Abstract
Background:
Cardiac arrhythmias can occur during pregnancy. Owing to radiation exposure and other uncertain risks for the mother and fetus, catheter ablation has rarely been performed and is often delayed until the postpartum period. We reported 2 pregnant women who were experiencing severe arrhythmias and were successfully ablated without fluoroscopic guidance. We also carried out a literature review of cases of pregnant women who underwent zero-fluoroscopy ablation.
Methods and Results:
One woman had drug-resistant and poorly tolerated frequent premature ventricular contraction (PVC) and ventricular tachycardia (VT). The other one had persistent and hardly terminated supraventricular tachycardia (SVT) via a right accessory pathway. The 2 patients were successfully underwent zero-fluoroscopy ablation guided by Ensite NavX system. The procedure time was 42 and 71 minutes, respectively.
Conclusion:
Catheter ablation of SVT or PVC/VT in pregnant patients can be safely and effectively performed with a completely zero-fluoroscopy approach guided by the Ensite NavX system. In the case of a drug refractory, life-threatening arrhythmia during pregnancy, catheter ablation may be considered.
Keywords: cardiac arrhythmias, Ensite NavX, pregnancy, radiofrequency catheter ablation, zero-fluoroscopy
1. Introduction
Cardiac arrhythmias are common cardiac complications that may appear during pregnancy. Compared to nonpregnant women of childbearing age, an increased incidence of cardiac arrhythmias has been reported in pregnant women, which is likely because of a combination of hormonal, hemodynamic, and autonomic changes. [1] Supraventricular tachycardia (SVT) is the most common sustained arrhythmia with a reported incidence of 13 to 24 per 1000 pregnancies. [2] Arrhythmias are significantly more frequent in patients with a history of arrhythmias with structural heart disease ahead of pregnancy. Pregnancy may trigger an exacerbation of preexisting arrhythmias, whereas other arrhythmias may occur for the first time. Fortunately, most maternal cardiac arrhythmias in pregnancy are benign and severe arrhythmias requiring aggressive therapies are rare. [3] For benign arrhythmias, conservative therapies are preferred. However, not all cardiac arrhythmias can be conservatively treated in pregnancy. In fact, more than 30% of the time in patients with tachycardia is at risk for progression to dilated cardiomyopathy. [4] Moreover, blood pressure is lower than normal during tachycardia, which can lead to poor placental perfusion and pose a risk to fetal health. The influence of arrhythmias during pregnancy on fetal and neonatal outcomes is harmful. Adverse fetal events have been reported in 20% of pregnancies, which included respiratory distress syndrome, small fetus for gestational age, prematurity, and congenital heart diseases. [5] Therefore, hemodynamically unstable or dangerous rhythm disturbances that jeopardize the life of mother or fetus should be treated promptly. There are 2 main medical treatment options for symptomatic arrhythmias, including antiarrhythmic medications and catheter ablation. [6] Drugs might be a choice for pregnant patients with symptomatic tachyarrhythmia; however, antiarrhythmic medications also carry risks and most drugs are classified as class C or D, except sotalol (class B), by the US Food and Drug Administration (FDA). The 3 commonly used drugs, such as amiodarone, phenytoin, and atenolol, are class D because of their adverse maternal and fetal effects. The potential adverse risks of antiarrhythmic medications on the fetus include low birth weight, fetal demise, teratogenicity, and proarrhythmicity effects. [7] Current recommendations also suggest avoiding the use of these drugs, especially during the first trimester if possible, and that medications with the longest safety record should be used first. It is important to optimize maternal health and to manage the mother's symptoms properly. A careful risk-benefit analysis of long-term medication therapy should be studied on an individual basis and discussed by the whole treatment team. Usually the minimum recommended dose should be applied first, and accompanied with periodical monitoring of clinical responses. [8]
Although radiofrequency (RF) catheter ablation is a highly successful procedure in the nonpregnant patients, there has been a reluctance to carry out ablation in pregnant women because radiation exposure can be potentially harmful to a mother and her fetus. In the case of drug refractory, life-threatening, poorly tolerated SVT and VT during pregnancy, higher risk strategies, such as catheter ablation procedure, should be considered. [9] Fortunately, in recent years, 3-dimensional navigation system allows for the visualization of catheters with minimal or without fluoroscopy. In some centers, nonfluoroscopic catheter ablation of cardiac arrhythmias is now routinely performed by using 3-dimensional navigation system.[ 10 11] These techniques reduce the threshold to perform ablation in pregnancy, thus avoiding the potentially harmful effects of antiarrhythmic drugs. [12] We present 2 pregnant patients who underwent successful catheter ablation of cardiac arrhythmias without x-ray exposure using Ensite NavX system.
2. Case presentation
Two women (aged 33 and 22 years old, respectively) with drug refractory arrhythmia during pregnancy (31 and 25 weeks of gestation, respectively) were referred to our hospital for catheter ablation. They both had arrhythmia episodes before pregnancy, which became more frequent or incessant and drug resistant during pregnancy. The 2 patients had structurally normal hearts. Table 1 summarizes their baseline characteristics.
Table 1.
The characteristics of patients.
Patient 1 was a 33-year-old pregnant primigravida at the 31st week of pregnancy with severe symptoms of premature ventricular contraction (PVC) (Fig. 1A) and ventricular tachycardia (VT) refractory to β-blockers. Patient 1 had suffered from palpitations since childhood, but during pregnancy the symptoms became aggravated. A 24-hour Holter monitor recorded PVCs more than 50% of the time.
Figure 1.
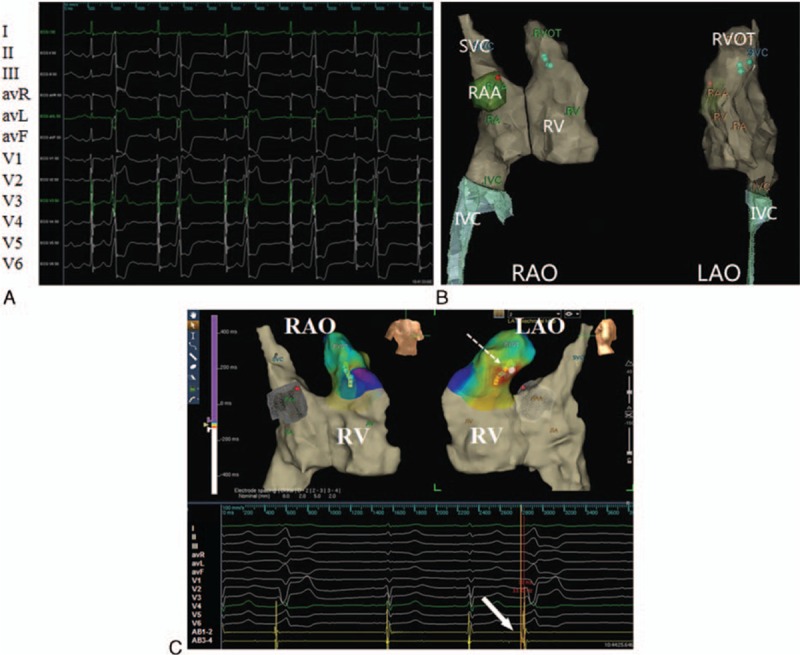
(A) Surface electrocardiograms show frequent premature ventricular complexes before radiofrequency catheter ablation. (B) Path and geometry-relevant structure during catheter insertion. The name of surface leads and AB electrodes were shown in the left panel of the figure. (C) Electrophysiology study was performed without X-ray exposure guided by the Ensite NavX system. Upper panel: The yellow dot denotes the His bundle; the lowest green dot marks the tip of AB placed at target site in the septum of the right ventricular outflow tract; the red dot denotes 2 sites where ablation was attempted for 10 seconds but failed (LAO, left anterior oblique view, RAO = right anterior oblique view). Lower panel: The local ventricular activation at the tip of AB is 33 milliseconds earlier than that at surface electrocardiography recordings; radiofrequency ablation at the upper green dot abolishes the ventricular premature beat and ventricular tachycardia; from the top down are the unipolar recordings from the ablation catheter (AB), surface lead I, II, III, avR, avL, avF, and V1 to V6, and the bipolar recordings from distal pair of the AB.
Patient 2 was a 22-year-old pregnant woman at the 25th week of pregnancy with a monozygotic twin gestation and a history of palpitation. The 12-lead electrocardiogram showed preexcitation with a positive delta wave in lead V4, V5, II, III, aVF and a negative delta wave in lead V1, suggesting a right accessory pathway (Fig. 2A). The tachycardia was up to 200 beats per minute with dizzy spells. The arrhythmia was frequent, and recurred after adenosine administration or transesophageal overdrive suppression.
Figure 2.
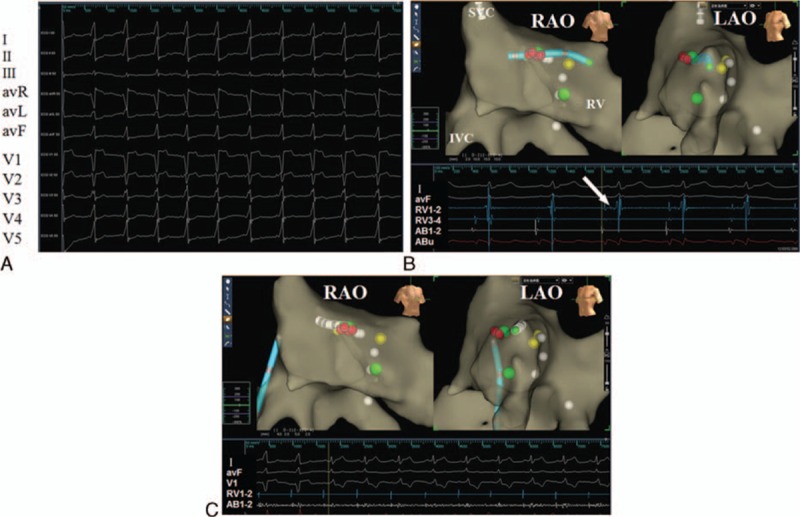
(A) Surface electrocardiograms show an evident delta wave due to an accessory pathway before radiofrequency catheter ablation. (B) Electrophysiology study was performed without X-ray exposure guided by the Ensite NavX system. Upper panel: The yellow dot denotes the His bundle; the green dot denotes the suspected location of 2 accessory pathways; the tip of tetrapolar catheter was placed near the His bundle to verify the right anatomic location after the tentative ablation and before the final ablation delivery (LAO, left anterior oblique view, RAO = right anterior oblique view). Lower panel: Surface electrocardiography recordings showed the occurrence of supraventricular tachycardia (SVT); the white arrow indicates the potential of the His bundle recorded by ablation catheter (AB). (C) Radiofrequency ablation at the upper green dot abolishes the accessory pathway. The name of surface leads, RV, and AB electrodes are shown in the left panel of the figure.
A multidisciplinary consultation with the electrophysiologist, obstetrician, and medical physicist reached the consensus that fast or incessant arrhythmias pose a potential risk to the fetuses and mothers. Catheter ablation was recommended for the treatment. Since the patients and their relatives worried about the risk of radiation exposure to the fetuses, the patients finally chose to undergo catheter ablation using the Ensite NavX system with a zero-fluoroscopy approach. The 2 patients were informed of the potential risks to both themselves and their fetuses during the procedure. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China. Written informed consents were obtained from 2 patients before the procedures.
2.1. Procedure
Antiarrhythmic medications were discontinued for at least 5 half lives before the procedure. After performing local infiltration with 1% lidocaine, we obtained venous access from the femoral vein. Three orthogonal pairs of electrode patches were placed on the skin of the patient. Transthoracic electrical fields are created by emitting low-amplitude electrical signals through these patches. The right femoral vein was canulated with a 6 or 8 F sheath and electrophysiology catheters were introduced. An electrophysiological study was performed using standard pacing protocols.[ 13 14] The entire procedure was done without any fluoroscopy and catheter navigation was guided by the Ensite NavX system, a nonfluoroscopic navigation. [10] Mapping catheters were placed at the right ventricle apex, His bundle, or coronary sinus (CS); and appropriate geometry was drawn to allow for completion of the procedure (Fig. 1B).
The mapping points were as follows: for Patient 1, the virtual geometry of the targeted area in the right ventricle was reconstructed after a rough mapping (Fig. 1C); for Patient 2, the tricuspid valve was labeled with 5 white points according to electrophysiological characteristics before the ablation (Fig. 2B and C).
End points were as follows: for Patient 1, abolishment of right ventricular outflow tract premature and VT; for Patient 2, no evidence of accessory pathway and noninducibility of tachycardia.
2.2. Literature review
We conducted a search of the PubMed, MedlinePlus, Embase, and Ovid databases until December 2015 using the following search terms: “pregnancy” and “catheter ablation” and “zero-fluoroscopy” or “without fluoroscopy.” No language restrictions were applied. Studies were reviewed for case reports that pregnant patients were performed on catheter ablation of cardiac arrhythmias. After screening titles and corresponding contents, 8 published studies were identified as meeting the search criteria.
3. Results
3.1. Results of ablation
The techniques of catheter introduction, mapping, and ablation were carried out according to the established criteria. [15] The 2 patients were able to lie down for the time required to complete the procedure. Catheter ablation guided by Ensite NavX system and without fluoroscopy was performed on 2 pregnant women.
3.2. Premature ventricular contractions (Patient 1)
The site of successful termination of the arrhythmia was approximately in the right outflow tract (Fig. 1). A single RF application (70 seconds) abolished the arrhythmia. The procedure time was 41 minutes. There were no episodes of PVC and VT during follow up (Table 2).
Table 2.
Results of the procedures and the follow up.
3.3. Atrioventricular reciprocating tachycardia (Patient 2)
Two catheters were introduced in CS and right atrium (RA). Atrial pacing protocols demonstrated a right accessory pathway consistent with atrioventricular reciprocating tachycardia (AVRT). Based on local unipolar and bipolar signals, 2 RF applications, lasting at most 5 seconds were attempted but failed due to instable tissue contact, and then a Swartz R0 (St. Jude Medical, St. Paul, MN) sheath was introduced via the navigation of ablation catheter (AB) inside. Finally, 3 RF applications (70, 70, and 90 seconds, respectively) were delivered in the approximately 10:30 o’clock region of tricuspid annulus and the accessory pathway was successfully ablated. The procedure time was 71 minutes (Table 2).
3.4. Follow-up
Patient 1 delivered a healthy male child by Cesarean section. Patient 2 delivered healthy female twins at 35 weeks of gestation by Cesarean delivery without complications. Both mothers and children had an uneventful postoperative course. There were no complications related to ablation either in the mothers or children. During 6 to 12 months of follow-up, the 2 women showed no evidence of recurrence and Holter monitoring revealed no SVT or PVC/VT (Table 2).
3.5. Literature review
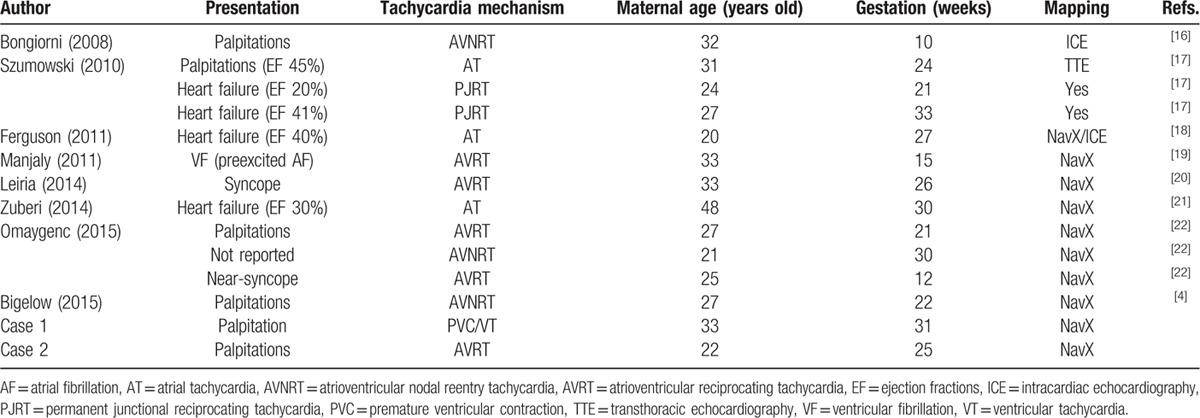
A summary of other cases reported before and our cases are shown in Table 3. Table 3 lists the patient characteristics. Eight published studies that reported 12 separate pregnant patients undergoing catheter ablation were identified.[ 4 16 17 18 19 20 21 22] The studies had 3 patients with atrial tachycardia, 3 patients with atrioventricular nodal reentry tachycardia (AVNRT), 4 patients with AVRT, and 2 patients with persistent junctional reciprocating tachycardia. The mean maternal age was 29 years old (range: 20–48), and catheter ablation was performed at a mean gestational age of 23.4 weeks (range: 10–33). Ablations were successfully performed using RF energy. After the procedure, all women and fetuses were in good general condition and had an uneventful postoperative course. There were no reported recurrence of arrhythmia and complications related to the procedure.
Table 3.
Characteristics of pregnant patients from reported cases and our cases.
4. Discussion
We performed successful catheter ablation of cardiac arrhythmias in 2 patients without fluoroscopy under the guidance of Ensite NavX system. In our cases, the 2 patients’ burden of SVT or PVC/VT was severe enough to pose risks to both the mothers and fetuses. Standard antiarrhythmic drugs failed to control tachycardia, therefore catheter ablation became the final choice.
According to the American Heart Association/American College of Cardiology and the European Society of Cardiology guidelines, the recommendation of catheter ablation to treat pregnant women with SVT is IIB; the level of evidence is “C.” Catheter ablation is recommended for drug refractory, poorly tolerated SVT and to be performed in the second trimester. Actually, the mainly concerned issue about the safety of procedure is radiation dose and risk. [23] As for catheter ablation to treat pregnant women with ventricular arrhythmia, there is no relevant recommendation, [24] which may also be due to the lack of enough clinical data. Potential risks to the mother and fetus from catheter ablation during pregnancy include anesthesia-related risk, pacing-induced maternal tachycardia, and radiation exposure. [25] Medical care for the maternal patient during procedure prompts technical challenges and safety concerns to the electrophysiology staff. Also, the anesthesia care should be individualized to the unique patient so as to prioritize the safety of the mother and fetus. Collaboration with a group of experienced anesthesia and obstetrics is generally considered necessary to guarantee maternal and fetal safety. [18] In order to avoid aortocaval compression by the gravid uterus, the mother should be lying in the left lateral tilt position rather than the supine position. [22] Frequent episodes may lead to a progressive deterioration in ventricular contractile function or a sudden reduction in cardiac output. Therefore, rapid pacing in tachycardia during the procedure should be minimized to avoid deleterious effects on fetal well-being. Generally, fetal cardiotocography was put to use for intraprocedural fetal monitoring. Changes in the fetal heart rate pattern in response to maternal physiologic condition might need to be identified and treated. [9] In our 2 patients, the procedures were performed solely with local anesthesia and without sedation, which avoided the risk of systemic hypotension and potential low placental perfusion.
Since conventional RF catheter ablation that required the use of fluoroscopy for the determination of cardiac anatomy and catheter navigation was applied as an effective method to eliminate the arrhythmogenic substrate in symptomatic patients with various types of arrhythmias, [26] the concern has remained to avoid ablation associated with ionizing radiation and complications. Ionizing radiation exposure to interventional electrophysiologist is an underestimated risk because of its unpredictable side effects. Andreassi et al [27] confirmed a significant correlation between years of work in a catheterization laboratory and the formation of micronuclei that were created by chromosomal breaks. A higher incidence of the left-sided brain tumors among this subgroup of clinicians suggested that the proximity of the left hemisphere to the x-ray source may be a culprit. [28] Ionizing radiation exposure is relevant not only to the interventional electrophysiologist but also to the patients. Radiation exposure presents even more risks for individuals with special conditions such as patients with immune system dysfunction and pregnant women. [29] Major concerns with catheter ablation during pregnancy are the risks of fluoroscopy to both the mother and the developing fetus. The potential side effects of radiation exposure to the developing fetus are various, including fetal death, major organ malformations, intrauterine growth restriction, microcephaly, and cognitive deficits. [30] Case–control studies have indicated that antenatal exposure of as little as 10 mGy may increase the risk of childhood cancer. [31] Radiation exposure to the fetus should be minimized particularly in the early pregnancy during neuronal development and organogenesis. [32] Minimizing radiation exposure to the pregnant patient is the responsibility of the electrophysiologists when performing interventional radiologic procedure. Abdominal shielding of the mother, extending completely around the pregnant abdomen, limits fetal radiation exposure to theoretical less than 1 mGy [33] for the conceptus during catheter ablation procedure. To emphasize the importance of minimizing of radiation exposure during cardiac intervention procedure, the American College of Cardiology strongly recommends that all catheterization laboratories apply “ALARA” (as low as reasonably achievable) principle, which intends to protect both the staff and patient. [34] Accordingly, to help realize this goal, the use of 3-dimensional navigation system for catheter ablation has grown.
Electroanatomical navigation system has been applied for over a decade for localization and ablation of tachycardia substrates. [12] Ensite NavX system was developed as a nonfluoroscopic 3-dimensional navigation system to guide procedures, and it has facilitated a significant decrease, and in some circumstances, complete elimination of X-ray exposure during catheter ablation. [35] Elimination of fluoroscopy removes all the short- and long-term risks of teratogenicity and oncogenicity, especially in pregnant and pediatric patients. In addition to reduced ionizing radiation, nonfluoroscopic navigation system provides additional advantages compared with conventional fluoroscopy. The navigation system performs accurate 3-dimensional reconstructions of the geometry of both vessels and cardiac chambers, and visualizes all diagnostic and therapeutic catheters in real time and in 2 simultaneous views, leading to more precise spatial localization of the catheter. [36] The navigation system elucidates detailed individual variations in anatomy and electrogram distributions, and tags important sites, such as catheter locations and lesion sites, which can be accurately revisited. The system also can establish shadows of the catheter, which can be used to reposition the catheter in the case of dislodgment. [11] We successfully performed a zero-fluoroscopy approach using the Ensite NavX system for ablation of incessant arrhythmia in pregnant patients without compromising ablation time, number of RF applications, or complications related to the procedure. In fact, catheter ablation of cardiac arrhythmia (except arrhythmias originating in the left atrium) is now routinely performed without radiation exposure utilizing 3-dimensional navigation system in our center (unpublished data). Many studies have demonstrated that interventional cardiologists had experienced an alarming incidence (40%–75%) of spinal complaints. [37 38 39] An additional benefit of a zero-fluoroscopy approach is the removal of protective lead apparel by the medical staff, thus showing a significant reduction in physician injury associated with wearing heavy lead garments. [40]
However, a zero-fluoroscopy approach has some limitations. It is not applicable to patients with arrhythmias arising from the aortic cusps, with newly implanted intracardiac leads, and with epicardial arrhythmias. [40] It is also a little more costly than a conventional fluoroscopic approach due to the additional electrodes used. However, the increased costs may not outweigh the advantages, such as decreased radiation exposure and cancer risk. Based on economic considerations, Casella et al [10] conformed that the increase in life expectancy and in period of life without cancer made minimally fluoroscopic approach economically affordable at a rough economical analysis. The cost may be cut down in the future through more widespread use of this technology. The transition from a fluoroscopy to nonfluoroscopic approach needs a learning curve, a fact that is mentioned in previous studies. [41] Learning nonfluoroscopic ablation manipulation can be fulfilled quickly, especially as most electrophysiologists are already using 3-dimensional navigation system in ablation procedures. After a period of adjustment, electrophysiologists will become more familiar with the procedures and gain more experience over time. A shorter learning curve can be anticipated with adult patients. [42] In conclusion, compared with the conventional fluoroscopic procedure, 3-dimensional navigation system makes catheter ablation more effective, with an almost complete absence of radiation, with lower RF energy, fewer RF applications, shorter procedure time, reduced spinal injuries, reduced risk of inadvertent atrioventricular block, and greater safety for staff and pregnant patients.
5. Conclusions
We demonstrated that catheter ablation of SVT or PVC/VT in pregnant patients can be safely and effectively performed with a completely zero-fluoroscopy approach guided by the Ensite NavX system. Three-dimensional navigation system has the potential to change the traditional treatment strategy for clinically severe cardiac arrhythmias in pregnant women, away from medications and towards a cure. However, catheter ablation with advanced modern technology should be considered as a last resort for life-threatening and drug-resistant arrhythmias in pregnant patients.
Footnotes
Abbreviations: AB = ablation catheter, ALARA = as low as reasonably achievable, AVNRT = atrioventricular nodal reentry tachycardia, AVRT = atrioventricular reciprocating tachycardia, CS = coronary sinus, FDA = Food and Drug Administration, LAO = left anterior oblique view, PVCs = premature ventricular contractions, RA = right atrium, RAO = right anterior oblique view, RF = radiofrequency, RV = right ventricle electrode, SVT = supraventricular tachycardia, VT = ventricular tachycardia.
Funding: This work was supported by funds from the Nature Science Foundation Committee projects of China (no. 81400369 and 81570308) and the Science and Technology Department of Hubei Province (no. 2015CFA077).
The authors have no conflicts of interest to disclose.
References
- 1. Moore JS, Teefey P, Rao K, et al. Maternal arrhythmia: a case report and review of the literature. Obstet Gynecol Surv 2012; 67:298–312. [DOI] [PubMed] [Google Scholar]
- 2. Robins K, Lyons G. Supraventricular tachycardia in pregnancy. Br J Anaesth 2004; 92:140–143. [DOI] [PubMed] [Google Scholar]
- 3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol 2014; 38:285–288. [DOI] [PubMed] [Google Scholar]
- 4. Bigelow AM, Crane SS, Khoury FR, et al. Catheter ablation of supraventricular tachycardia without fluoroscopy during pregnancy. Obstet Gynecol 2015; 125:1338–1341. [DOI] [PubMed] [Google Scholar]
- 5. McAnulty JH. Arrhythmias in pregnancy. Cardiol Clin 2012; 30:425–434. [DOI] [PubMed] [Google Scholar]
- 6. Joglar JA, Page RL. Management of arrhythmia syndromes during pregnancy. Curr Opin Cardiol 2014; 29:36–44. [DOI] [PubMed] [Google Scholar]
- 7. Joglar JA, Page RL. Antiarrhythmic drugs in pregnancy. Curr Opin Cardiol 2001; 16:40–45. [DOI] [PubMed] [Google Scholar]
- 8. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol 2014; 7:961–967. [DOI] [PubMed] [Google Scholar]
- 9. Driver K, Chisholm CA, Darby AE, et al. Catheter ablation of arrhythmia during pregnancy. J Cardiovasc Electrophysiol 2015; 26:698–702. [DOI] [PubMed] [Google Scholar]
- 10. Casella M, Dello Russo A, Pelargonio G, et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: the NO-PARTY multicentre randomized trial. Europace 2015; Nov 10. pii: euv344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giaccardi M, Del Rosso A, Guarnaccia V, et al. Near-zero x-ray in arrhythmia ablation using a 3-dimensional electroanatomic mapping system: a multicenter experience. Heart Rhythm 2016; 13:150–156. [DOI] [PubMed] [Google Scholar]
- 12. Anselmino M, Sillano D, Casolati D, et al. A new electrophysiology era: zero fluoroscopy. J Cardiovasc Med (Hagerstown) 2013; 14:221–227. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez-Gomez JM, Morina-Vazquez P, Morales Edel R, et al. Exclusion of fluoroscopy use in catheter ablation procedures: six years of experience at a single center. J Cardiovasc Electrophysiol 2014; 25:638–644. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Patel D, Wang DW, et al. beta1-Adrenoceptor blocker aggravated ventricular arrhythmia. Pacing Clin Electrophysiol 2013; 36:1348–1356. [DOI] [PubMed] [Google Scholar]
- 15. Macias R, Uribe I, Tercedor L, et al. A zero-fluoroscopy approach to cavotricuspid isthmus catheter ablation: comparative analysis of two electroanatomical mapping systems. Pacing Clin Electrophysiol 2014; 37:1029–1037. [DOI] [PubMed] [Google Scholar]
- 16. Bongiorni MG, Di Cori A, Soldati E, et al. Radiofrequency catheter ablation of atrioventricular nodal reciprocating tachycardia using intracardiac echocardiography in pregnancy. Europace 2008; 10:1018–1021. [DOI] [PubMed] [Google Scholar]
- 17. Szumowski L, Szufladowicz E, Orczykowski M, et al. Ablation of severe drug-resistant tachyarrhythmia during pregnancy. J Cardiovasc Electrophysiol 2010; 21:877–882. [DOI] [PubMed] [Google Scholar]
- 18. Ferguson JD, Helms A, Mangrum JM, et al. Ablation of incessant left atrial tachycardia without fluoroscopy in a pregnant woman. J Cardiovasc Electrophysiol 2011; 22:346–349. [DOI] [PubMed] [Google Scholar]
- 19. Manjaly ZR, Sachdev B, Webb T, et al. Ablation of arrhythmia in pregnancy can be done safely when necessary. Eur J Obstet Gynecol Reprod Biol 2011; 157:116–117. [DOI] [PubMed] [Google Scholar]
- 20. Leiria TL, Martins Pires L, Lapa Kruse M, et al. Supraventricular tachycardia and syncope during pregnancy: a case for catheter ablation without fluoroscopy. Rev Port Cardiol 2014; 33:805.e1–805.e5. [DOI] [PubMed] [Google Scholar]
- 21. Zuberi Z, Silberbauer J, Murgatroyd F. successful non-fluoroscopic radiofrequency ablation of incessant atrial tachycardia in a high risk twin pregnancy. Indian Pacing Electrophysiol J 2014; 14:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Omaygenc MO, Karaca IO, Guler E, et al. Radiofrequency catheter ablation of supraventricular tachycardia in pregnancy: ablation without fluoroscopic exposure. Heart Rhythm 2015; 12:1057–1061. [DOI] [PubMed] [Google Scholar]
- 23. Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary. a report of the American college of cardiology/American heart association task force on practice guidelines and the European society of cardiology committee for practice guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol 2003; 42:1493–1531. [DOI] [PubMed] [Google Scholar]
- 24. Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 2006; 48:e247–e346. [DOI] [PubMed] [Google Scholar]
- 25. Bombelli F, Lagona F, Salvati A, et al. Radiofrequency catheter ablation in drug refractory maternal supraventricular tachycardias in advanced pregnancy. Obstet Gynecol 2003; 102 (5 pt 2):1171–1173. [DOI] [PubMed] [Google Scholar]
- 26. Andrade JG, Rivard L, Macle L. The past, the present, and the future of cardiac arrhythmia ablation. Can J Cardiol 2014; 30 (12 suppl):S431–S441. [DOI] [PubMed] [Google Scholar]
- 27. Andreassi MG, Cioppa A, Botto N, et al. Somatic DNA damage in interventional cardiologists: a case-control study. FASEB J 2005; 19:998–999. [DOI] [PubMed] [Google Scholar]
- 28. Roguin A, Goldstein J, Bar O, et al. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol 2013; 111:1368–1372. [DOI] [PubMed] [Google Scholar]
- 29. Fazel R, Krumholz HM, Wang Y, et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009; 361:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams PM, Fletcher S. Health effects of prenatal radiation exposure. Am Fam Physician 2010; 82:488–493. [PubMed] [Google Scholar]
- 31. Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol 1997; 70:130–139. [DOI] [PubMed] [Google Scholar]
- 32. Brent RL. Protection of the gametes embryo/fetus from prenatal radiation exposure. Health Phys 2015; 108:242–274. [DOI] [PubMed] [Google Scholar]
- 33. Damilakis J, Theocharopoulos N, Perisinakis K, et al. Conceptus radiation dose and risk from cardiac catheter ablation procedures. Circulation 2001; 104:893–897. [DOI] [PubMed] [Google Scholar]
- 34. Ernst S, Castellano I. Radiation exposure and safety for the electrophysiologist. Curr Cardiol Rep 2013; 15:402–427. [DOI] [PubMed] [Google Scholar]
- 35. Casella M, Pelargonio G, Dello Russo A, et al. “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavX mapping system: personal experience and review of the literature. J Interv Card Electrophysiol 2011; 31:109–118. [DOI] [PubMed] [Google Scholar]
- 36. Shurrab M, Laish-Farkash A, Lashevsky I, et al. Three-dimensional localization versus fluoroscopically only guided ablations: a meta-analysis. Scand Cardiovasc J 2013; 47:200–209. [DOI] [PubMed] [Google Scholar]
- 37. Birnie D, Healey JS, Krahn AD, et al. Prevalence and risk factors for cervical and lumbar spondylosis in interventional electrophysiologists. J Cardiovasc Electrophysiol 2011; 22:957–960. [DOI] [PubMed] [Google Scholar]
- 38. Klein LW, Tra Y, Garratt KN, et al. Occupational health hazards of interventional cardiologists in the current decade: results of the 2014 SCAI membership survey. Catheter Cardiovasc Interv 2015; 86:913–924. [DOI] [PubMed] [Google Scholar]
- 39. Nair GM, Nery PB, Redpath CJ, et al. Radiation safety and ergonomics in the electrophysiology laboratory: update on recent advances. Curr Opin Cardiol 2016; 31:11–22. [DOI] [PubMed] [Google Scholar]
- 40. Razminia M, Manankil MF, Eryazici PL, et al. Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. J Cardiovasc Electrophysiol 2012; 23:1078–1086. [DOI] [PubMed] [Google Scholar]
- 41. Gist K, Tigges C, Smith G, et al. Learning curve for zero-fluoroscopy catheter ablation of AVNRT: early versus late experience. Pacing Clin Electrophysiol 2011; 34:264–268. [DOI] [PubMed] [Google Scholar]
- 42. Tuzcu V. A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing Clin Electrophysiol 2007; 30:519–525. [DOI] [PubMed] [Google Scholar]


