Supplemental Digital Content is available in the text
Keywords: fibrosis, GRADE, histological outcome, NAFLD, NASH, network meta-analysis
Abstract
Background:
The prevalence of nonalcoholic fatty liver disease (NAFLD) has significantly increased over the last decades. Despite existence of several interventions, there remains unclear which interventions work the best.
Methods:
A systematic review and network meta-analysis of randomized trials comparing efficacy of all treatment options in NAFLD were performed to determine comparative efficacy and safety of interventions in the management of NAFLD. Several electronic databases were searched up to Nov 15, 2015. Outcomes include liver histological outcomes (i.e., fibrosis), all-cause mortality, cirrhosis, and safety. A network meta-analysis was applied to estimate pooled risk ratios (RR). Quality of evidence was assessed using GRADE criteria.
Results:
A total of 44 studies (n = 3802) were eligible. When compared with placebo, obeticholic acid (OCA) was the only intervention that significantly improved fibrosis with RR (95% CI) of 1.91 (1.15, 3.16), while pentoxyfylline (PTX) demonstrated improved fibrosis without statistical significance with RR (95% CI) of 2.27 (0.81, 6.36). Only thiazolidinedione (TZD) and vitamin E use resulted in significant increase in resolution of NASH, while OCA, TZD, and vitamin E significantly improved other outcomes including NAS, steatosis, ballooning, and inflammation outcomes. Quality of evidence varied from very low (i.e., metformin, PTX on mean change of ballooning grade) to high (OCA, TZD, vitamin E on improving histological outcomes). Limitations of this study were lack of relevant long-term outcomes (e.g., cirrhosis, death, safety), possible small study effect, and few head-to-head studies.
Conclusions:
Our study suggests potential efficacy of OCA, TZD, and vitamin E in improving histologic endpoints in NAFLD. These findings are however based on a small number of studies. Additional studies are awaited to strengthen this network meta-analysis.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as excessive fat (i.e., triglyceride) accumulation in the liver without secondary hepatic fat accumulation such as significant alcohol consumption, use of steatogenic medication, or hereditary disorders. [1] It is histologically categorized into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFL is defined as the presence of hepatic steatosis without hepatocellular injury, whereas NASH is characterized by NAFL with hepatocellular ballooning injury with or without fibrosis. [1] NAFLD is now the most common cause of chronic liver disease worldwide [2] and affects 15% to 30% of the general population but is more prevalent (about 50%–90%) in patients with diabetes, metabolic syndrome, and severe obesity.[ 1 3] Current evidence suggests that 68% of adults in the United States are overweight, estimating that 75 to 100 million individuals may have NAFLD. [4] NAFLD is associated both with an increased risk of liver-related complications, for examples, liver fibrosis (41%), cirrhosis (20%–25%), end-stage liver disease (5.4%), and cardiovascular disease (CVD).[ 4 5]
The pathophysiologic mechanisms in NAFLD remain incompletely understood; therapy is therefore empiric and has mainly emphasized treatment of the associated conditions (e.g., diabetes, obesity, hyperlipidemia) including lifestyle modifications (e.g., weight loss, diet, and exercise). Both nonpharmacological and pharmacological interventions seem to play important roles [6] and have been investigated, [7 8 9 10 11 12 13] but it remains unclear which interventions are the most efficacious for NAFLD managements.
A traditional pair-wise meta-analysis could answer which treatment is better than placebo, but not for comparison of multiple treatment options.[ 14 15] Applying a network meta-analysis by borrowing data from common comparators may lead us to indirectly compare multiple interventions and thus answer this question. Therefore, we conducted a systematic review and a network meta-analysis to compare the efficacy and safety of multiple interventions in the management of NAFLD.
2. Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions, [16] and was conducted following a priori established protocol in Prospero (CRD42015025051). [17] We used GRADE criteria for network meta-analysis to assess quality of evidence.[ 18 19]
2.1. Data sources and searches
We identified randomized controlled trials (RCTs) published up to November 15, 2015 and compared different interventions for NAFLD from the following databases: PubMed, the Cochrane Library Central Register of Controlled Trials (CENTRAL), Embase, CINAHL, Web of Science, Scopus, ClinicalTrials.gov, and WHO registry. We developed and modified search algorithms properly for each database by combining relevant search terms following Cochrane for systematic reviews of RCTs suggestions. [20] Uses of search strategies were clearly described in Appendix Table 1. Reference lists of relevant studies were also screened. Two investigators (RS, BC) independently reviewed the titles and abstracts and the full articles were evaluated if a decision could not be made for selections. Disagreements were resolved by discussion with NC.
2.2. Study selection
RCTs were included if they met the following inclusion criteria: biopsy-proven NAFLD; any type of nonpharmacological and pharmacological interventions, single or combined interventions as sole, or adjunct therapy; a placebo or active comparator; and use of biopsy-based histological outcomes. Studies were excluded if insufficient data, or Traditional Chinese Medicine (TCM) or probiotics interventions.
2.3. Data extraction and quality assessment
Data were independently extracted by 2 investigators (RS, BC) using the standardized data extraction forms. These included patient characteristics, time to follow-up, histological characteristics, types of interventions, and outcomes (definitions and measurements). The risk of bias (ROB) was assessed using Cochrane risk of bias tool, [20] which consisted of 6 items (i.e., sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other biases). Each item was rated as high, low, or unclear.
2.4. Type of interventions
Types of interventions were categorized into 9 main groups and combinations of them including antioxidants (Antiox), metformin (Met), pentoxifyline (PTX), polyunsaturated fatty acid (PUFA), thiazolidinedione (TZD), ursodeoxycholic acid (UDCA), vitamin E plus C (vitamin E/vitamin C), weight/lipid control (pharmacology and nonpharmacology), others (obeticholic acid; OCA, Metadoxine, Betaine, Valsartan, and Tryptophan/phospholipid).
2.5. Outcomes of interest
The primary outcomes were improvement of fibrosis, death either overall or related to liver and cardiovascular disease deaths, and cirrhosis. Secondary outcomes were improvement of ballooning degeneration, steatosis, lobular inflammation, and NAS, mean changes in NAS, ballooning, steatosis, and lobular inflammation, adverse effects. If outcomes were repeatedly assessed, we considered only at the end of the study. The denominator (the number of patients at risk) was based on intention-to-treat analysis. The patients without follow-up biopsy (or with lack of information on follow-up histological findings) were defined as treatment failures.
2.6. Quality of evidence
GRADEpro GDT software online version (http://www.guidelinedevelopment.org/ (access Sep 2015)) was used to evaluate quality of evidence from direct and network meta-analysis. Quality of evidence was categorized into 4 levels including, high, moderate, low, and very low.[ 18 19] Details on grading are provided in Appendix Table 2. The quality of evidence for each pooled outcome was graded based on 5 domains including risk of bias, inconsistency, indirectness, imprecision, and publication bias.
2.7. Data synthesis and analysis
A pairwise meta-analysis with a random-effects model [21] was used to estimate treatment effects, pooled risk ratios (RR), or weighted mean differences (WMD) along with 95% confidence intervals (CI) for dichotomous and continuous outcomes, respectively. Heterogeneity was assessed using χ 2 test and I 2.[ 20 22] If there was evidence of heterogeneity, we attempted to explore its sources (i.e., risk of bias criteria, study characteristics, and patient characteristics) by performing subgroup analyses.
A network meta-analysis was applied to indirectly compare intervention effects for all NAFLD managements with the following steps: coefficients (i.e., lnRR) along with variance–covariance of comparisons were estimated for each study using placebo or common interventions as comparators. Then, these lnRRs were pooled across studies using a multivariate meta-analysis with restricted maximum likelihood function. Between-study variance and covariance were taken into account using exchangeable method. Inconsistency assumption (i.e., agreement of direct and indirect effects) was checked by estimating inconsistency factor (IF) using design-by-treatment and node splitting technique model. [23] In addition, the IF was tested using Z test indicating inconsistency if the IF is significantly different from 0. The surface under the cumulative ranking curve (SUCRA) was performed based on Bayesian approach to measure the ranking and the uncertainty. A probability of being best intervention was also estimated.
An adjusted funnel plot was constructed to examine small study effects. [24] A sensitivity analysis was performed accordingly based on size of included RCTs. [25] We also performed prespecified subgroup/sensitivity analyses according to patient characteristics (i.e., age, obesity, DM, dosage and characteristics of interventions, period of follow-up (i.e., 6 months, 12 months, >12 months), procedure of staging outcome (i.e., NASH CRN [26] or Brunt method) [27] and study characteristics (i.e., study design, sample size, quality of study). All analyses were performed using STATA 14.0 (Stata Corp, College Station, TX). A P value ≤0.05 was considered statistically significant.
2.8. Ethical approval
Ethical approval was not required for this study. It is a systematic review and meta-analysis which was not affected patients directly.
3. Results
A total of 3216 relevant articles were identified (Appendix Figure 1). After duplication removal, 1896 articles were eligible for screening based on titles and abstracts, 1774 articles were excluded, leaving 122 articles for review. Finally, 44 RCTs involving a total of 3802 patients were included in our study.
3.1. Characteristics of included studies and quality of studies
Uses of intervention and comparator of 44 included studies[ 8 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70] are summarized in Table 1 . Among them, 35, 8, and 1 study were 2-arm,[ 8 28 29 30 31 33 35 37 38 39 42 43 44 45 46 47 48 49 50 51 52 54 55 56 59 60 61 62 63 64 66 67 68 69 70] 3-arm,[ 32 34 40 41 53 57 58 65] and 4-arm [36] RCTs, respectively. Among 2-arm RCTs, 31 and 4 RCTs were placebo and active controls, respectively. Among 31 placebo controls, following various interventions were used: 8 studies for weight/lipid controls,[ 42 43 48 49 50 54 64 70] 3 studies for TZD,[ 29 31 55] and PTX,[ 56 67 69] 4 studies for Met,[ 39 62 63 66] and PUFA,[ 30 33 45 52] 2 studies for antioxidants,[ 47 68] UDCA,[ 44 46] vitamin E/vitamin C,[ 38 51] and other groups including betaine, [28] metadoxine, [61] and OCA, [8] respectively. Among 4 trials of 2-arm RTCs with active controls, their interventions and active comparators were as follows: telmisartan versus valsartan, [35] vitamin E versus bicyclol, [37] vitamin E versus vitamin E/TZD, [59] and TZD versus PTX. [60]
Table 1.
Characteristic of studies included in the network meta-analysis.

Among 3-arm RCTs, comparators were placebo in 6 RCTs,[ 32 34 40 41 57 58] and active controls in 2 RCTs (Met vs TZD vs Met/TZD, [53] and TZD vs TZD/Met vs TZD/losartan). [65] The interventions for these 6 RCTs were Met or TZD, [40] PUFA (low/high dose), [57] UDCA or UDCA/vitamin E, [34] vitamin E or Met, [41] vitamin E or TZD, [58] and tryptophan/phospholipid or melatonin/phospholipid. [32] Only 1 was 4-arm trial, which compared effects of UDCA, Alpha-lipoic acid (ALA), UDCA/ALA, and placebo. [36]
A total sample size in the 44 RCTs ranged from 16 to 283 patients (median = 58), duration of study/time at outcome measurement ranged from 4 to 24 months (median = 12). Most RCTs were conducted in the United States (N = 20), followed by European (EU) (N = 18), and Asian countries (N = 5). Most RCTs (34/44 studies) studied NASH patients and some (10/44) were in NAFLD patients. All RCTs studied in adults (aged 33–62 years, median = 48), except 2 RCTs studied in children.[ 41 51] Patients with obesity were included in most RCTs (37/44), only diabetes in a few RCTs,[ 31 33] and diabetes (ranged 9% to 53%) mixed with general in 20/44 RCTs.
Quality of included studies based on Cochrane risk of bias (ROB) tool was assessed, which suggested that 39%, 34%, and 27% of studies were low, unclear, and high quality, respectively (Appendix Figure 2). Most domains had at least 75% low risk of bias, except blinding had only 53%.
The primary outcomes were improvement of fibrosis, death (overall death, death related to liver, and cardiovascular diseases) and cirrhosis. A total of 21 RCTs reported improvement of fibrosis, 4 reported deaths but none reported reverse or development of cirrhosis during study period (Tables 1 and 2 ). Among 44 RCTs, NASH CRN's technique was used for grading histological outcomes by a blinded histologist in most studies (80%; 35/44). Figure 1A–F presents network map of all interventions for network meta-analysis in improvement of fibrosis, resolution of NASH, improvement of NAFLD activity score (NAS), steatosis, ballooning degeneration, and lobular inflammation, respectively.
Table 1 (Continued).
Characteristic of studies included in the network meta-analysis.
Figure 1.
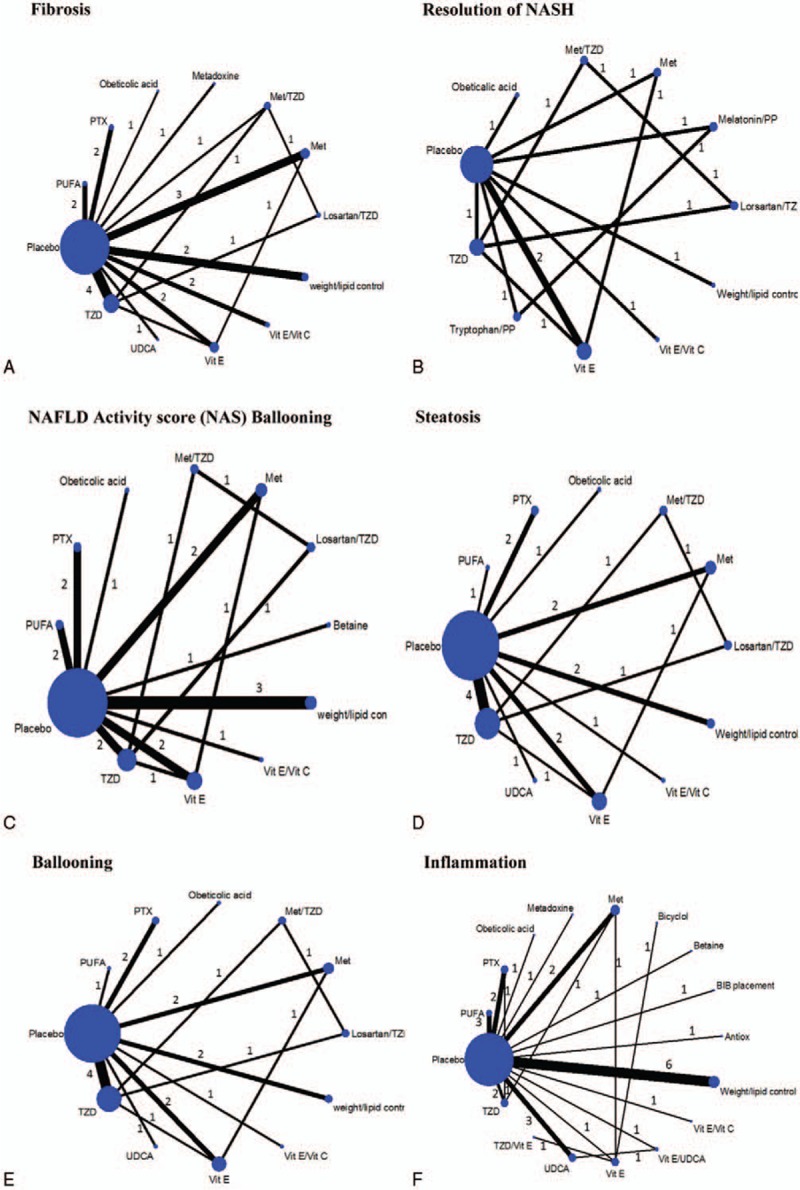
Network map of binary outcomes for improvement of histological outcomes. A, Fibrosis. B, Resolution of NASH (nonalcoholic steatohepatitis). C, NAFLD activity score (NAS). D, Steatosis. E, Ballooning degeneration. F, Lobular inflammation. Met = metformin, PP = phospholipid, PTX = pentoxifyline, PUFA = polyunsaturated fatty acid, TZD = thiazolidinedione, UCDA = ursodeoxycholic acid, Vit C = vitamin C, Vit E = vitamin E.
Table 2 (Continued).
Adverse events and rates of participant dropout of all include studies.
Table 2 (Continued).
Adverse events and rates of participant dropout of all include studies.
3.2. Death outcome
Only 4 studies reported death outcome.[ 8 41 46 58] All of them follow up patients more than 1 year. Among 2 studies investigating vitamin E, only 2 cases of death were reported in among vitamin E users (2/142).[ 41 58] Cause of death was cirrhosis with sepsis for 1 patient but another was not specified. One study reported death of 2 patients receiving OCA (2/141), 1 died from sepsis with congestive heart failure (CHF), and another one from myocardial infraction (MI), respectively. [8] In a study of UCDA, 1 patient died from myocardial infarction but it was not specified as a user of UCDA or placebo [46] (Table 2 ).
3.3. Improvement of fibrosis
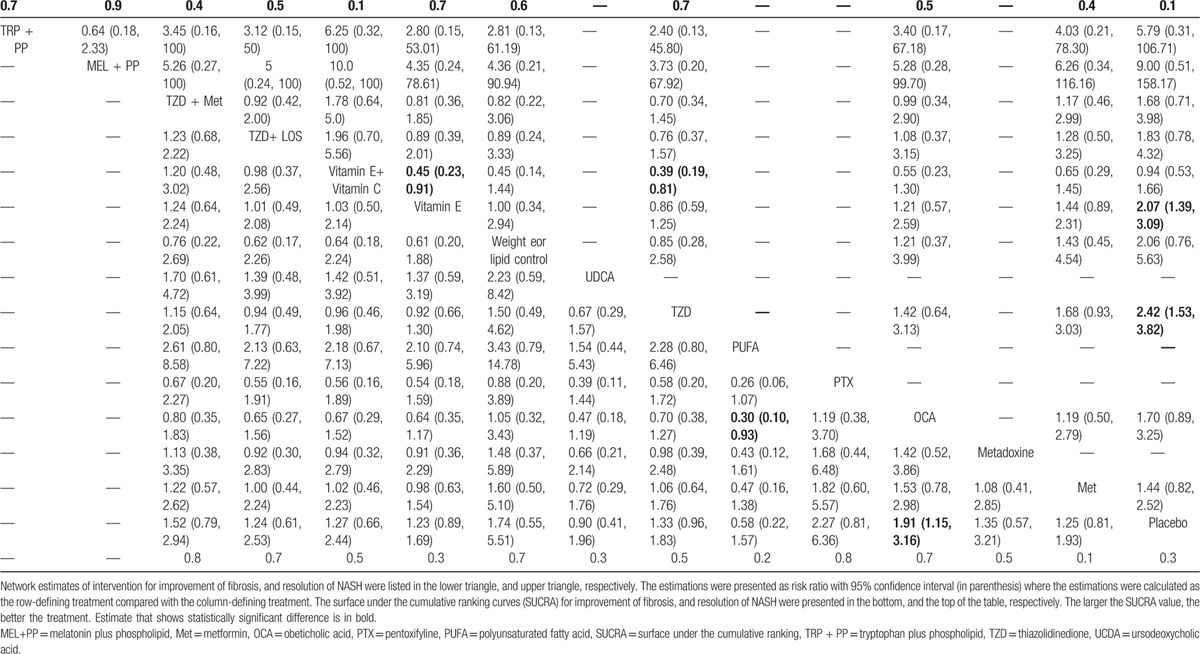
A total of 21 studies[ 8 29 30 31 33 38 39 40 41 42 46 48 51 55 58 61 65 66 67 69 70] (n = 1939 patients) reported improvement in fibrosis from 12 interventions (Fig. 1A). Results of direct comparisons showed that only OCA and TZD significantly improved fibrosis relative to placebo, with a pooled RR of 1.91 (1.15, 3.16) and 1.42 (1.01, 1.99), respectively (Appendix Table 3). A network meta-analysis indicated that only OCA remained significant similar effect to the direct one with pooled RR of 1.91(1.15, 3.16) (Fig. 2). PTX, TZD plus Met, weight/lipid control were effective when compared with placebo but these were not significant with a pooled RRs of 2.27 (0.81, 6.36), 1.52 (0.79, 2.94), and 1.74 (0.55, 5.51), respectively. The summary results of network meta-analysis and ranking are reported in Table 3. PTX showed a trend for better than other interventions with the RRs ranging from 1.19 to 3.85, but it was not significant (Table 3).). The ranking of interventions for this outcome can be also found in Appendix Figure 3A.
Figure 2.
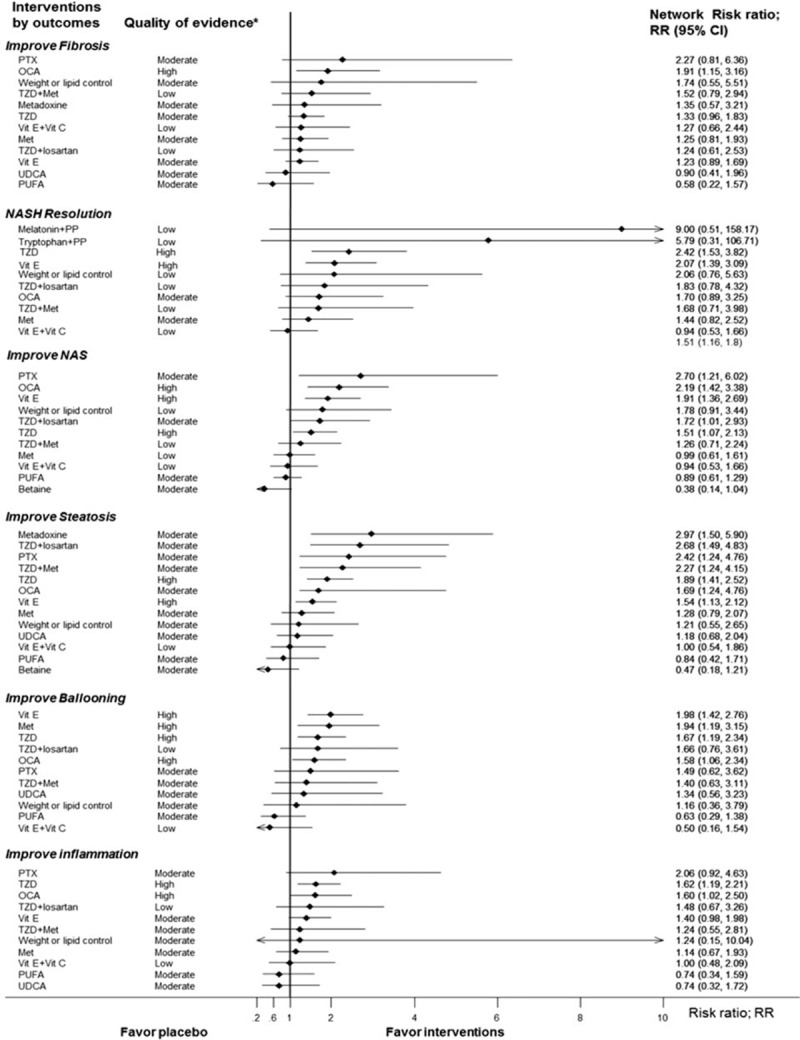
Forest plot summary of network estimates of interventions compared with placebo (cointervention: advise of weight and diet control) on histological outcomes. A, Fibrosis. B, Resolution of NASH (nonalcoholic steatohepatitis). C, NAFLD activity score (NAS). D, Steatosis. E, Ballooning degeneration. F, Lobular inflammation. ∗Quality of evidence was graded based on GRADE Working Group: High = we are very confident that the true effect lies close to that of the estimate of the effect, Moderate = we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different, Low = our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect, Very low = we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. NAS = nonalcoholic fatty liver disease (NAFLD) activity score, NASH = nonalcoholic steatohepatitis, PP = phospholipid, PTX = pentoxifyline.
Table 2.
Adverse events and rates of participant dropout of all include studies.
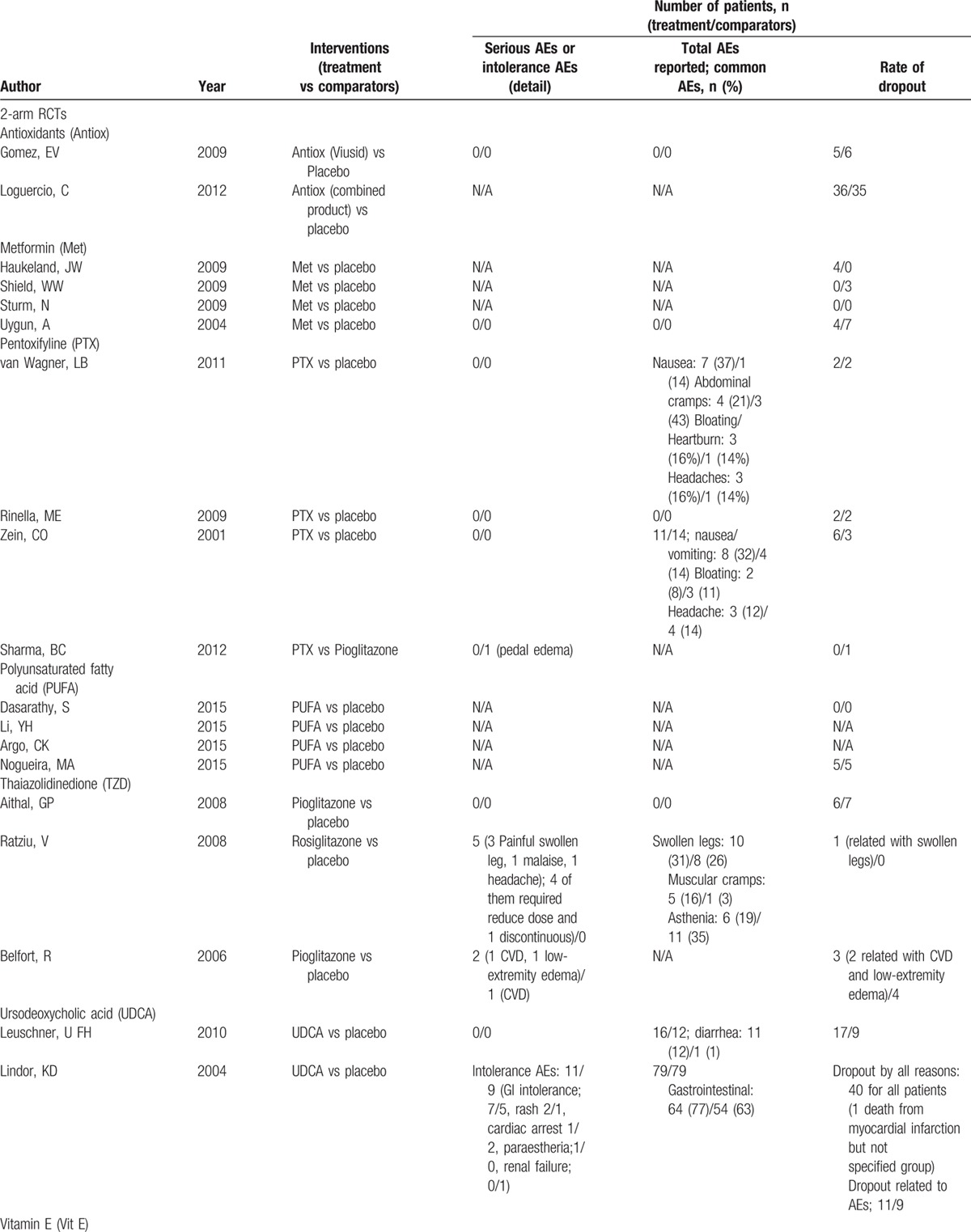
Table 3.
Network meta-analysis estimated effects of intervention for NAFLD therapy on improvement of fibrosis (lower triangle) and resolution of NASH (upper triangle).
3.4. Resolution of NASH
Seven studies (n = 1007)[ 8 32 41 51 54 58 65] reported resolution of NASH for 11 interventions (Fig. 1B). The direct evidence demonstrated statistically significant higher resolution of NASH for TZD and vitamin E when compared with placebo, with RRs of 2.28 (1.35, 3.87) and 2.07 (1.39, 3.09), respectively (Appendix Table 3). These RRs were not much changed by a network meta-analysis; melatonin/phospholipid and tryptophan/phospholipid additionally showed a trend for efficacy when compared with placebo with a pooled RR of 9.00 (0.51, 158.17), and 5.79 (0.31, 106.71), respectively (Table 3). The ranking of interventions for this outcome can be also found in Appendix Figure 3B.
3.5. Improvement of NAFLD activity score (NAS)
Fifteen studies (n = 1590)[ 8 28 33 39 41 42 48 51 54 55 57 58 65 67 69] reported improvement of NAS (Fig. 1C). The direct effects of PTX, OCA, TZD, and vitamin E were statistically significant in improvement of NAS when compared with placebo, with RRs of 2.70 (1.21, 6.03), 2.19 (1.42, 3.28), 1.56 (1.08, 2.26), and 2.24 (1.52, 3.31), respectively (Appendix Table 3). A network meta-analysis yielded similar effects but additionally indicated that TZD/losartan showed a significantly higher likelihood of NAS score improvement than placebo with RR of 1.72 (1.01, 2.93) (Fig. 2). The summary results of network meta-analysis and ranking were shown in Appendix (Appendix Table 4 and Appendix Figure 3C).
3.6. Improvement of steatosis, ballooning, and lobular inflammation
The summary results of meta-analysis of steatosis, ballooning, and lobular inflammation improvement are reported in Appendix Table 3, while network meta-analysis results are reported in Fig. 2. The direct evidence and network meta-analysis provided similar interventions effects on improvement of steatosis. When compared with placebo, the network meta-analysis RRs for PTX, OCA, TZD, metadoxine, and vitamin E were 2.42 (1.24, 4.76), 1.69 (1.24, 4.76), 1.89 (1.41, 2.52), 2.97 (1.50, 5.90), and 1.54 (1.13, 2.12), respectively. For the improvement of ballooning, both pairwise and network meta-analysis results demonstrated that OCA and vitamin E were significantly better than placebo. The network meta-analysis RRs of those were 1.58 (1.06, 2.34), and 1.98 (1.42, 2.76), respectively (Fig. 2). For improvement of lobular inflammation, both evidences demonstrated that TZD and OCA were significantly better than placebo. The network meta-analysis RRs of those were 1.62 (1.19, 2.21) and 1.60 (1.02, 2.50), respectively (Fig. 2). The ranking of these outcomes can be found in Appendix Figure 3D–F.
3.7. Mean change in fibrosis stage, NAS, steatosis, ballooning, and lobular inflammation
All interventions included in network meta-analysis for estimating mean changes of fibrosis stage, NAS, steatosis, ballooning, and lobular inflammation are presented in Appendix Figure 4. Pairwise (direct) meta-analysis of mean changes in fibrosis stage, NAS, steatosis, ballooning, and lobular inflammation are reported in Appendix Table 5, while network meta-analysis results of these outcomes are reported in Appendix Figure 5. Direct comparisons of weighted mean difference of changes in fibrosis grade indicated that PTX, OCA, antioxidant plus UDCA significantly decreased fibrosis grade when compared with placebo, with WMDs of −0.60 (0.95, −0.25), −0.30 (−0.52, −0.08), −0.29 (−0.35, −0.23), respectively (Appendix Table 5). The results remained unchanged in network meta-analysis (Appendix Table 6). Both direct and network meta-analysis results of mean change of NAS, steatosis, ballooning, and lobular inflammation tended to be the same as improvement outcomes (Appendix Table 5, Appendix Figure 5). The ranking efficacy of these outcomes is presented in Appendix Figure 6.
3.8. Inconsistency tests
There was no evidence of inconsistency between direct and indirect effects for most outcomes except mean changes in fibrosis stage and NAS (χ 2 = −74.62, P value <0.001 for fibrosis change; χ 2 = 89.33, P value <0.001 for NAS), respectively (Appendix Table 7). The pooled estimates of these outcomes were then based on an inconsistency model. [71]
3.9. Assessment of small-study effects
Small-study effects were assessed using adjusted funnel plots (Appendix Figure 7), indicating small-study effect might be present particularly for fibrosis, NAS, steatosis, ballooning outcomes. Distribution of sample size of all included RCTs was then explored for each outcome. A sensitivity analysis was performed by including only RCTs where their sample sizes exceeded the 25th percentile. Therefore, 10, 5, 6, 9, 8, and 7 trials were included in sensitivity analyses for improvement of fibrosis, resolution of NASH, NAS, steatosis, ballooning, and lobular inflammation, respectively. Results for the primary outcome are described in Appendix Table 8, while secondary outcomes are described in Appendix Table 9. These suggested that most rankings remained the same except the ranking of PTX, weight or lipid control, betaine, tryptophan/phospholipid, and melatonin/phospholipid were omitted.
3.10. Quality of evidence
Evidence quality was graded for both network (Fig. 2, Appendix Figure 5) and pairwise (Appendix Table 3, Appendix Table 5) meta-analyses, indicating high quality evidence for OCA in improvement of fibrosis, NAS, ballooning and lobular inflammation, and TZD and vitamin E in resolution of NASH, improvement of NAS, steatosis, ballooning, and lobular inflammation, respectively. Evidence quality for PTX in improvement of NAS and steatosis was moderate (Fig. 2).
3.11. Sensitivity and subgroup analyses
Most results from sensitivity and subgroup analyses were comparable with those in main analyses for most interventions (data not shown). The effects of TZD, vitamin E, and PTX on improvement of steatosis disappeared in pooling high quality RCTs (Appendix Table 8). Subgroup analysis showed no significant effects on improvement of any histological outcomes if follow-up time less than 1 year, whereas the effects of PTX on improvement of NAS and steatosis, and vitamin E on lobular inflammation were reversed from the main results (Appendix Table 8).
3.12. Adverse events
Adverse events were reported in 34 studies (77%) but only 11 studies reported treatment-related serious/intolerance adverse events. Five of 11 studies reported serious or intolerance adverse events including cardiovascular diseases and peripheral edema related to pioglitazone or rosiglitazone (TZD) more than placebo. Gastrointestinal adverse events including nausea/vomiting, abdominal cramps, bloating, and heartburn were commonly reported in patients who used PTX, PUFA, and betaine than those in patients who used placebo. For other interventions, both serious/intolerance and common adverse events were infrequent and comparable to placebo (Table 2 ).
4. Discussion
We conducted a systematic review and network meta-analysis of all published RCTs with biopsy-proven NAFLD to provide a critical summary of evidence of all interventions for NAFLD therapy. Our findings demonstrated that several interventions significantly improved histological outcomes, such as fibrosis and resolution of NASH. Given an increasing trend of NAFLD prevalence globally, our review is timely and clinically relevant for guiding clinical practice of NAFLD management.
OCA was the only intervention that significantly improved fibrosis with a high quality of evidence and suggested other interventions (i.e., PTX, TZD plus metformin, TZD plus losartan) might potentially be effective. TZD and vitamin E resulted in resolution of NASH with high quality of evidence. PTX, OCA, vitamin E, and TZD were effective in improving the NAS score with a moderate quality for PTX and high quality of evidence for the rest.
Our findings were different from those reported in a previous network meta-analysis, which supported efficacy of PTX [72] not OCA, in improvement of fibrosis. Since the evidence of OCA is supported by a single RCT, while PTX is based on 2 small RCTs with low event rates, the estimated CIs for both treatments were wide. Therefore, there is a need for more RCTs assessing the long-term outcomes and safety of both for NAFLD.
Vitamin E and TZD were supported by high quality of evidence in resolution of NASH. These findings reinforce the current recommendation of American Association for the Study of Liver Diseases (AASLD) guidelines for the use of natural vitamin E (800 IU/day) and pioglitazone in nondiabetic adults with biopsy-proven NASH. [1] However, concern has been raised about risks of vitamin E therapy. [73 74 75 76] Currently, pioglitazone is the only TZD available in clinical practice, because rosiglitazone is not available in Europe and highly restricted in the United States. [77] The long-term safety of pioglitazone regarding cardiovascular disease (especially chronic heart failure) limits widespread use. [78] It is important to note that majority of the patients in our included trials is nondiabetic, limiting the applicability of these interventions in diabetic patients.
Our study had a number of advantages over previously published meta-analysis studies of NAFLD.[ 9 72 79 80 81] We included both NAFL and NASH patients, assessed both nonpharmacological and pharmacological interventions, and considered only biopsy-proved histological outcomes. Contrastingly, the previous studies only considered NASH patients, assessed only pharmacological interventions, [72] or considered surrogate outcomes (e.g., liver fatty content by ultrasound, ALT, AST, insulin sensitivity).[ 9 79 80 81] The number of RCTs or patients included in our study was larger than the previous published report, [72] that is, 44 and 3802 versus 9 and 964, respectively. A unique feature of our study is the inclusion of reports in patients with NASH and NAFLD, which provides a more global assessment of therapeutic interventions in this disease state.[ 4 82]
Limitations of our study are the heterogeneity from inclusion of various interventions and patient characteristics and the fact that a large number (60%) of the included studies were rated as unclear/high ROB, although sensitivity and subgroup analyses showed similar results to the main findings. In addition, more relevant long-term outcomes (e.g., cirrhosis, death, safety) could be not assessed because none of included studies reported. Furthermore, number of included studies and subjects were very small and thus yielded imprecise estimation of some treatment effects. These results are thus needed to update when there are more RCTs available.
In conclusion, we observed that of the interventions studies thus far, OCA was effective for improving fibrosis and NAS score, while TZD and vitamin E were effective for resolution of NASH and NAS score. Large comparative RCTs and cost-effectiveness analyses are warranted to investigate the effects of interventions on histological and clinical outcomes.
Supplementary Material
Acknowledgments
The authors thank Loguercio C, and his colleagues for support with some data from their previously published study.
Footnotes
Abbreviations: AEs = adverse events, ALA = alpha-lipoic acid, Antiox = antioxidant, BET = betaine, BIB = gastric surgery, F/U follow-up, LY program = lifestyle change program, MEL + PP = melatonin plus phospholipid, Met = metformin, N/A = not available, NAFL = nonalcoholic fatty liver, NAFLD = nonalcoholic fatty liver disease which is composed of NAFL and NASH, NAS = NAFLD activity score, NASH = nonalcoholic steatohepatitis, Pio = pioglitazone, PP = phospholipid, PTX = pentoxifyline, PUFA = polyunsaturated fatty acid, Rosi = rosiglitazone, Tel = telmisartan, TRP + PP = tryptophan plus phospholipid, TRP = tryptophane, TZD = thiazolidinedione, UCDA = ursodeoxycholic acid, Val = valsartan, Vit C + Vit E = vitamin C plus vitamin E, Vit E = vitamin E, vs = versus.
RS, BC, PP, SS, AT, and NC declare no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. KVK has received support for research related to NASH from Gilead Sciences, Galectin Pharmaceuticals, Intercept, Immuron, Tobira, Mochida and NGM BioPharma and has served as a paid advisor to Intercept, Enanta, Verlyx, and Gilead Sciences, and also been a consultant of Gilead Sciences.
Study concept and design: RS, PP, NC, AT.
Acquisition of data: RS, BC.
Analysis and interpretation of data: RS, SS, BC, AT, KVK, NC.
Drafting of the manuscript: RS, NC, AT.
Critical revision of the manuscript for important intellectual content: RS, SS, BC, PP, AT, KVK, NC.
Approval of the final manuscript: RS, SS, BC, PP, AT, KVK, NC.
Review registration: PROSPERO CRD 42015025051.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1. Chalasani N, Younossi Z, Lavine J, et al. AASLD practice guideline. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 5:2005–2023. [DOI] [PubMed] [Google Scholar]
- 2. Watanabe S, Hashimoto E, Ikejima K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatol Res 2015; 45:363–377. [DOI] [PubMed] [Google Scholar]
- 3. Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol 2015; 13:2062–2070. [DOI] [PubMed] [Google Scholar]
- 4. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015; 313:2263–2273. [DOI] [PubMed] [Google Scholar]
- 5. Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015; 13: 643-654.e641-649; quiz e639-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012; 142:1592–1609. [DOI] [PubMed] [Google Scholar]
- 7. Musso G, Gambino R, Cassader M, et al. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 2010; 52:79–104. [DOI] [PubMed] [Google Scholar]
- 8. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015; 385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Musso G, Cassader M, Rosina F, et al. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 2012; 55:885–904. [DOI] [PubMed] [Google Scholar]
- 10. Parker HM, Johnson NA, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012; 56:944–951. [DOI] [PubMed] [Google Scholar]
- 11. Mahady SE, Webster AC, Walker S, et al. The role of thiazolidinediones in non-alcoholic steatohepatitis—a systematic review and meta-analysis. J Hepatol 2011; 55:1383–1390. [DOI] [PubMed] [Google Scholar]
- 12. Boettcher E, Csako G, Pucino F, et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2012; 35:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeng T, Zhang CL, Zhao XL, et al. Pentoxifylline for the treatment of nonalcoholic fatty liver disease: a meta-analysis of randomized double-blind, placebo-controlled studies. Eur J Gastroenterol Hepatol 2014; 26:646–653. [DOI] [PubMed] [Google Scholar]
- 14. Mills EJ, Ioannidis JP, Thorlund K, et al. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA 2012; 308:1246–1253. [DOI] [PubMed] [Google Scholar]
- 15. Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013; 159:130–137. [DOI] [PubMed] [Google Scholar]
- 16. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Inter Med 2015; 162:777–784. [DOI] [PubMed] [Google Scholar]
- 17. Hutton B, Salanti G, Chaimani A, et al. The quality of reporting methods and results in network metaanalyses: an overview of reviews and suggestions for improvement. PLoS One 2014; 9:e92508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014; 349:g5630. [DOI] [PubMed] [Google Scholar]
- 19. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401–406. [DOI] [PubMed] [Google Scholar]
- 20. Higgins J, Altman D, Sterne J. Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins JPT, editor. Available at: http://www.cochrane-handbook.org. The Cochrane Collaboration. 2011 (accessed June 10, 2015). [Google Scholar]
- 21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 22. Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009; 50:206–217. [DOI] [PubMed] [Google Scholar]
- 23. Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29:932–944. [DOI] [PubMed] [Google Scholar]
- 24. Mavridis D, Salanti G. Exploring and accounting for publication bias in mental health: a brief overview of methods. Evid Based Ment Health 2014; 17:11–15. [DOI] [PubMed] [Google Scholar]
- 25. Dechartres A, Altman DG, Trinquart L, et al. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA 2014; 312:623–630. [DOI] [PubMed] [Google Scholar]
- 26. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 27. Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94:2467–2474. [DOI] [PubMed] [Google Scholar]
- 28. Abdelmalek MF, Sanderson SO, Angulo P, et al. Betaine for nonalcoholic fatty liver disease: results of a randomized placebo-controlled trial. Hepatology 2009; 50:1818–1826. [DOI] [PubMed] [Google Scholar]
- 29. Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008; 135:1176–1184. [DOI] [PubMed] [Google Scholar]
- 30. Argo CK, Patrie JT, Lackner C, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol 2015; 62:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006; 355:2297–2307. [DOI] [PubMed] [Google Scholar]
- 32. Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease—14 months follow up. J Physiol Pharmacol 2014; 65:75–82. [PubMed] [Google Scholar]
- 33. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol 2015; 49:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dufour JF, Oneta CM, Gonvers JJ, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2006; 4:1537–1543. [DOI] [PubMed] [Google Scholar]
- 35. Georgescu EF, Ionescu R, Niculescu M, et al. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol 2009; 15:942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gianturco V, Troisi G, Bellomo A, et al. Impact of combined therapy with alpha-lipoic and ursodeoxycolic acid on nonalcoholic fatty liver disease: double-blind, randomized clinical trial of efficacy and safety. Hepatol Int 2013; 7:570–576. [DOI] [PubMed] [Google Scholar]
- 37. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig 2014; 34:1–7. [DOI] [PubMed] [Google Scholar]
- 38. Harrison SA, Torgerson S, Hayashi P, et al. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2003; 98:2485–2490. [DOI] [PubMed] [Google Scholar]
- 39. Haukeland JW, Konopski Z, Eggesbo HB, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol 2009; 44:853–860. [DOI] [PubMed] [Google Scholar]
- 40. Idilman R, Mizrak D, Corapcioglu D, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2008; 28:200–208. [DOI] [PubMed] [Google Scholar]
- 41. Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011; 305:1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012; 56:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee YM, Low HC, Lim LG, et al. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc 2012; 76:756–760. [DOI] [PubMed] [Google Scholar]
- 44. Leuschner UF, Lindenthal B, Herrmann G, et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology 2010; 52:472–479. [DOI] [PubMed] [Google Scholar]
- 45. Li YH, Yang LH, Sha KH, et al. Efficacy of poly-unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World J Gastroenterol 2015; 21:7008–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 2004; 39:770–778. [DOI] [PubMed] [Google Scholar]
- 47. Loguercio C, Andreone P, Brisc C, et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med 2012; 52:1658–1665. [DOI] [PubMed] [Google Scholar]
- 48. Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015; 61:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malaguarnera M, Gargante MP, Russo C, et al. L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis—a randomized and controlled clinical trial. Am J Gastroenterol 2010; 105:1338–1345. [DOI] [PubMed] [Google Scholar]
- 50. Nelson A, Torres DM, Morgan AE, et al. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol 2009; 43:990–994. [DOI] [PubMed] [Google Scholar]
- 51. Nobili V, Manco M, Devito R, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology 2008; 48:119–128. [DOI] [PubMed] [Google Scholar]
- 52. Nogueira MA, Oliveira CP, Ferreira Alves VA, et al. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2016; 35:578–586. [DOI] [PubMed] [Google Scholar]
- 53. Omer Z, Cetinkalp S, Akyildiz M, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2010; 22:18–23. [DOI] [PubMed] [Google Scholar]
- 54. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010; 51:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 2008; 135:100–110. [DOI] [PubMed] [Google Scholar]
- 56. Rinella ME, Koppe S, Brunt E, et al. Pentoxifylline improves ALT and histology in patients with NASH, a double blind placebo controlled trial. AGA. Abstract No. 563. 2009(563):A88–89. [Google Scholar]
- 57. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014; 147:377–384. [DOI] [PubMed] [Google Scholar]
- 58. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sanyal AJ, Mofrad PS, Contos MJ, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2004; 2:1107–1115. [DOI] [PubMed] [Google Scholar]
- 60. Sharma BC, Kumar A, Garg V, et al. A randomized controlled trial comparing efficacy of pentoxifylline and pioglitazone on metabolic factors and liver histology in patients with non-alcoholic steatohepatitis. J Clin Exp Hepatol 2012; 2:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shenoy KT, Balakumaran LK, Mathew P, et al. Metadoxine versus placebo for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. J Clin Exp Hepatol 2014; 4:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shields WW, Thompson KE, Grice GA, et al. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): a pilot trial. Ther Adv Gastroenterol 2009; 2:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sturm N, Bronowicki JP, Maynard-Muet M, et al. Metformin plus pentoxifylline versus prescriptive diet in non-alcoholic steatohepatitis (NASH): a randomized controlled pilot trial. Gastroenterol Clin Biol 2009; 33:984–986. [DOI] [PubMed] [Google Scholar]
- 64. Takeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia 2014; 57:878–890. [DOI] [PubMed] [Google Scholar]
- 65. Torres DM, Jones FJ, Shaw JC, et al. Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis in humans: a 12-month randomized, prospective, open-label trial. Hepatology 2011; 54:1631–1639. [DOI] [PubMed] [Google Scholar]
- 66. Uygun A, Kadayifci A, Isik AT, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2004; 19:537–544. [DOI] [PubMed] [Google Scholar]
- 67. Van Wagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol 2011; 10:277–286. [PubMed] [Google Scholar]
- 68. Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, et al. Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2009; 30:999–1009. [DOI] [PubMed] [Google Scholar]
- 69. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology 2011; 54:1610–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006; 4:639–644. [DOI] [PubMed] [Google Scholar]
- 71. Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013; 159:130–137. [DOI] [PubMed] [Google Scholar]
- 72. Singh S, Khera R, Allen AM, et al. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: a systematic review and network meta-analysis. Hepatology 2015; 62:1417–1432. [DOI] [PubMed] [Google Scholar]
- 73. Berry D, Wathen JK, Newell M. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clin Trials 2009; 6:28–41. [DOI] [PubMed] [Google Scholar]
- 74. 2007; Bjelakovic G, Nikolova D, Gluud LL, et al. Mortality in randomized trials of antioxidant supplements of primary and secondary prevention: systematic review and meta-analysis JAMA. 297:842–857. [DOI] [PubMed] [Google Scholar]
- 75. Dietrich M, Jacques PF, Pencina MJ, et al. Vitamin E supplement use and the incidence of cardiovascular disease and all-cause mortality in the Framingham Heart Study: does the underlying health status play a role? Atherosclerosis 2009; 205:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gerss J, Kopcke W. The questionable association of vitamin E supplementation and mortality—inconsistent results of different meta-analytic approaches. Cell Mol Biol 2009; 55 (Suppl:OL):1111–1120. [PubMed] [Google Scholar]
- 77. Mitka M. FDA eases restrictions on the glucose-lowering drug rosiglitazone. JAMA 2013; 310:2604. [DOI] [PubMed] [Google Scholar]
- 78. Lincoff A, Wolski K, Nicholls S, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus. A meta-analysis of randomized trials. JAMA 2007; 298:1180–1188. [DOI] [PubMed] [Google Scholar]
- 79. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol 2014; 20:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ji HF, Sun Y, Shen L. Effect of vitamin E supplementation on aminotransferase levels in patients with NAFLD, NASH, and CHC: results from a meta-analysis. Nutrition 2014; 30:986–991. [DOI] [PubMed] [Google Scholar]
- 81. Xiang Z, Chen YP, Ma KF, et al. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: a systematic review. BMC Gastroenterol 2013; 13:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sanyal AJ, Friedman SL, McCullough AJ, et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology 2015; 61:1392–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


