are several limitations in the present study. First, this was not a population-based study, but rather a retrospective study that included participants who underwent a colonoscopy as a part of a regular health examination in 2 centers in Korea. As a result, there was likely some degree of selection bias. Moreover, a large majority of the study subjects were men and <50 years of age. Therefore, interpretation of our findings requires careful consideration when applied to other populations or settings. However, this uniqueness of our cohort made it possible to identify the risk factors for metachronous adenoma in a young population and confirm that MetS was associated with a high risk of metachronous adenoma even in a young population. Considering the increasing incidence of CRC in young adults <50 years of age, [45] our study provides significant information about metachronous adenoma in a young population. Second, the follow-up period might be too short to evaluate the risk for the occurrence of adenoma, especially advanced adenoma. Some percentage of adenomas found at the time of surveillance colonoscopy might have been missed in the initial colonoscopy. Third, MetS was determined using overall obesity (BMI ≥25 kg/m2) instead of abdominal obesity because waist circumference measurements were not available for all participants. Finally, data on dietary factors, physical activity, and economic status, which could be confounders, were not analyzed.
In conclusion, patients’ characteristics, including MetS, obesity, older age, and male sex, as well as baseline adenoma characteristics, were independent risk factors for adenoma occurrence at follow-up surveillance colonoscopy. These patient-related risk factors, in addition to adenoma characteristics, may be considered in determining surveillance colonoscopy intervals. Further prospective, long-term, longitudinal studies are required to establish guidelines for individualized surveillance intervals based on both patient characteristics and adenoma characteristics.
Footnotes
Abbreviations: ACRN = advanced colorectal neoplasm, aHR = adjusted hazard ratio, BMI = body mass index, BP = blood pressure, CIs = confidence intervals, CRC = colorectal cancer, CRN = colorectal neoplasia, DM = diabetes mellitus, FBG = fasting blood glucose, HDL-C = high-density lipoprotein cholesterol, HGD = high-grade dysplasia, HRs = hazard ratios, MetS = metabolic syndrome, NSAIDs = nonsteroidal anti-inflammatory drugs, US = ultrasonography.
The authors have no conflicts of interest to disclose.
References
- 1. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2. Song YK, Park YS, Seon CS, et al. Alcohol drinking increased the risk of advanced colorectal adenomas. Intest Res 2015; 13:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng SC, Wong SH. Colorectal cancer screening in Asia. Br Med Bull 2013; 105:29–42. [DOI] [PubMed] [Google Scholar]
- 4. Lee HS, Jeon SW. Is retroflexion helpful in detecting adenomas in the right colon?: a single center interim analysis. Intest Res 2015; 13:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin OS, Kozarek RA, Cha JM. Impact of sigmoidoscopy and colonoscopy on colorectal cancer incidence and mortality: an evidence-based review of published prospective and retrospective studies. Intest Res 2014; 12:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857. [DOI] [PubMed] [Google Scholar]
- 7. Yang D-H, Hong SN, Kim Y-H, et al. Korean guidelines for post-polypectomy colonoscopic surveillance. Intest Res 2012; 10:89–109. [DOI] [PubMed] [Google Scholar]
- 8. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010; 59:666–689. [DOI] [PubMed] [Google Scholar]
- 9. de Jonge V, Sint Nicolaas J, van Leerdam ME, et al. Systematic literature review and pooled analyses of risk factors for finding adenomas at surveillance colonoscopy. Endoscopy 2011; 43:560–572. [DOI] [PubMed] [Google Scholar]
- 10. van Heijningen EM, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology 2013; 144:1410–1418. [DOI] [PubMed] [Google Scholar]
- 11. Nusko G, Mansmann U, Kirchner T, et al. Risk related surveillance following colorectal polypectomy. Gut 2002; 51:424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Enckevort CC, de Graaf AP, Hollema H, et al. Predictors of colorectal neoplasia after polypectomy: based on initial and consecutive findings. Neth J Med 2014; 72:139–145. [PubMed] [Google Scholar]
- 13. Simunic M, Perkovic N, Rosic-Despalatovic B, et al. Colonoscopic polypectomies and recommendations on the colonoscopy follow-up intervals depending on endoscopic and histopathological findings. Acta Inform Med 2013; 21:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laiyemo AO, Doubeni C, Badurdeen DS, et al. Obesity, weight change, and risk of adenoma recurrence: a prospective trial. Endoscopy 2012; 44:813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin CC, Huang KW, Luo JC, et al. Hypertension is an important predictor of recurrent colorectal adenoma after screening colonoscopy with adenoma polypectomy. J Chin Med Assoc 2014; 77:508–512. [DOI] [PubMed] [Google Scholar]
- 16. Chiu HM, Lee YC, Tu CH, et al. Effects of metabolic syndrome and findings from baseline colonoscopies on occurrence of colorectal neoplasms. Clin Gastroenterol Hepatol 2015; 13:1134–1142.e1138. [DOI] [PubMed] [Google Scholar]
- 17. Giovannucci E, Rimm EB, Stampfer MJ, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst 1994; 86:183–191. [DOI] [PubMed] [Google Scholar]
- 18. Liu CS, Hsu HS, Li CI, et al. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol 2010; 10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim BC, Shin A, Hong CW, et al. Association of colorectal adenoma with components of metabolic syndrome. Cancer Causes Control 2012; 23:727–735. [DOI] [PubMed] [Google Scholar]
- 20. Park HW, Byeon JS, Yang SK, et al. Colorectal neoplasm in asymptomatic average-risk Koreans: the KASID Prospective Multicenter Colonoscopy Survey. Gut Liver 2009; 3:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferlitsch M, Reinhart K, Pramhas S, et al. SEx-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA 2011; 306:1352–1358. [DOI] [PubMed] [Google Scholar]
- 22. Anderson JC, Attam R, Alpern Z, et al. Prevalence of colorectal neoplasia in smokers. Am J Gastroenterol 2003; 98:2777–2783. [DOI] [PubMed] [Google Scholar]
- 23. Pommergaard HC, Burcharth J, Rosenberg J, et al. Advanced age is a risk factor for proximal adenoma recurrence following colonoscopy and polypectomy. Br J Surg 2016; 103:e100–e105. [DOI] [PubMed] [Google Scholar]
- 24. Jang ES, Kim JW, Jung YJ, et al. Clinical and endoscopic predictors of colorectal adenoma recurrence after colon polypectomy. Turk J Gastroenterol 2013; 24:476–482. [DOI] [PubMed] [Google Scholar]
- 25. Soweid AM, Kobeissy AA, Jamali FR, et al. A randomized single-blind trial of standard diet versus fiber-free diet with polyethylene glycol electrolyte solution for colonoscopy preparation. Endoscopy 2010; 42:633–638. [DOI] [PubMed] [Google Scholar]
- 26. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 27. Wen CP, David Cheng TY, Tsai SP, et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 2009; 12:497–506. [DOI] [PubMed] [Google Scholar]
- 28. Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007; 102:2708–2715. [DOI] [PubMed] [Google Scholar]
- 29. Kim CW, Yun KE, Jung HS, et al. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J Hepatol 2013; 59:351–357. [DOI] [PubMed] [Google Scholar]
- 30. Tsilidis KK, Brancati FL, Pollak MN, et al. Metabolic syndrome components and colorectal adenoma in the CLUE II cohort. Cancer Causes Control 2010; 21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 2011; 60:829–836. [DOI] [PubMed] [Google Scholar]
- 32. Stadlmayr A, Aigner E, Steger B, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med 2011; 270:41–49. [DOI] [PubMed] [Google Scholar]
- 33. Taniguchi L, Higurashi T, Uchiyama T, et al. Metabolic factors accelerate colorectal adenoma recurrence. BMC Gastroenterol 2014; 14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacobs ET, Martinez ME, Alberts DS, et al. Association between body size and colorectal adenoma recurrence. Clin Gastroenterol Hepatol 2007; 5:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. So H, Han S, Park HW, et al. Metabolic factors affect the occurrence of colorectal neoplasm on surveillance colonoscopies. J Gastroenterol Hepatol 2016; 31:1273–1279. [DOI] [PubMed] [Google Scholar]
- 36. Bergman RN, Kim SP, Catalano KJ, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006; 14 (suppl 1):16s–19s. [DOI] [PubMed] [Google Scholar]
- 37. Giorgino F, Laviola L, Eriksson JW. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol Scand 2005; 183:13–30. [DOI] [PubMed] [Google Scholar]
- 38. Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 2001; 60:329–339. [DOI] [PubMed] [Google Scholar]
- 39. Mazidi M, Rezaie P, Kengne AP, et al. Gut microbiome and metabolic syndrome. Diabetes Metab Syndr 2016; In press. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40. Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut 2016; 65:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci 2013; 104:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol 2006; 169:1505–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev 1994; 3:687–695. [PubMed] [Google Scholar]
- 44. Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007; 132:2208–2225. [DOI] [PubMed] [Google Scholar]
- 45. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg 2015; 150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


