Abstract
This meta-analysis examined whether early decompressive craniectomy (DC) can improve control of intracranial pressure (ICP) and mortality in patients with traumatic brain injury (TBI).
Medline, Cochrane, EMBASE, and Google Scholar databases were searched until May 14, 2015, using the following terms: traumatic brain injury, refractory intracranial hypertension, high intracranial pressure, craniectomy, standard care, and medical management. Randomized controlled trials in which patients with TBI received DC and non-DC medical treatments were included.
Of the 84 articles identified, 8 studies were selected for review, with 3 randomized controlled trials s having a total of 256 patients (123 DCs, 133 non-DCs) included in the meta-analysis. Patients receiving DC had a significantly greater reduction of ICP and shorter hospital stay. They also seemed to have lower odds of death than patients receiving only medical management, but the P value did not reach significance (pooled odds ratio 0.531, 95% confidence interval 0.209–1.350, Z = 1.95, P = 0.183) with respect to the effect on overall mortality; a separate analysis of 3 retrospective studies yielded a similar result.
Whereas DC might effectively reduce ICP and shorten hospital stay in patients with TBI, its effect in decreasing mortality has not reached statistical significance.
INTRODUCTION
Traumatic brain injury (TBI) is associated with elevated intracranial pressure (ICP), primarily as a result of cerebral edema, and can lead to a decrease of cerebral blood flow and brain stem herniation, and is the most common cause of death and disability after severe TBI.1 The treatment objectives after TBI include preventing and reversing elevation of ICP to maintain satisfactory cerebral perfusion pressure (CPP) and prevent further brain injury.1 Elevated ICP may be treated initially to maintain normothermia and sedation with moderate hypocapnia, mannitol, and hypertonic saline.1 When these measures fail, second-line therapies are added, which include barbiturates, hyperventilation, moderate hypothermia, and ventriculostomy.1
Decompressive craniectomy (DC) can reduce ICP and increase CPP, but its use and timing remain controversial.2,3 DC has generally been used as a last resort to control ICP when medical therapies failed.1,4,5 Whereas some studies found DC associated with unfavorable outcomes,6 others found DC and medical management both could lead to similar outcomes.7,8 DC may be associated with improved prognosis and survival,9–13 and the time from injury to DC might be the variable with the greatest influence on outcomes.14,15 Younger age and higher initial Glasgow Coma Scale (GCS) scores were also associated with favorable outcomes in patients who receive DC.16 Many have suggested that DC should be performed as soon as possible after trauma, to prevent secondary injuries due to uncontrolled intracranial hypertension.10,12,13,17–20
The purpose of this study was to perform a meta-analysis of several randomized controlled trials (RCTs) to determine if early DC can significantly improve control of ICP and overall mortality rate in patients with TBI.
MATERIALS AND METHODS
Literature Search Strategy and Selection Criteria
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines. Medline, Cochrane, EMBASE, and Google Scholar databases were searched until May 14, 2015, using combinations of the following search terms: traumatic brain injury, refractory intracranial hypertension, high intracranial pressure, craniectomy, standard care, and medical management. Reference lists of relevant studies were hand-searched.
Inclusion criteria were as follows: RCTs and 2-arm studies (only RCTs were included in the meta-analysis); patients with TBI who received DC as an intervention; and patients who reported at least one of the outcomes. Letters, comments, editorials, case reports, proceedings, personal communications, 1-arm studies, and studies in which no quantitative outcome data were reported were excluded. Studies in which the patients had dilated and/or unreactive pupils, mass lesions, spinal cord injury, or cardiac arrest at the scene of the injury were also excluded. Studies were identified by the search strategy by 2 independent reviewers. When there was uncertainty regarding eligibility, a third reviewer was consulted.
Data Extraction
Information and data extracted from the eligible studies included the name of the first author, year of publication, study design, number of participants in each treatment group, participants’ age and sex, details of treatment received, ICP before and after treatment measured at different time points, and overall mortality.
Outcome Measures and Data Analysis
The primary outcome measure was overall mortality, and the secondary outcome was ICP reduction. For overall mortality, odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated and compared between DC and non-DC groups. An OR >1 indicates that DC is associated with higher risk of death, whereas an OR <1 indicates that DC is associated with a lower risk of death as compared to non-DC patients. For ICP, differences in means between DC and non-DC groups were calculated. A chi-square test of homogeneity was performed by using Cochran Q statistic and I2. For the Q statistic, a value of P < 0.10 was considered to indicate statistical significance for heterogeneity. I2 illustrates the percentage of the total variability in effect estimates among trials that is due to heterogeneity rather than to chance. Random-effects models (DerSimonian–Laird method) of analysis were used if heterogeneity was detected (I2 > 50% or Q statistic P < 0.10). Otherwise, fixed-effects models were used. Sensitivity analysis for overall mortality was performed based on the leave-one-out approach. Pooled ORs and differences in means were calculated, and a 2-sided value of P <0.05 was considered statistically significant. All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
Quality Assessment
The Delphi list21 was used to assess quality of the 3 included studies. The quality assessment was performed by 2 separate reviewers, with a third reviewer acting as a consultant for any uncertainty.
Ethic Review
Meta-analyses do not involve humans and do not require IRB review.
RESULTS
Literature Search
A flow diagram of study selection is shown in Figure 1. A total of 84 articles were identified in the database search, and after removal of duplicates and those not meeting the inclusion criteria, 16 full-text articles were assessed for eligibility. Of these 16 articles, 8 were subsequently excluded, the reasons for which are shown in Figure 1. Thus, 8 studies6–13 were included in the systematic review, with 3 RCT studies6,11,13 and 3 retrospective studies7,10,12 grouped separately to perform meta-analysis.
FIGURE 1.
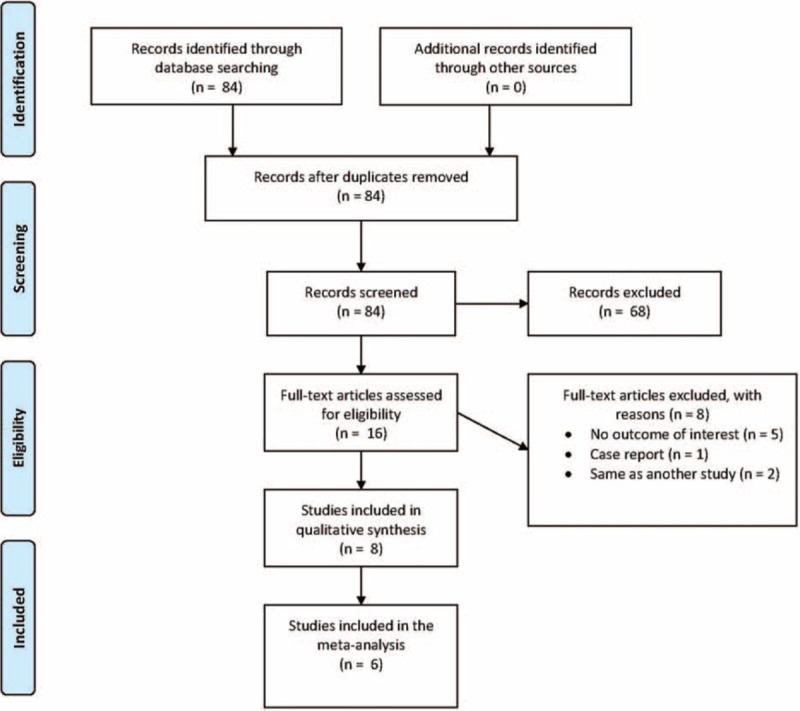
Flow diagram of study selection.
Study Characteristics
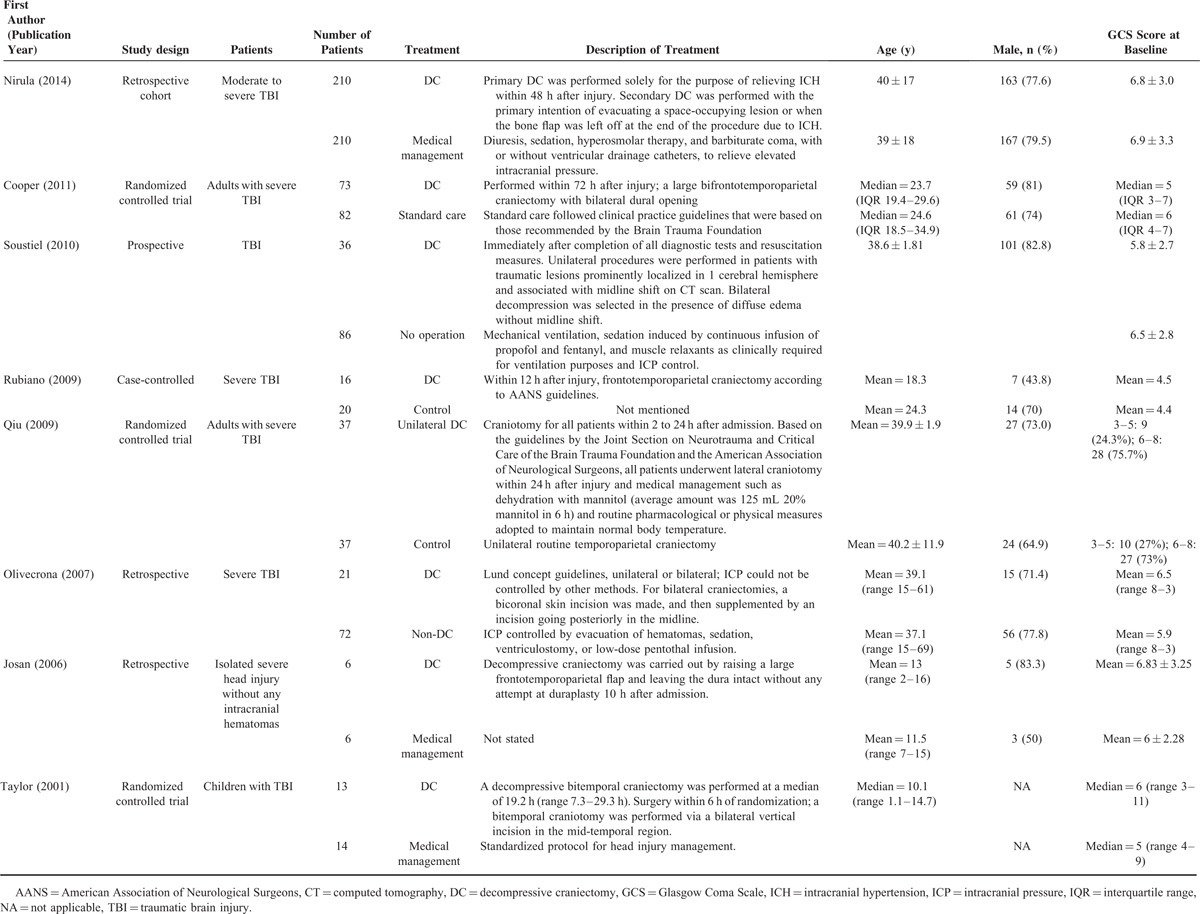
Of the 8 studies, there were 3 RCTs, 3 retrospective studies, 1 prospective study, and 1 case-control study. The basic characteristics of the 8 studies are summarized in Table 1. The number of patients in the studies ranged from 12 to 420, and the total number of patients included in the meta-analysis was 256 (123 DC, 133 non-DC patients). The mean or median age of patients ranged from 10.1 to 45.4 years, and the majority were male. The mean or median GCS score at baseline ranged from 5 to 7.2.
TABLE 1.
Baseline Characteristics of Studies Included in the Qualitative Synthesis
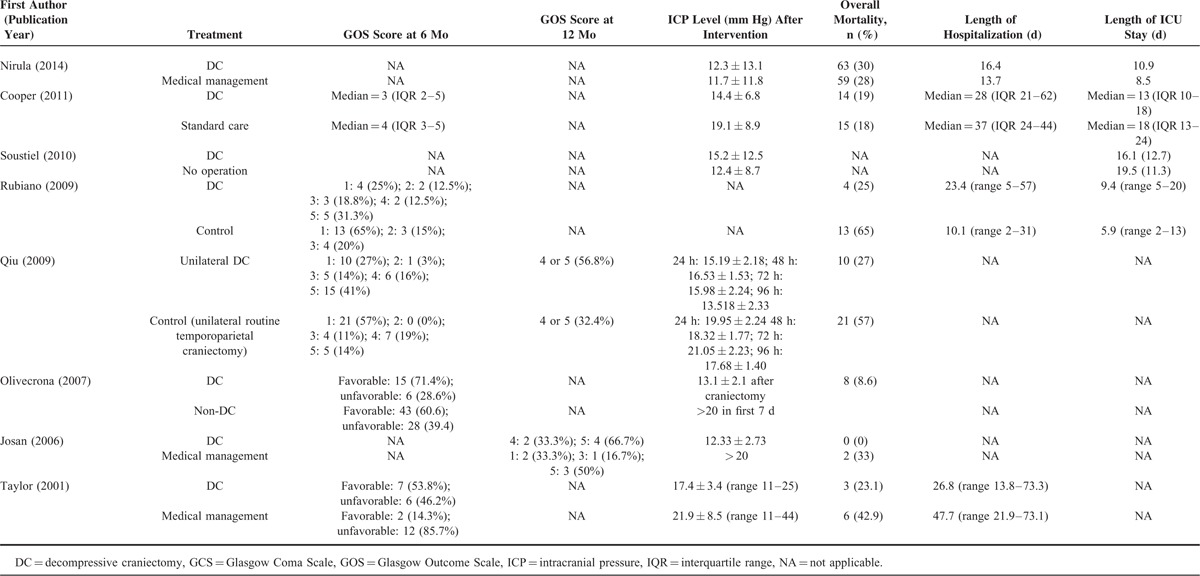
Decompressive craniectomy-related outcomes are summarized in Table 2. The overall mortality rates ranged from 0% to 65%. The proportion of patients with a favorable functional outcome (Glasgow Outcome Scale [GOS] score of 4 or above at 6 months) ranged from 14.3% to 71.4%, and the proportion was generally higher in the DC group than in the non-DC group across studies. Patients in most studies who received DC had lower ICP levels than those who did not receive DC.
TABLE 2.
Summary of Decompressive Craniectomy-related Outcomes
Outcome Measures: Overall Mortality, ICP Reduction, and Hospital Stay
Three RCTs were included in the meta-analysis for the effect of DC on overall mortality and ICP reduction after intervention.3,11,13 Two studies were included to examine the association between DC and hospital stay. We also performed an analysis for the effect of DC on overall mortality, with data from 3 retrospective studies for comparison.
For the effect on overall mortality rate, there was significant heterogeneity when data from the 3 RCT studies were pooled (Q = 4.43, df = 2, P = 0.109, I2 = 54.8%); therefore, a random-effects model of analysis was used. Patients who underwent DC had approximately half the risk of death as compared with those who had not undergone DC; however, the P value did not reach statistical significance (pooled OR 0.531, 95% CI 0.209–1.350, Z = 1.95, P = 0.183; Fig. 2A).
FIGURE 2.
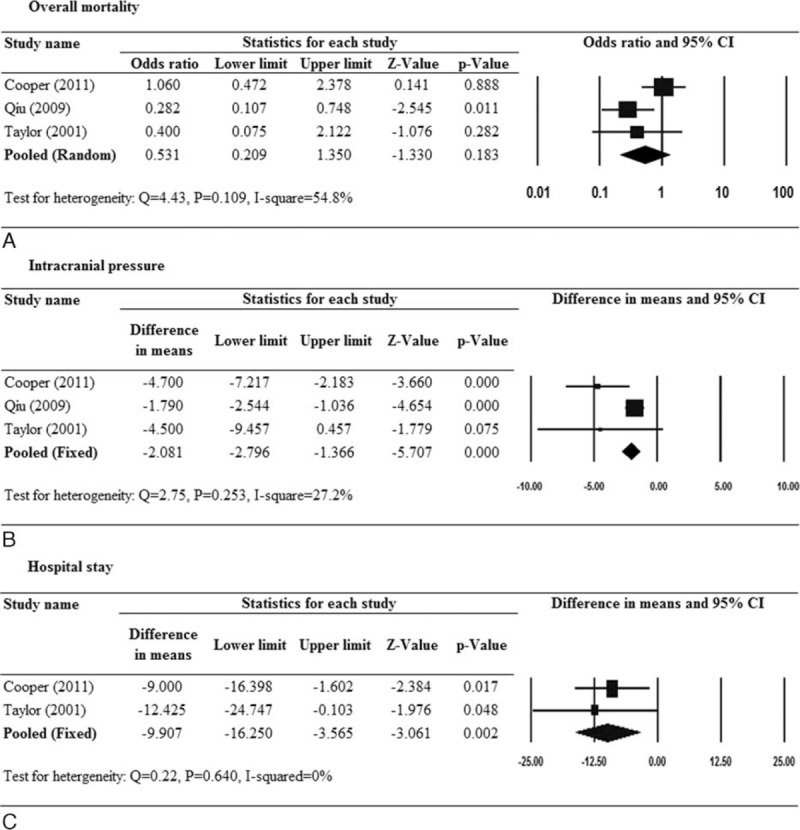
Forest plots for the effect of decompressive craniectomy versus medical management with respect to (A) overall mortality, (B) intracranial pressure, and (C) hospital stay. Only randomized controlled trials were included.
As a comparison, we performed a separate analysis for effect on overall mortality using data reported by 3 retrospective studies.7,10,12 There was significant heterogeneity among the studies (Q = 6.79, P = 0.034, I2 = 70.5%); therefore, a random-effects model of analysis was used. The pooled results showed a lower odds of death with DC, but the difference did not reach significance (pooled OR 0.422, 95% CI 0.091–1.961, Z = −1.101, P = 0.271) (forest plot not shown).
For the effect on ICP reduction, there was no significant heterogeneity across the 3 RCTs (Q = 2.75, P = 0.253, I2 = 27.2%); therefore, pooled estimates were generated by a fixed-effect model. A significant reduction of ICP was found in the DC group as compared with the non-DC group (pooled difference in means −2.081, 95% CI −2.796 to −1.366, P < 0.001; Fig. 2B).
For hospital stay, 2 RCTs were included in the analysis and a fixed-effects model was used since there was little heterogeneity among the studies (Q = 0.22, P = 0.640, I2 = 0%). The pooled results showed that the hospital stay was about 10 days less in the DC group as compared with the non-DC group (pooled difference in means −9.907, 95% CI −16.250 to −3.565, P = 0.002; Fig. 2C).
Sensitivity Analysis
The results of sensitivity analysis for overall mortality and ICP reduction are shown in Figure 3. When the study by Cooper et al6 was removed, the effect of DC on mortality became significant (pooled OR 0.308, 95% CI 0.133–0.715, P = 0.006; Fig. 3A). For ICP reduction and hospital stay, none of the included studies alone had a significant impact on the direction and magnitude of the association (Fig. 3B and C).
FIGURE 3.
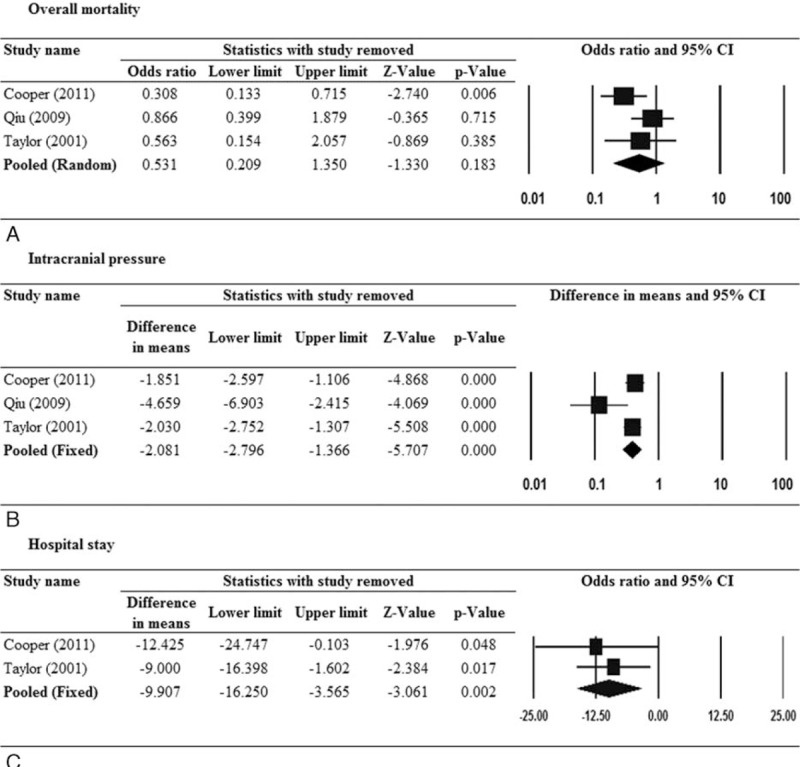
Sensitivity analysis for the effect of decompressive craniectomy versus medical management with respect to (A) overall mortality, (B) intracranial pressure, and (C) hospital stay. Only randomized controlled trials were included.
DISCUSSION
This meta-analysis of 3 RCTs showed that for patients with TBI, DC lowers ICP to a greater degree than conventional management, but its effect in reducing mortality rate was less clear. While the findings suggest that DC was associated with decreased mortality, the results did not reach statistical significance unless 1 of the 3 RCTs was removed. Similarly, analysis of 3 retrospective studies indicated that DC was not associated with lower mortality. While DC appeared to be associated with a decreased hospital stay, only 2 RCTs were available for analysis.
In theory, DC is believed to reduce ICP by allowing edematous brain tissue to expand and thus improve perfusion, ultimately leading to a reduction of the area of damaged tissue and neurological deficits. Few studies, however, have examined the physiology of this theory. In a seminal study, Schaller et al22 performed a standard left-side craniectomy in cats and examined regional cerebral blood flow (CBF), cerebral metabolic rate of oxygen (CMRO2), and cerebral metabolic rate of glucose (CMRglc) in the brain tissue underneath the craniectomy at 2, 20, and 28 hours. The results showed that CBF significantly decreased (P < 0.01) and oxygen extraction fraction (OEF) (P < 0.05) significantly increased, and CMRO2 and CMRglc were decreased only in regions with most severe CBF reduction, and the effects were present for at least 20 hours regardless of whether or not a corrective cranioplasty was performed.
One of the larger RCTs to examine the effect of DC in patients with TBI was the DECRA (Decompressive Craniectomy in Patients with Severe Traumatic Brain Injury) trial performed from 2002 to 2010.6 Adults with severe diffuse TBI and intracranial hypertension refractory to first-tier medical management were randomized to receive either standard care or bifrontotemporoparietal DC. Whereas patients in the DC group had shorter duration with ICP above the treatment threshold, fewer interventions needed to reduce ICP, and fewer days in the intensive care unit (ICU), the DC group had worse extended GOS scores (OR 1.84, 95% CI 1.05–3.24, P = 0.03), and a greater risk of an unfavorable outcome (OR 2.21, 95% CI 1.14–4.26, P = 0.02). Rates of death at 6 months were similar in the DC (19%) and standard-care groups (18%). The DECRA trial was criticized by some for more severe primary TBI sustained in patients of the DC arm, the fact that the ICP treatment threshold of >20 mm Hg for >15 minutes did not reflect clinical practice, and that there was a high cross-over rate from the standard care to the DC group.23 Another RCT by Qiu et al11 randomized 74 patients with unilateral acute post-traumatic brain swelling to receive either unilateral DC or unilateral routine temporoparietal craniectomy. The degree of ICP-lowering was greater in the DC group, mortality rates at 1 month after treatment were 27% in the DC group and 57% in the temporoparietal craniectomy group (P = 0.010), and good neurological outcome (GOS score of 4–5) rates 1 year after injury were 56.8% and 32.4%, respectively (P = 0.035). The third RCT included in the meta-analysis compared outcomes of 13 children with TBI who received early DC with those of 14 who were managed medically, and reported a larger mean ICP reduction in the DC group than in medical management group at 48 hours after the procedure (8.89 mm Hg vs 3.69 mm Hg, respectively).13 Mortality rate was also significantly lower in the DC group than in the medical management group (23.1% vs 42.9%, respectively).
There were 5, two-arm studies included in the qualitative synthesis,6–8,10,12 and they reported mixed results with respect to ICP reduction and overall outcomes of DC versus medical management. Two reported lower mortality with DC as compared with medical management,10,12 1 reported similar mortality,6 and 2 did not provide complete the mortality data.8,9 Several studies suggested that DC was associated with improved outcomes in patients with TBI.17,19,20,24 For example, Akyuz et al17 compared the outcomes of TBI patients who received DC as a first-tier or second-tier treatment, and found that patients in whom DC was used as a first-tier treatment had better GOS scores.
The international multicenter RESCUEicp (Randomized Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of Intra-Cranial Pressure) trial comparing optimal medical management with DC for the management of intracranial hypertension (>25 mm Hg) refractory to first-line treatment following TBI, has recently completed the enrollment goal of 400 patients, and hopes to determine the role of DC in patients with TBI when ICP continues to increase.25 Whereas goals such as ICP reduction and improvement of CPP are critical with any treatment for TBI, long-term functional outcomes are also important for being related to quality of life after recovery. Honeybul et al26 assessed the outcomes beyond 3 years after TBI in patients who received DC and found that substantial physical recovery beyond 19 months did not occur.
There are few other systematic reviews or meta-analyses that examined the effectiveness of DC in patients with TBI. A Cochrane review in 2006 did not find enough evidence to either confirm or refute effectiveness of DC in adults at that time.27 A literature review in 2010 on children who received DC reported an overall favorable outcome achieved in 106 of 172 patients (62%), with a favorable outcome achieved in 25 of 36 patients without TBI versus 81 of 136 patients with TBI (69% vs 60%).28 A meta-analysis by Bor-Seng et al29 examining the role of DC in reducing ICP and increasing CPP for patients with TBI included 20 studies with a total of 479 patients. They showed postoperative ICP to be significantly lower than preoperative ICP immediately, 24 hours, and 48 hours after DC, and also reported postoperative CPP to be significantly higher than preoperative values. The study, however, did not report long-term clinical results such as functional outcomes or mortality rates.
Clearly, certain patients with TBI may benefit from DC. For example, Gouello et al30 showed that patients with a higher GCS after TBI had better outcomes after DC. However, as study has shown, it has not been clearly established which group of patients will clearly benefit from the procedure and which patients will not. A large part of the difficulty studying this topic is patient selection, and all of the many potential variables. For example, patients who receive DC may have a higher ICP than those in whom DC is not performed, leading to selection bias.
There are limitations to the current analysis. Foremost, the number of high-quality studies examining the use of DC after TBI is limited. There was significant heterogeneity in the included studies, especially with respect to the types of DC performed, the medical management administered, and the time points at which ICP was measured and reported. The study by Cooper et al6 had an overt influence on the pooled results of overall mortality, and in that study, DC was performed within 72 hours after TBI as compared with 2 to 24 hours in the study by Qiu et al,11 and a median of 19.2 hours in the study by Taylor et al.13 Furthermore, the ranges of the CIs were large, primarily as a result of the small sample sizes in the studies. Complications associated with DC were not examined, and it has been shown that occurrence of complications after DC is associated with an increased risk of prolonged hospital or rehabilitation facility stay.28 Publication bias was not assessed because more than 10 studies are required to detect funnel plot asymmetry.31 Furthermore, patients selected for DC tend to have a worse prognosis, so studies (especially nonrandomized ones) are prone to have selection bias which may have impacts on the findings.
CONCLUSIONS
The results of this meta-analysis suggest that the benefits of DC in cases of TBI are not significant enough for DC to be recommended over conventional medical management. However, the results must be interpreted with caution as the number of high-quality studies was limited, there was marked heterogeneity of the included studies, and the lack of statistical significance was marginal.
Footnotes
Abbreviations: AANS = American Association of Neurological Surgeons, CI = confidence interval, CT = computed tomography, DC = decompressive craniectomy, DECRA = Decompressive Craniectomy in Patients with Severe Traumatic Brain Injury, GCS = Glasgow Coma Scale, GOS = Glasgow Outcome Scale, ICH = intracranial hypertension, ICP = intracranial pressure, ICU = intensive care unit, IQR = interquartile range, OR = odds ratio, TBI = traumatic brain injury.
The authors declare that they have no conflicts of interest to report.
REFERENCES
- 1.Brain Trauma Foundation Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007; 24 Suppl 1:1–106. [DOI] [PubMed] [Google Scholar]
- 2.Kolias AG, Kirkpatrick PJ, Hutchinson PJ. Decompressive craniectomy: past, present and future. Nat Rev Neurol 2013; 9:405–415. [DOI] [PubMed] [Google Scholar]
- 3.Bohman LE, Schuster JM. Decompressive craniectomy for management of traumatic brain injury: an update. Curr Neurol Neurosci Rep 2013; 13:392.doi: 10.1007/s11910-013-0392-x. [DOI] [PubMed] [Google Scholar]
- 4.Maas AI, Dearden M, Teasdale GM, et al. EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Wien) 1997; 139:286–294. [DOI] [PubMed] [Google Scholar]
- 5.Rekate HL. Head injuries: management of primary injuries and prevention of secondary damage. A consensus conference on pediatric neurosurgery. Childs Nerv Syst 2001; 17:632–634. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 2011; 364:1493–1502. [DOI] [PubMed] [Google Scholar]
- 7.Nirula R, Millar D, Greene T, et al. Decompressive craniectomy or medical management for refractory intracranial hypertension: an AAST-MIT propensity score analysis. J Trauma Acute Care Surg 2014; 76:944–952. [DOI] [PubMed] [Google Scholar]
- 8.Olivecrona M, Rodling-Wahlström M, Naredi S, et al. Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma 2007; 24:927–935. [DOI] [PubMed] [Google Scholar]
- 9.Soustiel JF, Sviri GE, Mahamid E, et al. Cerebral blood flow and metabolism following decompressive craniectomy for control of increased intracranial pressure. Neurosurgery 2010; 67:65–72. [DOI] [PubMed] [Google Scholar]
- 10.Rubiano AM, Villarreal W, Hakim EJ, et al. Early decompressive craniectomy for neurotrauma: an institutional experience. Ulus Travma Acil Cerrahi Derg 2009; 15:28–38. [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu W, Guo C, Shen H, et al. Effects of unilateral decompressive craniectomy on patients with unilateral acute post-traumatic brain swelling after severe traumatic brain injury. Crit Care 2009; 13: R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josan VA, Sgouros S. Early decompressive craniectomy may be effective in the treatment of refractory intracranial hypertension after traumatic brain injury. Childs Nerv Syst 2006; 22:1268–1274. [DOI] [PubMed] [Google Scholar]
- 13.Taylor A, Butt W, Rosenfeld J, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst 2001; 17:154–162. [DOI] [PubMed] [Google Scholar]
- 14.Münch E, Horn P, Schürer L, et al. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery 2000; 47:315–322. [DOI] [PubMed] [Google Scholar]
- 15.Polin RS, Shaffrey ME, Bogaev CA, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery 1997; 41:84–92. [DOI] [PubMed] [Google Scholar]
- 16.Limpastan K, Norasetthada T, Watcharasaksilp W, et al. Factors influencing the outcome of decompressive craniectomy used in the treatment of severe traumatic brain injury. J Med Assoc Thai 2013; 96:678–682. [PubMed] [Google Scholar]
- 17.Akyuz M, Ucar T, Acikbas C, et al. Effect of early bilateral decompressive craniectomy on outcome for severe traumatic brain injury. Turk Neurosurg 2010; 20:382–389. [DOI] [PubMed] [Google Scholar]
- 18.Palmer S, Bader MK, Qureshi A, et al. The impact on outcomes in a community hospital setting of using the AANS traumatic brain injury guidelines. Americans Associations for Neurologic Surgeons. J Trauma 2001; 50:657–664. [DOI] [PubMed] [Google Scholar]
- 19.Patel N, West M, Wurster J, et al. Pediatric traumatic brain injuries treated with decompressive craniectomy. Surg Neurol Int 2013; 4:128.doi: 10.4103/2152-7806.119055. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DR, Yang SH, Sung JH, et al. Significance of intracranial pressure monitoring after early decompressive craniectomy in patients with severe traumatic brain injury. J Korean Neurosurg Soc 2014; 55:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhagen AP, de Vet HC, de Bie RA, et al. The delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus. J Clin Epidemiol 1998; 51:1235–1241. [DOI] [PubMed] [Google Scholar]
- 22.Schaller B, Graf R, Sanada Y, et al. Hemodynamic and metabolic effects of decompressive hemicraniectomy in normal brain. An experimental PET-study in cats. Brain Res 2003; 982:31–37. [DOI] [PubMed] [Google Scholar]
- 23.Honeybul S, Ho KM, Lind CR. What can be learned from the DECRA study. World Neurosurg 2013; 79:159–161. [DOI] [PubMed] [Google Scholar]
- 24.Soukiasian HJ, Hui T, Avital I, et al. Decompressive craniectomy in trauma patients with severe brain injury. Am Surg 2002; 68:1066–1071. [PubMed] [Google Scholar]
- 25.Hutchinson PJ, Corteen E, Czosnyka M, et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study (www.RESCUEicp.com). Acta Neurochir Suppl 2006; 96:17–20. [DOI] [PubMed] [Google Scholar]
- 26.Honeybul S, Janzen C, Kruger K, et al. Decompressive craniectomy for severe traumatic brain injury: is life worth living? J Neurosurg 2013; 119:1566–1575. [DOI] [PubMed] [Google Scholar]
- 27.Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev 2006; 1: CD003983. [DOI] [PubMed] [Google Scholar]
- 28.Güresir E, Schuss P, Seifert V, et al. Decompressive craniectomy in children: single-center series and systematic review. Neurosurgery 2012; 70:881–888. [DOI] [PubMed] [Google Scholar]
- 29.Bor-Seng-Shu E, Figueiredo EG, Amorim RL, et al. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J Neurosurg 2012; 117:589–596. [DOI] [PubMed] [Google Scholar]
- 30.Gouello G, Hamel O, Asehnoune K, et al. Study of the long-term results of decompressive craniectomy after severe traumatic brain injury based on a series of 60 consecutive cases. Scientific World Journal 2014; 2014:207585.doi: 10.1155/2014/207585. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomized controlled trials. BMJ 2011; 343:d4002. [DOI] [PubMed] [Google Scholar]


