Abstract
The risk factors associated with postoperative pulmonary complications (PPCs) following laparoscopic gastrectomy have not been well studied. We sought to identify the risk factors for PPCs following gastric cancer surgery.
A retrospective analysis was performed on all gastric cancer patients in a prospective database who underwent a laparoscopic gastrectomy from 2004 to 2014. The potential risk factors for PPCs were evaluated.
PPCs occurred in 6.8% (83/1205) of patients and included pneumonia in 56 (67.5%) patients, pleural effusion in 26 (31.3%) patients, and pulmonary embolism in 1 (1.2%) patient. The multivariate analysis identified the following significant risk factors for PPCs: advanced age (odds ratio [OR] = 1.043, 95% confidence interval [CI] = 1.021%, 1.066%), chronic obstructive pulmonary disease (COPD) (OR = 17.788, 95% CI = 2.618%, 120.838%), total gastrectomy (OR = 2.781, 95% CI = 1.726%, 4.480%), time to first diet (OR = 1.175, 95% CI = 1.060%, 1.302%), and postoperative hospital stay (OR = 1.015, 95% CI = 1.002%, 1.028%). The risk factors for pneumonia included advanced age (OR = 1.036, 95% CI = 1.010%, 1.063%), total gastrectomy (OR = 3.420, 95% CI = 1.960%, 5.969%), and time to first diet (OR = 1.207, 95% CI = 1.703%, 1.358%). Only pancreatectomy was a risk factor for pleural effusion (OR = 9.082, 95% CI = 2.412%, 34.206%).
The frequency of PPCs in patients with gastric cancer who underwent laparoscopic surgery was relatively high. Patients with cardiac and pulmonary comorbidities and those who undergo total gastrectomy and combined resection should be considered at high risk.
Keywords: gastric cancer laparoscopic surgery, postoperative pulmonary complications
1. Introduction
Gastric cancer is one of the most frequent types of cancer and the most common cause of cancer-related deaths globally.[ 1 2] Within Asia, China has the highest incidence of advanced gastric cancer. Surgeons who perform minimally invasive laparoscopic techniques have been at the forefront of this area of medicine and have perfected the technique for gastric cancer resection. To date, radical gastrectomy with an effective D2 lymphadenectomy has remained the primary option for therapy.
Despite the advantages of minimally invasive surgery, [3] concerns have focused on improving postoperative outcomes. In gastric cancer patients undergoing laparoscopic gastrectomy surgery, postoperative pulmonary complications (PPCs) are a significant contributor to postoperative morbidity and mortality because healing of the affected wound is closely related to tissue hypoxia. [4 5 6] However, most studies have focused on general complications, which have their own criteria, and postoperative complications remain a clinically relevant issue. [7] PPCs are most common after upper abdominal surgery, including laparoscopic gastrectomy. Pulmonary function decreases because of reduced diaphragmatic activity and microatelectasis, which may lead to PPCs.
PPCs have become increasingly common adverse events; therefore, the identification and mitigation of perioperative risk factors in patients undergoing laparoscopic gastrectomy surgery for gastric cancer should enable further improvements in outcomes and decrease the length of hospital stay. To date, no studies have been published that have examined the incidence of PPCs in gastric cancer patients who have undergone laparoscopic surgery.
The purpose of this study was to identify the risk factors for postoperative PCCs in patients with gastric cancer who undergo laparoscopic surgery.
2. Methods
2.1. Data collection
We identified all patients who underwent laparoscopic gastrectomy surgery for gastric cancer between January 2004 and December 2014, which included 1205 patients in the Department of General Surgery, Nanfang Hospital. The inclusion criteria consisted of analyzed PPC events that occurred following elective laparoscopic gastrectomy surgery. Data regarding patient demographics, clinicopathological characteristics, extent of lymph node (D1+ or D2) involvement, type of surgery, and intraoperative and postoperative outcomes were prospectively obtained from our departmental database and retrospectively analyzed with a focus on specific factors that might influence the risk of PCCs. We excluded patients who underwent laparoscopic exploration because of peritoneal dissemination and those who exhibited either unresectable disease or distant adjuvant organ invasion. We also excluded patients who underwent open laparotomy surgery and those younger than 18 years. All patients provided written informed consent. The study was approved by the ethics committee of Nanfang Hospital.
All laparoscopic gastrectomy resections and lymph node dissections were performed according to Japanese guidelines. [8] TNM staging was conducted according to the American Joint Committee on Cancer, 7th edition, which was in accordance with the UICC classification. [9] Patients with pathological stages II, III, and IV disease received postoperative chemotherapy.
Postoperative morbidity and mortality were based on any events, complications, or deaths that occurred during the first 30 days following surgery. All postoperative adverse events were recorded and classified according to the Clavien–Dindo classification of severity. [10]
2.2. Data analysis
Investigated data included age, sex, body mass index (BMI), Charlson Comorbidity Index (CCI), Easter Cooperative Oncology performance status (ECOG), comorbidities, TNM stage, pathological stage, surgical procedure, lymph node dissection, operative time, additional procedures, estimated blood loss, intraoperative complications, and postoperative outcomes.
2.3. Definition and evaluation of PPCs
PPCs and adverse events were recorded postoperatively in all patients and were defined as the development of pneumonia, pleural effusion, or pulmonary embolism within 30 days after laparoscopic gastrectomy surgery.
Pneumonia was defined based on the signs and symptoms of PPCs (shortness of breath, cough, chest pain, and fever); blood tests and x-ray or CT scans were used to confirm the diagnosis of pneumonia.[ 11 12] Blood tests or x-rays were used for clinical evaluation before discharge (postoperative day 7–9).
Pleural effusion was defined as evidence of excessive fluid in the pleural space in the absence of clinical symptoms associated with abnormal findings detected by a simple chest radiography examination that required percutaneous intervention.
Pulmonary embolism was defined as the presence of clinical chest pain or blood gas abnormalities detected by simple chest radiography or a CT scan.
2.4. Statistical analysis
Data are expressed as means ± standard deviations, medians (quartile ranges), or frequencies (percentages). The Pearson χ 2 test, Fishere exact test, Student t test, and the Mann-Whitney U test were used for statistical analyses. Both univariate and multivariate logistic regression models were used to generate odds ratios (ORs). A value of P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (SPSS version 20.0; IBM, Inc, Chicago, IL).
3. Results
3.1. Patient demographics
We identified all patients who underwent a laparoscopic gastrectomy for treatment of gastric cancer between January 2004 and December 2014, which included 1205 patients in the Department of General Surgery, Nanfang Hospital. Data regarding patient demographics, clinicopathological characteristics, and preoperative and intraoperative variables were prospectively collected and retrospectively analyzed. The subjects included 822 men (68.2%) and 383 women (31.8%), with a mean age of 55 ± 12 years. Nineteen (1.6%) patients had congestive heart failure (CHF), and 5 (0.4%) had preexisting chronic obstructive pulmonary disease (COPD) before surgery. No significant differences were observed among the 2 groups with respect to BMI, CCI, ECOG, lymph node dissection, estimated blood loss, and intraoperative transfusion. The distribution of PPCs is shown in Table 1.
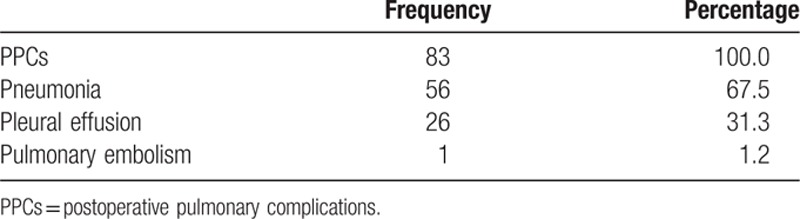
Table 1.
Frequency table for three PPC subtypes.
3.2. PPCs and postoperative outcomes
Eighty-three (6.8%) patients exhibited PCCs, and the types of complications were significantly different. Several risk factors that were strongly associated with PPCs are shown in Table 2. The most common complication experienced by the majority of our patients was pneumonia (67.5%, 56/83 patients), followed by pleural effusion (31.3%, 26/83 patients) and pulmonary embolism (1.2%, 1/83 patients).
Table 2.
Univariate and multivariate analyses of the risk factors for PPCs.
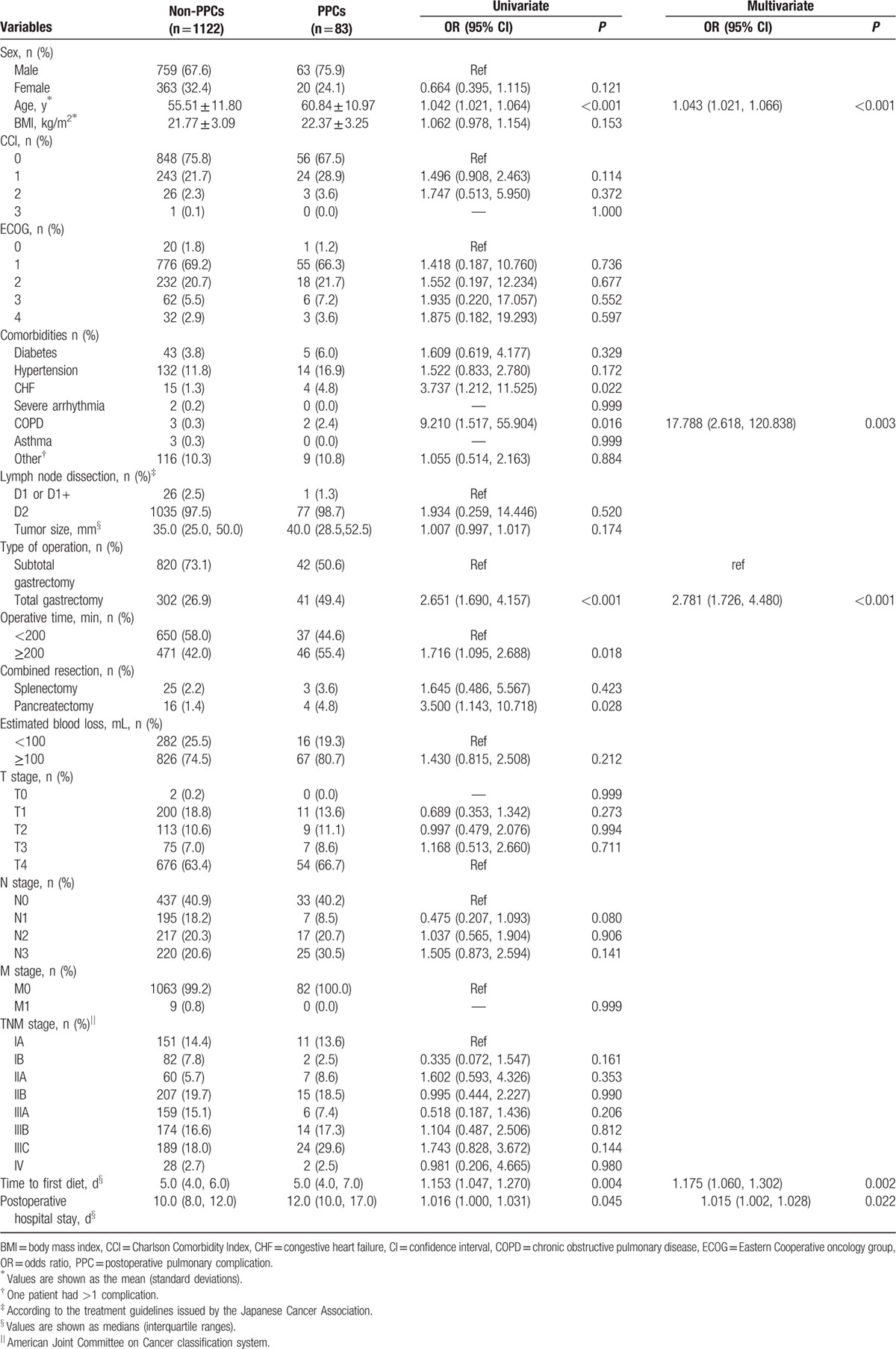
Table 3 summarizes the postoperative morbidity and mortality. Overall, 27.7% of patients experienced PPCs and 11.5% experienced non-PPCs (P < 0.001). Significant differences were found for renal complications (4.8% vs 0.9%, P = 0.007), hepatic complications (4.8% vs 1.2%, P = 0.025), intra-abdominal abscesses (8.4% vs 2.5%, P = 0.006), and abdominal bleeding (6.0% vs 0.9%, P < 0.001) in patients with and without PPCs. One death occurred (0.1%) during hospitalization in the non-PPC group, but no significant difference was observed between the 2 groups.
Table 3.
Postoperative complications of the PPC group.
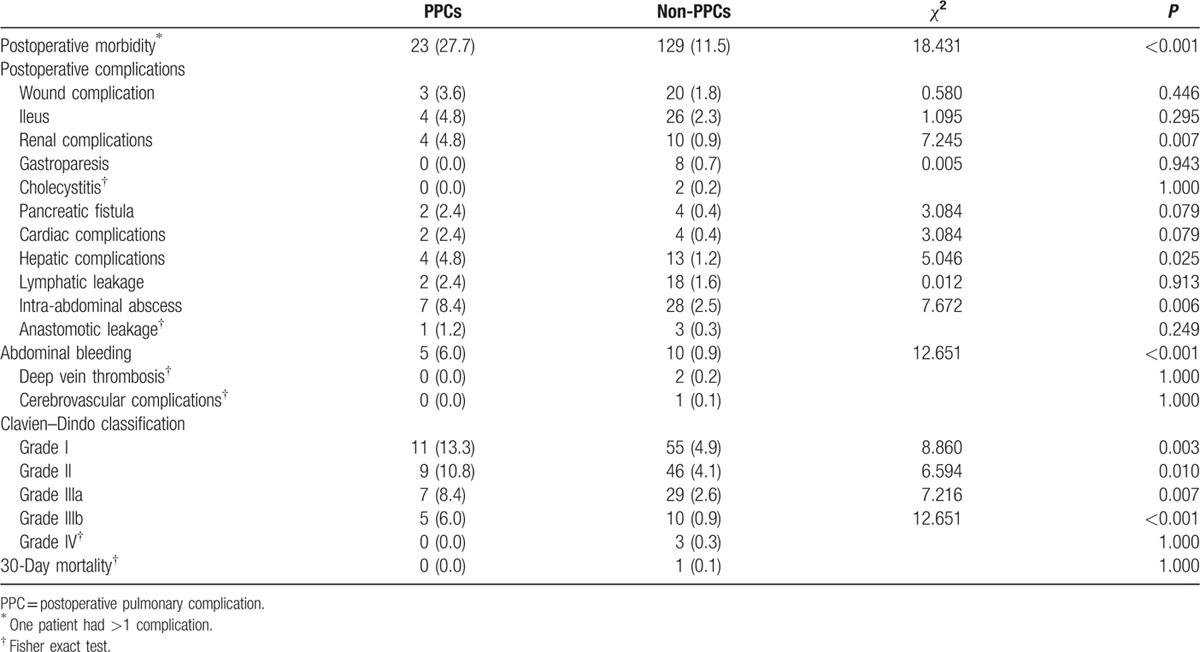
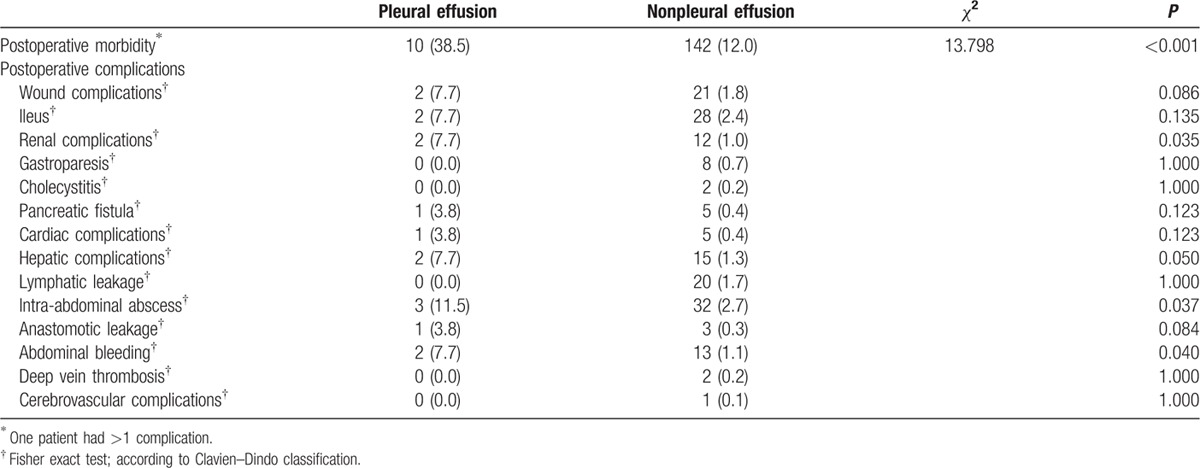
Table 4 shows the complications in patients with pneumonia (23.2%) compared to patients without pneumonia (12.1%) (P = 0.014). Abdominal bleeding was more common in the pneumonia group (5.4% vs 1.0%, P = 0.026). Postoperative outcomes for patients with pleural effusion are shown in Table 5, and the overall complication rate was significantly different between patients with and without pleural effusion (38.5% vs 12.0%, P < 0.001). Renal complications (7.7% vs 1.8%, P = 0.035), hepatic complications (7.7% vs 1.3%, P = 0.050), and abdominal bleeding (7.7% vs 1.1%, P = 0.040) exhibited significant differences between patients with and without pleural effusion.
Table 4.
Postoperative complications in the pneumonia group.
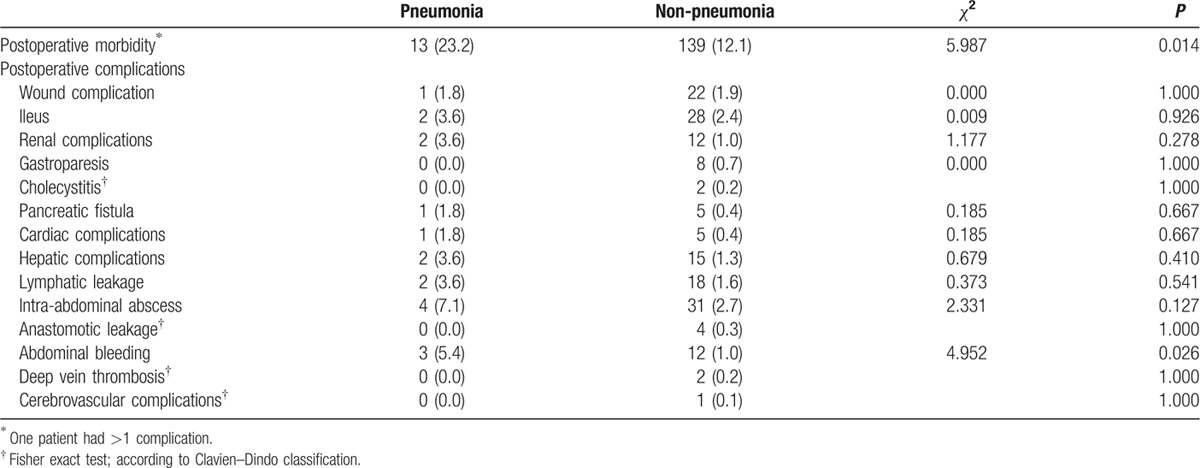
Table 5.
Postoperative complications of the pleural effusion group.
3.3. Risk factors related to PPCs
The types of risk factors in our study were diverse. After both univariate and multivariate analyses were conducted, advanced age (>60 years), preexisting CHF, preexisting COPD, the operative method (laparoscopic total gastrectomy), prolonged surgery (≥200 min), additional procedures (pancreatectomy), time to first diet, and postoperative hospital stay were identified as risk factors for PPCs (Table 2). The multivariate logistic regression model determined 5 independent risk factors for PPCs, including advanced age (OR = 1.043, 95% confidence interval [CI] = 1.021%, 1.066%; P < 0.001), preexisting COPD (OR = 17.788, 95% CI = 2.618%, 120.838%; P = 0.003), operative method (total gastrectomy) (OR = 2.781, 95% CI = 1.726%, 4.480%; P < 0.001), time to first diet (OR = 1.175, 95% CI = 1.060%, 1.302%; P = 0.002), and postoperative hospital stay (OR = 1.015, 95% CI = 1.002%, 1.028%; P = 0.022).
Table 6 summarizes the results of both the univariate and multivariate analyses of the risk factors for pneumonia. Advanced age, operative method (laparoscopic total gastrectomy), and time to first diet were significant risk factors associated with pneumonia according to the univariate analysis. Three predictive risk factors for pneumonia were identified in the multivariate logistic analysis, including advanced age (OR = 1.033, 95% CI = 1.008%, 1.059%; P = 0.010), total gastrectomy (OR = 3.420, 95% CI = 1.960%, 5.969%; P < 0.001), and time to first oral diet (OR = 1.207, 95% CI = 1.073%, 1.358%; P = 0.002).
Table 6.
Univariate and multivariate analyses of the risk factors for pneumonia.
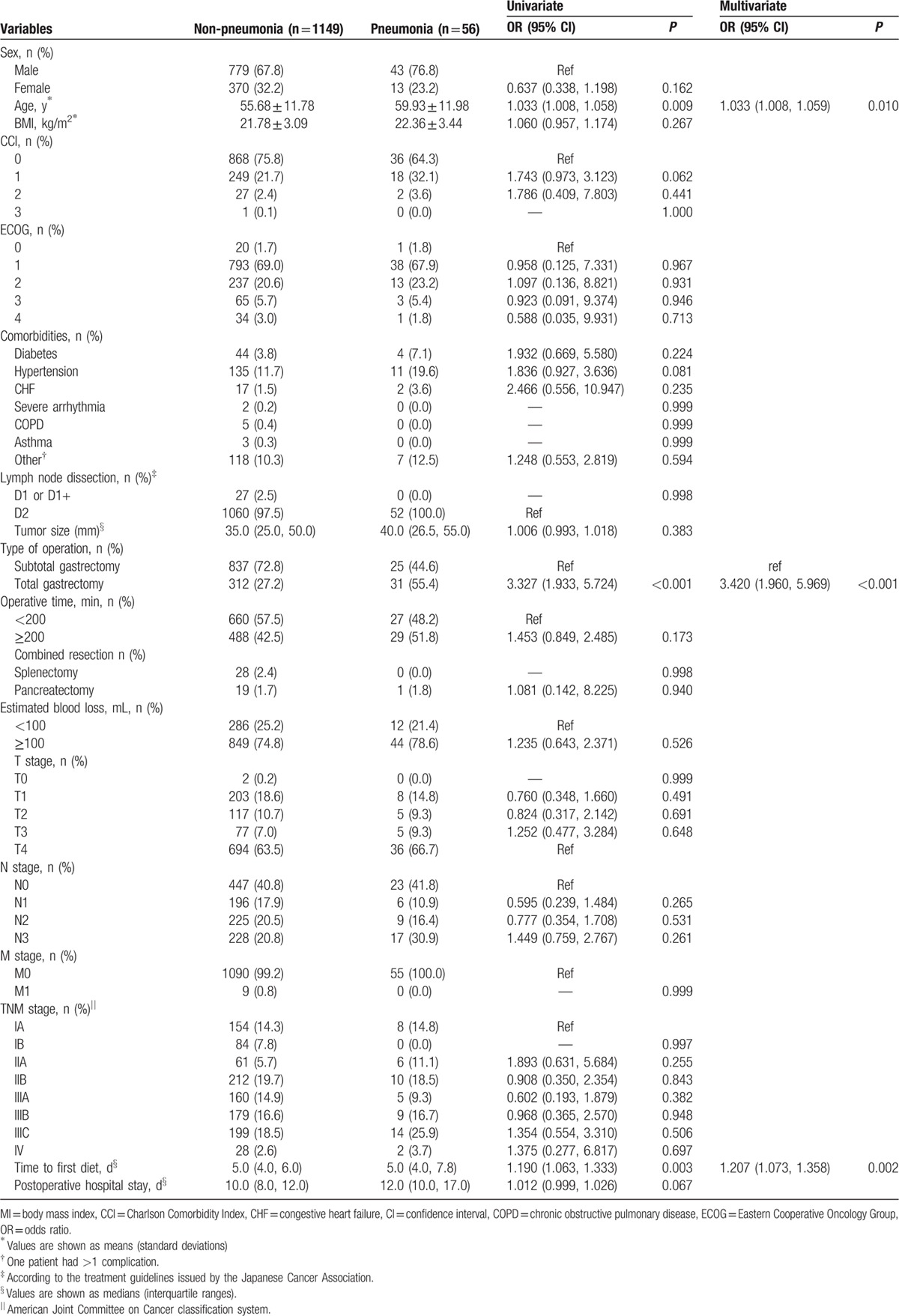
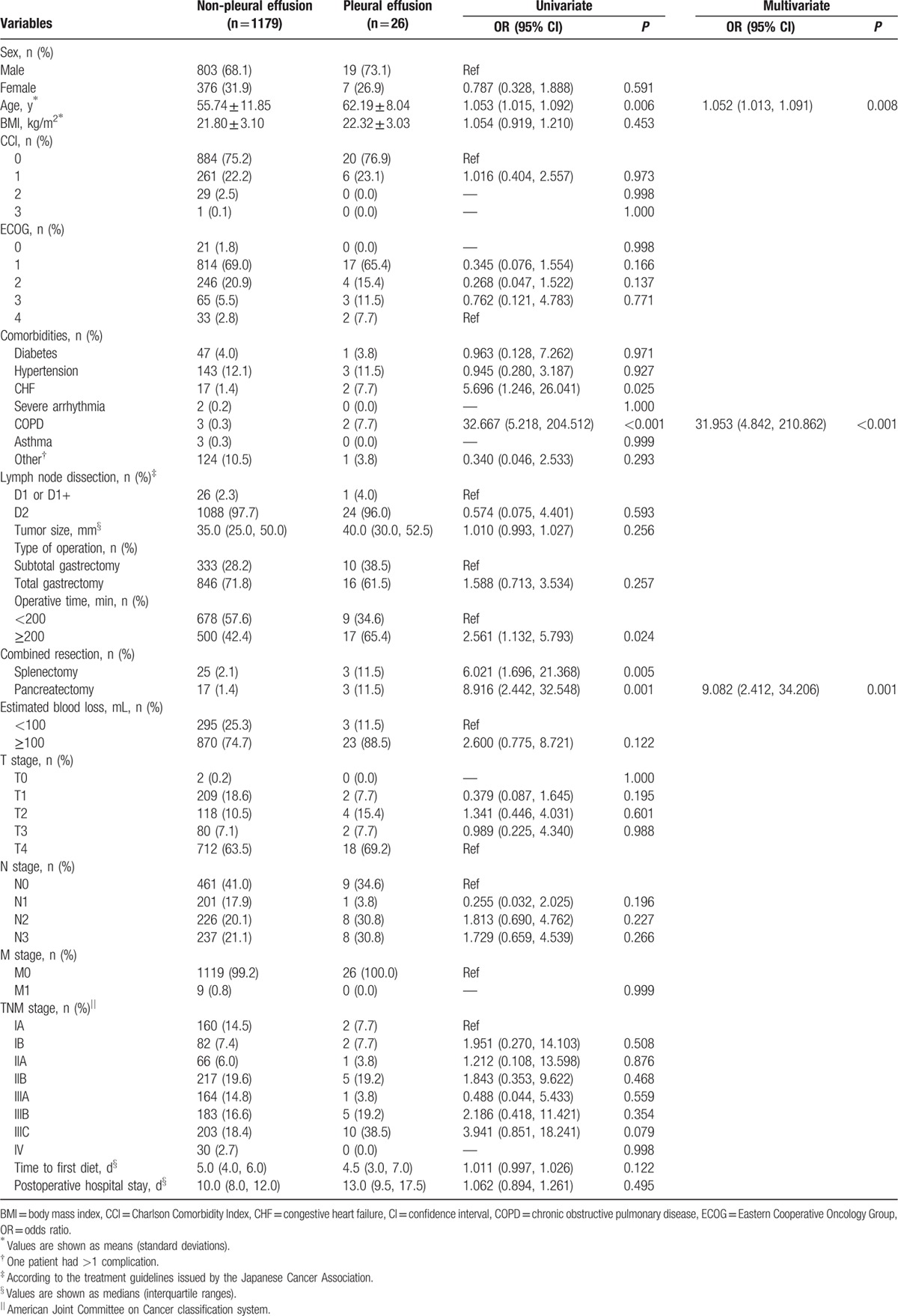
Table 7 summarizes the results of the univariate and multivariate analyses of risk factors for pleural effusion, which identified advanced age, preexisting CHF, preexisting COPD, prolonged surgery (>200 min), and combined resection (splenectomy and pancreatectomy) as risk factors. The predictive risk factors for pleural effusion identified in the multivariate logistic analysis included advanced age (OR = 1.052, 95% CI = 1.013%, 1.091%; P = 0.008), pre-existing COPD (OR = 31.953, 95% CI = 4.842%, 210.862%; P < 0.001), and additional pancreatectomy (OR = 9.082, 95% CI = 2.412%, 34.206%; P = 0.001).
Table 7.
Univariate and multivariate analyses of the risk factors for pleural effusion.
4. Discussion
In this study, we focused on PCCs in gastric cancer patients, and we identified several risk factors for PPCs following a laparoscopic gastrectomy, including advanced age, preexisting COPD, total gastrectomy, time to first oral diet, and length of hospital stay. Our study is important because we determined the incidence of PPCs (6.8%) and revealed the correlated risk factors for PPCs in gastric cancer patients following laparoscopic gastrectomy. Some of the risk factors that we identified are preventable. PPCs increase morbidity and mortality. [13] Multiple risk factors have been closely correlated with the development and incidence of PPCs. [14]
In our retrospective study, we emphasized the definition of each PCC in gastric cancer patients who underwent laparoscopic surgery and categorized the different complications to assess the risk factors. We also demonstrated that significant predictors vary among types of complications. Many types of PCCs have been correlated with a longer postoperative hospital stay. Therefore, the prevention of PPCs following laparoscopic gastrectomy for gastric cancer may decrease both the morbidity rate and healthcare costs.
Several studies in the literature have evaluated the incidence of morbidity and mortality or postoperative outcomes following gastric cancer surgery in general and have compared open and laparoscopic approaches, with all complications included in these analyses. Although these studies analyzed specific postoperative outcomes, [15 16 17 18] to our knowledge, no previous study has specifically examined the incidence of PCCs and the risk factors associated with laparoscopic gastrectomy surgery for gastric cancer. These critical gaps suggest the need for a specific analysis focused on laparoscopic gastrectomy with respect to PPCs because the risk factors cannot be determined based on other studies that have focused on other types of upper abdominal surgery.[ 6 19 20] Moreover, significant heterogeneity is present in the existing literature regarding the definition of PPCs. Therefore, our study identified the risk factors for PPCs, including infection (pneumonia), abnormal complications (pleural effusion), and thromboembolic complications (pulmonary embolism). We also investigated various preoperative, intraoperative, and postoperative risk factors that may lead to the development of PPCs.
Advanced age was identified as a risk factor for PPCs in previous studies. [21 22 23] The results of our study revealed advanced age as a predictive risk factor related to PCCs and morbidity following laparoscopic gastrectomy. This finding is similar to and corroborates previous studies that have found increased age to be a risk factor for the development of PCCs.[ 24 25] However, in our study, the probability of developing PCCs was high in older individuals (60 years) and in individuals with preexisting cardiopulmonary disease. The factors that might influence and lead to poorer outcomes in some older groups of individuals may be correlated with the presence and incidence of COPD comorbidity and significant postoperative complications in this group of patients.
Patients with COPD have a greater risk of developing atelectasis, pneumonia, bronchitis, and fever during the postoperative period. [26] Several studies have reported that comorbidities, such as COPD, increase the risk of perioperative PCCs.[ 20 27] In our study, the multivariate analysis identified preexisting COPD as a risk factor associated with an increased prevalence of PPCs. This finding is consistent with previous studies.[ 28 29] Sex, ECOG performance status, and the CCI measurements did not affect the development of PPCs. Among other patient characteristics, BMI was not a risk factor, which is inconsistent with the existing literature.[ 20 30] Mitigation strategies should focus on the refinement of patient selection and the improvement of outcomes in patients with a preexisting cardiopulmonary status in this group of high-risk patients.
The results of our study also indicate that a longer operative time and the type of operative procedure were predictive risk factors for the development of PCCs. Both factors might lead to patients developing significant morbidity and undergoing longer hospitalization. In addition, both factors might have a strong relationship with tumor stage or other complications, such as bleeding. We also analyzed the TNM stage of the tumor to determine whether these factors increase the risk of PCCs, and we found no correlations. We aimed to determine whether a prolonged operative time would increase the risk of PPC development. It is notable that surgeries lasting >3 hours are correlated with a likelihood of PPCs,[ 31 32] and the results of our study showed that this is a risk factor. These findings are consistent with the existing literature, which identifies prolonged surgery as a risk factor for PPCs.[ 31 32] We believe this might be because of the complexity of the surgical procedure, tumor size, and complex anatomy of the abdomen.
Our study found that patients with gastric cancer, particularly those who underwent laparoscopic total gastrectomy, had more risk factors for developing PPCs and a longer operative time than those who underwent a subtotal gastrectomy. This finding is similar to the results reported by Papenfuss et al, [33] who showed that a total gastrectomy carries significant morbidity and is related to a long operative time. In addition to the magnitude of the operation itself, resection around the esophagus might be a risk factor for PPCs. However, we did not identify a difference between laparoscopic and laparotomy procedures because that was not the intent of our study, and all patients in this study underwent laparoscopic surgery. Studies have not indicated a consensus regarding the advantages of laparoscopic approaches in terms of PPCs. Laparoscopic approaches have several advantages, including less operative pain, faster recovery, reduction in direct trauma to the respiratory muscles, and better short-term quality of life than open gastrectomy[ 34 35] ; however, preoperative PCCs have been shown to be related to increased pneumoperitoneum, which significantly affects respiratory pressure and pulmonary resistance.[ 14 36 37 38] In our study, the univariate analysis indicated that patients who underwent a laparoscopic gastrectomy with an additional procedure had an increased risk of PPCs, and this finding is supported by previous studies.[ 39 40] Attention should be paid to the surgical technique and patients who require additional resections because it is known that these are significant risk factors that could negatively influence patient management, particularly for those who require chemotherapy. We should address these issues to minimize their potential negative impact on patients.
Importantly, we found that the time to first diet was a predictive risk factor for PPC development. It has been noted that patients who undergo laparoscopic gastrectomy surgery have nutritional difficulties. Therefore, attention should focus on optimizing the nutritional status of these patients both before and after surgery.
We examined the length of the hospital stay as an independent risk factor, and the multivariate analysis indicated that it was a risk factor for PPCs. In addition, in both groups, a prolonged length of hospital stay after surgery was a significant predictor of PPC development, which was consistent with our cohort study. A prolonged hospital stay may occur because of differences in the healthcare hospital culture or other influential factors.
Data suggest that the immobilization of patients following surgery has an influence on and may lead to PPC development and may contribute to thromboembolism, as was also reported by Brooks et al. [6] A pulmonary embolism occurred in 1 patient in our study; however, we did not analyze that patient because the number of patients in this study was small, and it was statistically impossible to compare 1 patient with the entire cohort.
This study has several limitations that must be acknowledged. First, it is a single-center study. Therefore, the results may be biased by a lack of data on important variables, such as the American Society of Anesthesiologist classification of physical health, nasogastric tube decompression related to PPCs, and nutritional status. Another important limitation is that we were unable to assess perioperative pulmonary function in COPD patients to investigate how this affected respiratory capacity; simple pulmonary spirometry analysis alone may be sufficient to avoid this complication. The impact of this factor is not measureable and could be controlled because of the retrospective nature of the study. Only randomized clinical trials may provide more definitive evidence.
When considering the laparoscopic gastrectomy procedure, patients with gastric cancer should carefully weigh the benefits and risks of the various treatment options. Papenfuss et al [33] reported that the incidence of morbidity was significantly increased in patients who underwent a total gastrectomy compared to patients who underwent a subtotal gastrectomy. We cannot report the relative advantages of different laparoscopic procedures. However, we will conduct a study that will provide information regarding the relative advantages of different surgical methods with regard to fewer complications, quality of life, and patient satisfaction.
Our study findings may not be generalizable outside of our institution. Regardless, the results reported in this study may serve as a useful benchmark for future studies.
In conclusion, we have identified the risk factors and the frequency of PPCs in patients with gastric cancer following laparoscopic gastrectomy surgery, which is a relatively common procedure. Advanced age, preexisting COPD, total gastric resection, time to first diet, and postoperative hospital stay are factors associated with a high risk of PPCs. Attention should be paid to these patients, and strategies should be developed to improve and reduce the incidence of PPCs.
Footnotes
Abbreviations: BMI = body mass index, CCI = Charlson Comorbidity Index, COPD = chronic obstructive pulmonary disease, ECOG = Easter Cooperative Oncology performance status, GC = gastric cancer, PPC = postoperative pulmonary complication, TNM = tumor node metastasis, UICC = Union for International Cancer Control.
The authors report no conflicts of interest.
This work was supported by the Guangdong Provincial Science and Technology Key Project (No.2014A020215014), the Research Fund of Public Welfare in the Health Industry, the National Health and Family Planning Commission of China (No. 201402015), and the Key Clinical Specialty Discipline Construction Program.
RN, HL, and LZ contributed equally to this work and should be considered as co-first authors.
References
- 1. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 3. Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc 2009; 23:1759–1763. [DOI] [PubMed] [Google Scholar]
- 4. Arozullah AM, Khuri SF, Henderson WG, et al. Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001; 135:847–857. [DOI] [PubMed] [Google Scholar]
- 5. Kanat F, Golcuk A, Teke T, et al. Risk factors for postoperative pulmonary complications in upper abdominal surgery. ANZ J Surg 2007; 77:135–141. [DOI] [PubMed] [Google Scholar]
- 6. Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest 1997; 111:564–571. [DOI] [PubMed] [Google Scholar]
- 7. Sah BK, Zhu ZG, Chen MM, et al. Gastric cancer surgery and its hazards: post operative infection is the most important complication. Hepato Gastroenterol 2008; 55:2259–2263. [PubMed] [Google Scholar]
- 8. Sano T, Kodera Y. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112. [DOI] [PubMed] [Google Scholar]
- 9. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. Hoboken: Wiley-Blackwell; 2009. [Google Scholar]
- 10. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pellegrino R, Viegi G, Brusasco V. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–968. [DOI] [PubMed] [Google Scholar]
- 12. Celli BR, MacNee W, Agusti A. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23:932–946. [DOI] [PubMed] [Google Scholar]
- 13. Sabaté S, Mazo V, Canet J. Predicting postoperative pulmonary complications: implications for outcomes and costs. Curr Opin Anaesthesiol 2014; 27:201–209. [DOI] [PubMed] [Google Scholar]
- 14. Kocabas A, Kara K, Ozgur G, et al. Value of preoperative spirometry to predict postoperative pulmonary complications. Respir Med 1996; 90:25–33. [DOI] [PubMed] [Google Scholar]
- 15. Lee JH, Park do J, Kim HH, et al. Comparison of complications after laparoscopy-assisted distal gastrectomy and open distal gastrectomy for gastric cancer using the Clavien-Dindo classification. Surg Endosc 2012; 26:1287–1295. [DOI] [PubMed] [Google Scholar]
- 16. Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report–a phase III multicenter, prospective, randomized trial (KLASS Trial). Ann Surg 2010; 251:417–420. [DOI] [PubMed] [Google Scholar]
- 17. Lee JH, Son SY, Lee CM, et al. Morbidity and mortality after laparoscopic gastrectomy for advanced gastric cancer: results of a phase II clinical trial. Surg Endosc 2013; 27:2877–2885. [DOI] [PubMed] [Google Scholar]
- 18. Lee SW, Nomura E, Bouras G, et al. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg 2010; 211:33–40. [DOI] [PubMed] [Google Scholar]
- 19. Barisione G, Rovida S, Gazzaniga GM, et al. Upper abdominal surgery: does a lung function test exist to predict early severe postoperative respiratory complications? Eur Respir J 1997; 10:1301–1308. [DOI] [PubMed] [Google Scholar]
- 20. Smetana GW, Lawrence VA, Cornell JE. American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:581–595. [DOI] [PubMed] [Google Scholar]
- 21. Gretschel S, Estevez-Schwarz L, Hünerbein M, et al. Gastric cancer surgery in elderly patients. World J Surg 2006; 30:1468–1474. [DOI] [PubMed] [Google Scholar]
- 22. Park DJ, Lee HJ, Kim HH, et al. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg 2005; 92:1099–1102.doi: 10.1002/bjs.4952. [DOI] [PubMed] [Google Scholar]
- 23. Persiani R, Antonacci V, Biondi A, et al. Determinants of surgical morbidity in gastric cancer treatment. J Am Coll Surg 2008; 207:13–19. [DOI] [PubMed] [Google Scholar]
- 24. Hall JC, Tarala RA, Hall JL, et al. A multivariate analysis of the risk of pulmonary complications after laparotomy. Chest 1991; 99:923–927. [DOI] [PubMed] [Google Scholar]
- 25. Lee KG, Lee HJ, Yang JY, et al. Risk factors associated with complication following gastrectomy for gastric cancer: retrospective analysis of prospectively collected data based on the Clavien-Dindo system. J Gastrointest Surg 2014; 18:1269–1277. [DOI] [PubMed] [Google Scholar]
- 26. Kroenke K, Lawrence VA, Theroux JF, et al. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med 1992; 152:967–971. [PubMed] [Google Scholar]
- 27. Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med 2006; 144:575–580. [DOI] [PubMed] [Google Scholar]
- 28. Hsieh CH. Laparoscopic cholecystectomy for patients with chronic obstructive pulmonary disease. J Laparoendosc Adv Surg Tech A 2003; 13:5–9. [DOI] [PubMed] [Google Scholar]
- 29. Wittgen CM, Naunheim KS, Andrus CH, et al. Preoperative pulmonary function evaluation for laparoscopic cholecystectomy. Arch Surg 1993; 128:880–886. [DOI] [PubMed] [Google Scholar]
- 30. McAlister FA, Bertsch K, Man J, et al. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med 2005; 171:514–517. [DOI] [PubMed] [Google Scholar]
- 31. Christensen EF, Schultz P, Jensen OV, et al. Postoperative pulmonary complications and lung function in high-risk patients: a comparison of three physiotherapy regimens after upper abdominal surgery in general anesthesia. Acta Anaesthesiol Scand 1991; 35:97–104. [DOI] [PubMed] [Google Scholar]
- 32. Westbrook PR, Stubbs SE, Sessler AD, et al. Effects of anesthesia and muscle paralysis on respiratory mechanics in normal man. J Appl Physiol 1973; 34:81–86. [DOI] [PubMed] [Google Scholar]
- 33. Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol 2014; 21:3008–3014. [DOI] [PubMed] [Google Scholar]
- 34. Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 2008; 248:721–727. [DOI] [PubMed] [Google Scholar]
- 35. Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005; 19:168–173. [DOI] [PubMed] [Google Scholar]
- 36. Lawrence VA, Cornell JE, Smetana GW. American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med 2006; 144:596–608. [DOI] [PubMed] [Google Scholar]
- 37. Mitchell CK, Smoger SH, Pfeifer MP, et al. Multivariate analysis of factors associated with postoperative pulmonary complications following general elective surgery. Arch Surg 1998; 133:194–198. [DOI] [PubMed] [Google Scholar]
- 38. Canet J, Mazo V. Postoperative pulmonary complications. Minerva Anestesiol 2010; 76:138–143. [PubMed] [Google Scholar]
- 39. Martin RC, Jaques DP, Brennan MF, et al. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg 2002; 236:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ozer I, Bostanci EB, Orug T, et al. Surgical outcomes and survival after multiorgan resection for locally advanced gastric cancer. Am J Surg 2009; 198:25–30. [DOI] [PubMed] [Google Scholar]


