Abstract
Although the prognostic significance of the histologic patterns in lung adenocarcinoma is being identified, no significant prognostic indicators in lung squamous carcinoma are accepted as a standard universally. The aim of this study was to evaluate the histologic characteristics incorporating the defined invasion types and distinguish the features that can reflect prognosis.
We reviewed all slices of 132 patients with lung squamous carcinoma. The cases were classified according to the World Health Organization (WHO) classification and were evaluated for tumor budding, single cell invasion, large cell invasion, cytologic atypia degree, mitotic count, number of buds, tumor nest size, fibrosis, and necrosis.
In univariate analysis, overall survival was associated significantly with age (P = 0.023), lymph nodes metastasis (P < 0.001), distant organ metastasis (P < 0.001), pleural invasion (P < 0.001), tumor budding (P = 0.003), single cell invasion (P = 0.001), mitotic count (P < 0.001), and the cytologic atypia degree (P = 0.009). However, the subtypes of 2004 WHO classification showed no association with outcome (P = 0.209). In multivariate analysis, the independent significant prognostic indicators of lung squamous carcinoma were tumor budding (hazard ratio [HR] = 0.466, P = 0.005), single cell invasion (HR = 0.447, P = 0.003), mitotic count (HR = 0.502, P = 0.048) and cytologic atypia degree (HR = 0.479, P = 0.024).
Lung squamous carcinomas with the invasion types were associated with a poor prognosis.
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths in both sexes worldwide.1 Despite advances in treatment, such as complete surgical resection, the prognosis of lung cancer is generally poor,2 with recurrence rates of 15% to 30% and 5-year survival rates of 60% to 70%.3 In the current World Health Organization (WHO) classifications of lung carcinomas, squamous cell carcinomas are classified into keratinizing, nonkeratinizing, and basaloid subtypes.4 Nevertheless, these histologic subtypes within lung squamous cell carcinoma (LSCC) have not been shown to have enough prognostic or clinical significance that can affect treatment decisions.5
According to previous research, methods to treat LSCC were unitary, and customized chemotherapy for unresectable or recurrent lung cancers was more frequently used for adenocarcinoma than for LSCC.6,7 Recently, with the development of drugs treating nonsmall cell lung carcinoma (NSCLC), different kinds of histologic subtype can partly influence the treatment decision. However, the treatment strategies, which can be used for LSCC, are limited. For example, bevacizumab is contraindicated in patients with LSCC because it can increase the risk of fatal hemoptysis.8–10 There are still no alternative histologic features, and grading system has been identified that clinicians could use to predict patient prognosis. Nevertheless, prognostic indicators for lung squamous carcinoma are important to refine prognostic profiles that can potentially select patients who will benefit most from aggressive treatment. Thus, the search for additional prognostic factors in the assessment of LSCC has been a major research focus recently.
Some significant invasive patterns such as tumor budding have been considered as a poor prognostic factor in other carcinomas.11–15 Recently, several studies identified tumor budding as an unfavorable prognostic indicator in patients with lung adenocarcinoma and LSCC.16–18 Tumor budding is defined as small separate clusters of tumor cells composed of <5 tumor cells within the stromal tissue at the invasive margin of the tumor.19 Another invasive pattern which is being focused on is the single cell invasion. There is one primary study identified single cell invasion as a poor prognostic indicator in patients with LSCC.20 More evidence is necessary to prove invasion types such as the tumor budding and single cell invasion as prognostic factors in patients with LSCC.
There is still no enough evidence to demonstrate the existence of the connection between histologic subtypes of LSCC and clinical outcome of patients with LSCC. Except of basaloid variant and small cell variant, previous research has shown to have correlation with slightly more favorable prognosis.17 Based on these previous studies, we pondered evaluating more prognostic factors to estimate the LSCC and analyzing whether they are associated with survival.
The aim of this study was to examine whether different kind of invasion types are correlated with LSCC clinical outcome and whether the evaluating method has clinical significance.
MATERIALS AND METHODS
Patient Characteristics
The study group included 132 consecutive patients with LSCC treated with radical surgical resection in Shandong Provincial Hospital between 2007 and 2008 (Table 1). The cases were selected sequentially. And Ethics Committee in Shandong Provincial Hospital approved the study. All those patients’ tumor slides were available for histologic evaluation. Clinical data were collected, and the tumor subtypes were defined according to the WHO classification criteria.21 The cases were staged according to the 7th Edition of the American Joint Committee on Cancer manual.22 The clinicopathologic characteristics included age, sex, tumor size, TNM classification, pathologic stages, histologic differentiation degree, and pleural invasion.
TABLE 1.
Patient Demographics and Clinical Features
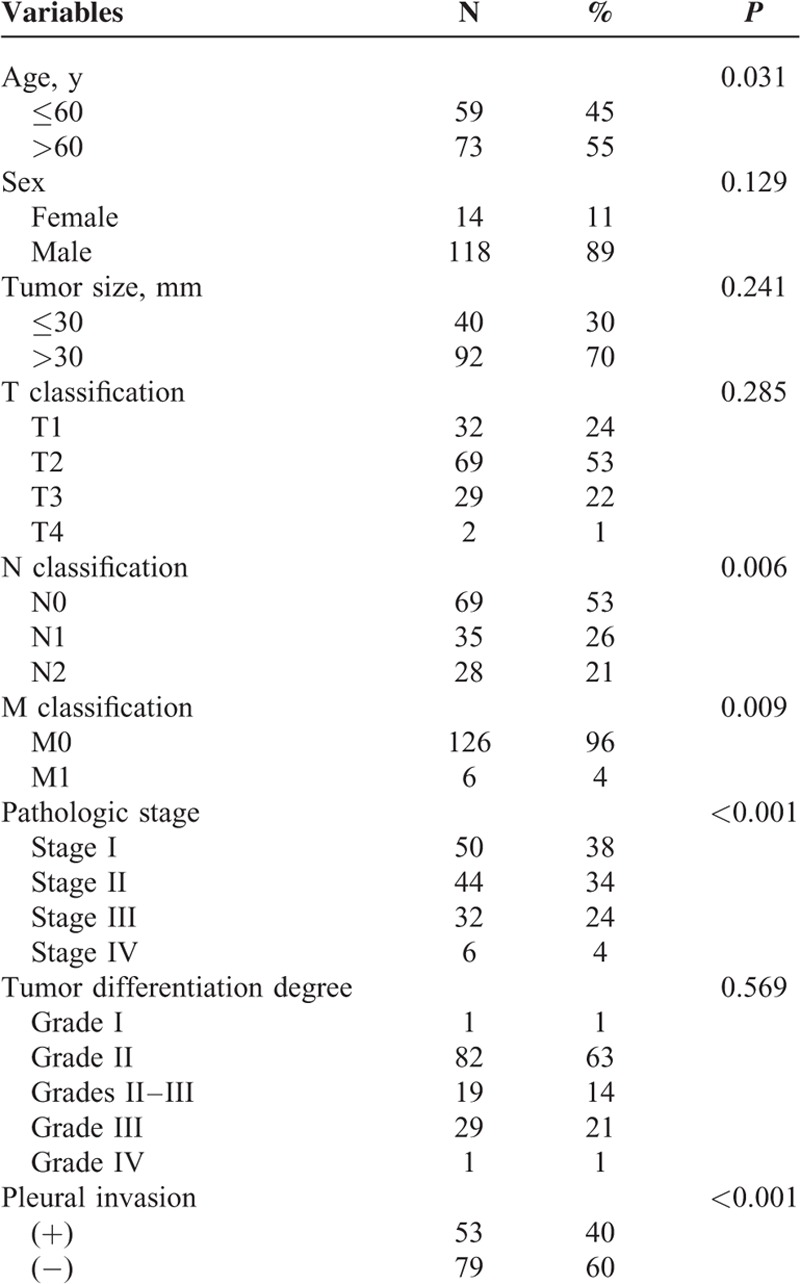
Histopathologic Analysis of LSCC
All hematoxylin and eosin slides of tumor for each case were reviewed. Each case was evaluated for fibrosis, necrosis, mitotic count, degree of cytologic atypia, and keratin pearl. In addition, invasion types (tumor budding type, large cell invasion type, and single cell invasion type) were reviewed. Some characteristics related to the tumor budding invasion type were also recorded, such as the minimal tumor nest size, the border of the nest, and the number of the buds.
The tumor budding was defined as the presence of small discrete clusters of tumor cells at the invasive edge (Figure 1A). After scanning the entire slides at ×100 magnification (10× objective lens), we can find the most invasive part by counting the number of the minimal tumor buds on the edge. To semiquantify budding, we counted the maximal number of the minimal tumor bud in the most invasive field at ×400 magnification (40× objective lens). Five high-power fields (HPF) were counted, and the average number was used for analysis in order to avoid error. We took 10 as the cut point and assessed all the slices that were evaluated as the tumor budding invasive type. In addition, we counted the number of the cells that composed the minimal tumor nest (MTN) and used 1, 5, and 15 as the cut point.
FIGURE 1.
Microscopic findings of lung squamous carcinoma (hematoxylin and eosin stain). Tumor budding is defined as a cluster of tumor cells composed of <5 tumor cells at the invasive margin of the tumor. Single cell invasion showed by arrows. Large cell invasion (arrows) was classified using the cut point of 4 lymphocytes (arrows) nearby in diameter. Mitosis was showed by arrow. Cytologic atypia was evaluated according to the size and shape of tumor cells. The severe degree was defined that the largest cell was twice larger than the smallest one. Fibrosis was evaluated using the cut point of 50%. Necrosis was evaluated using the cut point of 10%.
The single cell was defined as the single tumor cell within the stromal tissue at the invasive margin of the tumor (Figure 1B). It was a special component of tumor budding. In order to evaluate the relationship between single cell invasion and patient prognosis, we classified it as an independent subtype.
The large cell was defined as tumor cell whose nuclei diameter was the quadruple of a small lymphocyte nearby (Figure 1C). We scanned all the slices at ×100 magnification first and then assessed them at ×400 magnification in order to be more accurate. It was very simple to define this kind of invasion type, and the large cell was obvious even at ×100 magnification.
Mitotic count was evaluated at ×400 magnification (objective 40×). For each case, we examined between 30 and 50 HPF and recorded the average number of mitosis in 10 HPF. Using 15 as the cut point, we assessed all the slices (Figure 1D).
Cytologic atypia was apparent in LSCC. After scanning the fields of the tumor with the highest degree of atypia at ×100 magnification (objective 10×), we classified all the slices into 3 degrees (Figure 1E). The severe degree included slices in which tumor cells were bizarre and nucleoli were varied. Even some nucleoli were at least twice as large as others. The mild degree included slices in which the nucleoli were almost uniform in size. The moderate degree included slices in which nucleoli size and shape was between the severe and the moderate.
The percentage of fibrosis was evaluated at ×100 magnification (objective 10×) and used 50% as the cut point (Figure 1F). When the area of fibrosis was >50%, we recorded as positive. In addition, we used 10% as the cut point for necrosis (Figure 1G).
Statistical Analysis
Overall survival (OS) was defined from the date on which the first time diagnosis was made or the date of surgery until the date of death. If patients were still alive, the endpoint was the recent fellow-up date. Relationships between 2 features were analyzed using the χ2 tests. About the univariate survival analysis, we made the survival curves using the Kaplan-Meier method. We regarded the results had statistical significance only when the P values were <0.05. Then we did the multivariate analysis using the Cox proportional hazard model about the significant features in univariate survival analysis.
RESULTS
Patient Demographics and Clinical Features
We reviewed 132 patients with LSCC. Until the last time we knew their prognosis, 71 (53%) patients were alive. The clinicopathologic characteristics of the 132 patients with LSCC are summarized in Table 1. The age of the patients ranged from 38 to 80 years, and the average age was 56 years. The histologic differentiation degree of the patients was almost between grade II and grade III (98%). Ninety-six percent had the pathologic stage from stages I to III (stage I 38%, stage II 34%, and stage III 24%). Forty-one (31%) patients had tumor whose size was <30 mm. The tumor status was T1 in 33 patients (25%), T2 in 70 patients (52%), T3 in 29 patients (22%), and T4 in 2 patients (1%). Sixty-three patients (47%) had lymph nodes metastasis. Six patients (4%) were observed distant organs metastasis. Forty percent patients had pleural invasion. Using the χ2 tests, the relationships between these features and the outcomes could be shown, and the significant ones were lymph nodes metastasis (N0 vs N1 + N2, P = 0.006), distant organs metastasis (M0 vs M1, P = 0.009), pathologic stage (P < 0.001), and plural invasion (positive vs negative, P < 0.001).
Histopathologic Features
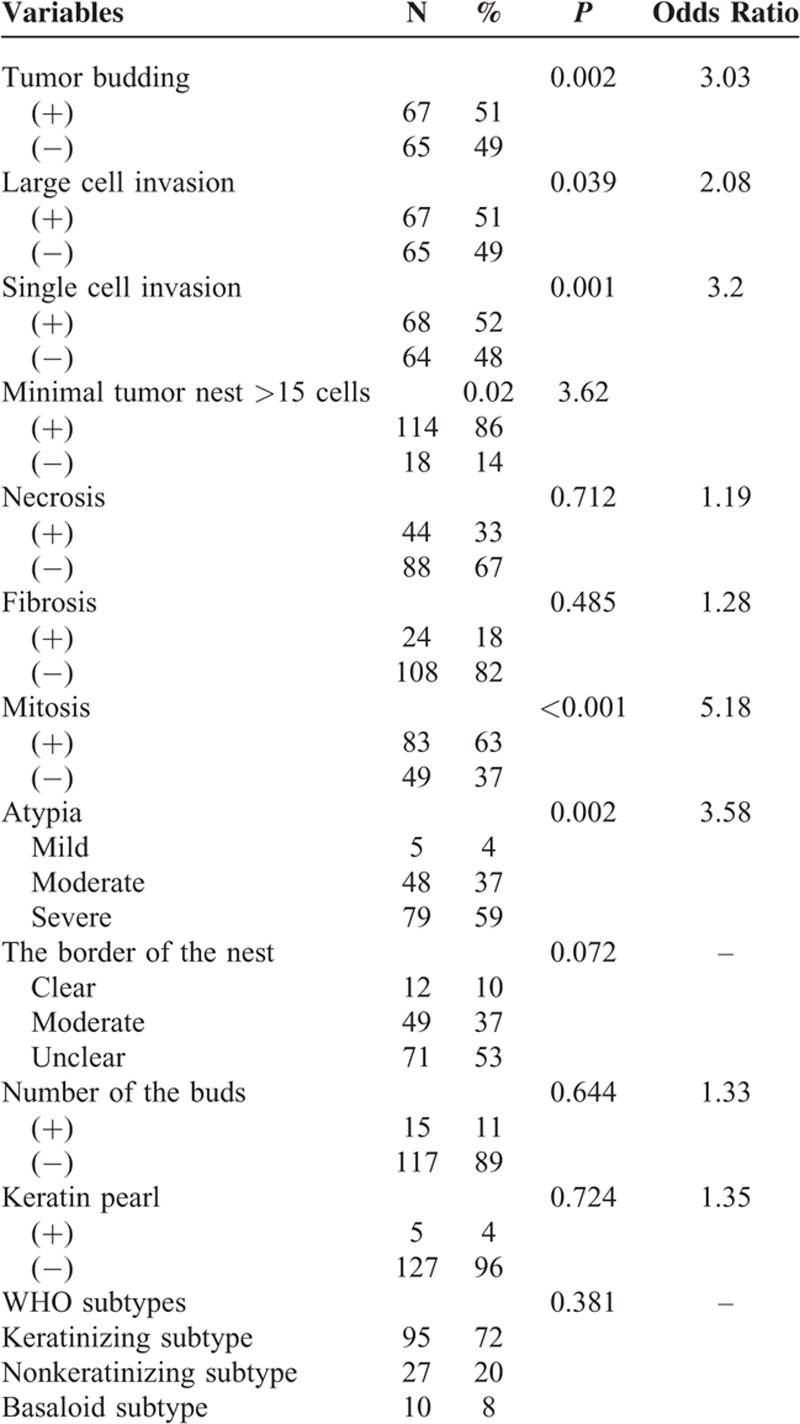
The histopathologic characteristics and the results of a univariate analysis are shown in Table 2. The slices of 132 patients with LSCC were evaluated. Tumor budding was observed in 67 (51%) of them. Large cell was observed in 67 (51%) of them whose nuclei size was 4 times bigger than the lymphocyte nearby. The single cell invasion was observed in 68 (52%) of them. In addition, 44 (33%) had necrosis using 10% as the cut point. Twenty-four (18%) were recorded fibrosis positive with the cut point of 50%. The mitosis in the slices was counted and 83 (63%) were regarded as positive using 15/10 HPF as cut point. The atypia was classified into 3 degrees: mild, moderate, and severe. Five patients (4%) were considered as mild degree whose tumor cells were relatively uniform in size and shape; 48 patients (37%) were considered as moderate; and 79 patients (59%) were considered as severe with apparent variety in size and shape of tumor cells. We categorized the 132 patients with LSCC recording to the new WHO classification standard [4] and the 2004 WHO classification. Meanwhile, 112 (84%) were papillary type, 10 (8%) were basaloid type, and 10 (8%) were clear cell type. Ninety-five (72%) were keratinizing type, 27 (20%) were nonkeratinizing type, and 10 (8%) were basaloid type. The associations between these characteristics and the prognosis were shown using χ2 tests. The significant features were tumor budding (P = 0.002), large cell (P = 0.039), single cell invasion (P = 0.001), mitosis count (P < 0.001), and atypia degree (P = 0.001). The other characteristics did not show significance according to statistical analysis.
TABLE 2.
Patient Histopathologic Features
Associations Between the Invasion Types and the Clinicopathologic Characteristics
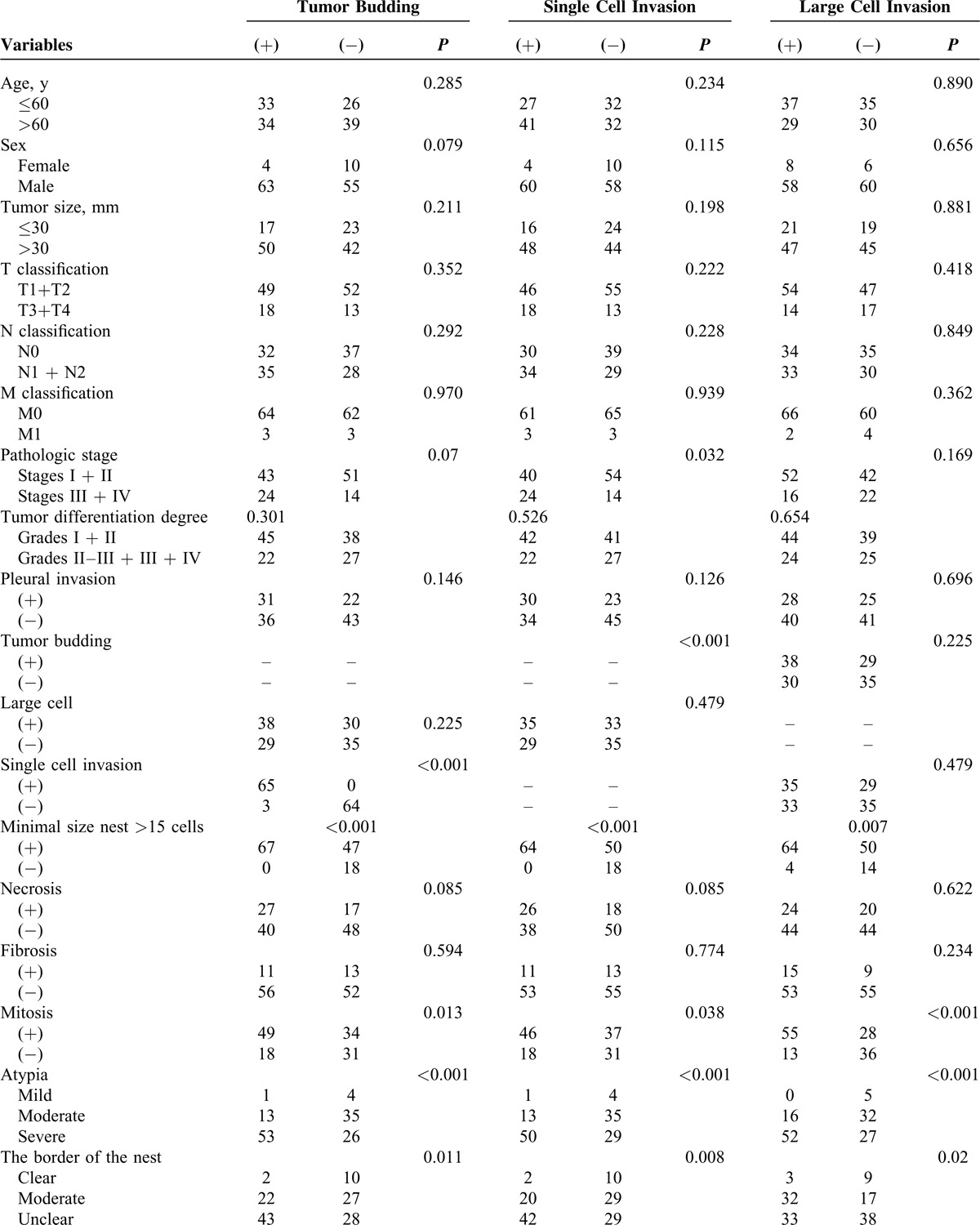
The associations between the invasion types and the clinical characteristics are summarized in Table 3 . Three invasion types (tumor budding invasion type, single cell invasion type, and large cell invasion type) were evaluated. Tumor budding was defined as the presence of a cluster of tumor cells, and the number of the cells was <5. Using χ2 tests, the tumor budding showed significant association with some histopathologic features: mitosis (P = 0.013), atypia (P < 0.001), and the border of the nest (P = 0.011). According to the same statistical analysis, single cell invasion showed significant relationship with mitosis (P = 0.038), atypia (P < 0.001), and the border of the nest (P = 0.008). The same as large cell invasion, the significant association was shown between this invasion type and mitosis (P < 0.001), atypia (P < 0.001), and the buds number (P = 0.019).
TABLE 3.
Associations Between Prognostic Factors and Clinicopathologic Factors
Associations Between the WHO Subtypes and the Clinical Characteristics
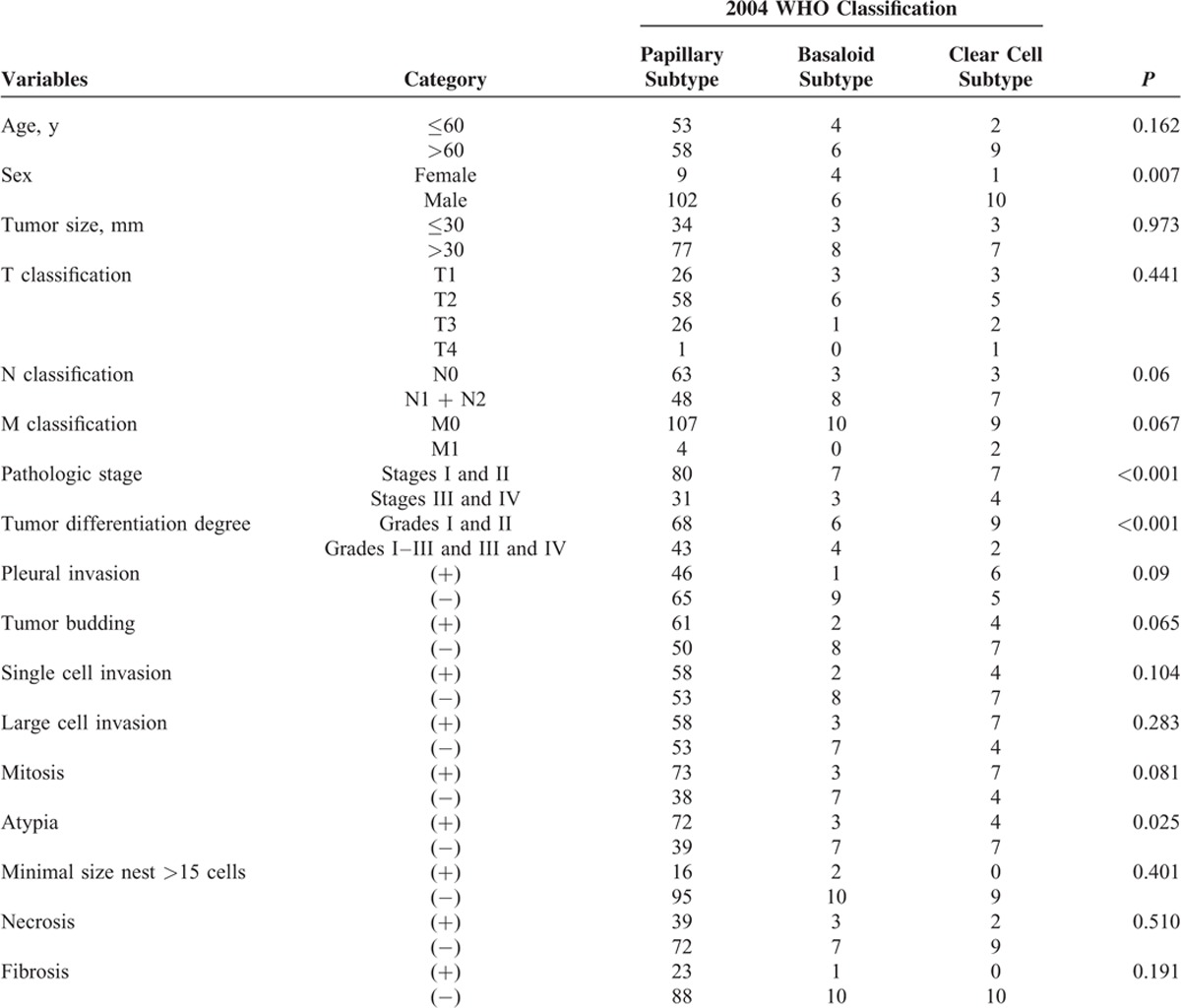
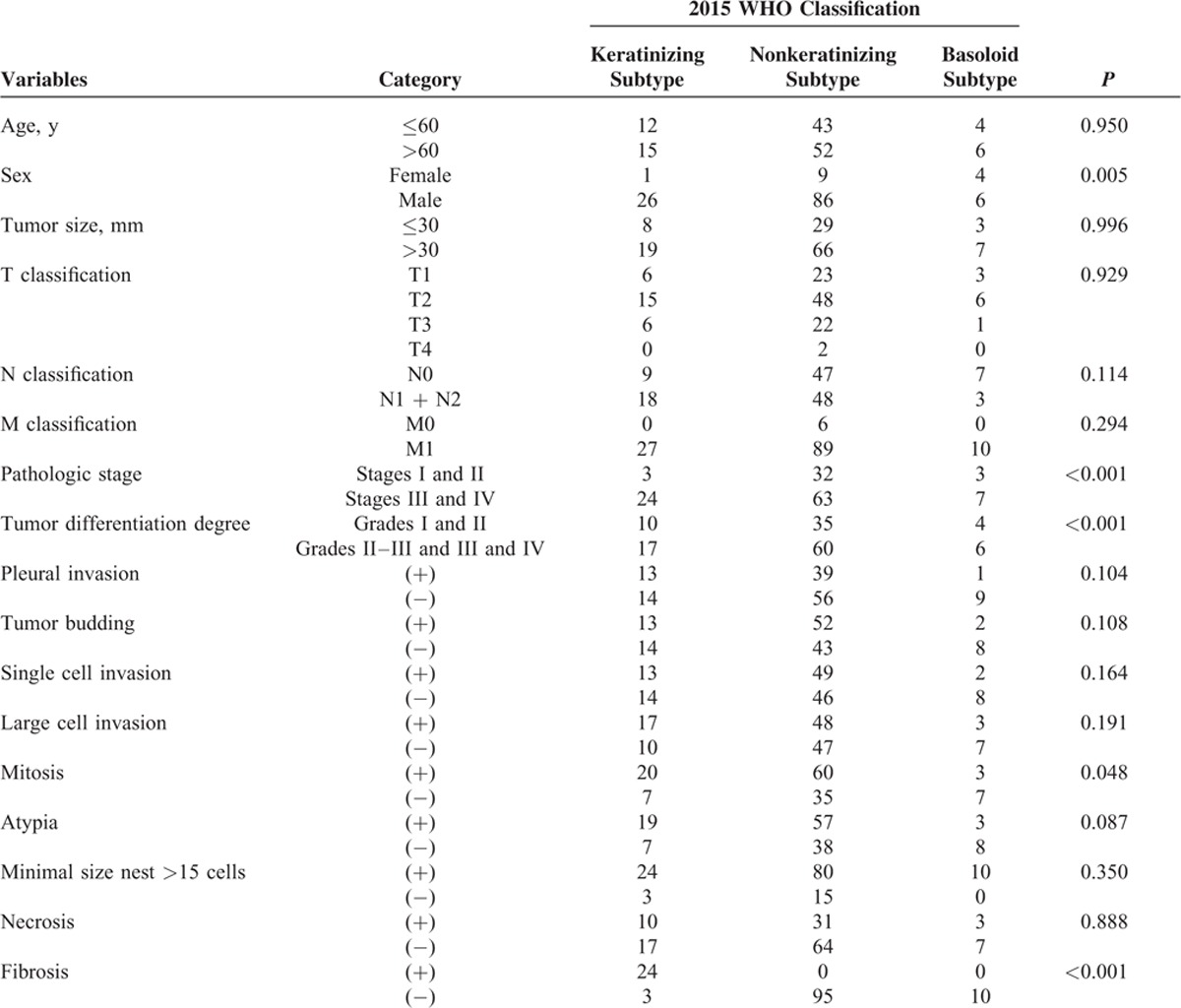
The associations between the WHO subtypes and the clinical characteristics are summarized in Tables 4 and 5. According to the 2004 WHO classification, cases were categorized into 4 subtypes: papillary subtype, basaloid subtype, clear cell subtype, and small cell subtype. Using the χ2 tests, some clinicopathologic features were associated with WHO subtypes, such as sex (P = 0.007), pathologic stage (P < 0.001), tumor differentiation degree (P < 0.001), pleural invasion (P = 0.009), and cytologic atypia (P = 0.025). According to the 2015 WHO classification, the previous papillary subtype, small cell subtype, and clear cell subtype were replaced by keratinizing and nonkeratinizing subtypes. Our research showed that the sex, pathologic stage, tumor differentiation degree, mitosis, and fibrosis had statistical significance with the new WHO classification.
TABLE 3 (Continued).
Associations Between Prognostic Factors and Clinicopathologic Factors
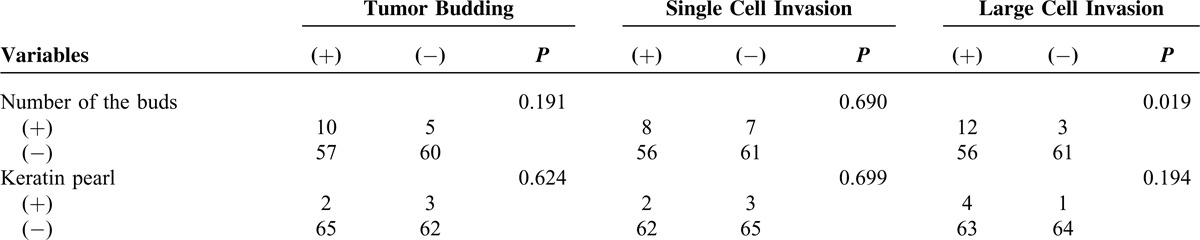
TABLE 4.
Associations Between Histopathologic Features and 2004 WHO Classification
Survival Analysis
Univariate Analysis of Clinical and Pathologic Characteristics
The univariate analysis of clinical and pathologic characteristics was summarized in Table 6. The 5-year OS was shorter in cases with lymph nodes metastasis (41%) than those absent (79%, P < 0.001). The 5-year OS was shorter in cases with distant organs metastasis (33%) than those without (62%, P < 0.001). Similarly, the cases with pleural invasion had shorter 5-year OS (30%) than those without (76%, P < 0.001).
TABLE 5.
Associations Between Histopathologic Features and NEW 2015 WHO Classification
First, we investigated the correlations between the WHO subtypes and the OS rate. The finding was that the relationship did not have statistical significance (P = 0.381). Patients with a basaloid subtype tumor had a slightly better 5-year OS than those with nonbasaloid tumors (68% vs 58%). Then our focus was turned to the invasion types. The patients with tumor budding invasion had poor prognosis. The 5-year OS of cases with tumor budding was shorter (45%) than the cases without (72%, P = 0.003; Figure 2A). The mean of survival time was 52 months with tumor budding and 68 months without tumor budding. Similar as tumor budding, the cases with single cell invasion had shorter 5-year OS (44%) than those absent (72%, P = 0.001; Figure 2B). According to the χ2 tests, the large cell invasion type was associated with poor follow up status (P = 0.037). However, the univariate analysis using χ2 tests, the association between the large cell invasion, and the 5-year OS had no statistical significance (P = 0.104; Figure 2C). Using a cut point of 15/10 HPF, the patients with a higher grade mitosis count had poor outcomes. The 5-year OS of the cases with a higher grade mitosis count was 47%, and the cases without was 79% (P < 0.001; Figure 2D). The degree of atypia was classified into 3 groups: mild, moderate, and severe. The 5-year OS of the mild group was not able to be recorded because of a too small patient number. The 5-year OS of the moderate group was 68%, and the sever group was 52% (P = 0.009; Figure 2E).
FIGURE 2.
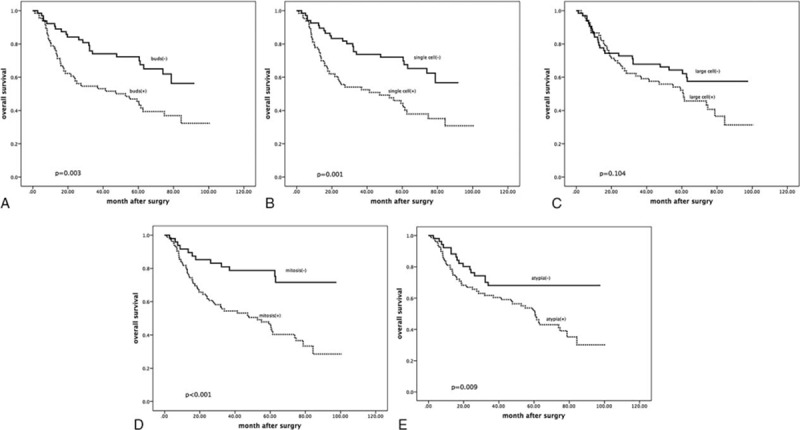
Tumor budding and cumulative survival of patients with lung squamous carcinoma. The 5-year OS of cases with tumor budding was shorter (47%) than the cases without (72%, P = 0.003). The mean of survival time was 53 months with tumor budding and 69 months without tumor budding. Patients with single cell invasion had shorter 5-year OS (46%) than those absent (72%, P = 0.001). Patients with single cell invasion had shorter 5-year OS (52%) than those absent (66%), but there was no statistical significance (P = 0.059). Patients with high mitosis count degree had shorter 5-year OS (48%) than those absent (78%, P < 0.001). Patients with severe cytologic atypia degree had shorter 5-year OS (54%) than those with moderate degree (63%, P = 0.035).
Multivariate Analysis of Clinical and Pathologic Characteristics
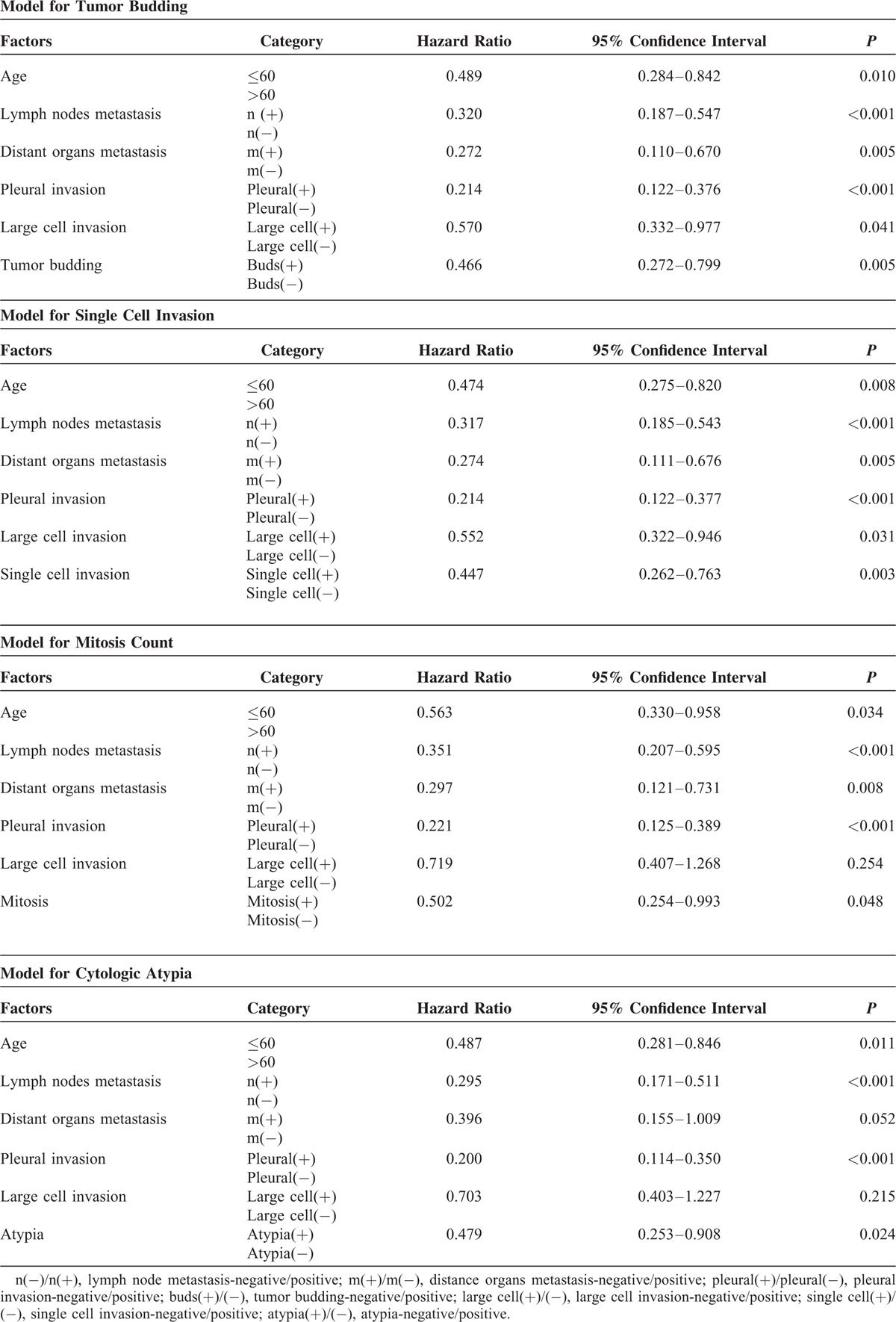
The multivariate analysis of clinical and pathologic characteristics was summarized in Table 7. Using the Cox proportional hazard model, we analyze the features that showed statistical significance in univariate analysis. Because the single cell invasion was a specific component of tumor budding and the correlation between them might influence the result, we analyze these features separately. The tumor budding invasion (hazard ratio [HR] = 0.466, P = 0.005), single cell invasion (HR = 0.447, P = 0.003), mitosis (>15/HPF, HR = 0.502, P = 0.048), and higher atypia degree (severe degree, HR = 0.479, P = 0.024) were independent factors of decreased OS.
TABLE 6.
Survival Analysis–Univariate Analysis of Clinical and Pathologic Characteristics
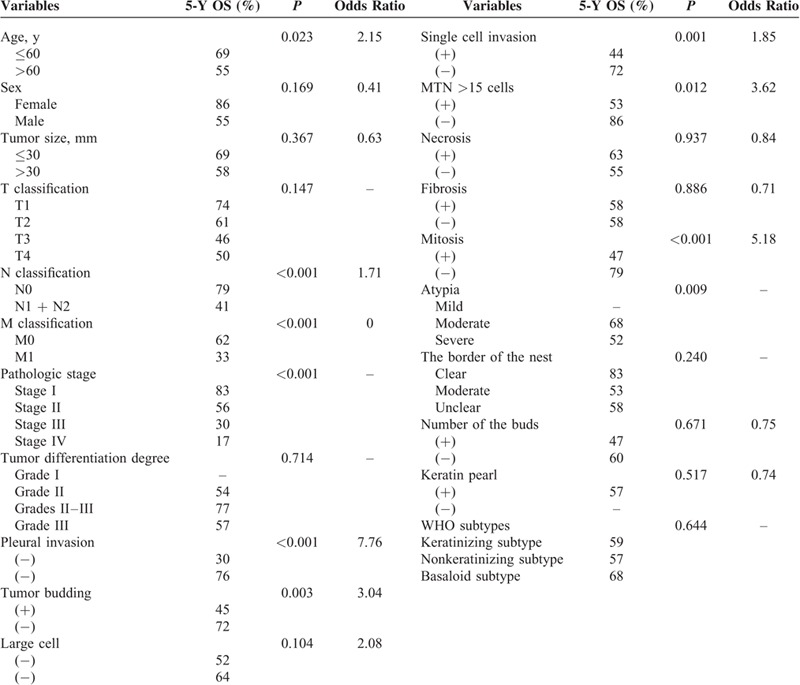
TABLE 7.
Multivariate Prognostic Analysis of 5-Year Overall Survival in All Stages (n = 134)
DISCUSSION
In the present study, we made an effort to analyze the different invasion types of patients with LSCC in order to clarify the independent prognosis indicators of LSCC. We demonstrated that tumor budding, single cell invasion, large cell invasion, mitosis, and cytologic atypia degree were associated with poor prognosis. To the best of our knowledge, although the 2004 WHO classification has been published and used for years, the papillary and clear cell carcinoma subtypes have no clear correlation with the clinical outcomes. The small cell variant and basaloid variant have association with poor prognosis. In the contrast, the invasion types probably have clinical significance.
The previous study has found that tumor budding has correlation with worse OS in many kinds of tumors, such as colorectal cancer, tongue squamous carcinoma, and esophagus squamous carcinoma.11,12,14,19 In addition, the recent study also showed the tumor budding was a poor prognostic indicator in lung adenocarcinoma and squamous carcinoma.16–18 As a study used actual budding number per HPF at ×200 magnification,18 we used 10 buds/HPF at ×400 magnification. For accuracy, we counted 5 fields and recorded the average. In addition, this method provided good reproducibility and reduced the error rate. However, the budding number had no statistical significance. It is interesting that previous studies showed different results about tumor budding. Some were significant17 and others were not.18 The factors influenced the results maybe due to variations in human races, disease stage, treatment difference, or interobserver difference definition in budding count patterns. Ethnic difference maybe an important factor because the study whose result was similar with us reviewed patients from Japan and the other was from the United States.
As tumor budding showed relationship with the poor prognosis, the mechanism of tumor budding making effort on the metastasis, and migration is a significant question to be studied. In the gastrointestinal carcinomas, the visible correlate of cells undergoing epithelial-mesenchymal transition (EMT) is thought to be the presence of single cell or clusters of cells which is composed of <5 tumor cells at the invasive edge, termed tumor budding.23–25 Recent studies showed that in epithelial carcinomas the appearance of the EMT in the tumor microenvironment is related to cancer cell migration and metastasis.23,24,26 These evidence prompt that tumor budding is correlated with tumor aggressiveness. A research showed that tumor budding could be a source of circulating cancer cells, leading to the establishment of metastasis at distant sites in the process of mesenchymal-epithelial transition.27 In addition, we conjectured that the isolated single tumor cell detached from the primary tumor probably be regarded as anoikis-resistant cells, and, consequently, has relationship with the invasiveness and metastasis of the malignant carcinoma. The anoikis-resistant cells loss the normal characteristic of apoptosis under the condition of losing the cell–cell or cell–matrix interaction. Instead, they have a capability to resist anoikis and survive in the blood and lymphatic circulation and form secondary tumor eventually.28 It is interesting to do further research on the relationship between tumor budding and anoikis-resistance. All in all, as to the lung squamous carcinoma, more researches are needed to explore the mechanism of the tumor aggressiveness.
With regard to the single cell invasion, previous studies have demonstrated that it was a poor prognostic indicator in LSCC.17,20 Our study made the same conclusion. As the single cell was a kind of group of MTN, the number of tumor cells in MTN was counted with cut point of 1, 5, and 15. And we analyzed the large tumor nest composed of >15 tumor cells. A previous study discovered that the size of MTN was a significant prognostic factor.20 They defined MTN as large (>6 tumor cells), small (2–5 tumor cells), or single cell. Similar as our research, the large MTN had better prognosis.
Using the optimal cut point of 4 lymphocytes, our study found that the large cell invasion was related to poor prognosis. According to the χ2 tests, the large cell invasion was a poor prognostic factor (P = 0.035). However, using the Kaplan-Meier method and compared using the log-rank test, the univariate analysis result showed that large cell invasion had no statistical significance (P = 0.104). The reasons due to this phenomenon may be that the sample of our study was not large enough. It is necessary to do more investigation using more cohorts and demonstrate this kind of invasion type correlated with worse outcome.
The nuclear grading system we analyzed contained the mitosis and the cytologic atypia. Although the prognostic value of nuclear grades in lung adenocarcinomas has been demonstrated,29 the research in lung squamous carcinoma is limited. Previous study in adenocarcinoma showed that the higher mitosis degree had better prognosis.30 However, our study showed that the mitosis and the atypia degree were both related to worse OS (mitosis P < 0.001, atypia P = 0.009). This kind of phenomenon might indicate the difference in mechanism of lung adenocarcinoma and LSCC. Therefore, we considered that the nuclear grading system for LSCC might be developed and published as a method to guide clinical treatment in the future.
A previous study clarified that fibrous stroma was associated with poor prognosis in LSCC.31 However, according to our study, the fibrosis was not associated with prognosis. This difference may be due to variations in ethnic difference of patients, the location of the tumor, or the definition for fibrosis. More study was needed to demonstrate this conclusion.
In addition, the sample size of our research was not big enough. Based on this study of indicating the correlation between invasion types and prognosis of lung squamous carcinoma, we are trying to collect more lung squamous carcinoma cases to support our research and do more deeply exploration on finding significant independent prognostic factors.
In conclusion, we clearly showed that invasion type (tumor budding, single cell invasion) and the nuclear grading system (mitotic count, cytologic atypia) were independent prognostic indicators of LSCC. Although the cut points for histologic features were well defined, additional studies are needed to evaluate interobserver reproducibility and to prove its efficacy. Based on our study, more studied are needed to help establish a new classification or grading system for LSCC that can indicate the clinical treatment strategies in the future.
Footnotes
Abbreviations: EMT = epithelial-mesenchymal transition, HPF = high-power fields, LSCC = lung squamous carcinoma, MTN = minimal tumor nest, NSCLC = nonsmall cell lung carcinoma, OS = Overall survival, WHO = World Health Organization.
The work was supported by Provincial science and technology development plan of Shandong (2012GG0021836), Provincial Natural Science Foundation of Shandong (ZR2013HZ001) and the National Natural Science Foundation of China (project 81301728).
YZ and HS contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin 2007; 57:43–66. [DOI] [PubMed] [Google Scholar]
- 2.Beadsmoore CJ, Screaton NJ. Classification, staging and prognosis of lung cancer. Eur J Radiol 2003; 45:8–17. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007; 2:706–714. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD. The 2015 WHO classification of lung tumors. Pathologe 2014; 35 suppl 2:188. [DOI] [PubMed] [Google Scholar]
- 5.Schiller JH, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92–98. [DOI] [PubMed] [Google Scholar]
- 6.Hong J, et al. Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small cell lung cancer. Korean J Intern Med 2010; 25:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi A, et al. Pemetrexed in the treatment of advanced non-squamous lung cancer. Lung Cancer 2009; 66:141–149. [DOI] [PubMed] [Google Scholar]
- 8.Sandler A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 9.Hainsworth JD, et al. BRIDGE: an open-label phase II trial evaluating the safety of bevacizumab + carboplatin/paclitaxel as first-line treatment for patients with advanced, previously untreated, squamous non-small cell lung cancer. J Thorac Oncol 2011; 6:109–114. [DOI] [PubMed] [Google Scholar]
- 10.Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. J Thorac Oncol 2012; 7:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, et al. Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med 2011; 40:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanishi Y, et al. Correlation between tumor budding and post-resection prognosis in patients with invasive squamous cell carcinoma of the thoracic esophagus. World J Surg 2011; 35:349–356. [DOI] [PubMed] [Google Scholar]
- 13.Ohike N, et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am J Surg Pathol 2010; 34:1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno H, et al. Tumour ’budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002; 40:127–132. [DOI] [PubMed] [Google Scholar]
- 15.Sarioglu S, et al. Tumor budding as a prognostic marker in laryngeal carcinoma. Pathol Res Pract 2010; 206:88–92. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi Y, et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol 2010; 5:1361–1368. [DOI] [PubMed] [Google Scholar]
- 17.Kadota K, et al. Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J Thorac Oncol 2014; 9:1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuda R, et al. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol Med Rep 2012; 6:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitrovic B, et al. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol 2012; 25:1315–1325. [DOI] [PubMed] [Google Scholar]
- 20.Maeshima AM, et al. Histologic prognostic factors for small-sized squamous cell carcinomas of the peripheral lung. Lung Cancer 2006; 52:53–58. [DOI] [PubMed] [Google Scholar]
- 21.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005; 40:90–97. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 23.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 2010; 1:651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013; 13:97–110. [DOI] [PubMed] [Google Scholar]
- 25.Prall F. Tumour budding in colorectal carcinoma. Histopathology 2007; 50:151–162. [DOI] [PubMed] [Google Scholar]
- 26.Kevans D, et al. Epithelial-mesenchymal transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int J Surg Pathol 2011; 19:751–760. [DOI] [PubMed] [Google Scholar]
- 27.Koelzer VH, et al. Tumor budding in upper gastrointestinal carcinomas. Front Oncol 2014; 4:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liotta LA, Kohn E. Anoikis: cancer and the homeless cell. Nature 2004; 430:973–974. [DOI] [PubMed] [Google Scholar]
- 29.Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer 2010; 116:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadota K, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol 2012; 25:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi Y, et al. Fibrous stroma is associated with poorer prognosis in lung squamous cell carcinoma patients. J Thorac Oncol 2011; 6:1460–1467. [DOI] [PubMed] [Google Scholar]


