Supplemental Digital Content is available in the text
Keywords: atherosclerosis, risk factors, venous thromboembolism
Abstract
Background:
Previous studies have shown that idiopathic pulmonary embolism is positively associated with other cardiovascular events, such as myocardial infarction and stroke, suggesting a potentially important association between atherosclerosis risk factors and venous thromboembolism (VTE). We performed a meta-analysis to evaluate the correlation between risk factors for atherosclerosis and VTE.
Methods:
In December 2014, we searched MEDLINE and EMBASE for studies evaluating the associations between VTE and risk factors for atherosclerosis and pooled outcome data using random-effects meta-analysis. In addition, we analyzed publication bias.
Results:
Thirty-three case-control and cohort studies with a total of 185,124 patients met the inclusion criteria. We found that participants with body mass index (BMI) ≥30 kg/m2 had a significantly higher prevalence of VTE than those with BMI <30 kg/m2 in both case-control studies (odds ratio [OR] = 2.45, 95% confidence interval [CI]: 1.78–3.35) and cohort studies (relative risk [RR] = 2.39, 95% CI: 1.79–3.17). VTE was more prevalent in patients with hypertension than without hypertension (OR = 1.40, 95% CI: 1.06–1.84; RR = 1.36, 95% CI: 1.11–1.67). The findings were similar for VTE prevalence between patients with and without diabetes (OR = 1.78, 95% CI: 1.17–2.69; RR = 1.41, 95% CI: 1.20–1.66). Current smoking was significantly associated with VTE prevalence in case-control studies (OR = 1.34, 95% CI: 1.01–1.77), but not in cohort studies (RR = 1.29, 95% CI: 0.96–1.72). In addition, we found that total cholesterol and triglyceride concentrations were significantly higher in patients with VTE than without VTE (weighted mean differences [WMD] = 8.94 mg/dL, 95% CI: 3.52–14.35 mg/dL, and WMD = 14.00 mg/dL, 95% CI: 8.85–19.16 mg/dL, respectively). High-density lipoprotein cholesterol concentrations were significantly lower in patients with VTE than without VTE (WMD = −2.03 mg/dL, 95% CI: −3.42 to −0.63 mg/dL). Higher quality studies were more homogeneous, but confirmed the same significant associations.
Conclusions:
Based on our systematic review and meta-analysis, we observed a significant association between VTE and the risk factors for atherosclerosis. These results may make an important contribution to clinical practice regarding VTE treatment.
1. Introduction
Venous thromboembolism (VTE), which comprises pulmonary embolism (PE) and deep venous thrombosis (DVT), is common, and requires early diagnosis and treatment because of its association with high mortality and morbidity.[ 1 2] Even though Virchow's triad of factors contributing to thrombosis—vascular endothelial damage, hypercoagulation, and venous stasis—has been widely known for many years, [3] the role of PE as one of the leading causes of death is complex, multifactorial, and interactive. Venous and arterial thrombotic disorders have long been considered separate pathophysiological states, arterial thrombosis originating from platelet activation, and VTE from coagulation factors. However, the concept that VTE and atherosclerosis are 2 entirely distinct entities has recently been challenged. [4] Studies have shown that idiopathic PE (20%) is associated with other cardiovascular events such as myocardial infarction and stroke. [5] Furthermore, some studies have demonstrated a potential association between VTE and atherosclerosis.[ 6 7] Some studies have also shown that these 2 vascular complications share multiple risk factors such as age, obesity, smoking, diabetes mellitus, blood hypertension, dyslipidemia, and metabolic syndrome. [8] A 20-year cohort study has shown that patients with DVT and PE have a substantially increased risk of myocardial infarction and stroke during the first year after the thrombotic event, [9] whereas some studies have reported negative results for some risk factors. [10 11 12 13] Because there is still controversy about the relationship between VTE and risk factors for atherosclerosis, the most recent meta-analysis on this topic was published in 2008, [14] and several large case-control and prospective cohort studies have been reported since then, we performed a systematic review of published reports and a meta-analysis to update and reassess the strength of the evidence concerning risk factors for atherosclerosis and VTE. Clear evidence for an association between VTE and traditionally recognized risk factors for atherosclerosis would likely improve prevention of VTE by validating treatment of those risk factors.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was followed in our study. [15] Ethical approval was not necessary for our meta-analysis because the results for publication only involved de-identified pooled data from individual studies which ethics approval has been received.
2.1. Data sources and search strategy
A systematic search was performed on December 29, 2014 by searching PubMed from 1974 to December 2014 and EMBASE (OvidSP) from 1980 to December 2014. The detail of search strategy was shown in Table 1. For the first search, titles alone were reviewed based on text key words. Then, the abstracts of suitable titles and full-text conforms to the abstracts were obtained and reviewed. We extracted the data from the suitable full-text reports as described in the following section. Parts of additional suitable reports in our study were supplemented when discovered by citation tracking.
Table 1.
Search Strategy: Searching MEDLINE and EMBASE (OvidSP) on December 25, 2014.

2.2. Study selection
All published studies that evaluated the prevalence or severity of atherosclerosis risk factors in patients with VTE and met the following criteria were identified: English language articles; age from childhood to adulthood; prospective studies that recruited patients who had been diagnosed with VTE and included definitions of hypertension, dyslipidemia, hypercholesterolemia, and hypertriglyceridemia; concentrations of high-density lipoprotein cholesterol (HDL-C); body mass index (BMI); and the presence or absence of diabetes mellitus. In the selected studies, VTE (deep vein thrombosis and/or PE) had been diagnosed by at least one of the following standard means: Doppler echocardiography, deep lower limb compression ultrasonography, Doppler venous ultrasound or venography, computed tomography pulmonary angiography, and radioisotope studies such as pulmonary ventilation-perfusion scans. [16] For atherosclerosis, outcomes that were reported for coronary arteriosclerotic cardiopathy, myocardial infarction, angina, acute coronary syndrome, or coronary disease were included.
All studies in which the entire cohort of patients with VTE had a concomitant, known, major risk factor (e.g., studies of patients undergoing major surgery or trauma, involving pregnant women only, patients with antiphospholipid antibody syndrome) were excluded.
2.3. Data extraction and quality assessment
References were screened and data extracted independently by 2 authors using a predetermined data collection template. In the case of disagreement about inclusion of studies and interpretation of data, a third investigator was consulted and consensus reached by discussion. The following data were recorded: publication characteristics, location of study, inclusion and exclusion criteria, sample size, and patients’ characteristics. The following risk factors for atherosclerosis were collected from each included study: number and proportion of patients, BMI, blood pressure, cholesterol or triglyceride (TG) concentrations, HDL-C concentrations, diabetes mellitus, and smoking. If information on the proportion of patients with and without a particular risk factor was not available, mean levels and standard deviations were extracted for both cases and controls.
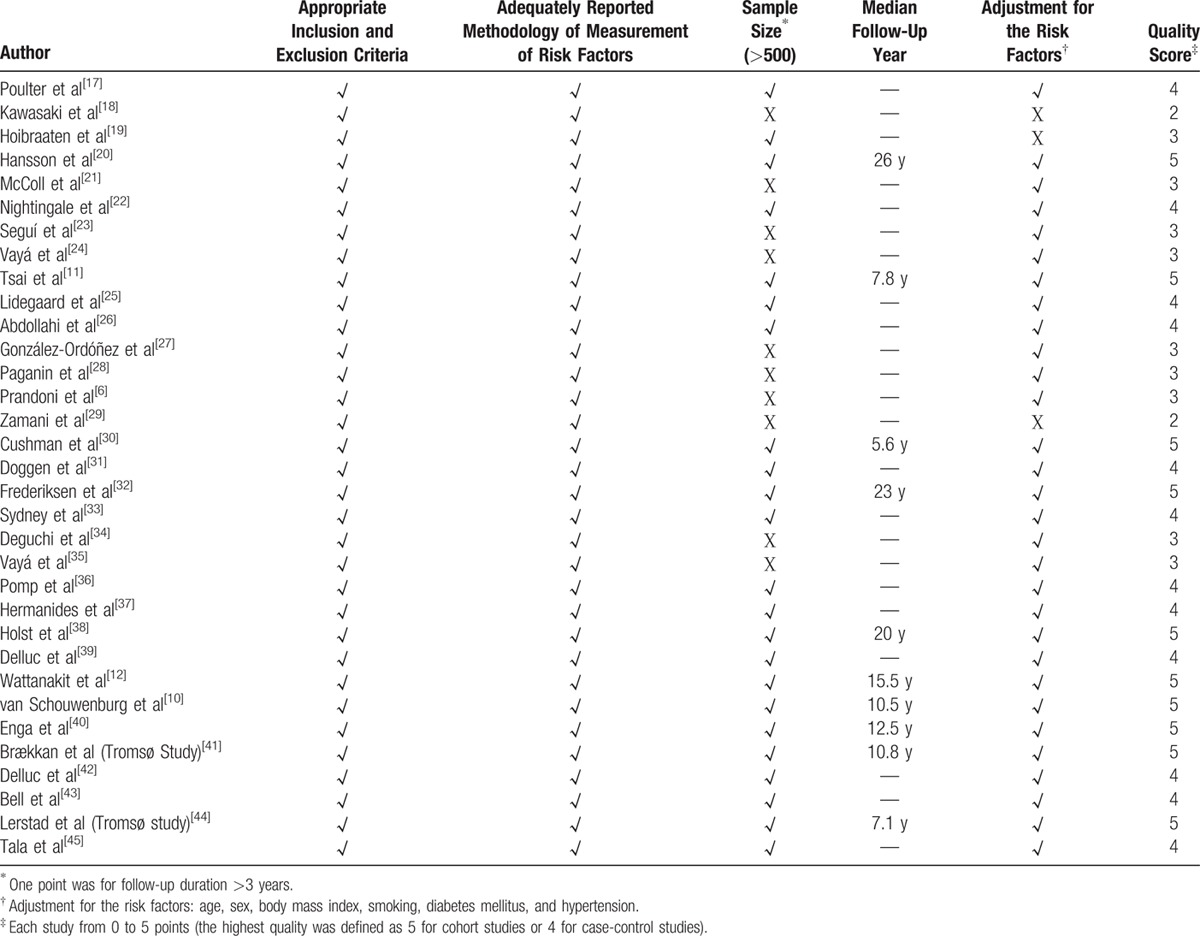
The quality of all included studies was assessed using a scoring system that resulted in total scores for each study from 0 to 5 points (the highest quality was defined as 5 for cohort studies or 4 for case-control studies). In the system we created, one point is allocated for each of the following items: appropriate inclusion and exclusion criteria; adequately reported methodology for measuring risk factors; sample size >500; follow-up duration >3 years; and adjustments made for risk factors such as age, sex, BMI, diabetes mellitus, hypertension, and smoking (Table 2).
Table 2.
Scoring System of Quality Assessment.
2.4. Data synthesis and analysis
Pooled odds ratio (OR), relative risk (RR) or weighted mean difference (WMD), and 95% confidence interval (CI) were calculated using the DerSimonian-Laird random-effects model, which takes study heterogeneity into account to generate the estimates. The extent of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic. [46] Meta-analyses were stratified by study design. Potential publication bias was assessed with the Egger test and is presented graphically by funnel plots of the natural log of the OR, RR, or WMD versus its standard error. [47] STATA 11 (Stata, College Station, TX) was used for statistical computations. A 2-sided P < 0.05 was considered significant. Sensitivity analyses were based on the quality of the studies to assess the robustness of our primary results.
3. Results
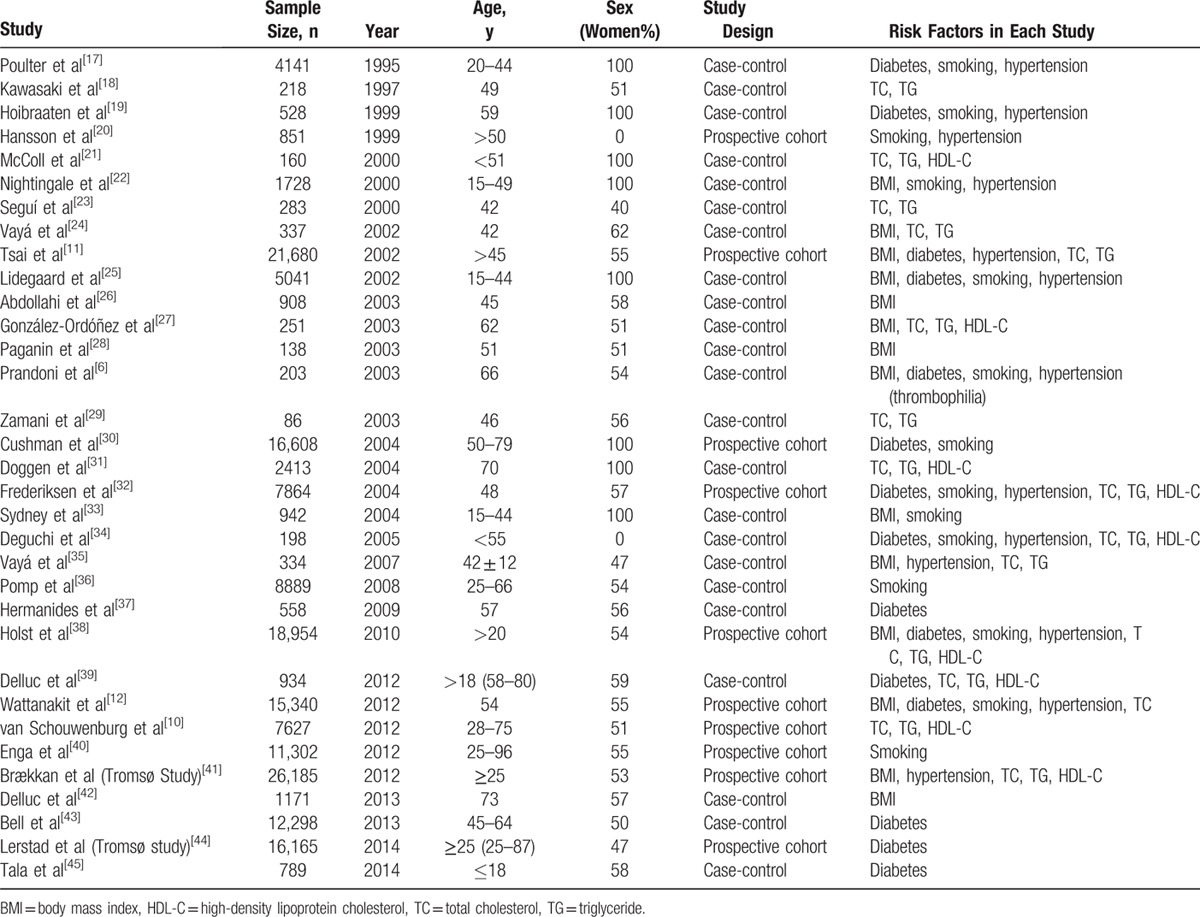
Figure 1 shows the process for selecting reports. Of 3490 reports selected in the initial search by scanning titles and abstracts, 48 seemed to meet the inclusion criteria. These were selected for detailed assessment, which resulted in exclusion of a further 15 studies for the following reasons: 3, no comparable data[ 2 48 49] ; 4, no control group [50 51 52 53]; 2, only autopsies[ 54 55] ; 2, no objective criteria for diagnoses [56 57 58]; and 3, duplicate data.[ 24 30 59] Finally, 33 studies with a total of 185,124 patients[ 6 10 11 12 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45] were included in this meta-analysis. Relevant characteristics of these 33 studies are shown in Table 3. The number of subjects per study ranged between 86 and 26,185.[ 29 41] The mean age of participants varied widely from study to study because of the varied inclusion criteria, which ranged widely from children with diabetes mellitus only [45] to adults with various risk factors depending on the studies’ inclusion criteria. Seven studies investigated only women[ 17 19 21 22 30 31 33] and 2 studies investigated only men.[ 20 34] Five studies involved oral contraceptives[ 17 19 22 25 26] and 3 studies inherited risk factors.[ 6 23 34] There were 10 prospective cohort studies [ 10 11 12 20 30 32 38 40 41 44] with durations of follow-up ranging from 5.6 [30] to 26 years. [20] Twenty-three case-control studies included healthy subjects.[ 6 17 18 19 21 22 23 24 25 26 27 28 29 31 33 34 35 36 37 39 42 43 45] Two studies included only patients with DVT.[ 18 26] Three of the 33 studies identified no associations between VTE and risk factors for atherosclerosis; these were all cohort studies.[ 10 32 44] It was not possible to compare the prevalence of cardiovascular risk factors between patients with unprovoked and provoked VTE because there were too few studies for which this information was available.
Figure 1.
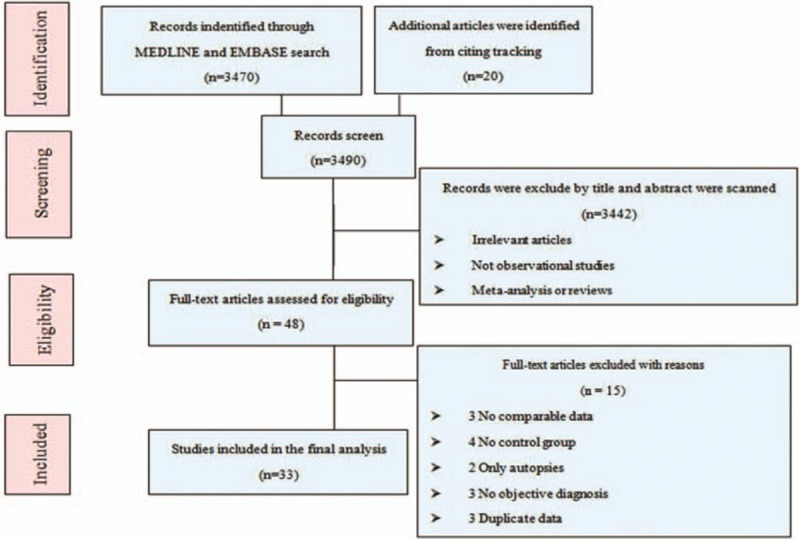
Study selection flow diagram adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Table 3.
Characteristics of Included Trials in This Meta-Analysis.
3.1. Association between BMI and VTE
In this meta-analysis, 10 case-control studies and 4 cohort studies with 19,608 subjects with thrombosis and 68,521 controls included data on BMI. As shown in Figure 2, participants with BMI ≥30 kg/m2 had a significantly higher prevalence of VTE than those with BMI <30 kg/m2 in both case-control (OR = 2.45, 95% CI: 1.78–3.35, I2 = 79.7%) and cohort studies (RR = 2.39, 95% CI: 1.79–3.17, I2 = 72.7%). When lower quality case-control studies were excluded, the 5 remaining high-quality case-control studies[ 22 25 26 33 42] still reported a significant difference between these 2 BMI categories (OR = 2.43, 95% CI: 1.72–3.44, I2 = 82.6%).
Figure 2.
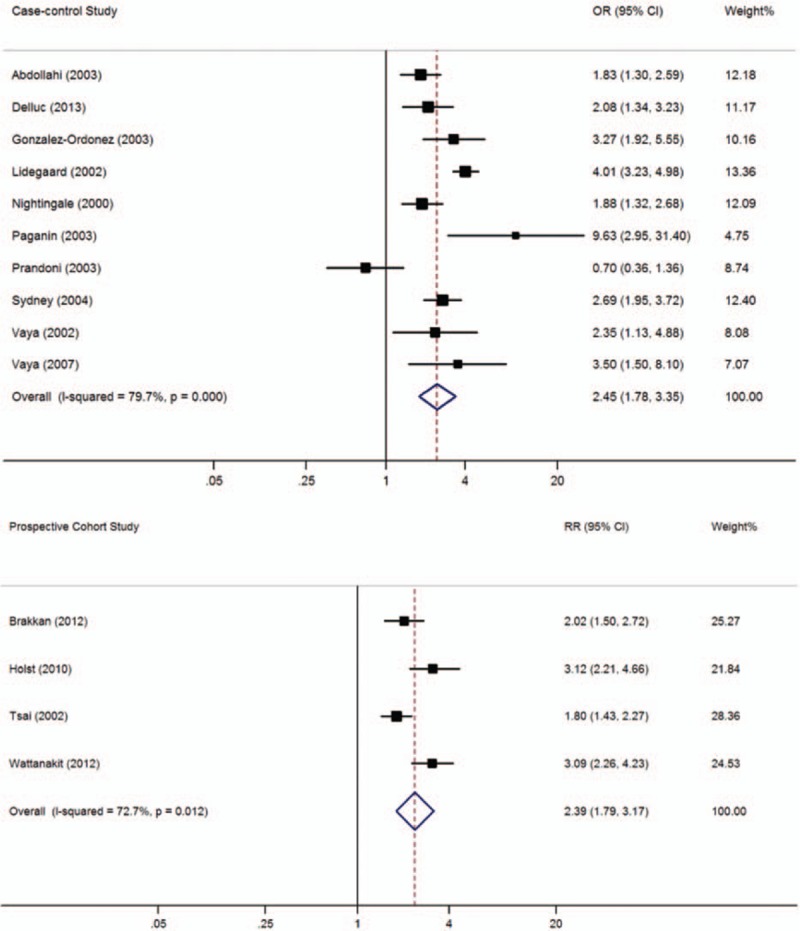
Meta-analysis of the effect of obesity (body mass index ≥30 kg/m2) on venous thromboembolism (based on 10 case-control and 4 cohort studies). Squares represent point estimates for effect size expressed as an OR/RR with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, OR = odds ratio, RR = relative risk.
3.2. Association between diabetes and VTE
In this meta-analysis, 9 case-control and 6 cohort studies with 44,809 subjects with thrombosis and 83,207 controls included data on subjects with diabetes mellitus. As shown in Figure 3, the prevalence of VTE was significantly higher among participants with diabetes than in those without diabetes (case-control studies: OR = 1.78, 95% CI: 1.17–2.69, I2 = 66.4%; cohort studies: RR = 1.41, 95% CI: 1.20–1.66, I2 = 0.0%). When lower quality case-control studies were excluded, the 6 remaining high-quality case-control studies[ 17 25 37 39 43 45] still showed a significant difference between participants with and without diabetes (OR = 1.99, 95% CI: 1.28–3.11, I2 = 63.9%).
Figure 3.
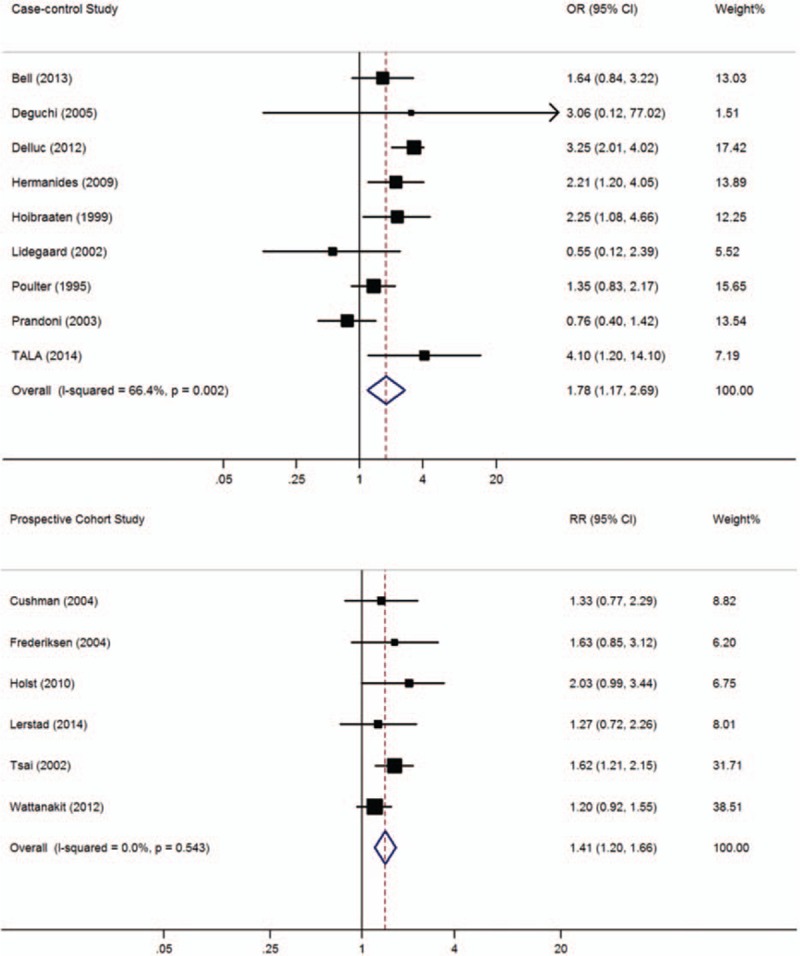
Meta-analysis of the effect of diabetes mellitus on venous thromboembolism (based on 9 case-control and 6 cohort studies). Squares represent point estimates for effect size expressed as an OR/RR with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, OR = odds ratio, RR = relative risk.
3.3. Association between hypertension and VTE
In this meta-analysis, 7 case-control and 6 cohort studies with 23,639 thrombosis patients and 74,249 controls included data on hypertension. Figure 4 shows that the prevalence of VTE was significantly higher among participants with hypertension than in those without hypertension (case-control studies: OR = 1.40, 95% CI: 1.06–1.84, I2 = 49.9%; cohort studies: RR = 1.36, 95% CI: 1.11–1.67, I2 = 71.7%). When lower quality case-control studies were excluded, the 3 remaining high-quality case-control studies[ 17 22 25] still reported a significant difference between subjects with and without hypertension (OR = 1.70, 95% CI: 1.36–2.13, I2 = 0.0%).
Figure 4.
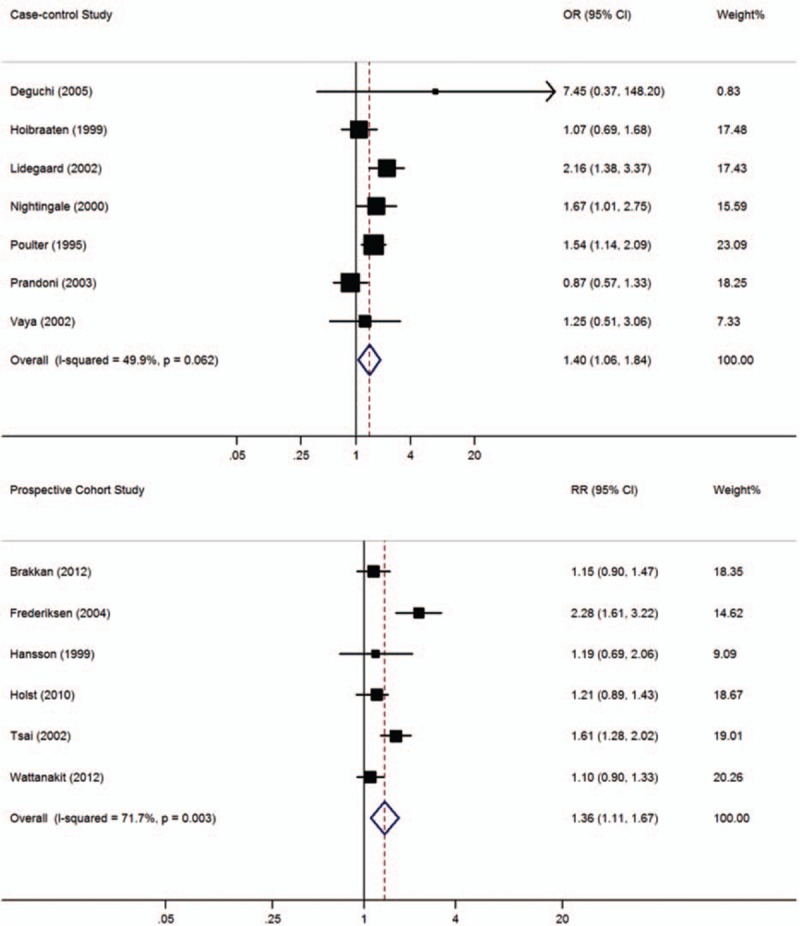
Meta-analysis of the effect of hypertension on venous thromboembolism (based on 7 case-control and 6 cohort studies). Squares represent point estimates for effect size expressed as an OR/RR with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, OR = odds ratio, RR = relative risk.
3.4. Association between current smoking and VTE
In this meta-analysis, 8 case-control and 6 cohort studies with 16,555 patients with thrombosis and 71,946 controls included data on smoking. As shown in Figure 5, a significant association between current smoking and VTE prevalence was found in case-control studies (OR = 1.34, 95% CI: 1.01–1.77, I2 = 87.1%), but not in cohort studies (RR = 1.29, 95% CI: 0.96–1.72, I2 = 75.2%). When lower quality case-control studies were excluded, the 5 remaining high-quality case-control studies[ 17 22 25 33 36] still showed a significant difference between current smokers and nonsmokers (OR = 1.48, 95% CI: 1.04–2.11, I2 = 92.0%).
Figure 5.
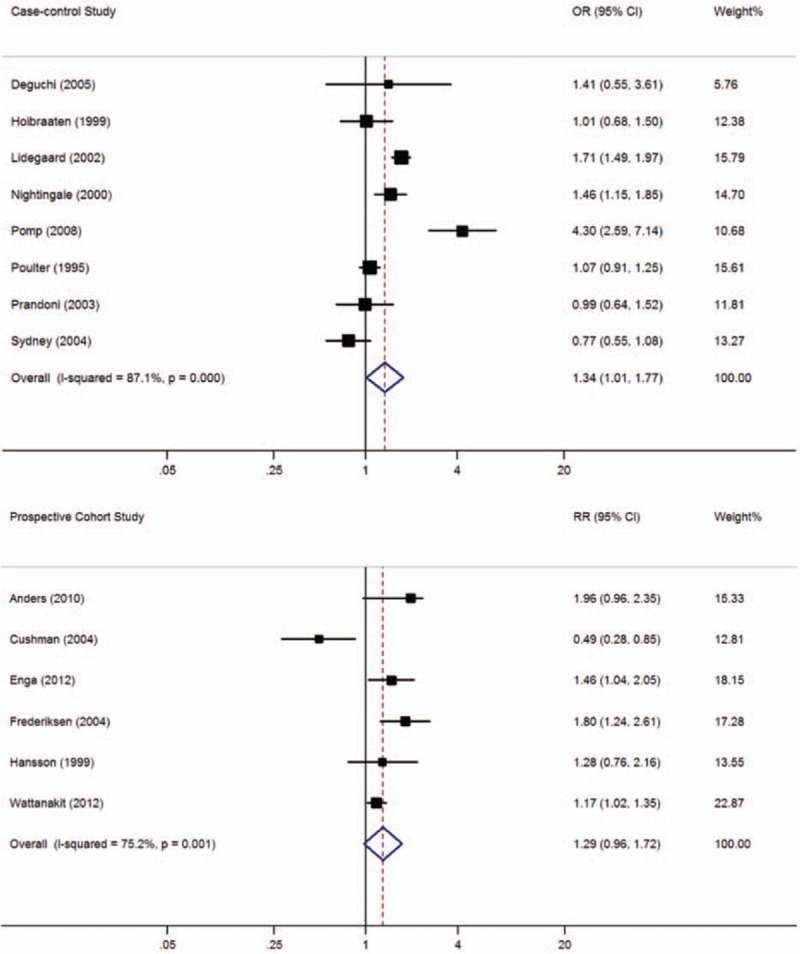
Meta-analysis of the effect of smoking on venous thromboembolism (based on 8 case-control and 6 cohort studies). Squares represent point estimates for effect size expressed as an OR/RR with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, OR = odds ratio, RR = relative risk.
3.5. Association between total cholesterol and VTE
In this meta-analysis, 10 case-control and 6 cohort studies with 11,044 subjects with thrombosis and 79,974 controls included data on total cholesterol (TC) (Fig. 6). Overall, higher mean TC concentrations were reported in subjects with VTE than in those without VTE (WMD = 8.94 mg/dL, 95% CI: 3.52–14.35 mg/dL, I2 = 95.2%). Subgroup analysis indicated that significantly higher TC concentrations among subjects with VTE were observed in cohort studies (WMD = 15.89 mg/dL, 95% CI: 10.33–21.44 mg/dL, I2 = 95.0%), but not in case-control studies (WMD = 3.34 mg/dL, 95% CI: −7.16 to 13.85 mg/dL, I2 = 92.5%). When lower quality case-control and cohort studies were excluded, the 2 remaining high-quality case-control[ 31 39] and 6 cohort studies[ 10 11 12 32 38 41] still showed a significant difference between subjects with and without high TC concentrations (WMD = 12.90, 95% CI: 7.13–18.66, I2 = 96.0%).
Figure 6.
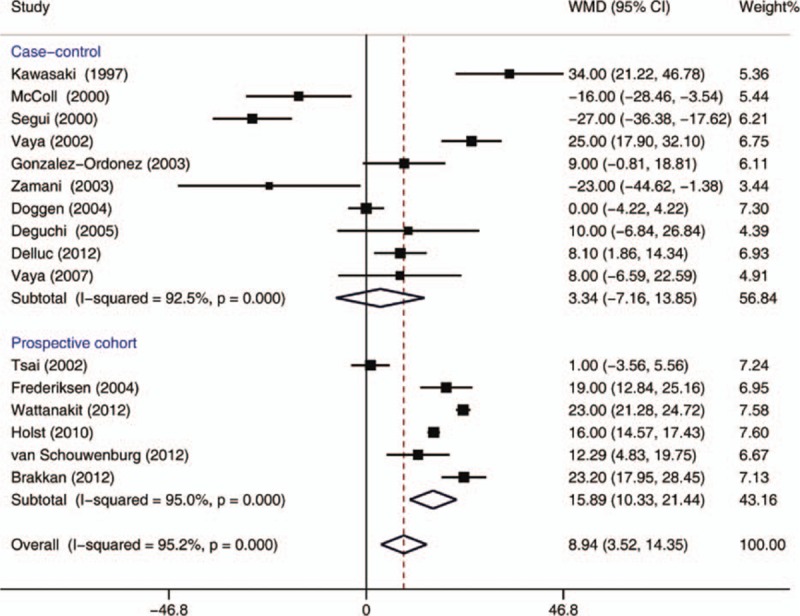
Meta-analysis of the effect of total cholesterol concentrations (mg/dL) on venous thromboembolism (based on 10 case-control and 6 cohort studies). Squares represent point estimates for effect size expressed as a WMD with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, WMD = weighted mean difference.
3.6. Association between HDL-C and VTE
In this meta-analysis, 5 case-control and 4 cohort studies with 6589 patients with thrombosis and 51,484 controls included data on HDL-C concentrations (Fig. 7). Mean HDL-C concentrations were significantly lower in subjects with VTE than in those without VTE (WMD = −2.03 mg/dL, 95% CI: −3.42 to −0.63 mg/dL, I2 = 73.5%). Subgroup analysis showed that significantly lower HDL-C concentrations among VTE patients were observed in case-control studies (WMD = −1.95 mg/dL, 95% CI: −3.36 to −0.54 mg/dL, I2 = 14.7%), but not in cohort studies (WMD = −1.91 mg/dL, 95% CI: −4.36 to 0.54 mg/dL, I2 = 87.5%). When lower quality case-control and cohort studies were excluded, the remaining 2 high-quality case-control[ 31 39] and 4 cohort studies[ 10 32 38 41] still showed a significant difference between subjects with and without high HDL-C concentrations (WMD = −1.75, 95% CI: −3.36 to −0.14, I2 = 81.5%).
Figure 7.
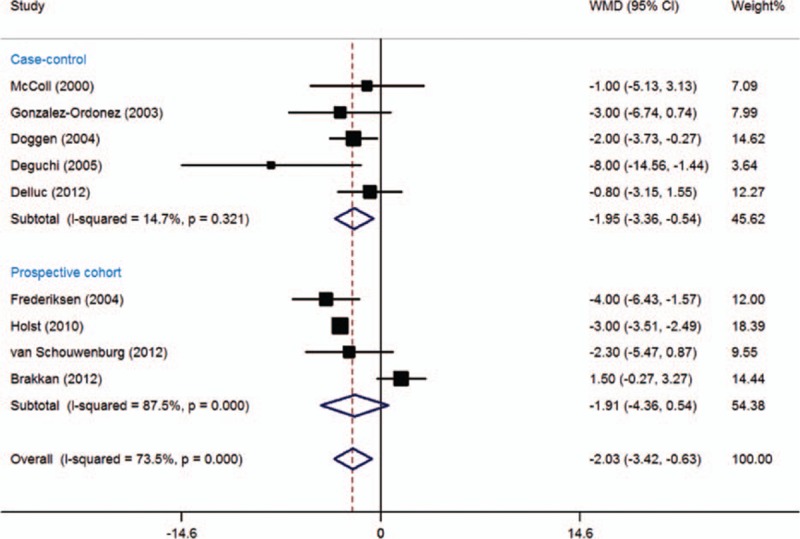
Meta-analysis of the effect of high-density lipoprotein cholesterol concentrations (mg/dL) on venous thromboembolism (based on 5 case-control and 4 cohort studies). Squares represent point estimates for effect size expressed as a WMD with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, WMD = weighted mean difference.
3.7. Association between TG and VTE
In this meta-analysis, 10 case-control and 5 cohort studies with 7200 subjects with thrombosis and 72,206 controls included data on TG concentrations (Fig. 8). A significantly higher mean TG concentration was reported among patients with VTE than in those without VTE (WMD = 14.00 mg/dL, 95% CI: 8.85–19.16 mg/dL, I2 = 57.3%). Subgroup analysis indicated that the significantly higher TG concentrations among VTE patients were observed in both case-control (WMD = 19.32 mg/dL, 95% CI: 10.56–28.08 mg/dL, I2 = 52.9%) and cohort studies (WMD = 7.42 mg/dL, 95% CI: 5.16–9.68 mg/dL, I2 = 0.0%). When lower quality case-control and cohort studies were excluded, the 2 remaining high-quality case-control[ 31 39] and 5 cohort studies[ 10 11 32 38 41] still showed significantly higher mean TG concentrations in subjects with VTE than without VTE (WMD = 7.46, 95% CI: 5.26–9.65, I2 = 0.0%).
Figure 8.
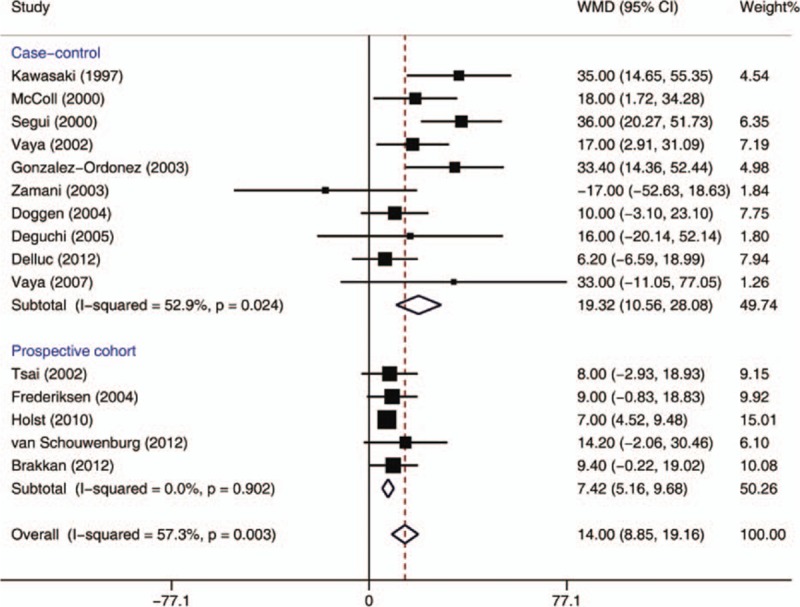
Meta-analysis of the effect of triglyceride concentrations (mg/dL) on venous thromboembolism (based on 10 case-control and 5 cohort studies). Squares represent point estimates for effect size expressed as a WMD with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, WMD = weighted mean difference.
3.8. Publication bias
There was no evidence of publication bias on the basis of either visual inspection of funnel plots (Supplementary Figures S1–S6) or Egger test for associations between VTE and BMI, diabetes, hypertension, current smoking, TC, or HDL-C (all P > 0.05) (Supplementary Figures E1–E6). However, a funnel plot (Supplementary Figure S7) and Egger test (Supplementary Figure E7) suggested the presence of publication bias for the association between VTE and TG (P = 0.020).
4. Discussion
4.1. Our principle finding and its possible underlying mechanism(s)
Our present meta-analysis demonstrated that the major risk factors for atherothrombotic disease are also significantly associated with VTE, which explains why atherosclerosis is an independent risk factor for VTE. [7] Among the traditionally recognized risk factors for atherosclerosis we evaluated, obesity, diabetes mellitus, hypertension, and hypercholesteremia and hypertriglyceridemia in particular had clear positive correlations with VTE, whereas HDL-C concentrations had a clear negative correlation with VTE. We found a significant association between current smoking and VTE prevalence in case-control studies, but not in cohort studies; however, in the latter the pooled RR was close to reaching significance.
Obesity has consistently been identified as a common risk factor for both arterial and VTE. The risk of VTE in subjects with obesity was about 2.39 to 2.45 times higher than that in those with BMI <30 kg/m2 in our meta-analysis. All 14 studies that provided BMI data verified that BMI was closely associated with the prevalence of VTE; the same result was also reported for the high-quality studies. Previous reports on the association between atherosclerotic risk factors and VTE have thus far been inconsistent.[ 11 20 32 47 60] Studies conducted by Fronek et al [61] and Sugerman et al [62] have shown that abdominal obesity is associated with increased intraabdominal pressure and reduced venous blood flow velocity, which may increase susceptibility to thrombosis. Moreover, other studies[ 63 64] have shown that visceral adipose tissue is highly active metabolically, releasing proinflammatory, proatherogenic, and prothrombotic substances that may contribute to thrombosis risk. According to one study, the biological mechanisms may be involved high concentrations of fibrinogen and some clotting factors, low-grade systemic inflammation, increased intra-abdominal pressure, and reduced venous return from the lower limbs. [65] However, whether obesity itself definitely increases the risk of VTE is uncertain because several risk factors such as pregnancy, [66 67 68] oral contraceptive use, [69] and hormone replacement therapy [70] may coexist with obesity in subjects with current and recurrent VTE.
Eight of the 15 studies about diabetes mellitus reported a positive association[ 6 11 17 19 30 37 43 45] with VTE, whereas 7 did not.[ 12 25 32 34 38 39 44] Our pooled analysis resulted in a weak correlation; however, 4 cohort studies did not identify an association between diabetes mellitus and VTE.[ 12 32 38 44] A possible explanation for this discrepancy is that risk factors often coexist in patients with VTE, for example, diabetes mellitus may be result from metabolic syndrome. [71] The causal relationship between hyperglycemia and VTE has been confirmed by several studies, the main mechanisms for the causality possibly involving activated factor VII activity, the increased thrombin-antithrombin complexes, and soluble tissue factor.[ 72 73] Whereas, study did not find the direct biological relationship between hyperglycemia and the coagulation system. [74]
Consistent with our study, several case-control studies have demonstrated an association between concentrations of serum lipids such as TG, TC, and lipoprotein (a) and VTE.[ 31 34 75 76] However, a number of large prospective studies have failed to confirm such associations, failing to support a role for serum lipids in the pathogenesis of VTE.[ 10 27 41 49] Our study showed that VTE was indeed associated with dyslipidemia; however, a funnel plot and Egger test suggested the presence of publication bias for the association between VTE and TG. This may be attributable to failure to publish studies with negative result or to other variables such as race and geographical region. Funnel figures, which only determine whether a graph is symmetrical, cannot conclusively identify publication bias. The negative association between HDL-C and the prevalence of VTE in our study indicates that HDL-C may play a protective role in regulating thrombosis.
In the early days of research into this topic, [60] hypertension was identified as a risk factor for PE in the Nurses’ Health Study; however, most more recent prospective studies have found no association between blood pressure and VTE.[ 11 20 38 49] In our study, 7 of the 13 articles providing data on hypertension reported a positive association between hypertension and VTE.[ 6 11 17 24 32 34 38] However, one reported a positive association in men, [34] only when combined with smoking and BMI ≥30 kg/m2. [38] In addition, one of these studies involved subjects using oral contraception. [17] These findings suggest that patients with hypertension have a tendency to develop VTE when they also have other risk factors.
Daily smoking, regardless of duration and amount, was not found to be a risk factor in several studies,[ 11 41 49] However, other studies have identified heavy cigarette smoking as a risk factor for VTE.[ 20 36 39] In the current meta-analysis, smoking was positively correlated with VTE in 5 cohort[ 12 20 32 38 40] and 2 case-control studies[ 25 36] among the 14 studies in which smoking was assessed. Smoking is a well-established risk factor for atherosclerotic disease; however, its role as an independent risk factor or effect modifier for VTE remains controversial. Several prospective studies have reported that smoking is an independent risk factor. [38] One possible explanation for these discrepancies is that some studies have not provided sufficiently detailed information, such as current smoking versus previous smoking status and differences between heavier and lighter smokers. Current research suggests that the mechanisms by which smoking induces hypercoagulation may be associated with the reduced fibrinolysis, inflammation, and increased blood viscosity. [77 78 79] One study has shown a correlation between VTE and higher plasma fibrinogen and factor VIII concentrations among smokers, [80] and others have shown that fibrinogen concentration drops rapidly to a normal level after cessation of smoking.[ 81 82] Yarnell and other authors have detected a positive relationship between the amount of current tobacco consumption and plasminogen activator inhibitor-1 concentration, which may also be related to VTE.[ 70 83 84]
In contrast with a previous report, [14] we found in this meta-analysis that smoking and HDL-C were risk factors for VTE in case-control studies, whereas TC was a risk factor for VTE only in the cohort studies.
4.2. Clinical significance
Because VTE is associated with multiple interacting factors, to minimize their influences on each other we eliminated cases of VTE diagnosed on autopsy [55] and the subjects with definite provoking factors such as joint replacement.[ 85 86] Despite this, a mechanistic link between VTE and the risk factors for atherosclerosis remains uncertain. Based on the current findings, we speculate that both vascular disorders are simultaneously triggered by biological stimuli responsible for activating coagulation and inflammatory pathways in both the arterial and venous systems (VTE and atherothrombosis share common risk factors and the common pathophysiological characteristics of inflammation, hypercoagulability, and endothelial injury). Meanwhile, it is reasonable to assume that adequate management of the risk factors for atherosclerosis would reduce the risk of VTE. Studies have shown that statins may be protective against VTE.[ 87 88] The obvious mechanism for such a protective effect is through improving lipid profiles; however, another possibility is that statins may directly affect endothelial function and coagulation. [89 90 91] Our findings are very important for informing further studies on the causes of VTE and new strategies for its primary prevention.
4.3. Study limitations
Our study has several potential limitations. First, we could not perform a sex-specific analysis because some variables were measured in only some of the studies and not in others for various reasons. Second, detailed information about VTE events was not always provided. Third, not all studies provided information on thrombophilia testing, making it difficult for us to distinguish the risk factors from hereditary thrombophilia. Fourth, several types of risk factors coexist in most cases, making it difficult to accurately analyze the influence of any one factor. Fifth, some of the summarized estimations for the association between the risk factor and VTE were highly heterogeneous (I2 > 70%) across the included studies, which may have affected the reliability of our results. Because it was not possible to adjust or stratify for potential confounders in the present meta-analysis, we could not resolve the heterogeneity by meta-regression. Lastly, the pooled ORs were calculated on study level and not individual level data, which means that ORs for meta-analyses were adjusted for different risk factors; we acknowledge that confounding may therefore have affected our findings.
5. Conclusions
This systematic review and meta-analysis showed significant associations between VTE and risk factors for atherosclerosis. Higher quality studies are needed in the future to clarify the nature of this association and whether VTE can be benefit from intensive treatment of established risk factors.
Supplementary Material
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DVT = deep venous thrombosis, HDL-C = high-density lipoprotein cholesterol, OR = odds ratio, PE = pulmonary embolism, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR = relative risk, TC = total cholesterol, TG = triglyceride, VTE = venous thromboembolism, WMD = weighted mean differences.
Funding: This study was funded by the Beijing Municipal Science and Technology Commission of China (Contract no. Z131107002213113). The fund provider had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Disclosure: The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med 2003; 163:1711–1717. [DOI] [PubMed] [Google Scholar]
- 2. Goldhaber SZ, Elliott CG. Acute pulmonary embolism: part I: epidemiology, pathophysiology, and diagnosis. Circulation 2003; 108:2726–2729. [DOI] [PubMed] [Google Scholar]
- 3. Brotman DJ, Deitcher SR, Lip GY, et al. Virchow's triad revisited. South Med J 2004; 97:213–214. [DOI] [PubMed] [Google Scholar]
- 4. Prandoni P. Venous and arterial thrombosis: two aspects of the same disease? Clin Epidemiol 2009; 1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becattini C, Agnelli G, Prandoni P, et al. A prospective study on cardiovascular events after acute pulmonary embolism. Eur Heart J 2005; 26:77–83. [DOI] [PubMed] [Google Scholar]
- 6. Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med 2003; 348:1435–1441. [DOI] [PubMed] [Google Scholar]
- 7. Hong C, Zhu F, Du D, et al. Coronary artery calcification and risk factors for atherosclerosis in patients with venous thromboembolism. Atherosclerosis 2005; 183:169–174. [DOI] [PubMed] [Google Scholar]
- 8. Becattini C, Vedovati MC, Ageno W, et al. Incidence of arterial cardiovascular events after venous thromboembolism: a systematic review and a meta-analysis. J Thromb Haemost 2010; 8:891–897. [DOI] [PubMed] [Google Scholar]
- 9. S⊘rensen HT, Horvath-Puho E, Pedersen L, et al. Venous thrombo-embolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet 2007; 960:1773–1779. [DOI] [PubMed] [Google Scholar]
- 10. van Schouwenburg IM, Mahmoodi BK, Gansevoort RT, et al. Lipid level do not influence the risk of venous thromboembolism. Thromb Haemost 2012; 108:923–929. [DOI] [PubMed] [Google Scholar]
- 11. Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: the Longitudinal Investigation of Thromboembolism Etiology. Arch Intern Med 2002; 162:1182–1189. [DOI] [PubMed] [Google Scholar]
- 12. Wattanakit K, Lutsey PL, Bell EJ, et al. Association between cardiovascular disease risk factors and occurrence of venous hromboembolism: a time-dependent analysis. Thromb Haemost 2012; 108:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parkin L, Sweetland S, Balkwill A, et al. Body mass index, surgery, and risk of venous thromboembolism in middle-aged women: a cohort study. Circulation 2012; 125:1897–1904. [DOI] [PubMed] [Google Scholar]
- 14. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008; 117:93–102. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 16. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008; 29:2276–2315. [DOI] [PubMed] [Google Scholar]
- 17. Poulter NR, Meirik O, Chang CL, et al. Venous thromboembolic disease and combined oral contraceptives: results of international multicentre case-control study. Lancet 1995; 346:1575–1582. [PubMed] [Google Scholar]
- 18. Kawasaki T, Kambayashi J, Ariyoshi H, et al. Hypercholesterolemia as a risk factor for deep-vein thrombosis. Thromb Res 1997; 88:67–73. [DOI] [PubMed] [Google Scholar]
- 19. Hoibraaten E, Abdelnoor M, Sandset PM. Hormone replacement therapy with estradiol and risk of venous thromboembolism: a population-based case-control study. Thromb Haemost 1999; 82:1218–1221. [PubMed] [Google Scholar]
- 20. Hansson PO, Eriksson H, Welin L, et al. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: “the Study of Men Born in 1913”. Arch Intern Med 1999; 159:1886–1890. [DOI] [PubMed] [Google Scholar]
- 21. McColl MD, Sattar N, Ellison J, et al. Lipoprotein (a), cholesterol and triglycerides in women with venous thromboembolism. Blood Coagul Fibrinolysis 2000; 11:225–229. [PubMed] [Google Scholar]
- 22. Nightingale AL, Lawrenson RA, Simpson EL, et al. The effects of age, body mass index, smoking and general health on the risk of venous thromboembolism in users of combined oral contraceptives. Eur J Contracept Reprod Health Care 2000; 5:265–274. [DOI] [PubMed] [Google Scholar]
- 23. Seguí R, Estellés A, Mira Y, et al. PAI-1 promoter 4G/5G genotype as an additional risk factor for venous thrombosis in subjects with genetic thrombophilic defects. Br J Haematol 2000; 111:122–128. [DOI] [PubMed] [Google Scholar]
- 24. Vayá A, Mira Y, Ferrando F, et al. Hyperlipidemia and venous thromboembolism in patients lacking thrombophilic risk factors. Br J Haematol 2002; 118:255–259. [DOI] [PubMed] [Google Scholar]
- 25. Lidegaard Ø, Edström B, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception 2002; 65:187–196. [DOI] [PubMed] [Google Scholar]
- 26. Abdollahi M, Cushman M, Rosendaal FR. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost 2003; 89:493–498. [PubMed] [Google Scholar]
- 27. González-Ordóñez AJ, Fernández-Carreira JM, Fernández-Alvarez CR, et al. The concentrations of soluble vascular cell adhesion molecule-1 and lipids are independently associated with venous thromboembolism. Haematologica 2003; 88:1035–1043. [PubMed] [Google Scholar]
- 28. Paganin F, Bourdé A, Yvin JL, et al. Venous thromboembolism in passengers following a 12-h flight: a case-control study. Aviat Space Environ Med 2003; 74:1277–1280. [PubMed] [Google Scholar]
- 29. Zamani A, Omrani GR, Lankarani KB. Hyperhomocysteinaemia, hyperlipidaemia and risk of venous thromboembolism in Shiraz. East Mediterr Health J 2003; 9:935–943. [PubMed] [Google Scholar]
- 30. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004; 292:1573–1580. [DOI] [PubMed] [Google Scholar]
- 31. Doggen CJ, Smith NL, Lemaitre RN, et al. Serum lipid levels and the risk of venous thrombosis. Arterioscler Thromb Vasc Biol 2004; 24:1970–1975. [DOI] [PubMed] [Google Scholar]
- 32. Frederiksen J, Juul K, Grande P, et al. Methylenetetrahydrofolate reductase polymorphism (C677T), hyperhomocysteinemia, and risk of ischemic cardiovascular disease and venous thromboembolism: prospective and casecontrol studies from the Copenhagen City Heart Study. Blood 2004; 104:3046–3051. [DOI] [PubMed] [Google Scholar]
- 33. Sydney S, Petitti DB, Soff GA, et al. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception 2004; 70:3–10. [DOI] [PubMed] [Google Scholar]
- 34. Deguchi H, Pecheniuk NM, Elias DJ, et al. Highdensity lipoprotein deficiency and dyslipoproteinemia associated with enous thrombosis in men. Circulation 2005; 112:893–899. [DOI] [PubMed] [Google Scholar]
- 35. Vayá A, Falcó C, Simó M, et al. Influence of lipids and obesity on haemorheological parameters in patients with deep vein thrombosis. Thromb Haemost 2007; 98:621–626. [PubMed] [Google Scholar]
- 36. Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am J Hematol 2008; 83:97–102. [DOI] [PubMed] [Google Scholar]
- 37. Hermanides J, Cohn DM, Devries JH, et al. Venous thrombosis is associated with hyperglycemia at diagnosis: a case-control study. J Thromb Haemost 2009; 7:945–949. [DOI] [PubMed] [Google Scholar]
- 38. Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism: results from the Copenhagen City Heart study. Circulation 2010; 121:1896–1903. [DOI] [PubMed] [Google Scholar]
- 39. Delluc A, Malécot JM, Kerspern H, et al. Lipid parameters, lipid lowering drugs and the risk of venous thromboembolism. Atherosclerosis 2012; 220:184–188. [DOI] [PubMed] [Google Scholar]
- 40. Enga KF, Braekkan SK, Hansen-Krone IJ, et al. Cigarette smoking and the risk of venous thromboembolism: the Troms⊘ Study. J Thromb Haemost 2012; 10:2068–2074. [DOI] [PubMed] [Google Scholar]
- 41. Brækkan SK, Hald EM, Mathiesen EB, et al. Competing risk of atherosclerotic risk factors for arterial and venous thrombosis in a general population: the Troms⊘ Study. Arterioscler Thromb Vasc Biol 2012; 32:487–491. [DOI] [PubMed] [Google Scholar]
- 42. Delluc A, De Moreuil C, Kerspern H, et al. Body mass index, a major confounder to insulin resistance association with unprovoked venous thromboembolism: result from the EDITH case-control study. Thromb Haemost 2013; 110:593–597. [DOI] [PubMed] [Google Scholar]
- 43. Bell EJ, Selvin E, Lutsey PL, et al. Glycemia (hemoglobin A1c) and incident venous thromboembolism in the Atherosclerosis Risk in Communities cohort study. Vasc Med 2013; 18:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lerstad G, Brodin EE, Enga KF, et al. Hyperglycemia, assessed according to HbA1c, and future risk of venous thromboembolism: the Troms⊘ study. J Thromb Haemost 2014; 12:313–319. [DOI] [PubMed] [Google Scholar]
- 45. Tala JA, Silva CT, Pemira S, et al. Blood glucose as a marker of venous thromboembolism in critically ill children. J Thromb Haemost 2014; 12:891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maguire MG, Tonascia J, Sartwell PE, et al. Increased risk of thrombosis due to oral contraceptives: a further report. Am J Epidemiol 1979; 110:188–195. [DOI] [PubMed] [Google Scholar]
- 49. Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol 2005; 162:975–982. [DOI] [PubMed] [Google Scholar]
- 50. Petrauskiene V, Falk M, Waernbaum I, et al. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia 2005; 48:1017–1021. [DOI] [PubMed] [Google Scholar]
- 51. Simmonds JP, O’Malley BP, Maskill MR, et al. Beta-thromboglobulin levels in relation to myocardial infarction: preliminary observations. Acta Haematol 1980; 64:172–175. [DOI] [PubMed] [Google Scholar]
- 52. Manfredini R, Gallerani M, Boari B, et al. Seasonal variation in onset of pulmonary embolism is independent of patients’ underlying risk comorbid conditions. Clin Appl Thromb Hemost 2004; 10:39–43. [DOI] [PubMed] [Google Scholar]
- 53. Ray JG, Lonn E, Yi Q, et al. Venous thromboembolism in association with features of the metabolic syndrome. Q J Med 2007; 100:679–684. [DOI] [PubMed] [Google Scholar]
- 54. Yoo HH, De Paiva SA, Silveira LV, et al. Logistic regression analysis of potential prognostic factors for pulmonary thromboembolism. Chest 2003; 123:813–821. [DOI] [PubMed] [Google Scholar]
- 55. Goldhaber SZ, Savage DD, Garrison RJ, et al. Risk factors for pulmonary embolism: the Framingham study. Am J Med 1983; 74:1023–1028. [DOI] [PubMed] [Google Scholar]
- 56. Whiteman MK, Cui Y, Flaws JA, et al. Low fibrinogen level: a predisposing factor for venous thromboembolic events with hormone replacement therapy. Am J Hematol 1999; 61:271–273. [DOI] [PubMed] [Google Scholar]
- 57. Beaumont V, Lemort N, Beaumont JL. Oral contraceptives, sex steroidinduced antibodies and vascular thrombosis: results from 1318 cases. Eur Heart J 1991; 12:1219–1224. [DOI] [PubMed] [Google Scholar]
- 58. Godsland IF, Winkler U, Lidegaard O, et al. Occlusive vascular diseases in oral contraceptive users. Epidemiology, pathology and mechanisms. Drugs 2000; 60:721–869. [DOI] [PubMed] [Google Scholar]
- 59. Lidegaard O. Smoking and use of oral contraceptives: impact on thrombotic diseases. Am J Obstet Gynecol 1999; 180:S357–S363. [DOI] [PubMed] [Google Scholar]
- 60. Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997; 277:642–645. [PubMed] [Google Scholar]
- 61. Fronek A, Criqui MH, Denenberg J, et al. Common femoral vein dimensions and hemodynamics including Valsalva response as a function of sex, age, and ethnicity in a population study. J Vasc Surg 2001; 33:1050–1056. [DOI] [PubMed] [Google Scholar]
- 62. Sugerman H, Windsor A, Bessos M, et al. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J Intern Med 1997; 241:71–79. [DOI] [PubMed] [Google Scholar]
- 63. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008; 29:2959–2971. [DOI] [PubMed] [Google Scholar]
- 64. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006; 444:881–887. [DOI] [PubMed] [Google Scholar]
- 65. Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011; 377:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ageno W, Piantanida E, Dentali F, et al. Body mass index is associated with the development of the post-thrombotic syndrome. Thromb Haemost 2003; 89:305–309. [PubMed] [Google Scholar]
- 67. Larsen TB, S⊘rensen HT, Gislum M, et al. Maternal smoking, obesity, and risk of venous thromboembolism during pregnancy and the puerperium: a population-based nested case-control study. Thromb Res 2007; 120:505–509. [DOI] [PubMed] [Google Scholar]
- 68. Jacobsen AF, Skjeldestad FE, Sandset PM. Ante-and postnatal risk factors of venous thrombosis: a hospital-based case-control study. J Thromb Haemost 2008; 6:905–912. [DOI] [PubMed] [Google Scholar]
- 69. Pomp ER, le Cessie S, Rosendaal FR, et al. Risk of venous thrombosis: obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol 2007; 139:289–296. [DOI] [PubMed] [Google Scholar]
- 70. Gary T, Hafner F, Froehlich H, et al. High factor VIII activity, high plasminogen activator inhibitor 1 antigen levels and low factor XII activity contribute to a thrombophilic tendency in elderly venous thromboembolism patients. Acta Haematol 2010; 124:214–217. [DOI] [PubMed] [Google Scholar]
- 71. Ageno W, Prandoni P, Romualdi E, et al. The metabolic syndrome and the risk of venous thrombosis: a case-control study. J Thromb Haemost 2006; 4:1914–1918. [DOI] [PubMed] [Google Scholar]
- 72. Ceriello A, Giugliano D, Quatraro A, et al. Blood glucose may condition factor VII levels in diabetic and normal subjects. Diabetologia 1988; 31:889–891. [DOI] [PubMed] [Google Scholar]
- 73. Stegenga ME, van der Crabben SN, Blümer RM, et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood 2008; 112:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Langouche L, Meersseman W, Vander Perre S, et al. Effect of insulin therapy on coagulation and fibrinolysis in medical intensive care patients. Crit Care Med 2008; 36:1475–1480. [DOI] [PubMed] [Google Scholar]
- 75. Marcucci R, Liotta AA, Cellai AP, et al. Increased plasma levels of lipoprotein(a) and the risk of idiopathic and recurrent venous thromboembolism. Am J Med 2003; 115:601–605. [DOI] [PubMed] [Google Scholar]
- 76. von Depka M, Nowak-Göttl U, Eisert R, et al. Increased lipoprotein(a) levels as an independent risk factor for venous thromboembolism. Blood 2000; 96:3364–3368. [PubMed] [Google Scholar]
- 77. Lee KW, Lip GY. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: a systematic review. Arch Intern Med 2003; 163:2368–2392. [DOI] [PubMed] [Google Scholar]
- 78. Miller GJ, Bauer KA, Cooper JA, et al. Activation of the coagulant pathway in cigarette smokers. Thromb Haemost 1998; 79:549–553. [PubMed] [Google Scholar]
- 79. Yarnell JW, Sweetnam PM, Rumley A, et al. Lifestyle and hemostatic risk factors for ischemic heart disease: the Caerphilly Study. Arterioscler Thromb Vasc Biol 2000; 20:271–279. [DOI] [PubMed] [Google Scholar]
- 80. Oger E, Lacut K, Van Dreden P, et al. High plasma concentration of factor VIII coagulant is also a risk factor for venous thromboembolism in the elderly. Haematologica 2003; 88:465–469. [PubMed] [Google Scholar]
- 81. Feher MD, Rampling MW, Brown J, et al. Acute changes in atherogenic and thrombogenic factors with cessation of smoking. J R Soc Med 1990; 83:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bakhru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med 2005; 2:e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yarnell JW, Sweetnam PM, Rumley A, et al. Lifestyle factors and coagulation activation markers: the Caerphilly Study. Blood Coagul Fibrinolysis 2001; 12:721–728. [DOI] [PubMed] [Google Scholar]
- 84. Zhao Z, Wang S, Ma W, et al. Diabetes mellitus increases the incidence of deep vein thrombosis after total knee arthroplasty. Arch Orthop Trauma Surg 2014; 134:79–83. [DOI] [PubMed] [Google Scholar]
- 85. Cohn DM, Hermanides J, DeVries JH, et al. Stress-induced hyperglycaemia and venous thromboembolism following total hip or total knee arthroplasty. Thromb Haemost 2012; 107:225–231. [DOI] [PubMed] [Google Scholar]
- 86. Yukizawa Y, Inaba Y, Watanabe S, et al. Association between venous thromboembolism and plasma levels of both soluble fibrin and plasminogen-activator inhibitor 1 in 170 patients undergoing total hip arthroplasty. Acta Orthop 2012; 83:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ray J. Dyslipidemia, statins and venous thromboembolism: a potential risk factor and a potential treatment. Curr Opin Pulm Med 2003; 9:378–384. [DOI] [PubMed] [Google Scholar]
- 88. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J 2010; 31:1248–1256. [DOI] [PubMed] [Google Scholar]
- 89. Squizzato A, Romualdi E, Ageno W. Why should statins prevent venous thromboembolism? A systematic literature search and a call for action. J Thromb Haemost 2006; 4:1925–1927. [DOI] [PubMed] [Google Scholar]
- 90. Biere-Rafi S, Hutten BA, Squizzato A, et al. Statin treatment and the risk of recurrent pulmonary embolism. Eur Heart J 2013; 34:1800–1806. [DOI] [PubMed] [Google Scholar]
- 91. Lassila R, Jula A, Pitkäniemi J, et al. The association of statin use with reduced incidence of venous thromboembolism: a population-based cohort study. BMJ Open 2014; 4:e005862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


