Abstract
We attempted to determine whether shoulder subluxation at the early stage of stroke can predict motor outcome in relation to the corticospinal tract (CST) state on diffusion tensor tractography.
Fifty-nine stroke patients with severe hemiparesis were recruited. The patients were classified according to the distance of shoulder subluxation (group A: ≥2 cm, group B: <2 cm) and the affected CST on diffusion tensor tractography at the first evaluation (CST type A—the CST was discontinued at the stroke lesion; CST type B—the integrity of the CST was preserved). Motor function of the patients was evaluated twice (first: beginning of rehabilitation—24.1 ± 16.6 days; second: discharge after first rehabilitation—58.5 ± 24.1 days) using the Medical Research Council score, Motricity Index, and Modified Brunnstrom Classification.
Regarding the improvement of the Medical Research Council for the finger extensor and upper Motricity Index, the order in terms of better recovery was as follows: group B–type B, group A–type B, group B–type A, and group A–type A (P < 0.05). The distance of shoulder subluxation showed significant correlation with improvement of the finger extensor (moderate negative correlation, r = −0.37) and improvement of the Modified Brunnstrom Classification (weak negative correlation, r = −0.29) (P < 0.05).
The presence of shoulder subluxation at the early stage of stroke can be a predictor of motor outcome of the affected upper extremity and the degree of shoulder subluxation can be a predictor of the motor function of the affected hand. Therefore, our results suggest that shoulder subluxation in relation to the affected CST state at the early stage of stroke can be a prognostic factor for motor outcome.
Keywords: corticospinal tract, diffusion tensor tractography, shoulder subluxation, stroke
1. Introduction
Stroke is a common cause of adult disability, and motor weakness is one of the most serious sequelae, occurring in >50% of stroke patients. [1] For successful stroke rehabilitation, accurate prediction of motor outcome at the early stage of stroke is essential, because it could provide useful information for clinicians in establishment of accurate rehabilitative strategies and prediction of final neurological deficits. [2] Many studies have reported on various prognostic factors following stroke; however, it has not clearly elucidated. [1 2 3 4 5 6 7 8 9 10 11 12 13]
The corticospinal tract (CST) is a main neural tract for motor function: its involvement was reported in average 69%, 73%, and 98% of motor function of all upper and lower extremity muscles, shoulder abductor, and finger extensor, respectively. [13 14 15 16] Therefore, many previous studies focused on evaluation of the CST status for prediction of motor outcome using clinical assessment, radiological measurement, electrophysiological assessment, functional neuroimaging technique, and diffusion tensor tractography (DTT), a technique derived from diffusion tensor imaging (DTI).[ 2 3 4 5 6 7 9 10 11 13 17 18] Among the above-mentioned evaluation tools, clinical assessment is important because of its easy applicability and cost-effectiveness. Most previous studies focused on the assessment of motor function using specific joint muscles.[ 3 4 5 6 10] However, when the motor weakness is so severe at stroke onset or the beginning of early rehabilitation, the evaluation of the motor power of specific joint muscles might not be precise and available. [4]
Shoulder subluxation is a common clinical feature at the early state of stroke: its presence was reported in up to 81% of stroke patients with hemiplegia. [19] The shoulder joint depends on the integrity of muscular and capsuloligamentous structures for its stability rather than bony conformation. [20] Weakness of muscles around the shoulder joint can lead to shoulder subluxation.[ 20 21] Little is known about relationship between shoulder subluxation at the early stage of stroke and motor outcome. Therefore, we hypothesized that the presence and degree of shoulder subluxation at the early stage of stroke might be a predictor of motor outcome in hemiplegic stroke patients.
In the current study, we attempted to determine whether shoulder subluxation at the early stage of stroke can predict motor outcome in relation to the CST state on DTT.
2. Subjects and methods
2.1. Subjects
Fifty-nine hemiplegic stroke patients were consecutively recruited (males: 34, females: 25, mean age: 60.7 ± 10.9 years, range: 35–75 years). Inclusion criteria were as follows: first ever stroke, age 20 to 75 years, severe weakness of the affected upper extremity to the extent of an inability to move without gravity at the time of DTI scanning, start of rehabilitation during the early stage (between 1 and 8 weeks after onset) of stroke, and performing DTI at the beginning of rehabilitation. This retrospective study was approved by the Institutional Review Board of a university hospital.
2.2. Clinical evaluation
The distance of shoulder subluxation between the lower margin of the acromion and the upper margin of the humerus head was measured using a caliper (Fig. 1).[ 20 22 23 24] The presence of shoulder subluxation was determined when distance was >2 cm.[ 22 23] The patients were classified according to 2 groups based on the presence of shoulder subluxation: group A—distance of shoulder subluxation was ≥2 cm; group B—distance of shoulder subluxation was <2 cm.[ 22 23] Among 59 patients, 33 patients were included in group A (55.9%, males: 19, females: 14, mean age: 59.4 ± 10.8 years, range: 39–75 years) and 26 patients in group B (44.1%, males: 15, females: 11, mean age: 62.4 ± 11.0 years, range: 35–75 years).
Figure 1.
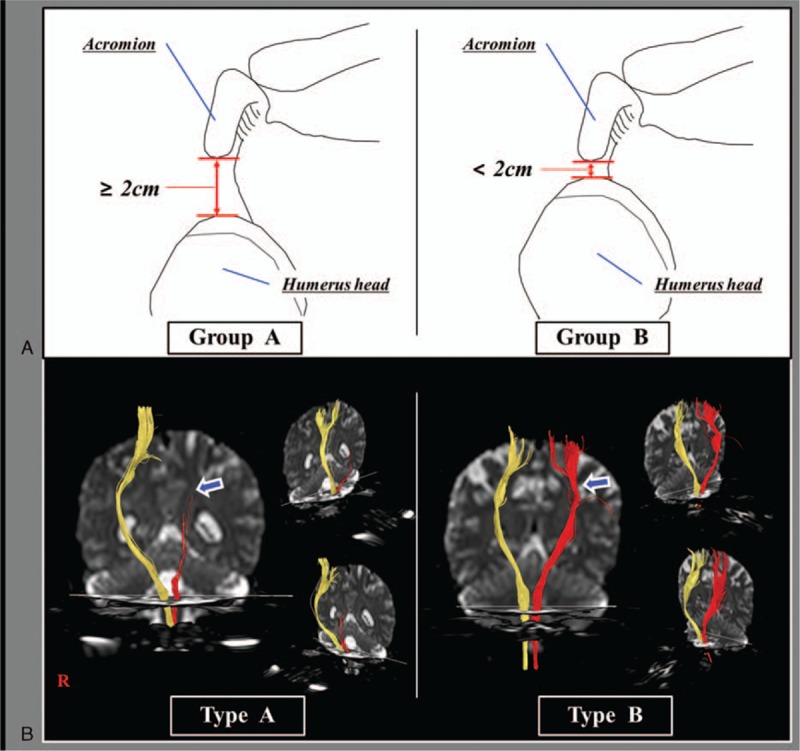
Classification of shoulder subluxation group and CST integrity on DTT. (A) Images of shoulder joint of group A (distance of shoulder subluxation was ≥2 cm) and group B (distance of shoulder subluxation was <2 cm); (B) DTT images of CST type A (the CST was discontinued at or around the lesion) and CST type B (the integrity of the CST was preserved from the cortex to the medulla). CST = corticospinal tract, DTT = diffusion tensor tractography.
Motor and hand function of the patients was evaluated twice; the first evaluation was performed at the beginning of rehabilitation at the rehabilitation department of our university hospital (mean days: 24.1 ± 16.6, range 7–56 days after onset) and the second evaluation was performed at the time of discharge at the rehabilitation department of our university hospital (mean days: 58.5 ± 24.1, range: 26–116 days after onset). The Medical Research Council (scores range 0–5 and higher scores indicate better motor function) scores and Motricity Index (MI: maximum scores 100 conversion from the Medical Research Council score, and higher scores indicate better motor function) were used to measure motor function.[ 25 26] The upper MI score is the average of the MI scores for upper limb (prehension, elbow flexor, and shoulder flexor), and the lower MI score is the average of the MI scores for lower limb (ankle dorsiflexor, knee extensor, and hip flexor). Average of the upper and lower MI scores is called total MI score. Modified Brunnstrom Classification (MBC) was used for categorization of function of the affected hand (1–6: higher scores indicate better hand function). [27] The validity and reliability of the Medical Research Council, MI, and MBC are well established. [25 26 27]
2.3. Fiber tracking
Using a 6-channel head coil with single-shot echo planar imaging on 1.5 T (Philips Ltd, Best, The Netherlands), DTI was acquired at an average of 24.1 ± 16.6 days after the onset of stroke. Seventy contiguous slices (number of excitations: 1, imaging reduction factor [SENSE (sensitivity encording: for reduction of the scanning time) factor]: 2, field of view: 240 × 240 mm2, reconstructed to matrix: 192 × 192, acquisition matrix: 96 × 96, parallel echo planar imaging factor: 59, TE: 72 milliseconds, TR: 10,398 milliseconds, b: 1000 s/mm2, and a slice thickness of 2.5 mm) were acquired. For analysis of the CST, the single-tensor model was used within the DTI task card software (Philips Extended MR Workspace 2.6.3). The first and second regions of interest were placed on the upper and lower pons on the color map of the axial slice (the anterior blue portion). [28] The patients were also classified according to 2 types based on the CST finding in the affected hemisphere: CST type A—the CST was discontinued at or around the stroke lesion; CST type B—the integrity of the CST was preserved at or around the lesion from the cortex to the medulla (Fig. 1). Among 59 patients, 33 patients were type A (55.9%, males: 20, females: 13, mean age: 58.4 ± 10.9 years, range: 39–75 years) and 26 patients type B (44.1%, males: 14, females: 12, mean age: 63.7 ± 10.2 years, range: 35–75 years). In group A, 26 patients (78.8%) of 33 patients were included in CST type A (group A–type A) and the other 7 patients (21.2%) in CST type B (group A–type B). Among 26 patients in group B, 7 patients (26.9%) were included in CST type A (group B–type A) and the remaining 19 patients (73.1%) in CST type B (group B–type B).
2.4. Statistical analysis
SPSS software (version 15.0; SPSS, Chicago, IL) was used for data analysis. The χ2 test and independent t test were used for determination of differences in demographic data between group A and group B. Regarding the clinical data, paired t test was performed for determination of differences in changes between first evaluation and second evaluation. One-way analysis of variance with Fisher least significant difference post hoc test was used for determination of differences between 2 CST types in each group at the first evaluation. The differences in the improvement between 4 groups were analyzed using 1-way analysis of covariance (severity at the first evaluation) with Fisher least significant difference post hoc test. Using Pearson correlation, the distance of shoulder subluxation was used in determination of correlation with the improvement of motor and hand function. The level of significance was set as P < 0.05.
3. Results
The demographic data for the patients are summarized in Table 1. No significant differences in age, durations (from onset to first evaluation and second evaluation), lesion side, and types of stroke were observed between the 2 groups (P > 0.05). In contrast, significant differences in the distance of shoulder subluxation and CST types were observed between groups A and B (P < 0.05).
Table 1.
Demographic data of the patients.

Table 2 shows average scores of the Medical Research Council, MI, and MBC in patients in the 2 groups and 2 types. Significant improvements from first to second evaluation were observed in all clinical evaluations in the patients of both groups and 2 types (group A–type A, group A–type B, group B–type A, and group B–type B) (P < 0.05). At the first evaluation, no significant differences in the Medical Research Council for finger extensor and MBC were observed in 4 comparisons between the 2 groups and 2 types (group A–type A, group A–type B, group B–type A, and group B–type B) (P > 0.05). However, the Medical Research Council for shoulder abductor of group B–type A and group B–type B were significantly higher than that of group A–type A (P < 0.05). The upper MI of group B–type B was also significantly higher than that of group A–type A and group A–type B, and the upper MI of group B–type A was significantly higher than that of group A–type A (P < 0.05). However, in the other comparisons no significant differences in the Medical Research Council for shoulder abductor and upper MI were observed between the 2 groups and 2 types (P > 0.05).
Table 2.
Changes of motor function and hand function according to state of shoulder subluxation and the types of diffusion tensor tractography finding.
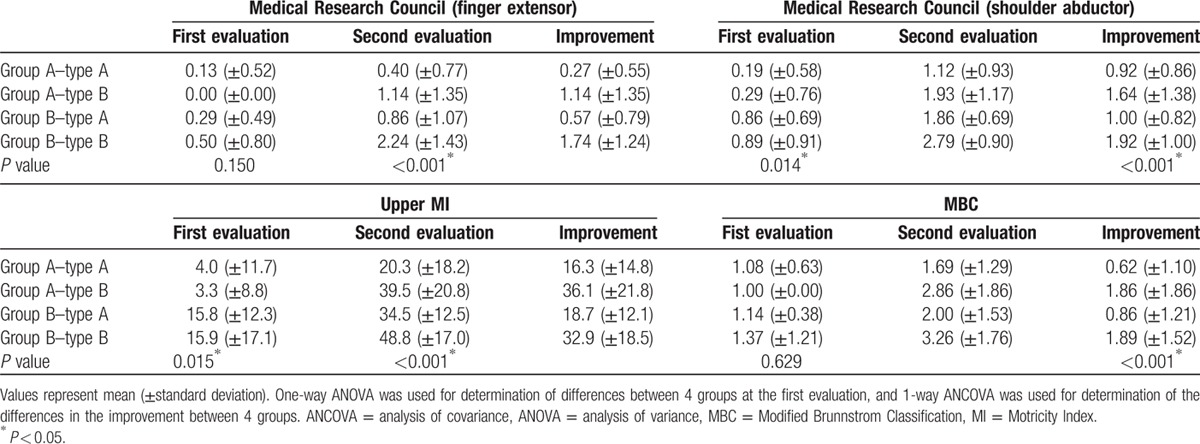
Regarding the improvement of the Medical Research Council for the finger extensor and upper MI, the order in terms of better recovery was as follows: group B–type B, group A–type B, group B–type A, and group A–type A (P < 0.05). With regard to improvement of the Medical Research Council for the shoulder abductor, group B–type B and group A–type B showed better recovery than group A–type A and group B–type A (P < 0.05). However, no significant difference was observed between the improvement of group B–type B and group A–type B, and between group A–type A and group B–type A (P > 0.05). In addition, in terms of the improvement of the MBC, group B–type B and group A–type B showed better recovery than group A–type A and group B–type A, and group B–type A showed better recovery than group A–type A (P < 0.05). However, no significant difference was observed between group A–type B and group B–type B (P > 0.05).
The distance of shoulder subluxation showed significant correlation with the improvement of the finger extensor (moderate negative correlation, r = −0.37), MBC (weak negative correlation, r = −0.29), and total MI (moderate negative correlation, r = −0.30) (P < 0.05). However, no significant correlation was observed with the improvement of the shoulder abductor (r = −0.18), elbow flexor (r = −0.23), hip flexor (r = 0.09), knee extensor (r = 0.10), ankle dorsiflexor (r = −0.13), upper MI (r = −0.22), and lower MI (r = −0.05) (P > 0.05).
4. Discussion
In the current study, we attempted to determine whether shoulder subluxation at the early stage of stroke can predict motor outcome in relation to the integrity of the affected CST on DTT. Our results can be summarized as follows. First, significant difference in the amount of improvement was observed in all clinical evaluations in 4 comparisons between 2 groups and 2 types; specifically, patients in group B–type B showed the best recovery and patients in group A–type A showed the worst recovery in terms of the Medical Research Council for the finger extensor and upper MI. These results indicated that patients with no shoulder subluxation and preserved integrity of the affected CST on DTT showed best motor outcome of the affected upper extremity including the finger extensor; in contrast, patients with shoulder subluxation and discontinued integrity of the affected CST on DTT showed the worst motor outcome. Second, the distance of shoulder subluxation showed negative correlation with the improvement of the finger extensor and the MBC. These results suggested that a more severe degree of shoulder subluxation resulted in poorer motor outcome of hand motor function. Therefore, our results can be summarized as follows: the presence of shoulder subluxation at the early stage of stroke can be a predictor of motor outcome of affected upper extremity including the hand and the degree of shoulder subluxation can be a predictor of the motor function of the affected hand.
Several neural tracts including the CST, corticoreticulospinal tract, and rubrospinal tract are involved in motor function in the human brain.[ 15 18 29 30 31] In general, the CST is known to be involved in control of distal musculature such as the finger; in contrast, the corticoreticulospinal tract is involved in control of the proximal musculature such as shoulder and hip.[ 14 18 31 32] However, a previous study reported that the CST is mainly involved in control of the proximal musculature as well as the distal musculature: the CST is involved in average 98% muscle power of finger extensor and average 73% muscle power of shoulder abductor. [15] Therefore, the fact that the CST is involved in control of the musculatures of shoulder and hand can provide a neurological background for our results that shoulder subluxation at the early stage of stroke can be a predictor of the motor outcome of the affected upper extremity. On the other hand, the severe weakness of patients who showed no shoulder subluxation (group B) and preserved integrity of the affected CST (type B) at the first evaluation was ascribed to the severe limb-kinetic apraxia as well as partial injury of the CST. [33 34 35 36] Therefore, information on shoulder subluxation and the integrity of the affected CST on DTT at an early stage of stroke can be useful for differential diagnosis for accompanying limb-kinetic apraxia and pure motor weakness. Furthermore, information on the presence of limb-kinetic apraxia can provide a useful guideline for rehabilitative management because limb-kinetic apraxia can be resolved by administration of dopaminergic drugs. [36 37 38]
A few studies have reported that assessment of motor function of the shoulder joint could predict motor outcome in stroke patients.[ 3 6 10] In 1998, Katrak et al demonstrated that patients who could shrug and abduct the affected shoulder at an early stage (within 28 days of onset) of stroke were more likely to show good recovery of hand movement at 1, 2, and 3 months in 71 hemispheric stroke patients. [3] By contrast, Smania et al reported that shoulder abduction and shrug at an early stage (within 7 days of onset) of stroke was not a reliable predictor of recovery of arm function in 48 hemorrhagic stroke patients. [6] Nijland et al recently reported that patients with some finger extensor and shoulder abductor ability within 2 days after stroke onset had a 98% probability of achieving some dexterity at 6 months in 188 stroke patients. [10] Therefore, to the best of our knowledge, this is the first study to examine the predictability of shoulder subluxation at the early stage of stroke for the motor outcome in stroke patients.
In this study, some limitations should be considered. The first limitation is the small number of patients. Second, there was a lack of long-term follow-up clinical data at 6 months after stroke such as distance of shoulder subluxation and motor function, when motor recovery had reached a plateau following stroke.[ 39 40] Last, fiber tracking technique is operator-dependent, and DTT can produce both false-positive and false-negative results throughout the white matter of the brain due to crossing fiber (regions of fiber complexity) or partial volume effect. [41] Therefore, conducting further studies to overcome the above limitations should be encouraged.
In conclusion, we attempted to determine whether shoulder subluxation at the early stage of stroke can predict motor outcome in relation to the CST state on DTT. It was found that, although the integrity of the affected CST on DTT at early stage of stroke was the better predictor of motor outcome of affected upper extremity including the hand, the presence and degree of shoulder subluxation at the early stage of stroke can be a predictor of motor outcome of affected upper extremity. Therefore, our results suggest that shoulder subluxation in relation to the affected CST state at the early stage of stroke can be a prognostic factor for motor outcome.
Footnotes
Abbreviations: CST = corticospinal tract, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, MBC = Modified Brunnstrom Classification, MI = Motricity Index.
This work was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
The authors have no conflicts of interest to disclose.
References
- 1. Duncan PW, Goldstein LB, Matchar D, et al. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 1992; 23:1084–1089. [DOI] [PubMed] [Google Scholar]
- 2. Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: a review. NeuroRehabilitation 2010; 27:367–372. [DOI] [PubMed] [Google Scholar]
- 3. Katrak P, Bowring G, Conroy P, et al. Predicting upper limb recovery after stroke: the place of early shoulder and hand movement. Arch Phys Med Rehabil 1998; 79:758–761. [DOI] [PubMed] [Google Scholar]
- 4. Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003; 34:2181–2186. [DOI] [PubMed] [Google Scholar]
- 5. de Groot-Driessen D, van de Sande P, van Heugten C. Speed of finger tapping as a predictor of functional outcome after unilateral stroke. Arch Phys Med Rehabil 2006; 87:40–44. [DOI] [PubMed] [Google Scholar]
- 6. Smania N, Paolucci S, Tinazzi M, et al. Active finger extension: a simple movement predicting recovery of arm function in patients with acute stroke. Stroke 2007; 38:1088–1090. [DOI] [PubMed] [Google Scholar]
- 7. Jang SH, Bai D, Son SM, et al. Motor outcome prediction using diffusion tensor tractography in pontine infarct. Ann Neurol 2008; 64:460–465. [DOI] [PubMed] [Google Scholar]
- 8. Tuttolomondo A, Pedone C, Pinto A, et al. Predictors of outcome in acute ischemic cerebrovascular syndromes: the GIFA study. Int J Cardiol 2008; 125:391–396. [DOI] [PubMed] [Google Scholar]
- 9. Lindenberg R, Renga V, Zhu LL, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 2010; 74:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nijland RH, van Wegen EE, Harmeling-van der Wel BC, et al. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke 2010; 41:745–750. [DOI] [PubMed] [Google Scholar]
- 11. Zhu LL, Lindenberg R, Alexander MP, et al. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010; 41:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011; 151:318–322. [DOI] [PubMed] [Google Scholar]
- 13. Jang SH, Kim K, Kim SH, et al. The relation between motor function of stroke patients and diffusion tensor imaging findings for the corticospinal tract. Neurosci Lett 2014; 572:1–6. [DOI] [PubMed] [Google Scholar]
- 14. Davidoff RA. The pyramidal tract. Neurology 1990; 40:332–339. [DOI] [PubMed] [Google Scholar]
- 15. Cho HM, Choi BY, Chang CH, et al. The clinical characteristics of motor function in chronic hemiparetic stroke patients with complete corticospinal tract injury. NeuroRehabilitation 2012; 31:207–213. [DOI] [PubMed] [Google Scholar]
- 16. Jang SH. The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med 2014; 46:193–199. [DOI] [PubMed] [Google Scholar]
- 17. Konishi J, Yamada K, Kizu O, et al. MR tractography for the evaluation of functional recovery from lenticulostriate infarcts. Neurology 2005; 64:108–113. [DOI] [PubMed] [Google Scholar]
- 18. Jang SH. The role of the corticospinal tract in motor recovery in patients with a stroke: a review. NeuroRehabilitation 2009; 24:285–290. [DOI] [PubMed] [Google Scholar]
- 19. Turner-Stokes L, Jackson D. Shoulder pain after stroke: a review of the evidence base to inform the development of an integrated care pathway. Clin Rehabil 2002; 16:276–298. [DOI] [PubMed] [Google Scholar]
- 20. Paci M, Nannetti L, Rinaldi LA. Glenohumeral subluxation in hemiplegia: an overview. J Rehabil Res Dev 2005; 42:557–568. [DOI] [PubMed] [Google Scholar]
- 21. Fitzgerald-Finch OP, Gibson II. Subluxation of the shoulder in hemiplegia. Age Ageing 1975; 4:16–18. [DOI] [PubMed] [Google Scholar]
- 22. Bohannon RW, Andrews AW. Shoulder subluxation and pain in stroke patients. Am J Occup Ther 1990; 44:507–509. [DOI] [PubMed] [Google Scholar]
- 23. Boyd EA, Torrance GM. Clinical measures of shoulder subluxation: their reliability. Can J Public Health 1992; 83 (suppl 2):S24–S28. [PubMed] [Google Scholar]
- 24. Gillen G, Burkhardt A. Stroke Rehabilitation: A Function-Based Approach. St. Louis: Mosby; 1998. [Google Scholar]
- 25. Medical Research Council. Memorandum No. 45—Aids to the Examination of the Peripheral Nervous System. London: H.M. Stationery Office; 1976. [Google Scholar]
- 26. Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol 1980; 19:382–389. [DOI] [PubMed] [Google Scholar]
- 27. Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther 1966; 46:357–375. [DOI] [PubMed] [Google Scholar]
- 28. Jang SH. Somatotopic arrangement and location of the corticospinal tract in the brainstem of the human brain. Yonsei Med J 2011; 52:553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kennedy PR. Corticospinal, rubrospinal and rubro-olivary projections: a unifying hypothesis. Trends Neurosci 1990; 13:474–479. [DOI] [PubMed] [Google Scholar]
- 30. Pettersson LG, Blagovechtchenski E, Perfiliev S, et al. Recovery of food-taking in cats after lesions of the corticospinal (complete) and rubrospinal (complete and incomplete) tracts. Neurosci Res 2000; 38:109–112. [DOI] [PubMed] [Google Scholar]
- 31. Matsuyama K, Mori F, Nakajima K, et al. Locomotor role of the corticoreticular–reticulospinal–spinal interneuronal system. Prog Brain Res 2004; 143:239–249. [DOI] [PubMed] [Google Scholar]
- 32. Jang SH. The recovery of walking in stroke patients: a review. Int J Rehabil Res 2010; 33:285–289. [DOI] [PubMed] [Google Scholar]
- 33. Leiguarda RC, Marsden CD. Limb apraxias: higher-order disorders of sensorimotor integration. Brain 2000; 123:860–879. [DOI] [PubMed] [Google Scholar]
- 34. Heilman KM. Apraxia. Continuum (Minneap Minn) 2010; 16:86–98. [DOI] [PubMed] [Google Scholar]
- 35. Hong JH, Lee J, Cho YW, et al. Limb apraxia in a patient with cerebral infarct: diffusion tensor tractography study. NeuroRehabilitation 2012; 30:255–259. [DOI] [PubMed] [Google Scholar]
- 36. Jang SH. Motor recovery by improvement of limb-kinetic apraxia in a chronic stroke patient. NeuroRehabilitation 2013; 33:195–200. [DOI] [PubMed] [Google Scholar]
- 37. Lee KC, Finley R, Miller B. Apraxia of lid opening: dose-dependent response to carbidopa–levodopa. Pharmacotherapy 2004; 24:401–403. [DOI] [PubMed] [Google Scholar]
- 38. Yamada S, Matsuo K, Hirayama M, et al. The effects of levodopa on apraxia of lid opening: a case report. Neurology 2004; 62:830–831. [DOI] [PubMed] [Google Scholar]
- 39. Newman M. The process of recovery after hemiplegia. Stroke 1972; 3:702–710. [DOI] [PubMed] [Google Scholar]
- 40. Page SJ, Gater DR, Bach YRP. Reconsidering the motor recovery plateau in stroke rehabilitation. Arch Phys Med Rehabil 2004; 85:1377–1381. [DOI] [PubMed] [Google Scholar]
- 41. Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci 2005; 360:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]


