Abstract
Background:
Albumin-bound paclitaxel (nab-paclitaxel, nab-PTX) plus gemcitabine (GEM) combination has demonstrated efficient antitumour activity and statistically significant overall survival of patients with metastatic pancreatic ductal adenocarcinoma (PDAC) compared with GEM monotherapy. This regimen is currently approved as a standard of care treatment option for patients with metastatic PDAC. It is unclear whether cremophor-based PTX combined with GEM provide a similar level of therapeutic efficacy in PDAC.
Methods:
We comprehensively explored the antitumour efficacy, effect on metastatic dissemination, tumour stroma and survival advantage following GEM, PTX and nab-PTX as monotherapy or in combination with GEM in a locally advanced, and a highly metastatic orthotopic model of human PDAC.
Results:
Nab-PTX treatment resulted in significantly higher paclitaxel tumour plasma ratio (1.98-fold), robust stromal depletion, antitumour efficacy (3.79-fold) and survival benefit compared with PTX treatment. PTX plus GEM treatment showed no survival gain over GEM monotherapy. However, nab-PTX in combination with GEM decreased primary tumour burden, metastatic dissemination and significantly increased median survival of animals compared with either agents alone. These therapeutic effects were accompanied by depletion of dense fibrotic tumour stroma and decreased proliferation of carcinoma cells. Notably, nab-PTX monotherapy was equivalent to nab-PTX plus GEM in providing survival advantage to mice in a highly aggressive metastatic PDAC model, indicating that nab-PTX could potentially stop the progression of late-stage pancreatic cancer.
Conclusions:
Our data confirmed that therapeutic efficacy of PTX and nab-PTX vary widely, and the contention that these agents elicit similar antitumour response was not supported. The addition of PTX to GEM showed no survival advantage, concluding that a clinical combination of PTX and GEM may unlikely to provide significant survival advantage over GEM monotherapy and may not be a viable alternative to the current standard-of-care nab-PTX plus GEM regimen for the treatment of PDAC patients.
Keywords: Nab-paclitaxel, paclitaxel, pancreatic cancer, gemcitabine, desmoplastic stroma, mouse models of human pancreatic cancer, combination chemotherapy
Pancreatic ductal adenocarcinoma (PDAC) is well known for its aggressive clinical course and remains as one of the most deadly malignancies in the world with a 5-year mortality of 95% (Hidalgo, 2010; Oettle, 2014). The unusually poor prognosis of the disease is due to the aggressively invasive and metastatic nature of these tumours, and their being impervious to chemotherapy and radiotherapy. A constant hallmark of PDAC is the presence of a dense desmoplastic reaction that consists largely of fibroblasts, myofibroblasts and extracellular matrix proteins including fibronectin and collagens (Maitra and Hruban, 2008). The stromal compartment is conscripted to provide protection to carcinoma cells and contributes to cancer progression and therapeutic resistance (Ijichi et al, 2011; Kadaba et al, 2013; Whatcott et al, 2013). Gemcitabine (GEM; Gemzar) has been established as the standard first-line chemotherapeutic drug for the treatment of patients with advanced PDAC (Burris et al, 1997). Despite laboratory evidence of robust combination activity, numerous phase III trials of GEM in combination with different cytotoxic or molecularly targeted agents have resulted in no substantial clinical improvement over the use of GEM alone (Van Cutsem et al, 2009; Colucci et al, 2010; Philip et al, 2010). Combinations focused to the tumour cell compartment have not proven clinically impactful, perhaps reflecting the invulnerability of this highly KRAS-mutated tumour to signaling inhibitors. This has led to an increased focus on targeting the desmoplastic stroma to improve clinical outcomes of patients with this deadly disease (Beatty et al, 2011; Provenzano et al, 2012; Kadaba et al, 2013).
We previously performed pilot studies in which several advanced PDAC patients were successfully treated on the basis of robust anticancer response in the xenografts (patient-derived xenografts; PDXs) derived from the resected primary tumours of those patients (Hidalgo et al, 2011; Villarroel et al, 2011). Preclinical and early clinical studies conducted by us confirmed that albumin-bound cremophor-free formulation of Paclitaxel (nab-PTX, Abraxane) exerts substantial antitumour activity in PDAC (Maitra et al, 2009; Von Hoff et al, 2011). Our previous studies, conducted in mice with established PDXs (subcutaneous) derived from 11 individual PDAC patients, confirmed that nab-PTX treatment in combination with GEM provided a high antitumour efficacy compared with GEM alone (Maitra et al, 2009; Von Hoff et al, 2011). This synergy was later confirmed by another group of researchers, utilising a genetically engineered mouse model of PDAC (Frese et al, 2012). Positive findings from these studies later led to a large randomised phase III clinical trial (N=861 metastatic PDAC patients) showed that nab-PTX plus GEM was superior in efficacy to GEM alone in terms of progression-free survival and overall survival in the treatment of metastatic PDAC (Von Hoff et al, 2013; Goldstein et al, 2015). In addition, our studies showed that one of the mechanisms by which nab-PTX works in PDAC is by eliminating the extensive desmoplastic reaction, which is considered as a key hallmark of PDAC and barrier to drug diffusion and treatment efficacy (Von Hoff et al, 2011; Xie and Xie, 2015).
Cremophor EL (CreEL)-based Paclitaxel (PTX, Taxol) is a key chemotherapy component for the treatment of several human malignancies (Von Hoff, 1997). Despite the clinical benefit achieved with solvent-based taxanes, the treatment often produce significant side effects (Gradishar et al, 2009). Nab-PTX is a cremophor-free and water-soluble albumin-based formulation of PTX consisting of 130nm albumin-paclitaxel nanoparticles. Nab-PTX treatment showed higher response rates and improved tolerability compared with solvent-based formulations in patients with advanced metastatic breast cancer and non-small cell-lung cancer (Montana et al, 2011; Viudez et al, 2014). Clinical experience with solvent-based traditional taxanes such as PTX and docetaxel in PDAC patients were disappointing (Saif et al, 2010).
Nab-PTX plus GEM combination is currently being implemented in national and international guidelines as a standard of care treatment option for patients with metastatic PDAC. It is unclear whether PTX when combined with GEM provide similar therapeutic efficacy in PDAC. Since PTX is widely available and the treatment cost of nab-PTX is relatively higher than PTX, clinicians, third party payers and regulatory agencies have a substantial interest in understanding whether these drugs share a similar level of pharmacological activities in PDAC.
In the present study, we utilised orthotopic models of human PDAC, which were shown to better recapitulate the histologic and metastatic characteristics of disease than the subcutaneous cell line xenograft models (Hoffman, 2015), and directly compared the anticancer activity, effect on tumour stroma modulation, metastatic spreading to distant organs and survival following GEM, PTX, nab-PTX and combinations of GEM plus PTX or nab-PTX. To our knowledge, this is the first study which used a large number of mice (300) with established orthotopic tumours, and comprehensively explored the therapeutic efficacy of PTX and nab-PTX alone and in combination with GEM in human PDAC models.
Materials and methods
Establishment of orthotopic PDX models of pancreatic cancer
Eight- to ten-week-old male athymic (nu/nu) nude mice (Harlan) were used for the study. Animals were maintained in a sterile environment and had access to autoclaved laboratory rodent diet and water ad libitum. All animal experiments were conducted following approval and in accordance with the Animal Care and Use Committee guidelines of the Johns Hopkins University, which is in line with guidelines for the welfare and use of animals in cancer research (Workman et al, 2010). Two human pancreatic cancer xenografts (Panc185 and Panc265) from the ‘PancXenoBank', a collection of patient-derived human pancreatic cancer xenografts established from the resected primary tumours of patients with PDAC, were used for the present study (Rubio-Viqueira et al, 2006). We used Panc185 tumour as a representative model of locally advanced primary PDAC and Panc265 tumour as a representative model of highly aggressive and metastatic PDAC. Briefly, subcutaneously maintained Panc185 and Panc265 tumours in mice were resected aseptically at the exponential growth phase and used as the source of tumours for surgical orthotopic implantation into the pancreas. Tumours were minced with a sterile razor and cut into cubes of ∼1.5 mm3. Mice were anaesthetised using isoflurane inhalation. A 1-cm left lateral abdominal incision was made on the splenic silhouette, without injury to underlying organs, with a sterile microscissor. The pancreas was identified and laterally externalised. A small cut was made at the middle of pancreas and a 1.5-mm3 tumour fragment, dipped in Matrigel (BD Biosciences, Bedford, MA, USA), was immediately implanted into the middle of the pancreas and sutured using 3-0 surgical sutures. The pancreas was returned into the peritoneal cavity. The abdominal wall and skin were sutured in two layers using 3-0 surgical sutures (Kim et al, 2009). Orthotopic tumour establishment was assessed initially by transabdominal palpations, confirmed by an ultrasound scan (Vevo 660 VisualSonics, Toronto, ON, Canada).
Drugs
Gemcitabine (Eli Lilly, Indianapolis, IN, USA) and cremophor-based paclitaxel (Bristol-Myers Squibb Company, Princeton, NJ, USA) were purchased from the Johns Hopkins Hospital Pharmacy (Baltimore, MD, USA). Nab-paclitaxel (Abraxis BioScience, LLC, a wholly owned subsidiary of Celgene Corporation) was supplied by Celgene Corporation (Summit, NJ, USA). All three drugs were reconstituted separately in normal saline, prepared fresh daily as required and administered to tumour harbouring mice within 1 h of preparation.
Evaluation of the in vivo efficacy of drug regimens in orthotopic PDAC harbouring mice
Mice were orthotopically implanted with Panc185 and Panc265 tumours. Engrafted tumours were allowed to grow over a period of 2–7 weeks (Panc265 and Panc185, respectively). In order to confirm orthotopic tumour growth in mouse pancreas, determine the weight of primary tumours and any metastatic dissemination (baseline), five animals in Panc185 and Panc265 were randomly selected and exploratory laparotomy was performed. Mice were screened visually for metastatic lesions in the spleen, liver, lungs, kidneys, lymph nodes and peritoneum using a × 2.5 lens. Primary tumour was excised from pancreas, weighed and measured using a digital caliper. Spleen, liver, lungs, kidneys, lymph nodes were excised. Tumours and tissues were formalin-fixed, paraffin-embedded, sectioned and were used for haematoxylin and eosin (H&E) staining.
In order to determine the efficacy of treatment regimens, mice with demonstrable primary tumours (200–300 mm3) were selected and randomly allocated into following arms: (1) Control (untreated); (2) GEM (100 mg kg−g, i.p. twice weekly for 4 weeks); (3) PTX (13.4 mg kg−1, i.v, once daily for 5 consecutive days, only on first week); (4) nab-PTX (22.3 mg kg−1, i.v, once daily for 5 consecutive days, only on first week); (5) PTX and GEM at the above-mentioned dose and schedule; (6) nab-PTX plus GEM at the above-mentioned dose and schedule (N=8–10 mice/group). Drug doses were selected based on previous studies (Desai et al, 2006; Rajeshkumar et al, 2011). Mice were evaluated for signs of discomfort or morbidity. Tumour growth was monitored by periodic transabdominal palpations. Animals were killed on day 28. Upon autopsy, mice were screened visually for metastatic lesions in the spleen, liver, lungs, kidneys, lymph nodes and peritoneum using a × 2.5 lens. After excision, the suspected metastatic lesions in organs were fixed in 10% buffered formalin, paraffin-embedded, sectioned and stained with H&E. Visible macroscopic metastatic lesions verified histologically were counted towards metastatic incidence. Primary tumours were excised from the pancreas, weighed and measured using a digital caliper. Tumour volume was calculated using the following formula: tumour volume=(length × width2)/2. Tumour pieces fixed in 10% buffered formalin were used for histological and immunohistochemical analysis.
Treatment effect on desmoplastic stroma, tumour cell proliferation and angiogenesis
We performed H&E staining on formalin-fixed, paraffin-embedded primary tumours and tissues (spleen, liver, lungs, kidneys and lymph nodes) harvested at the termination of experiment one (day 28). Tumours and tissues were sectioned (5μm) and de-paraffinised. H&E staining was conducted using standard procedures. Evaluable tumours from four to five separate animals were used for assessing the intratumour vascularity, tumour cell proliferation and stromal desmoplasia. Intratumour vascularity and tumour cell proliferation were assessed using anti-CD31antibody (Cat # DIA 310, Dianova GmbH, Hamburg, Germany) and Ki-67 staining (Cat # 790-4286, Ventana Medical Systems Inc., Tucson, AZ, USA), respectively, as previously described (Yabuuchi et al, 2013). Masson's trichrome (Rajeshkumar et al, 2015) and collagen IV staining (Cat # M0785, Dako, Carpinteria, CA, USA) were used to assess the extent of stromal desmoplasia. After staining, sections were observed under a light microscope and photographed using a digital camera.
Evaluation of the survival benefit of drug regimens in orthotopic PDAC harbouring mice
A separate study was conducted for assessing the survival advantage of treatment regimens. Mice with established orthotopic Panc185 and Panc265 tumours (200–300 mm3) were randomly allocated to six groups and treated with drug doses and schedule as mentioned in the in vivo efficacy study design (N=10 mice/group). Animals were monitored twice daily. Survival end points were reached and recorded when mice began to appear moribund with cachexia, abdominal distension, showed signs of hunched posture, hindlimb paralysis or laboured breathing, whichever occurred first. Mice were humanely killed by standard CO2 asphyxiation. Median survival was estimated and compared using Kaplan–Meier method.
Determination of the plasma and intratumour concentration of paclitaxel
Mice harbouring established Panc265 orthotopic tumours (∼200–300 mm3) were either untreated or treated with PTX (13.4 mg kg−1) or nab-PTX (22.3 mg kg−1), i.v, once daily for five consecutive days (N=9 mice per group). On day 5, blood and tumour samples were collected 2 h after the dosing of nab-PTX or PTX. Paclitaxel concentration was analysed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described elsewhere (Zhang et al, 2013). For the calibration curve, an aliquot of paclitaxel stock solution (0.2 mg ml−1 in acetonitrile) was spiked into plasma to make the initial plasma stock at 4000 ng ml−1, which was then diluted to make calibration standard samples at concentrations ranging from 2 to 4000 ng ml−1 in plasma. Quality control (QC) samples were made similarly with concentrations of low QC at 6 ng ml−1, medium QC at 100 ng ml−1 and high QC at 3200 ng ml−1. Dilution QC at 20 000 ng ml−1 was made by spiking the stock solution into blank plasma and then diluted 10-fold with blank plasma. Aliquots (50 μl) of plasma and tumour samples (including standards, blanks, QCs) were transferred into a 96-well plate. To each well, 150 μl of a solvent mixture of acetonitrile : methanol (9 : 1) containing 100 ng ml−1 of d5-paclitaxel (as the internal standard) was added. The plate was capped, vortex mixed and centrifuged. A 150 μl aliquot of the supernatant was transferred into a clean 96-well plate for LC-MS/MS analysis. Chromatographic separation was carried out using a Shimadzu LC20 system equipped with an Agilent Pursuit XRs 3 C18 column (100 × 2 mm). Mobile phases consisted of 5 mM ammonia acetate in water containing 0.1% formic acid (A) and 5 mM ammonia acetate in acetonitrol : water (9 : 1) mixture containing 0.1% formic acid (B), at a flow rate of 0.35 ml min−1. The column was eluted with a gradient of 25% B for 0.9 min, linearly increased to 100% B over 1.5 min and then maintained at 100% B for another 3.5 min. The LC elute was connected directly to a Sciex API4000 QTrap mass spectrometer (Ab Sciex, Framingham, MA, USA) equipped with an electrospray ionisation (ESI) ion source. The ratio of intratumoral vs plasma concentrations of paclitaxel was calculated and compared between nab-PTX and PTX treatment groups.
Evaluation of the acute effect of taxanes on tumour desmoplastic stroma
Mice harbouring established Panc185 and Panc265 orthotopic tumours (∼200–300 mm3) were either untreated or treated with PTX (13.4 mg kg−1) or nab-PTX (22.3 mg kg−1), i.v, once daily for five consecutive days (N=5 mice per group). Tumours were resected 24 h post the last dose of PTX or nab-PTX. Tumours were formalin-fixed, paraffin-embedded, sectioned and stained with Mason's Trichrome or Collagen IV. Sections were evaluated under a light microscope and photographed using a digital camera.
Statistical analysis
Data were analysed using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA). Primary tumour volumes were compared and analysed using Mann–Whitney U-test. Survival data were estimated and compared using Kaplan-Meier method and statistical significance was determined using Log-rank (Mantel-Cox) test. Differences and associations were considered statistically significant where P<0.05.
Results
Characteristics of orthotopic PDAC mouse models
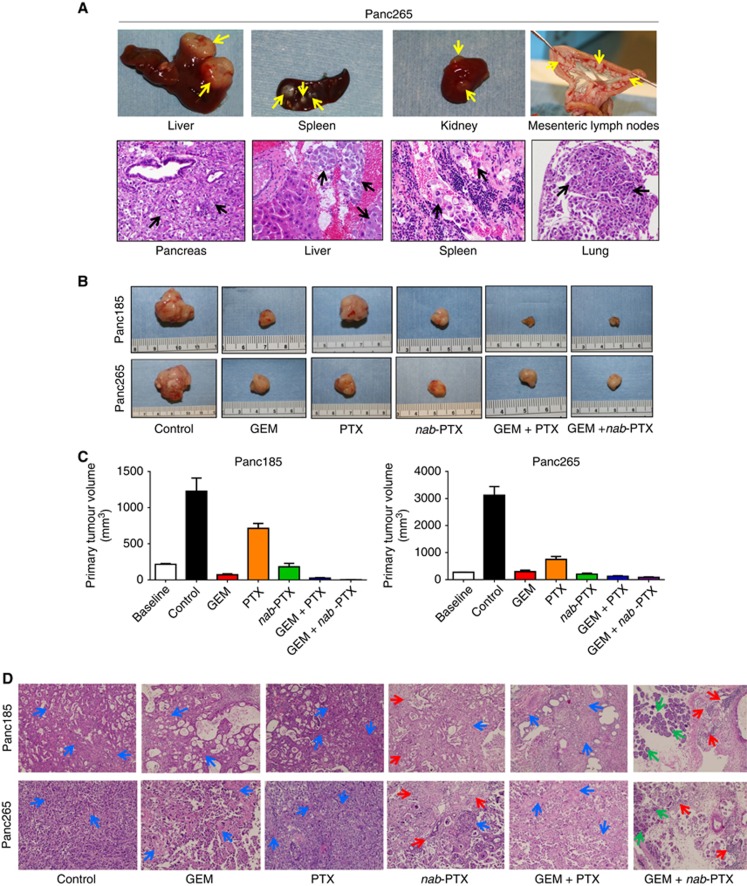
Upon orthotopic implantation in mouse pancreas, Panc185 tumours grew as locally advanced primary tumours, lacking metastatic dissemination to other organs. In contrast, Panc265 tumours showed aggressive tumour growth and metastatic spreading to liver, spleen, lungs, kidneys, lymph nodes and peritoneum (Figure 1A and B and Table 1). Cachexia was evident in control animals harbouring Panc265 tumours and their abdomens were enlarged due to tumour burden and ascites. There was a 11.47-fold increase in primary tumour volumes of control animals compared with baseline tumour volumes, indicating the highly aggressive phenotype of Panc265 tumours (Figure 1C and Supplementary Table S2). Both Panc185 and Panc265 tumours harbour activating mutations of KRAS oncogene and inactivating mutations in p53 tumour suppressor gene, the two most frequently found genetic alterations in human PDAC (Jones et al, 2008; Biankin et al, 2012; Rajeshkumar et al, 2015).
Figure 1.
Characteristics of orthotopically implanted PDAC tumours in mice and therapeutic efficacy of treatment regimens. Upon implantation in mouse pancreas, untreated Panc265 tumour showed more aggressive tumour growth and metastatic dissemination in mouse organs. (A) Photographs of resected liver, spleen and mesenteric lymph nodes from an untreated Panc265 tumour harbouring mouse showing prominent metastatic lesions in organs (upper panel). Yellow arrows point towards metastatic lesions. Representative photomicrographs (H&E, × 40) showing primary tumour growth in mouse pancreas, metastatic dissemination in liver, spleen and lungs. Black arrows point towards metastatic deposits in organs. (B) Gross morphology representative of the resected primary tumours at the termination of efficacy experiment (D28). Primary tumour volumes in different treatment regimens are shown in (C). Data represent mean primary tumour volumes ±s.e.m. N=9–10 mice per group in Panc185 and 8–10 mice per group in Panc265. Baseline mean tumour volumes (N=5) are shown on the left side of graphs. Statistical comparison of primary tumour volumes and P-values are shown in Supplementary Table S3. (D) Representative photomicrographs (H&E, × 20) of primary tumour sections. Histological evaluation of Panc185 and Panc265 tumours showed moderately differentiated and poorly differentiated pancreatic ductal adenocarcinoma, respectively. Blue arrows point towards desmoplastic stroma. Red arrows point towards areas of neutrophil infiltrate. Green arrows point towards normal pancreatic acinar cells.
Table 1. Treatment effect of regimens on metastatic dissemination to organs and peritoneum in the Panc265 PDAC model.
| Group | Spleen | Liver | Lungs | Kidneys | LN | Peritoneum |
|---|---|---|---|---|---|---|
| Vehicle | 10/10 | 4/10 | 1/10 | 5/10 | 10/10 | 8/10 |
| GEM | 9/10 | 1/10 | 0/10 | 0/10 | 6/10 | 3/10 |
| PTX | 10/10 | 0/10 | 0/10 | 0/10 | 3/10 | 1/10 |
| nab-PTX | 5/10 | 0/10 | 0/10 | 0/10 | 1/10 | 0/10 |
| GEM+PTX | 5/10 | 0/10 | 0/10 | 0/10 | 1/10 | 0/10 |
| GEM+nab-PTX | 3/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
Abbreviations: GEM=gemcitabine; LN=lymph nodes; nab-PTX=nab-paclitaxel; PTX=paclitaxel. N=8–10 mice/group.
Determination of the therapeutic efficacy of treatment regimens
We first determined the antitumour efficacy of each drugs alone and in combination with GEM. Mean primary tumour volumes, tumour weights and complete response of treatment groups are shown in Supplementary Tables S1 and S2. All treatment regimens tested in both Panc185 and Panc265 tumour models showed statistically significant reductions in primary tumour volume compared with control animals (Figure 1B and C and Supplementary Tables S1–S3). The primary tumour volumes increased substantially in the control and PTX-treated animals of Panc185 (P=0.0007 and 0.0007, respectively) compared with the baseline (pre-treatment) tumour volumes (Figure 1C and Supplementary Tables S1 and S3). GEM and nab-PTX monotherapy were highly effective in reducing the primary tumour volumes (P<0.0001) compared with the tumour volumes of control animals (Figure 1C and Supplementary Tables S1 and S3). Although the both combination treatments were remarkably effective in abolishing primary tumour growth, the rate of complete responses were higher in the GEM plus nab-PTX (89%) vs GEM plus PTX (22%) treatment, supporting the higher efficacy of GEM plus PTX regimen (Supplementary Table S1).
Baseline mean tumour volume of Panc265 tumours increased from 273 to 3123 mm3 (P=0.0007) and 751 mm3 (P=0.008) by day 28 in the control and PTX-treated groups, respectively (Figure 1C and Supplementary Tables S2 and S3). Treatment of the combination regimens resulted in statistically significant reductions in tumour size, but neither combination induced complete regressions (Supplementary Tables S2 and S3). GEM pus nab-PTX treatment produced statistically significant reduction in tumour volumes compared with GEM treatment (P=0.0004).
In both Panc185 and Panc265 tumour models, nab-PTX monotherapy resulted in primary tumour regression compared with baseline tumours (Figure 1C). Nab-PTX treatment was remarkably effective in blocking primary tumour progression compared with PTX monotherapy (3.944- and 3.645-fold; P<0.0001 and P=0.0001, respectively; Figure 1C and Supplementary Tables S1–S3). In both tumour models, one mouse each treated with nab-PTX achieved a complete response, whereas no complete responses were observed with PTX treatment (Supplementary Tables S1 and S2). Results of exploratory laparotomies conducted in mice before randomisation (baseline) confirmed the tumour growth confined only in pancreas.
Tumour histology
Histological evaluation of Panc185 and Panc265 tumours revealed moderately differentiated and poorly differentiated pancreatic ductal adenocarcinoma, respectively (Figure 1D). Tumours of control mice showed cribriform pattern of neoplastic cells admixed with necrotic debris, moderate to severe nuclear atypia, nuclear overcrowding, aberrant mitoses and abundant desmoplastic stroma (Figure 1D). While there was a reduction in neoplastic cells, desmoplastic stroma persisted in the tumours of animals treated with GEM or PTX. Depletion of desmoplastic stroma was clearly evident in the tumours of animals treated with nab-PTX alone and in combination with GEM. Tumours harvested from animals treated with nab-PTX alone and in combination with GEM displayed mild to moderate neutrophil infiltration in tumour microenvironment compared with control tumours and other treatment regimens. Nab-PTX alone and in combination with GEM treatment culminated in a sharp reduction of tumour cellularity and progressive loss of desmoplastic stroma. Islands of mouse pancreatic acinar cells were frequently noticed in the Panc185 and Panc265 tumours harvested from the animals administered with GEM plus nab-PTX (Figure 1D), indicating the therapy effectiveness of this regimen in destroying tumour cells and desmoplasia. H&E staining performed on the organs (spleen, liver, lungs, kidneys, lymph nodes) of mice killed prior to randomisation (baseline) did not show any metastatic infiltration (data not shown).
Effect of drug treatments on metastatic dissemination to organs and peritoneum
Panc265 tumours are highly metastatic when implanted orthotopically into mouse pancreas, showing metastatic dissemination in spleen, liver, lungs, kidneys, lymph nodes and peritoneum with frequencies ranging from 10 to 100% of animals (Figure 1A and Table 1). Treatment effects of agents alone and in combination with GEM on metastatic dissemination to distant organs and peritoneum are summarised in Table 1. All animals untreated or treated with PTX developed metastasis in at least one organ (Table 1). GEM treatment was ineffective in controlling metastatic spreading as evidenced by metastatic lesions in the spleen of nine out of ten (90%) mice (Table 1). However, nab-PTX prevented the metastatic spreading to spleen in 5 out of 10 mice (50%). Combined treatment of GEM and nab-PTX decreased metastatic tumour burden compared with either agent used alone (Table 1).
Impact of drug treatments on carcinoma cell proliferation, tumour vascularity and desmoplastic tumour stroma
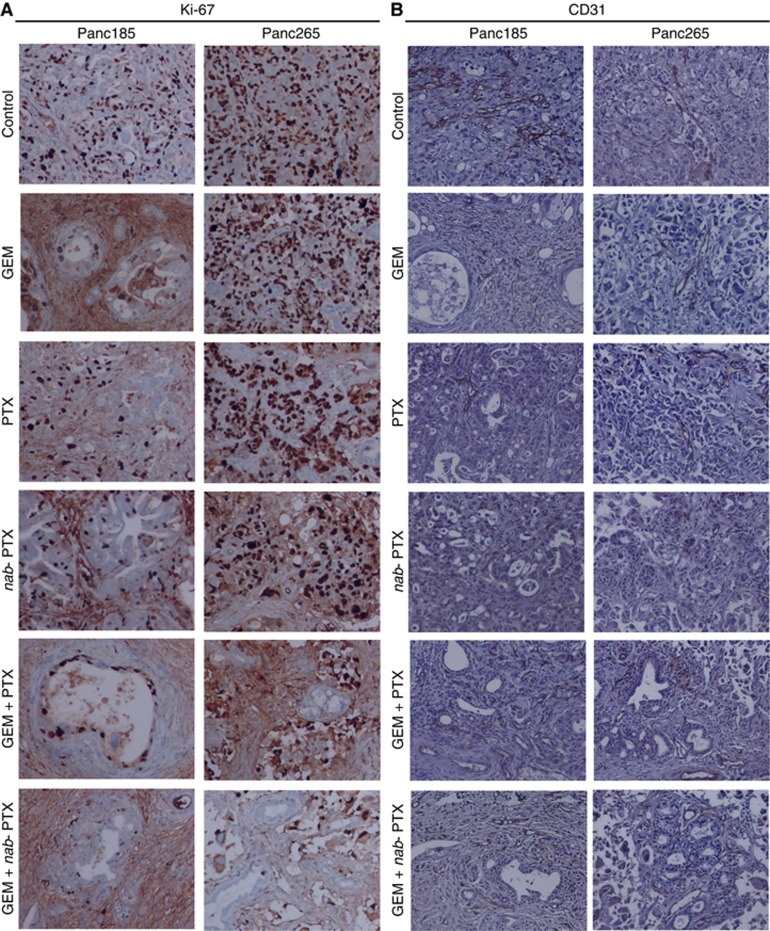
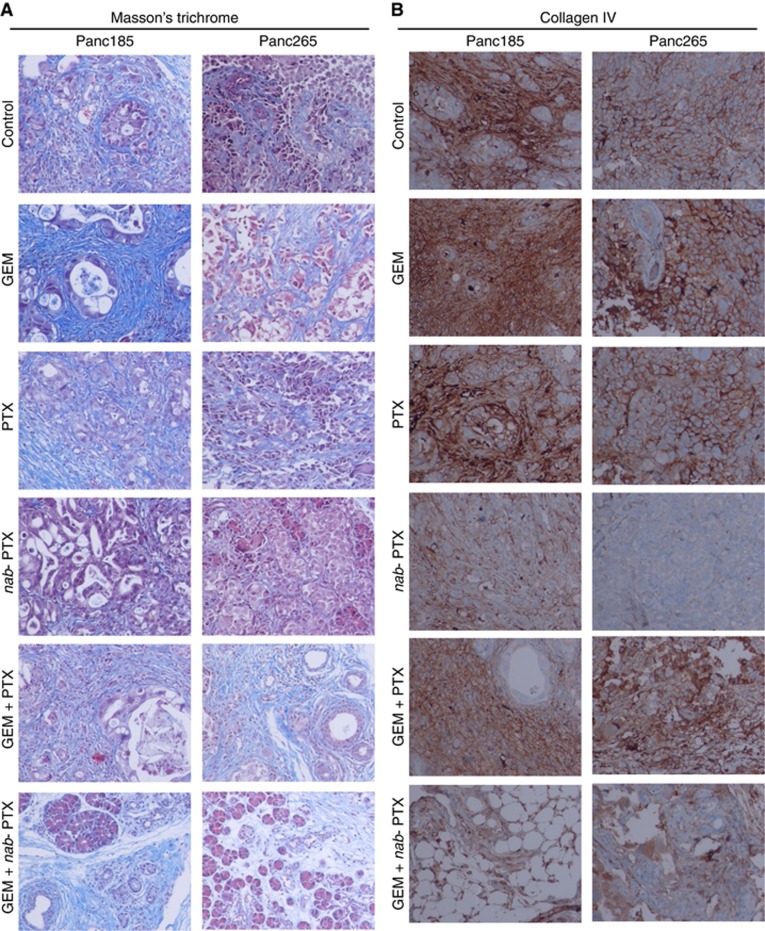
We determined the treatment effects of agents alone and in combination with GEM on tumour cell proliferation and desmoplastic stroma. Previous studies conducted by us revealed the presence of desmoplastic stroma in subcutaneously grown PDXs and metastatic lesions of human PDAC (Rajeshkumar et al, 2015; Whatcott et al, 2015). Tumours harvested from untreated animals of Panc265 PDX showed higher number of carcinoma cells expressing Ki-67, compared with tumours in Panc185, indicating the aggressiveness of Panc265 tumours (Figure 2A). The GEM plus nab-PTX combination effectively suppressed tumour cell proliferation in both Panc185 and Panc265 PDXs (Figure 2A). Comparable CD31-positive endothelial cell populations were noticed in the blood vessels of untreated and drug treated tumours, indicating that drug treatments did not alter the tumour vascularity (Figure 2B). Abundance of desmoplastic stroma, a hallmark of human PDAC, was evident in tumours harvested from untreated animals (Figure 3A and B). While GEM and PTX treatments were largely ineffective in reducing tumour stroma, nab-PTX monotherapy and in combination with GEM facilitated the destruction of dense tumour stroma (Figure 3A and B). These results are in agreement with our previous preclinical and clinical results (Maitra et al, 2009; Alvarez et al, 2013).
Figure 2.
Treatment effect of agents alone and in combinations on tumour cell proliferation and tumour vascularity. The nab-PTX plus GEM combination was highly effective in controlling tumour cell proliferation, as evidenced by reduced number of Ki-67-stained carcinoma cells. Tumours harvested from four to five mice of Panc185 and Panc265 on day 28 (first experiment) were processed and stained with Ki-67 or CD31 antibodies. Ki-67, a nuclear protein, which is expressed in the proliferating carcinoma cells is visualised as dark brown. There was no difference in various sized blood vessel density in tumours harvested from drug-treated animals compared with tumours in the untreated animals. CD31-stained endothelial cells in blood vessels are visualised as brown stain. (A, B) Representative photomicrographs of Ki-67 or CD31 stained tumour sections (× 20).
Figure 3.
Treatment effect of agents alone and in combinations on desmoplastic tumour stroma. Nab-PTX monotherapy or combined with GEM robustly depleted the dense tumour stroma as evidenced by less number of collagen fibres in the primary tumours. We performed immunohistochemistry on primary tumours harvested from untreated or treated with GEM, PTX, nab-PTX and combinations of GEM plus PTX or GEM plus nab-PTX (D28 samples, first experiment). The fibrotic tissue is visualised as blue (Mason's trichrome) or brown stain (Collagen IV). (A, B) Representative photomicrographs of Mason's trichrome and Collagen IV stained tumour sections (× 20).
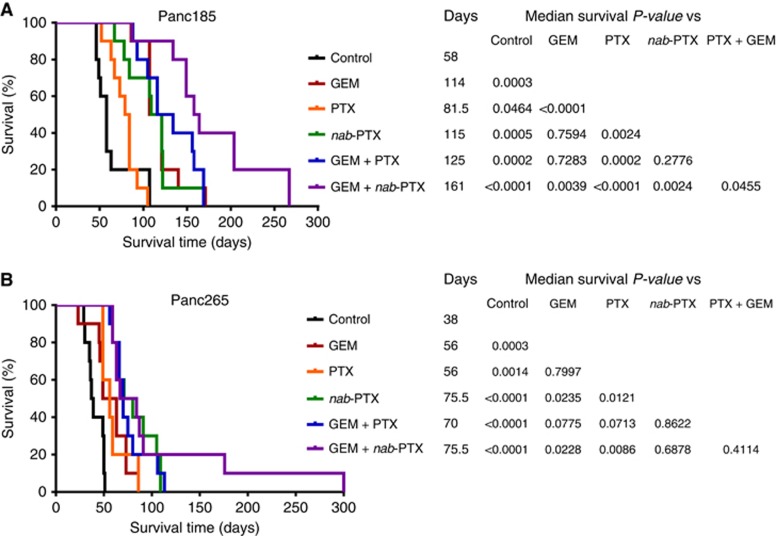
Therapeutic effect of regimens on the survival of mice harbouring human PDAC
We evaluated the impact of drug treatments on the survival of mice harbouring established PDAC. Kaplan–Meier survival curves of different treatment groups and the comparisons are shown in Figure 4 and Supplementary Figures S1 and S2. In the locally advanced model (Panc185), GEM monotherapy prolonged the median survival from 58 to 114 days (Figure 4A). PTX monotherapy also showed a positive, albeit smaller effect and extended median survival to 81.5 days. Nab-PTX monotherapy showed significant advantage compared with PTX monotherapy by extending the median survival to 115 days (P=0.0024; Figure 4A and Supplementary Figure S1).The combination of PTX and GEM showed a median survival of 125 days. The combination of nab-PTX and GEM was the most effective treatment. Median survival in this group was 161 days (Figure 4A), and the increase was statistically significant compared with PTX plus GEM treatment (P=0.0455; Figure 4A). Finally, the nab-PTX plus GEM combination treatment resulted in a statistically significant improvement in median survival compared with GEM treatment (P=0.0039; Figure 4A and Supplementary Figure S1).
Figure 4.
Treatment effect of regimens on the survival of mice with established PDAC. Nab-PTX monotherapy and in combination with GEM significantly improved the survival of mice with orthotopically implanted human PDAC. (A and B) Kaplan–Meir survival curves of mice with orthotopically implanted Panc185 tumour and Panc265 tumours. Briefly, tumour harbouring mice were treated with GEM, PTX, nab-PTX and combinations of GEM and PTX or GEM and nab-PTX as described in the study design. Survival data were analysed using GraphPad Prism 6 software. P-values for survival differences were calculated using the log-rank (Mantel-Cox) test. Tables shown on the right side of figure panels depict the median survival days and statistical significance. N=10 mice per group in both Panc185 and Panc265 PDXs.
Mice implanted with Panc265 tumours showed a more aggressive disease course with a median survival of 38 days in untreated mice (Figure 4B). Both GEM and PTX monotherapy resulted in the median survivals of 56 days (Figure 4B and Supplementary Figure S2). Nab-PTX treatment resulted in a near doubling in median survival to 75.5 days (Figure 4B), and the difference was statistically significant compared with PTX monotherapy (P=0.0121; Figure 4B and Supplementary Figure S2). Neither GEM plus PTX nor GEM plus nab-PTX combination was better than single agent nab-PTX (Figure 4B). The nab-PTX plus GEM combination treatment resulted in a statistically significant improvement in median survival compared with GEM treatment (P=0.0228; Figure 4B and Supplementary Figure S2). Remarkably, the survival of animals in the nab-PTX monotherapy was equivalent to those of animals received nab-PTX plus GEM treatment in the highly aggressive metastatic Panc265 PDAC model, indicating that nab-PTX could potentially stop the progression of late-stage pancreatic cancer and its utility as a backbone for combinations with existing or investigational agents.
Acute effect of taxanes on plasma and intratumour paclitaxel concentrations and desmoplastic stroma
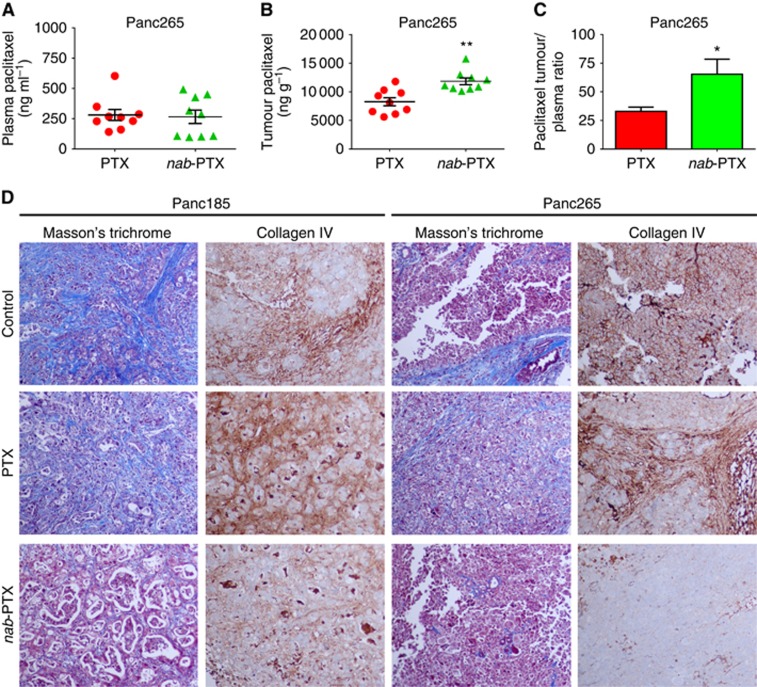
We determined the plasma and intratumour concentrations of paclitaxel following 5-day PTX or nab-PTX treatment in the Panc265 tumour model. Plasma paclitaxel concentrations in animals administered with PTX or nab-PTX were 280.9 and 265.8 ng ml−1, respectively. Plasma paclitaxel concentrations did not statistically differ between the two treatment groups (P=0.8357; Figure 5A). However, animals receiving nab-PTX treatment was associated with significantly higher (30.158%) tumour paclitaxel concentrations compared with PTX treatment (P=0.0014; Figure 5B). As shown in Figure 5C, the paclitaxel tumour plasma ratio was significantly higher (1.98-fold) in animals administered with nab-PTX compared with PTX treatment (P=0.0311). While PTX treatment failed to impart stromal depletion, nab-PTX treatment drastically lowered the levels of desmoplastic stroma in the tumour microenvironment (Figure 5D).
Figure 5.
Acute effect of taxanes on plasma and intratumour paclitaxel concentrations and desmoplastic stroma. Nab-PTX treatment resulted in higher intratumour paclitaxel concentration, higher paclitaxel tumour plasma ratio and facilitated depletion of tumour desmoplastic stroma, compared with PTX treatment. For the quantitation of plasma and intratumour paclitaxel, mice with established Panc265 orthotopic tumours were either untreated or treated with PTX/nab-PTX once daily, i.v. for five consecutive days as described in the Materials and Methods. Blood was collected and tumours were resected 2h post the last dose of PTX or nab-PTX on day 5 (N=9 mice/group). Tumour and plasma paclitaxel concentrations were analysed by LC/MS/MS. (A) Scatter plots showing plasma paclitaxel concentrations of animals administered with PTX or nab-PTX. Plasma paclitaxel concentrations of PTX was not statistically significant compared with nab-PTX (P=0.8357). As shown in panel B, nab-PTX treatment was associated with significantly higher (30.158%) tumour paclitaxel concentrations compared with PTX treatment (**P=0.0014). The paclitaxel tumour plasma ratio was significantly higher (1.98-fold) in animals administered with nab-PTX compared with PTX treatment (C, *P=0.0311). In order to determine the acute effect of taxanes on tumour stroma, mice with established Panc185 and Panc265 orthotopic tumours were either untreated or treated with PTX/nab-PTX once daily, i.v. for five consecutive days as described in the Materials and Methods. Tumours were resected 24 h post the last dose of PTX or nab-PTX on day 5 (N=5 mice/group). (D) Representative photomicrographs (× 20) of Masson's trichrome and Collagen IV staining performed on formalin-fixed paraffin-embedded primary tumour tissues. Treatment with nab-PTX resulted in a higher depletion of tumour desmoplastic stroma compared with PTX treatment. Fibrotic tissue is visualised as blue, nuclei are stained dark red/purple and cytoplasm is stained red/pink (Masson's trichrome). Collagen IV enriched fibrotic areas are visualised as brown stain.
Discussion
Advanced PDAC is deadly and difficult to treat with success (Wolfgang et al, 2013). The incidence of PDAC is increasing and is expected to be the second deadliest malignancy in the USA by 2020 (Garrido-Laguna and Hidalgo, 2015). Recently, an intensive cytotoxic regimen comprising oxaliplatin, irinotecan, fluorouracil and leucovorin (FOLFIRINOX) compared with GEM as first-line therapy in patients with metastatic PDAC has been shown to significantly improve the survival in patients with good performance status (Conroy et al, 2011). However, the safety profile of FOLFIRINOX was less favorable than that of GEM and many patients are not eligible to receive FOLFIRINOX because of poor performance status and advanced age (Ko, 2011).
The taxanes, PTX and docetaxel, were introduced more than two decades ago. These two drugs represented a revolution in cancer chemotherapy and have become core components of standard-of-care treatments in several cancer types such as breast, lung and ovary (Chiorean and Von Hoff, 2014). One of the drawbacks of PTX is the cremophor-ethanol, which has been shown to alter the pharmacokinetics of this agent and contribute to side effects such as hypersensitivity reactions in humans (Yared and Tkaczuk, 2012). In an effort to develop more effective and safe taxanes, nab-PTX was created as a solvent-free albumin-paclitaxel nanoparticle (Hawkins et al, 2008). Nab-PTX has demonstrated higher response rates and improved tolerability when compared with solvent-based formulations of PTX in patients with advanced metastatic breast cancer and advanced non-small-cell lung cancer and approved in certain settings and combinations for the treatment of these malignancies (Viudez et al, 2014).
Preclinical and early clinical trials conducted by us showed promising antitumour activity of nab-PTX and its potential to modulate dense desmoplastic stroma (Maitra et al, 2009; Von Hoff et al, 2011). These studies were followed by MPACT trial, a large randomised phase III study, which demonstrated the superior efficacy of nab-PTX in combination with GEM vs GEM alone (Von Hoff et al, 2013). Positive results from this study have led to the regulatory approval of this regimen, which is currently being implemented as a standard of care regimen for the treatment of patients with metastatic PDAC (Von Hoff et al, 2013). While the clinical efficacy of nab-PTX in PDAC is beyond any doubt, the mechanism of action is less clear. The extracellular matrix protein secreted protein, acidic and rich in cysteine (SPARC) had been put forward as a potential biomarker associated with superior nab-PTX activity. Despite support from a Phase I/II study (Von Hoff et al, 2011), a post hoc exploratory study of the MPACT trial did not detect any association between SPARC positivity in tumour tissue or plasma and treatment outcomes (Hidalgo et al, 2015). Two independent preclinical investigations using SPARC knockout mice and genetically engineered mice have corroborated this result (Alvarez et al, 2013; Neesse et al, 2014). Thus the notion that nab-PTX is effective in pancreatic cancer because the albumin binds to SPARC may not be correct (Hidalgo et al, 2015). A recent study demonstrated that efficacy of nab-PTX in metastatic breast cancer do not appears to be associated with expression of SPARC in primary or metastatic tumour tissue or in the serum (Schneeweiss et al, 2014). We recently showed that nab-PTX and GEM treatment effectively decreased the density of cancer-associated fibroblasts and induced a marked alteration in cancer stroma that results in primary tumour softening of patients with operable PDAC (Alvarez et al, 2013).
We performed studies to explore the efficacy and survival benefit of taxanes alone and in combination with GEM and determined the treatment effect on tumour cell proliferation, tumour desmoplasia and metastatic spreading to distant organs. We utilised orthotopic PDX models, which have been shown to better mimic metastasis than subcutaneously grown xenografts (Herreros-Villanueva et al, 2012; Hoffman, 2015; Hwang et al, 2016). Our results showed that PTX as a single agent was not significantly effective in reducing primary tumour burden or enhancing mouse survival compared with the activity of nab-PTX or GEM monotherapy. When compared with either agent used alone, combined treatment of GEM plus nab-PTX decreased metastatic tumour burden and increased median survival of animals (Table 1 and Figure 4). Masson's trichrome and Collagen IV staining performed on tumours harvested from untreated animals showed abundant levels of fibrotic tissue in tumour microenvironment (Figure 3). Visually striking reduction in fibrotic tissue was observed in tumour tissues harvested from the nab-PTX-treated animals compared with the tumours harvested from PTX-treated animals (Figure 3). As previously reported in mouse models and patient tissues (Maitra et al, 2009; Von Hoff et al, 2011), nab-PTX treatment effectively dismantled PDAC stroma (Figure 3). Nab-PTX treatment reduced the number of proliferating carcinoma cells compared with PTX treatment as evidenced by reduced number of carcinoma cells expressing Ki-67, a nuclear protein, expressed in proliferating cells (Figure 2A). GEM plus nab-PTX treatment was highly effective in reducing the Ki-67-positive carcinoma cells compared with GEM plus PTX treatment (Figure 2A).
In order to explore the potential mechanism of enhanced therapeutic efficacy of nab-PTX over PTX, we determined the plasma and intratumour concentrations of paclitaxel following nab-PTX or PTX treatment. We also evaluated the acute effect of taxanes on tumour desmoplastic stroma. Our results showed a 30.158% increase in tumour paclitaxel concentration and 1.98-fold higher paclitaxel tumour plasma ratio, following 5-day nab-PTX treatment compared with PTX treatment (Figure 5B and C).
Nab-PTX treatment was associated with higher depletion desmoplastic stroma in the tumour microenvironment compared with untreated or PTX-treated tumours. Considerable evidence from both preclinical and clinical studies demonstrated that multiple factors including favorable pharmacokinetics contribute to the augmented antitumour efficacy of nab-PTX over cremophor-based PTX (Gardner et al, 2008; Ma and Hidalgo, 2013). A previous preclinical study demonstrated that tumour paclitaxel area under the curve was 33% higher for nab-PTX treated mice compared with PTX treatment in a breast tumour model (Desai et al, 2006). Recent reports demonstrated that human pancreatic cancer cells display macropinocytosis and albumin internalisation occur through tumour macropinocytosis (Commisso et al, 2013; Kamphorst et al, 2015). Our in vivo studies did not investigate whether tumour macropinocytosis contribute to the uptake of albumin cocoon from nab-PTX or facilitate the bioavailability of paclitaxel in tumour microenvironment. Our data demonstrated that nab-PTX treatment resulted in a higher intratumour paclitaxel concentration, leading to enhanced killing of neoplastic cells and depletion of tumour desmoplastic stroma, which may be responsible for the significant therapeutic advantage of nab-PTX over PTX treatment in pancreatic cancer.
Our results convincingly demonstrated that nab-PTX treatment was remarkably effective in blocking primary tumour progression, depletion of dense tumour stroma and consistently achieved greater antitumour response, resulting in enhanced survival of tumour harbouring animals compared with PTX treatment. Combined treatment of GEM plus nab-PTX decreased metastatic tumour burden and increased overall survival of animals when compared with either agent used alone. Our findings clarified no benefit of adding PTX to GEM therapy for locally advanced and metastatic pancreatic cancer. Our studies confirmed that therapeutic efficacy of PTX and nab-PTX vary widely, and the contention that these agents elicit similar antitumour response was not supported. In this regard, clinical investigation of PTX alone and in combination with GEM in PDAC is not supported by our results.
Acknowledgments
We are very grateful to Elizabeth De Oliveira, Johns Hopkins University for excellent help in animal experiments. The study was supported by funding from Celgene Corporation (S738 to NVR) and a Stand Up To Cancer Dream Team Translational Cancer Research Grant (SU2C-AACR-DT0509). NVR, DDVH and MH received funding from Celgene Corporation. MH participated in Celgene sponsored symposiums and has received honoraria from Celgene Corporation.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Presented in part at 105th AACR Annual Meeting 2014; 5–9 April 2014; San Diego, CA, USA (Abstract LB-94).
ZT, SH, SB, DWP and CH are employees of Celgene Corporation. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Munoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, Plaza C, de Vicente E, Prados S, Tabernero S, Barbacid M, Lopez-Rios F, Hidalgo M (2013) Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer 109(4): 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331(6024): 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N. Australian Pancreatic Cancer Genome I Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM (2012) Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491(7424): 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6): 2403–2413. [DOI] [PubMed] [Google Scholar]
- Chiorean EG, Von Hoff DD (2014) Taxanes: impact on pancreatic cancer. Anticancer Drugs 25(5): 584–592. [DOI] [PubMed] [Google Scholar]
- Colucci G, Labianca R, Di Costanzo F, Gebbia V, Carteni G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolo M, Ciaparrone M, Cavanna L, Giuliani F, Maiello E, Testa A, Pederzoli P, Falconi M, Gallo C, Di Maio M, Perrone F, Gruppo Oncologico Italia M Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato D, Gruppo Oncologico Italiano di Ricerca C (2010) Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 28(10): 1645–1651. [DOI] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D (2013) Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497(7451): 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M Groupe Tumeurs Digestives of U, Intergroup P (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19): 1817–1825. [DOI] [PubMed] [Google Scholar]
- Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, Noker P, Yao R, Labao E, Hawkins M, Soon-Shiong P (2006) Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 12(4): 1317–1324. [DOI] [PubMed] [Google Scholar]
- Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA (2012) nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov 2(3): 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, Hawkins MJ, Sparreboom A, Figg WD (2008) Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res 14(13): 4200–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Laguna I, Hidalgo M (2015) Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol 12(6): 319–334. [DOI] [PubMed] [Google Scholar]
- Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem JL, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD (2015) nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst 107(2): dju413. [DOI] [PubMed] [Google Scholar]
- Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, Bhar P (2009) Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 27(22): 3611–3619. [DOI] [PubMed] [Google Scholar]
- Hawkins MJ, Soon-Shiong P, Desai N (2008) Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev 60(8): 876–885. [DOI] [PubMed] [Google Scholar]
- Herreros-Villanueva M, Hijona E, Cosme A, Bujanda L (2012) Mouse models of pancreatic cancer. World J Gastroenterol 18(12): 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362(17): 1605–1617. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Bruckheimer E, Rajeshkumar NV, Garrido-Laguna I, De Oliveira E, Rubio-Viqueira B, Strawn S, Wick MJ, Martell J, Sidransky D (2011) A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther 10(8): 1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M, Plaza C, Musteanu M, Illei P, Brachmann CB, Heise C, Pierce D, Lopez-Casas PP, Menendez C, Tabernero J, Romano A, Wei X, Lopez-Rios F, Von Hoff DD (2015) SPARC expression did not predict efficacy of nab-Paclitaxel plus gemcitabine or gemcitabine alone for metastatic pancreatic cancer in an exploratory analysis of the phase III MPACT Trial. Clin Cancer Res 21(21): 4811–4818. [DOI] [PubMed] [Google Scholar]
- Hoffman RM (2015) Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer 15(8): 451–452. [DOI] [PubMed] [Google Scholar]
- Hwang CI, Boj SF, Clevers H, Tuveson DA (2016) Preclinical models of pancreatic ductal adenocarcinoma. J Pathol 238(2): 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, Mohri D, Miyabayashi K, Asaoka Y, Maeda S, Ikenoue T, Tateishi K, Wright CV, Koike K, Omata M, Moses HL (2011) Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest 121(10): 4106–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321(5897): 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadaba R, Birke H, Wang J, Hooper S, Andl CD, Di Maggio F, Soylu E, Ghallab M, Bor D, Froeling FE, Bhattacharya S, Rustgi AK, Sahai E, Chelala C, Sasieni P, Kocher HM (2013) Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J Pathol 230(1): 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, Thompson CB, Rabinowitz JD (2015) Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res 75(3): 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE (2009) Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc 4(11): 1670–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AH (2011) FOLFIRINOX: a small step or a great leap forward? J Clin Oncol 29(28): 3727–3729. [DOI] [PubMed] [Google Scholar]
- MA WW, Hidalgo M (2013) The winning formulation: the development of paclitaxel in pancreatic cancer. Clin Cancer Res 19(20): 5572–5579. [DOI] [PubMed] [Google Scholar]
- Maitra A, Hruban RH (2008) Pancreatic cancer. Annu Rev Pathol 3: 157–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A, Rajeshkumar NV, Rudek M, Garrido-Laguna I, Laheru D, Iglesias J, Desai N, Von Hoff D, Hidalgo M (2009) nab-paclitaxel targets tumor stroma and results, combined with gemcitabine, in high efficacy against pancreatic cancer models. Mol Cancer Ther 8(12 Suppl): C246. [Google Scholar]
- Montana M, Ducros C, Verhaeghe P, Terme T, Vanelle P, Rathelot P (2011) Albumin-bound paclitaxel: the benefit of this new formulation in the treatment of various cancers. J Chemother 23(2): 59–66. [DOI] [PubMed] [Google Scholar]
- Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, Ellenrieder V, Jodrell DI, Tuveson DA (2014) SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut 63(6): 974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettle H (2014) Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev 40(9): 1039–1047. [DOI] [PubMed] [Google Scholar]
- Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, Khorana AA, Goldman B, Fenoglio-Preiser CM, Abbruzzese JL, Blanke CD (2010) Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 28(22): 3605–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21(3): 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar NV, De Oliveira E, Ottenhof N, Watters J, Brooks D, Demuth T, Shumway SD, Mizuarai S, Hirai H, Maitra A, Hidalgo M (2011) MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res 17(9): 2799–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar NV, Dutta P, Yabuuchi S, de Wilde RF, Martinez GV, Le A, Kamphorst JJ, Rabinowitz JD, Jain SK, Hidalgo M, Dang CV, Gillies RJ, Maitra A (2015) Therapeutic targeting of the Warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Res 75(16): 3355–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg PV, Kuramochi H, Tanaka K, Singh S, Salimi-Moosavi H, Bouraoud N, Amador ML, Altiok S, Kulesza P, Yeo C, Messersmith W, Eshleman J, Hruban RH, Maitra A, Hidalgo M (2006) An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res 12(15): 4652–4661. [DOI] [PubMed] [Google Scholar]
- Saif MW, Syrigos K, Penney R, Kaley K (2010) Docetaxel second-line therapy in patients with advanced pancreatic cancer: a retrospective study. Anticancer Res 30(7): 2905–2909. [PubMed] [Google Scholar]
- Schneeweiss A, Seitz J, Smetanay K, Schuetz F, Jaeger D, Bachinger A, Zorn M, Sinn HP, Marme F (2014) Efficacy of nab-paclitaxel does not seem to be associated with SPARC expression in metastatic breast cancer. Anticancer Res 34(11): 6609–6615. [PubMed] [Google Scholar]
- Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27(13): 2231–2237. [DOI] [PubMed] [Google Scholar]
- Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, De Jesus-Acosta A, Jones S, Maitra A, Hruban RH, Eshleman JR, Klein A, Laheru D, Donehower R, Hidalgo M (2011) Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther 10(1): 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viudez A, Ramirez N, Hernandez-Garcia I, Carvalho FL, Vera R, Hidalgo M (2014) Nab-paclitaxel: a flattering facelift. Crit Rev Oncol Hematol 92(3): 166–180. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD (1997) The taxoids: same roots, different drugs. Semin Oncol 24(4 Suppl 13): S13–3-S13-10. [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369(18): 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M (2011) Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 29(34): 4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatcott C, Han H, Posner RG, Von Hoff DD (2013) Tumor-stromal interactions in pancreatic cancer. Crit Rev Oncog 18(1–2): 135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD, Han H (2015) Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res 21(15): 3561–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH (2013) Recent progress in pancreatic cancer. CA Cancer J Clin 63(5): 318–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA Committee of the National Cancer Research Institute (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102(11): 1555–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D, Xie K (2015) Pancreatic cancer stromal biology and therapy. Genes Dis 2(2): 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A, Rajeshkumar NV (2013) Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett 335(1): 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yared JA, Tkaczuk KH (2012) Update on taxane development: new analogs and new formulations. Drug Des Dev Ther 6: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Marrano P, Kumar S, Leadley M, Elias E, Thorner P, Baruchel S (2013) Nab-paclitaxel is an active drug in preclinical model of pediatric solid tumors. Clin Cancer Res 19(21): 5972–5983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.