Supplemental Digital Content is available in the text
Abstract
Studies about the association between oral contraceptives use and liver cancer risk have generated controversial results. Therefore, a meta-analysis of cohort and case–control studies was performed to quantitatively summarize the existing evidence.
Eligible studies were identified by a computer search of PubMed and Embase databases and handed-search of reference lists, without any limitations. Study-specific risk estimates (RRs) with 95% confidence intervals (CIs) were combined with random-effects model.
A total of 17 articles were included in this meta-analysis. Overall, there was no statistically significant association between oral contraceptives use and liver cancer risk (RR: 1.23, 95% CI: 0.93–1.63). In a dose-analysis of meta-analysis, a linear relationship between oral contraceptives use and liver cancer risk (P for linearity = 0.391) was found, although this correlation was not statistically significant.
Oral contraceptives use was not positively associated with the risk of liver cancer.
INTRODUCTION
Primary liver cancer is the sixth most common diagnosed cancer.1 The vast majority of primary liver cancer is hepatocellular carcinoma, accounting for approximately 90% of the cases.2 The incidence of liver cancer varies worldwide, with high rates in East Asia and South Africa, and a relatively lower incidence in Western countries.2 Its etiology is only partially understood. The main risk factors for primary liver cancer are chronic infection with hepatitis B or C virus, alcohol consumption, and exposure to aflatoxin B1.2
About 9% of women who are at child-bearing age in the worldwide choose oral contraceptives. This number increases to 18% in developed countries.3 Since oral contraceptives are widely used, even a small effect of oral contraceptive use on the incidence of liver cancer may have a considerable impact on public health. Hepatocellular carcinoma occurs 2 to 3 times more often in men than women.4 Men also have poorer survival rates and higher recurrence rates after hepatocellular carcinoma treatment than do women.5 The gender differences in incidence or outcome indicate that sex hormones may involve in the carcinogenesis of hepatocellular carcinoma. A host of studies, beginning with that of Baum et al in 19736 reported that oral contraceptives play a role in the development of liver cancer. However, existing evidences are inconsistent. Therefore, a meta-analysis of case–control and cohort studies was undertaken to obtain a better understanding of the relationship between oral contraceptives use and liver cancer risk.
MATERIALS AND METHODS
Systematic Search
A computerized search of PubMed and Embase databases from inception to August 2015 was carried out to identify potentially eligible studies, with the string (“hormone” OR “oral contraceptive” OR “contraceptive” OR “birth control”) AND (“hepatocellular carcinoma” OR “hepatic carcinoma” OR “liver cancer” OR “liver tumors” OR “liver neoplasms”). No language limitation was imposed. The reference lists of all relevant studies were checked for further reports. In our paper, ethical approval is not necessary, as this study is a meta-analysis which is based on the published data.
Study Selection
Studies included in current meta-analysis should meet the following inclusion criteria: Case–control or cohort studies focused on the risk of liver cancer in users of oral contraceptives versus nonusers. (2) Relative risk estimates (RRs) with corresponding 95% confidence intervals (CIs) or other data (the distribution of cases and noncases across exposure categories) were reported in original studies. (3) When more than one study was published on the same population, the report presenting results based on a larger number of cases and controls was considered. Case reports, series of cases, reviews, meta-analyses, editorial, conference abstracts, and letter were excluded.
Data Collection
The following data were extracted in a standard format: first author of each study, year of publication, country, study design, calendar years of participants’ inclusion, number of participants (cases and controls or cohort size), RRs and corresponding 95% CIs, variables adjusted for in the analysis and/or matching variables.
Assessment of Methodological Quality
We evaluated the methodological quality of included studies by using the Newcastle-Ottawa Scale (NOS) (available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm). The NOS criteria includes subject selection (scores, 0–4), comparability of subject (scores, 0–2), and exposure or outcome (scores, 0–3). The NOS scores ranged from 0 to 9. In our meta-analysis, a score ≥7 indicates a good quality.
Statistical Analysis
Pooled RRs with 95% CIs with random-effects model described by DerSimonian and Laird method7 were calculated to estimate the association between oral contraceptives use and liver cancer risk by use of STATA 12.0 software (StataCorp, College Station, TX). Statistical heterogeneity among studies were estimated by Cochran Q and I2 tests (The I2 test describes the percentage of total variation across studies).8,9 Statistically significant heterogeneity was considered when P for Cochran Q < 0.1. Subgroup analyses were performed according to study design (case–control vs. cohort), geographic region (North America vs. Europe vs. Asia), adjust statue (adjusted vs. crude). The robustness of the overall results was evaluated by performing a sensitivity analysis with a method of excluding 1 study at a time. Additionally, we performed a sensitivity analysis based on those studies with a score ≥7 to investigate whether study quality had an influence on the overall association. Publication bias was assessed through funnel plots and Egger test.10,11 When some evidence for publication bias was observed, the trim-and-fill method was used to assess the stability.12
A dose–response analysis was performed according to the method proposed by Greenland and Longnecker13 and Orsini et al.14 The method requires that the distribution of cases and person-years or noncases and the adjusted RRs with 95% CIs for at least 3 exposure categories are reported. For studies that reported the ranges of year duration of oral contraceptives use, each class dose corresponding to the midpoint of upper and lower bound was assigned. If the upper level for the highest category was open-ended, the exposure doses were calculated as 1.2 times its exposure level. A potential nonlinear dose–response relationship between oral contraceptives use and liver cancer risk by modeling duration of oral contraceptives use was examined using restricted cubic splines with 3 knots at percentiles 25%, 50%, and 75% of the distribution.15 A P-value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline is equal to 0.15,16
RESULTS
Literature Search and Study Characteristics
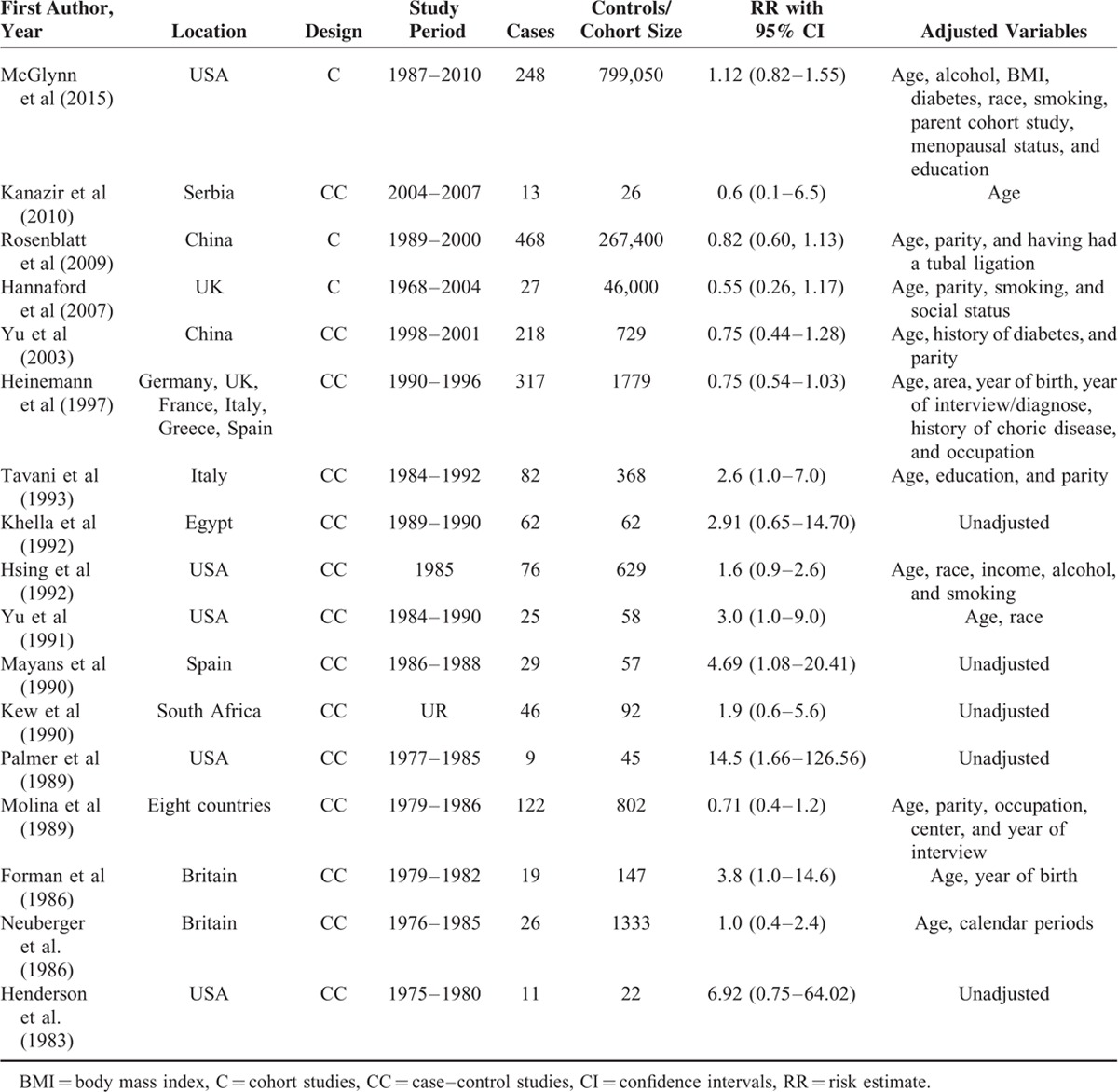
Figure 1 shows the literature search process. The search strategy yielded 4591 studies. After carefully examining these potential studies and checking the reference lists, a final total of 17 studies were identified for current analysis.5,17–32Table 1 shows the main characteristics. An overwhelming majority of studies were retrospective case–control studies (n = 14), regardless of controls from hospital or general population. Studies were conducted in various counties, involving the UK, Egypt, USA, China, Germany, France, Italy, Greece, Spain, Serbia, Chile, Colombia, Kenya, Israel, Nigeria, Philippines, South African, and Thailand. Studies varied in sample size. The number of cases in each study ranged from 9 to 468. Most studies reported RRs that were adjusted for age, alcohol, body mass index (BMI), diabetes, race, smoking, parent cohort study, menopausal status, parity, occupation, education, income, and year of interview/diagnose/birth. The NOS scores of included studies ranged from 5 to 9 (see Tables S1 and S2, http://links.lww.com/MD/A467, Supplemental Digital Content 1 and 2, http://links.lww.com/MD/A467).
FIGURE 1.
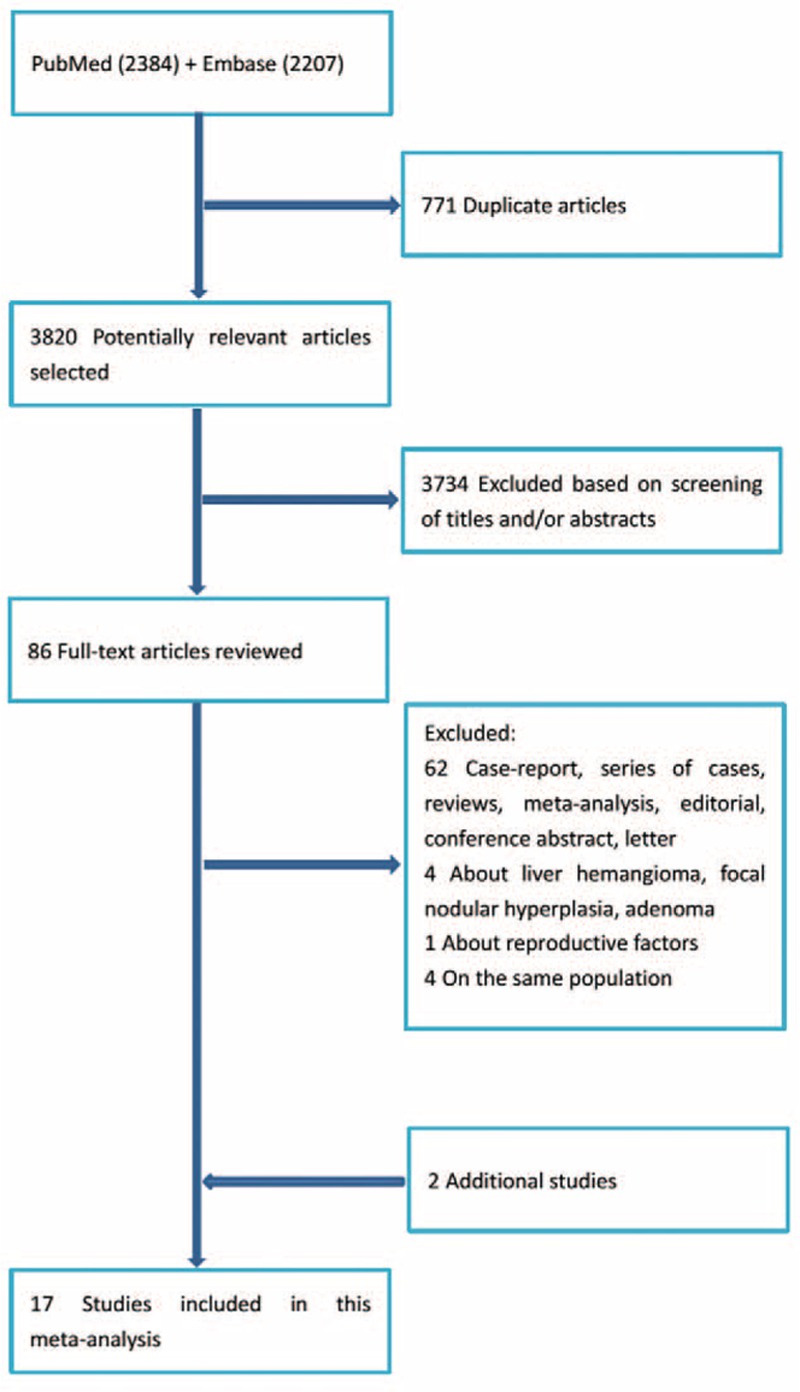
The literature search process.
TABLE 1.
Main Characteristics of Included Studies
Overall Association of Oral Contraceptives Use With the Risk of Liver Cancer
Figure 2 presents the RRs of liver cancer and oral contraceptives use. The summary RRs associated with women who have ever used oral contraceptives in comparison with never users were 1.55 (1.04–2.31), 0.88 (0.64–1.22), 1.23 (0.93–1.63) for case–control studies, cohort studies, all studies, respectively.
FIGURE 2.
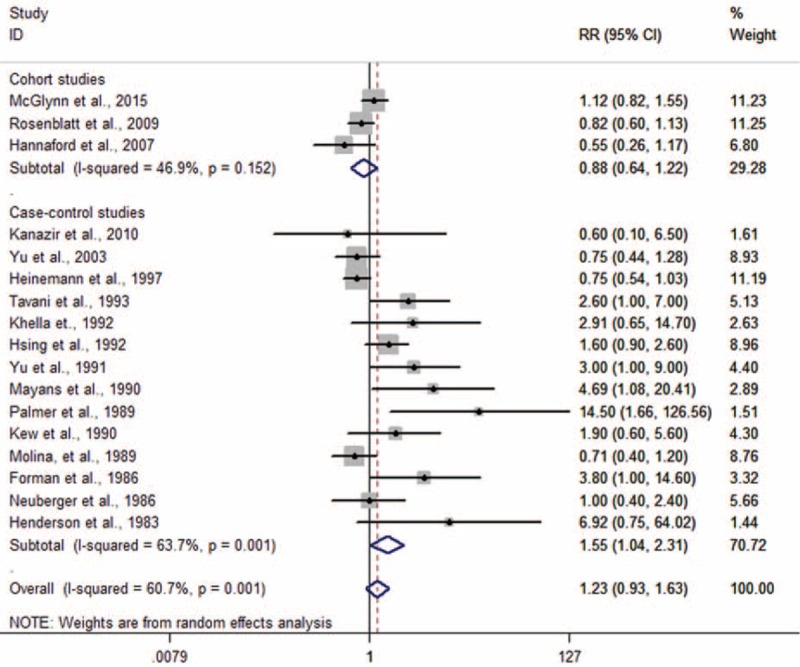
Forest plots of risk estimates of the relationship between oral contraceptives use and liver cancer risk.
Stratifying Analysis
Stratifying by geographic region, the summary RRs were 1.99 (1.08–3.68) for studies conducted in North America, 1.26 (0.70–2.26) for studies conducted in Europe, 0.80 (0.61–1.05) for studies conducted in Asia, and 2.20 (0.89–5.44) for studies conducted in Africa.
Stratifying by adjustment status, the pooled RRs were 1.02 (0.79–1.31) for studies reporting adjusted RRs and 3.52 (1.76–7.02) for studies providing crude RRs.
Heterogeneity Test
There was statistically significant heterogeneity across studies (P = 0.001, I2 = 60.7%). When conducting the subgroup analysis, the heterogeneity was also significant in case–control studies (P = 0.001, I2 = 63.7%), North America studies (P = 0.034, I2 = 61.6 %), Europe studies (P = 64.5, I2 = 0.010 %), and adjusted studies (P = 0.013, I2 = 54.4 %), but not in cohort studies (P = 0.152, I2 = 46.9 %), Asia studies (P = 0.778, I2 = 0.0%), Africa studies (P = 0.663, I2 = 0.0%), or unadjusted studies (P = 0.348, I2 = 9.1%).
Dose–Response Analysis
Sven studies were included in the dose analysis.5,18,20–23,25 A linear relationship between oral contraceptives use and liver cancer risk was observed (P for linearity = 0.391), although this correlation was not statistically significant (Fig. 3).
FIGURE 3.
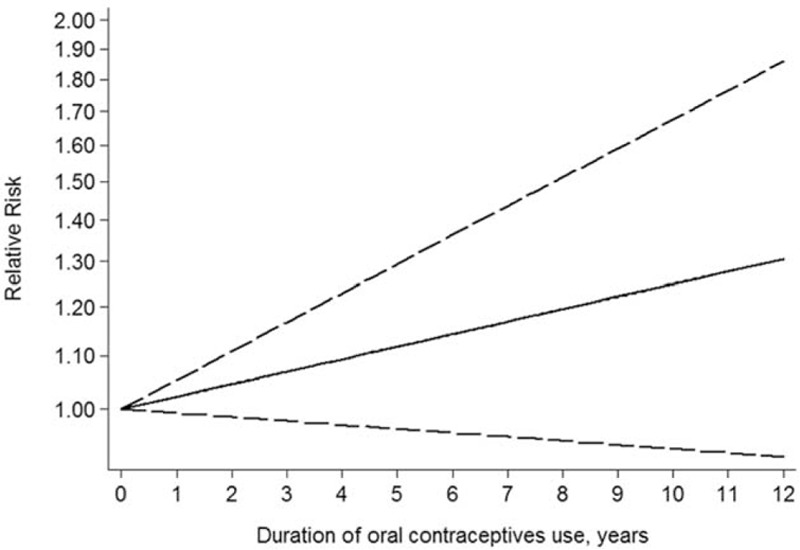
Dose–response relationship between oral contraceptives use and liver cancer risk.
Sensitivity Analysis
The results indicate that the each individual data has no significant influence on the overall results (Fig. 4).
FIGURE 4.
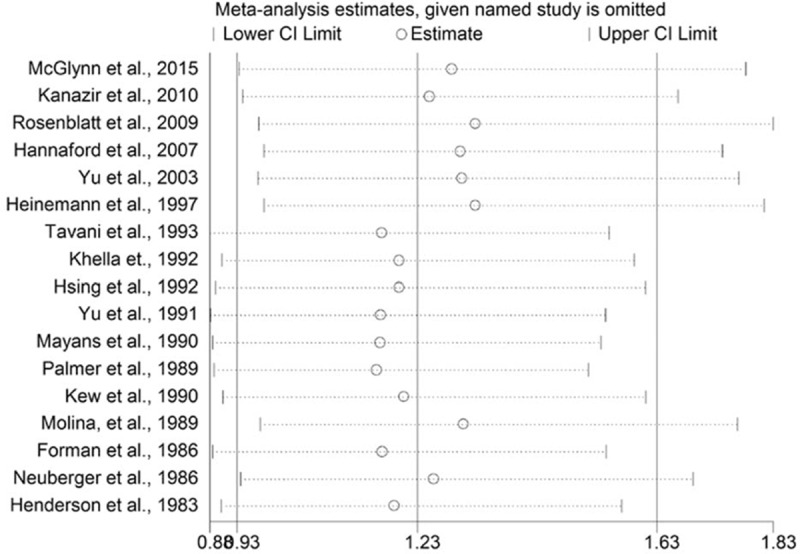
Sensitivity analyses through exclusion of 1 study at a time to reflect the influence of individual study to the overall results.
An alternative sensitivity analysis based on those studies with a score of 7 or more was conducted. The pooled RR was 0.98 (0.73–1.32).
Publication Bias
Both the Egger test (P = 0.004) and the Begg funnel plot (Fig. 5) showed some evidence for publication bias. Next, the “trim and fill” method performed. As shown in Figure 6, 5 potential missing studies were identified. Correspondingly, the recalculation of the pooled RR was 1.00 (0.74–1.36).
FIGURE 5.
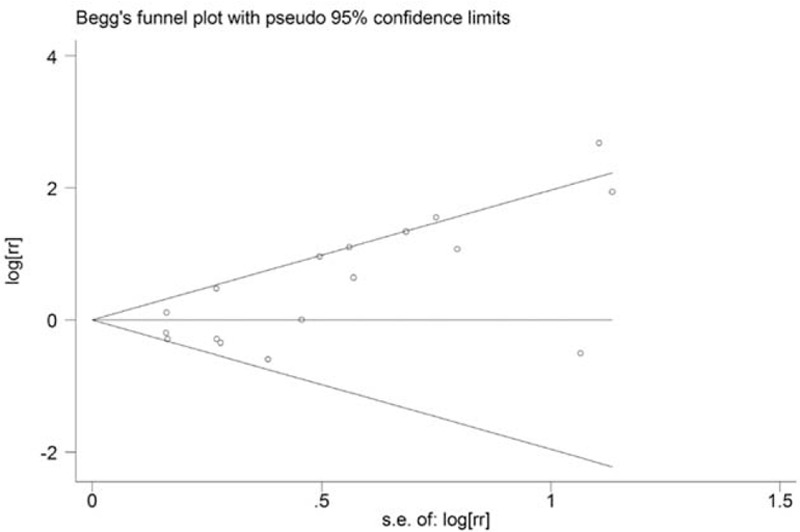
Funnel plots for the relationship between oral contraceptives use and liver cancer risk.
FIGURE 6.
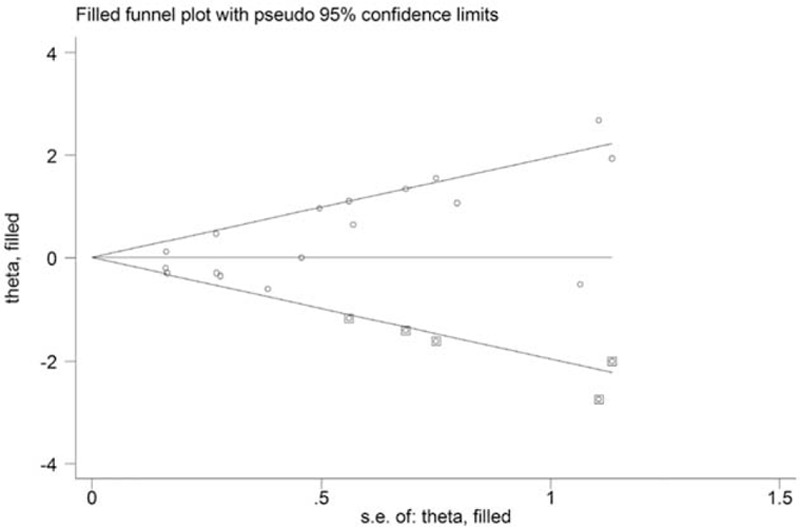
Funnel plots with “trim and fill” method for the relationship between oral contraceptives use and liver cancer risk.
DISCUSSION
Clinical and experimental evidence suggests that female hormones influence the risk of developing liver cancer. A great many epidemiologic studies investigated the association between oral contraceptives use and live cancer risk, with conflicting results reported. To obtain a better understanding of this issue, 2 meta-analyses were published in 2004 and 2007. However, the results from previous meta-analyses remain controversial. Yu and Yuan33 in a meta-analysis of 8 case–control studies found that compared with nonusers, oral contraceptives users were at higher risk of liver cancer (RR = 2.5, 95% CI: 1.7–3.5). In 2007, a meta-analysis of 12 case–control studies covering 739 cases and 5223 controls did not support a cause and effect relationship between oral contraceptives use and liver cancer risk (RR = 1.57, 95% CI: 0.96–2.54).34 Therefore, an undated dose–response meta-analysis was performed. The current meta-analysis summarizes the evidence of fourteen case–control and 3 cohort studies. The results suggested that oral contraceptives use was not positively associated with the risk of liver cancer. Meanwhile, subgroup analyses by study design and geographic region were performed, a statistically significant positive link was observed for case–control studies, North America, and crude RRs group, but not for cohort studies, Europe, Asia, Africa, or adjusted RRs group.
The effect of duration of oral contraceptives use on liver cancer risk is another interest concern, as the incidence of liver cancer increases with age and oral contraceptives use is limited to reproductive age. Thirteen of included studies evaluated the effect of time dependency of risk modulation.4,18–25,27–30 Variable definitions of duration were reported in original studies and nonsignificant increase or decrease in risk of liver cancer was shown in most studies. In present study, a dose–response analysis with the method proposed by Greenland and Longnecker and Orsini and colleagues was performed, which requires that the distribution of cases and person-years or noncases and the adjusted RRs with 95% for at least 3 exposure categories are reported. Seven studies meeting the inclusion criteria were included in dose–response analysis.5,18,20–23,25 The results showed that a linear trend of increasing risk of liver risk with longer duration use of oral contraceptives (P for linearity = 0.391), although this interaction was not statistically significant.
Composition of oral contraceptives formulations has greatly changed, since the introduction of oral contraception in the early 1960s was used to control unintended pregnancies. Today's formulations contain much lower estrogen doses and a greater variety of progestins than did the original pills.35,36 Among included studies, only 1 multicenter study with 317 cases and 1779 controls by Heinemann et al distinguished in the analyses exposure to the subgroup of oral contraceptives.21 Heinemann et al found that no significant alteration in risk of liver cancer risk in women who have ever used oral contraceptives containing cyproterone acetate, or for women ever using any other type of oral contraceptives.
There are some biological and experimental evidences that oral contraceptives may play a critical role in the carcinogenesis of liver cancer. In biological experiments, estrogen receptors were found in hepatocytes and highly expressed in hepatocellular carcinoma and can increase cellular proliferation and the rate of spontaneous mutation.37,38 In animals, estrogens and progestogens act as a inducer and promoter in development of liver tumors.32 Moreover, estrogens interact with insulin-like growth factor, which is an important part of pathway associated with carcinogenesis though prohibiting apoptosis and promoting cell proliferation.39
Some limitations of the current meta-analysis should be acknowledged when interpreted this findings. First, the current meta-analysis is unable to solve problems with confounding factors. Chronic infections with hepatitis B or C virus and alcohol consumption are established risk factors for liver cancer. Few studies in original data clarified its effect. Thus, inadequate control of the confounders might mask the true risk. Second, the vast majority of studies were retrospective case–control studies (n = 14), recall and/or reporting bias are concerns. Third, both the Egger test (P = 0.004) and the Begg funnel plots showed some evidence for publication bias. In further analysis with trim-and-fill method, 5 potential missing studies were identified. Corresponding, the results did not materially alter, suggesting the effect of potential publication bias did not yield noticeable harm.
In conclusion, oral contraceptives use does not have a significant positive effect on the risk of liver cancer. The possible linear risk on liver cancer depended on the duration of oral contraceptives use should be further clarified. Future prospective studies with particular attention to confounding factors, formulations of oral contraceptives, and duration period between oral contraceptives exposure and liver cancer are needed.
Footnotes
Abbreviations: BMI = body mass index, C = cohort studies, CC = case–control studies, NOS = Newcastle-Ottawa Scale.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Huang RX, Duan YY, Hu JA. Fish intake and risk of liver cancer: a meta-analysis. PLoS One 2015; 10:e0096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Zhang D, Feng N, et al. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology 2014; 147:1031–1042. [DOI] [PubMed] [Google Scholar]
- 3.Vinogradova Y, Coupland C, Hippisley-Cox J. Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ 2015; 350:h2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011; 15:223–243.vii-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlynn KA, Sahasrabuddhe VV, Campbell PT, et al. Reproductive factors, exogenous hormone use and risk of hepatocellular carcinoma among US women: results from the Liver Cancer Pooling Project. Br J Cancer 2015; 112:1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum JK, Bookstein JJ, Holtz F, et al. Possible association between benign hepatomas and oral contraceptives. Lancet 1973; 2:926–929. [DOI] [PubMed] [Google Scholar]
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 8.Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10:101–129. [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 13.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992; 135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 14.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006; 6:40–57. [Google Scholar]
- 15.Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA 2010; 303:1077–1083. [DOI] [PubMed] [Google Scholar]
- 16.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 1988; 80:1198–1202. [DOI] [PubMed] [Google Scholar]
- 17.Kanazir M, Boricic I, Delic D, et al. Risk factors for hepatocellular carcinoma: a case-control study in Belgrade (Serbia). Tumori 2010; 96:911–917. [PubMed] [Google Scholar]
- 18.Rosenblatt KA, Gao DL, Ray RM, et al. Oral contraceptives and the risk of all cancers combined and site-specific cancers in Shanghai. Cancer Causes Control 2009; 20:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannaford PC, Selvaraj S, Elliott AM, et al. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner's oral contraception study. BMJ 2007; 335:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu MW, Chang HC, Chang SC, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology 2003; 38:1393–1400. [DOI] [PubMed] [Google Scholar]
- 21.Heinemann LAJ, DoMinh T, Guggenmoos Holzmann, et al. Oral contraceptives and liver cancer. Results of the Multicentre International Liver Tumor Study (MILTS). Contraception 1997; 56:275–284. [PubMed] [Google Scholar]
- 22.Tavani A, Negri E, Parazzini F, et al. Female hormone utilisation and risk of hepatocellular carcinoma. Br J Cancer 1993; 67:635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsing AW, Hoover RN, McLaughlin JK, et al. Oral contraceptives and primary liver cancer among young women. Cancer Causes Control 1992; 3:43–48. [DOI] [PubMed] [Google Scholar]
- 24.Yu MC, Tong MJ, Govindarajan S, et al. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles County, California. J Natl Cancer Inst 1991; 83:1820–1826. [DOI] [PubMed] [Google Scholar]
- 25.Kew MC, Song E, Mohammed A, et al. Contraceptive steroids as a risk factor for hepatocellular carcinoma: a case/control study in South African black women. Hepatology 1990; 11:298–302. [DOI] [PubMed] [Google Scholar]
- 26.Mayans MV, Calvet X, Bruix J, et al. Risk factors for hepatocellular carcinoma in Catalonia, Spain. Int J Cancer 1990; 46:378–381. [DOI] [PubMed] [Google Scholar]
- 27.Palmer JR, Rosenberg L, Kaufman DW, et al. Oral contraceptive use and liver cancer. Am J Epidemiol 1989; 130:878–882. [DOI] [PubMed] [Google Scholar]
- 28.Molina R, Martinez L, Salas O, et al. Combined oral contraceptives and liver cancer. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Int J Cancer 1989; 43:254–259. [PubMed] [Google Scholar]
- 29.Forman D, Vincent TJ, Doll R. Cancer of the liver and the use of oral contraceptives. Br Med J (Clin Res Ed) 1986; 292:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuberger J, Forman D, Doll R, et al. Oral contraceptives and hepatocellular carcinoma. Br Med J (Clin Res Ed) 1986; 292:1355–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson BE, Preston-Martin S, Edmondson HA, et al. Hepatocellular carcinoma and oral contraceptives. Br J Cancer 1983; 48:437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khella AK, Faris L, Helmy S, et al. A hospital based case-control study of hepatocellular carcinoma. J Egypt Public Health Assoc 1992; 67:249–258. [PubMed] [Google Scholar]
- 33.Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology 2004; 127:S72–S78. [DOI] [PubMed] [Google Scholar]
- 34.Maheshwari S, Sarraj A, Kramer J, et al. Oral contraception and the risk of hepatocellular carcinoma. J Hepatol 2007; 47:506–513. [DOI] [PubMed] [Google Scholar]
- 35.Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol 2015; 25:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marnach ML, Long ME, Casey PM. Current issues in contraception. Mayo Clin Proc 2013; 88:295–299. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med 1992; 7:199–209. [DOI] [PubMed] [Google Scholar]
- 38.De Benedetti VM, Welsh JA, Yu MC, et al. p53 mutations in hepatocellular carcinoma related to oral contraceptive use. Carcinogenesis 1996; 17:145–149. [DOI] [PubMed] [Google Scholar]
- 39.Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep 2011; 13:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]


