Abstract
Churg–Strauss Syndrome (CSS) complicated with cardiogenic shock is rare. Few case reports have described successful treatment of this rare disease. However, no one has reported on the application of mechanical life support with extracorporeal membrane oxygenation (ECMO) to treat this life-threatening disease.
A 36-year-old female with limb numbness for >10 days, chest tightness for 2 days, and worsening dyspnea for 5 h presented in the emergency room. Vital signs showed a low blood pressure (104/60 mm Hg), increased heart rate (158 bpm), and respiration rate (28 bpm). Laboratory tests revealed that eosinophil was significantly increased (WBC: 34.46 × 109/L, neutrophil: 7.56 × 109/L[21.9%], eosinophil: 23.84 × 109/L[69.2%]), and serum myocardial enzymes was abnormal (CK 1049U/L, CKMB-mass 145.1 μg/L, cTnI 16.24 μg/L). Myocardial injury (tachycardia with ST elevation) and poor heart function (LVEF 31%) were found by electrocardiogram and transthoracic echocardiography. On the next day, cardiogenic shock had been developed as demonstrated by deteriorating the perfusion index.
Churg–Strauss Syndrome with cardiogenic shock.
A series of conservative therapy with drugs such as corticosteroids, anticoagulant, antiplatelet, nitrates, calcium antagonists, inotrope, and vasopressors were initiated on the day of admission. The treatment was ineffective and a cardiogenic shock developed on the next day. Thus, ECMO was initiated immediately to stabilize circulation and perfusion. At the same time, high-dose corticosteroids combined with immunosuppressive therapy were continuously used.
Symptoms of cardiogenic shock were gradually improved after ECMO treatment. Elevated values of cardiac enzymes were decreased and the dose of vasoactive drugs was reduced. Extracorporeal membrane oxygenation was discontinued after 8 days, and the patient was eventually weaned off the ventilator. The patient was discharged after 40 days treatment.
Once a CSS develops into a cardiogenic shock, the ECMO should be considered as an alternative therapeutics in that it stabilizes hemodynamic status, maintains effective tissue perfusion, and provides an opportunity for the recovery of cardiac function.
INTRODUCTION
Churg–Strauss syndrome (CSS, also known as eosinophilic granulomatosis with polyangitis [EGPA] or allergic granulomatosis) is a systemic vasculitis, which mainly involves small and medium-sized vessels, and could cause heart damage or even death.1 A serious cardiac lesion is a risk factor for poor prognosis of the patients with CSS.2 Mortality of the CSS patients with cardiogenic shock is high. Therefore, it is extremely important to promptly diagnose and treat the patients of CSS with cardiogenic shock. The current case report summarizes successful experience of rescuing a CSS patient complicated with cardiogenic shock by extracorporeal membrane oxygenation (ECMO).
CONSENT
Written informed consent was obtained from the patient before and after all procedures, and for the publication of this case report.
CASE REPORT
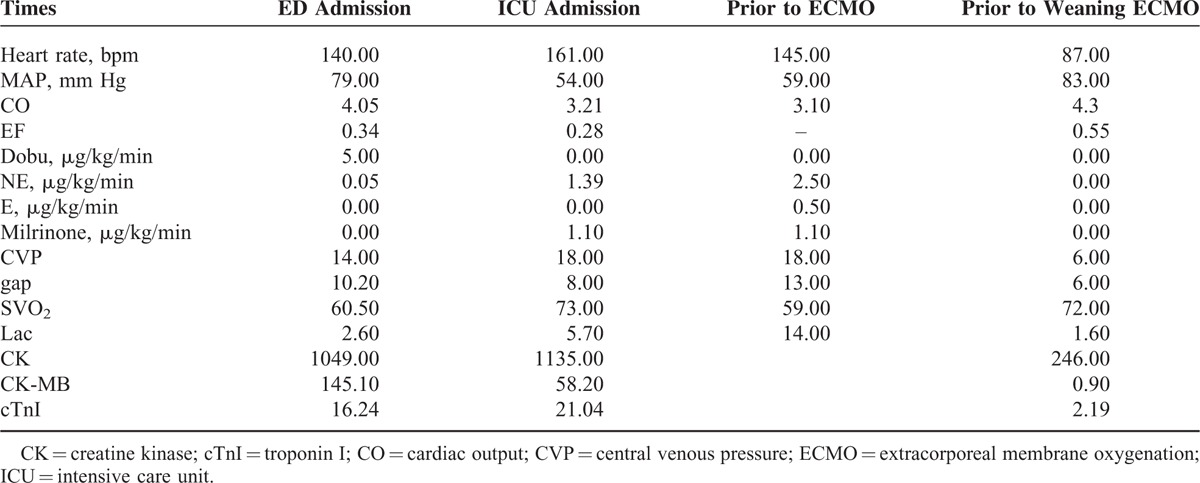
A 36-year-old female with limb numbness for >10 days, chest tightness for 2 days, and dyspnea for 5 h was admitted into the Emergency Department of Peking Union Medical College Hospital. The patient had a history of bronchial asthma and allergic rhinitis for 4 years without regular treatment, and she denied history of hypertension, diabetes, or coronary heart disease. Vital signs showed that body temperature was 36.7 °C, blood pressure was 104/60 mm Hg, heart rate was 158 beats/min, respiration rate was 28 beats/min, and pulse oxymetry was 96%. No obvious abnormalities were found by the physical examination. Laboratory tests revealed that white blood cell (WBC): 34.46 × 109/L, neutrophil:7.56 × 109/L (21.9%), eosinophil: 23.84 × 109/L (69.2%), platelet: 303 × 109/L, erythrocyte sedimentation rate (ESR): 43 mm/h, creatine kinase (CK, normal range 18–198 U/L): 1049U/L, CK-MB-mass (normal range 0–3.6 μg/L): 145.1 μg/L, cardiac troponin I (cTnI, normal range 0–0.056 μg/L): 16.24 μg/L, antineutrophil cytoplasmic antibodies (ANCA): negative. Tachycardia with ST segment elevated of V1, V2, V5, and V6 in the electrocardiogram (ECG) (Figure 1A). Dilated hypokinetic left ventricle with systolic dysfunction (LVEF: 31%), mitral valvular regurgitation, mild pulmonary hypertension, and a small pericardial effusion were detected by the transthoracic echocardiography. Acute coronary syndrome with hyper-eosinophilic involvement in heart and lung was preliminary diagnosed. The patients developed into aggravated chest tightness and difficult breathing even under the treatment with drugs, including anticoagulant, antiplatelet, nitrates, calcium antagonists, inotrope, and vasopressors. Therefore, the patient was endotracheally intubated and transferred to the intensive care unit (ICU) on the day of admission. After being transferred to the ICU, hemodynamics was monitored by the venous catheter and pulse indicator continuous cardiac output (PICCO). The following parameters were obtained: invasive arterial blood pressure 101/72 mm Hg, cardiac output (CO) 3.93L/min, central venous pressure (CVP) 16 cmH2O, lactate 2.9 mmol/l, ScvO2, central venous oxygen saturation, 73%, Pv-aCO2 8 mm Hg. As shown in Figure 2, chest x-ray found that unfixed pulmonary infiltrates in the process of the treatment. The patient was continuously treated with anticoagulant, antiplatelet, nitrates, calcium antagonists, dopamine, norepinephrine, milrinone, pulmicort, combivent, and methylprednisolone. However, the patient was not improved (Table 1) and developed into a shock on the second day as evidenced by deteriorating the perfusion index, 150 to 180 bpm of heart rate, premature ventricular contractions (PVCs), irregular ECG (Figure 1B), and 34% EF. The cardiac catheterization was not performed due to instability of blood circulation, and cardiac biopsy was not done due to family's refusal. The patient was preliminary diagnosed as CSS-induced cardiogenic shock and immediately treated with ECMO plus methylprednisolone 500 mg × 5 days and Tazocin. V-A mode was selected and the ECMO installed from artery and vein of the left femoral. The patients’ condition was then gradually improved, and amount of epinephrine, milrinone, and norepinephrine was gradually reduced and stopped to use on 4th or 5th day. A diuretic dehydration strategy was then applied. Extracorporeal membrane oxygenation flow was reduced from 3.5 to 2.9 L/min on 6th day, to 2.2 L/min on 7th day, and maintained through day 9 of admission. Meanwhile, methylprednisolone was reduced to 80 mg and 20 g immunoglobulin was intravenously given. On day 10, the ECMO was successfully taken off, the ventilator was weaned off, and the patient was extubated on 11th day. During the above treatment, electromyography was performed and showed that limb peripheral neuronal damage, which further supported diagnosis of CSS. The patient was then transferred to Department of Immunological Diseases, and glucocorticoid + cyclophosphamide was given for the further treatment of CCS. Meanwhile, inotropic support and diuretic therapy were given for additional 1 week, and drugs for controlling the ventricular rate and ventricular remodeling as well as trophic nerve drugs were used during this treatment period. The patient was finally discharged after one and half month treatment. After discharge, the patient was followed up in the outpatient clinic. The latest return was on March 23, 2015, and the patient did not have any discomfort or complaint. Laboratory tests showed that a number of eosinophil returned to normal, and echocardiogram showed LVEF59%.
FIGURE 1.
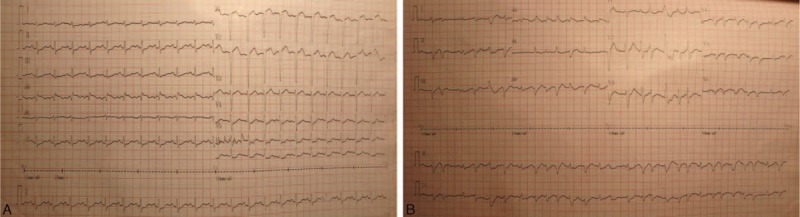
The ECG changes when admitted to the emergency room (panel A) and cardiogenic shock occurred (panel B). ECG = electrocardiogram.
FIGURE 2.
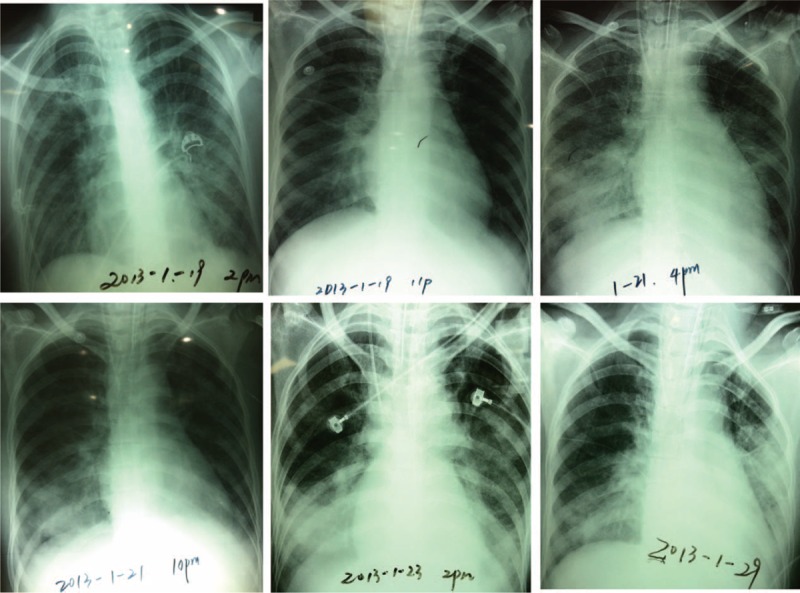
Chest x-ray showed unfixed pulmonary infiltrates in the process of the treatment.
TABLE 1.
Clinical Data Including Hemodynamics, Tissue Perfusion, Cardiac Enzymes, and the Usage of Vasoactive Drugs During Treatment
DISCUSSION
It is widely recognized that CSS is a necrotizing vasculitis characterized by eosinophil infiltration and granuloma formation. The heart is one of the major organs targeted by the disease, leading to increased risk of death and poor prognosis. It is reported that 13% to 47% of the CSS had cardiac involvement, and 50% of CSS mortality attributes to heart disease.1,3 Once the CSS complicated with cardiogenic shock, the risk of death raised rapidly. The following diagnostic criteria for the disease had been recommended by The American College of Rheumatology in 1990:4 (1) asthma; (2) eosinophil >10% of a differential white blood cell count; (3) presence of mononeuropathy or polyneuropathy; (4) unfixed pulmonary infiltrates; (5) presence of paranasal sinus abnormalities; (6) histological evidence of extravascular eosinophil. A patient shall be diagnosed as CSS if at least 4 of these 6 criteria are positive. Studies have shown that patients with ANCA negative are prone to have cardiac lesions.5 The case reported here met 4 of the 6 criteria in clinical manifestations, including history of asthma, increased eosinophil, polyneuropathy, and presence of paranasal sinus abnormalities.
Churg–Strauss syndrome patients with cardiac involvement often appeared as acute coronary syndromes, angina, and shock with negative ANCA. The pathogenesis of these clinical manifestations may be associated with vasculitis induced by eosinophil infiltration, which causes persistent occlusion or stenosis hemangioma, or may attribute to cytokines released by eosinophil, which causes coronary artery spasm, reversible multisite stenosis, or occlusion. Similar to the bronchospasm inasthma attack, coronary artery spasm in CSS was due to abnormally secreted acetylcholine by eosinophil infiltrating into artery wall, which binds to muscarinic receptors in the coronary artery smooth muscle, resulting invasoconstriction.
Cardiogenic shock in CSS is often resulted from left ventricular failure when acute myocardial infarction occurred, accounting for 79% of the cause of cardiogenic shock. It has been reported that 7% to 9% of patients with AMI combined cardiogenic shock, whose mortality was up to 70% to 85%.6,7 In the current case, the patient suffered from cardiogenic shock during the treatment in an emergency room. To our knowledge, only 5 cases with CSS complicated with cardiogenic shock had been reported to be successfully rescued by heart transplantation,8,9 microaxial blood pump,10 or even conservative treatment.11,12 Although successive experience in each case might be varying, prompt diagnosis and appropriate treatment are crucial to rescue a CSS patient with severe circulation failure. In this regard, ECMO could provide an effective respiratory and circulatory support in critically ill patients with cardiopulmonary failure. Application of ECMO in such a patient could maintain hemodynamic stability and provide an opportunity for the heart function recovery and primary disease treatment. Therefore, ECMO is an effective means for refractory cardiogenic shock or circulatory failure during the treatment of acute coronary syndrome and myocardial infarction.13–16
To our knowledge, the application of ECMO in the patients with CSS has not yet been reported. In the current case, an interventional treatment was not considered on the first day of admission in that it may induce or worsen bronchial and artery spasm. Therefore, only conservative therapy, including anticoagulant, antiplatelet, nitrates, and calcium antagonists, was given to the patients. However, when the patient's cardiac function was gradually deteriorated and developed into cardiogenic shock, the ECMO was immediately used to correct the ischemia and hypoxia of the tissue and organs, and to provide an opportunity for the recovery of cardiac function. Meanwhile, high-dose corticosteroids combined with immunosuppressive therapy were used to treat the primary disease.
In summary, once cardiogenic shock developed in a patient with CSS, ECMO should be considered as an alternative and effective therapy if an attempt with inotropic and vasopressors agents is unsuccessful. A prompt diagnosis and immediate application of ECMO was crucial step to rescue the patient with CSS and cardiogenic shock, and to avoid unnecessary heart transplantation, as reported in the current case report.
Footnotes
Abbreviations: ANCA = anti-neutrophil cytoplasmic antibodies, CK = creatine kinase, CO = cardiac output, CSS = Churg–Strauss Syndrome, cTnI = troponin I, CVP = central venous pressure, ECG = electrocardiogram, ECMO = extracorporeal membrane oxygenation, ICU = Intensive Care Unit, PICCO = pulse indicator continuous cardiac output, PVC = premature ventricular contraction, WBC = white blood cell.
Na Cui and Longxiang Su contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Noth I, Strek ME, Leff AR. Churg–Strauss syndrome. Lancet 2003; 361:587–594. [DOI] [PubMed] [Google Scholar]
- 2.Guillevin L, Pagnoux C, Seror R, et al. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011; 90:19–27. [DOI] [PubMed] [Google Scholar]
- 3.Bourgarit A, Le Toumelin P, Pagnoux C, et al. Deaths occurring during the first year after treatment onset for polyarteritis nodosa, microscopic polyangiitis, and Churg–Strauss syndrome: a retrospective analysis of causes and factors predictive of mortality based on 595 patients. Medicine 2005; 84:323–330. [DOI] [PubMed] [Google Scholar]
- 4.Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg–Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990; 33:1094–1100. [DOI] [PubMed] [Google Scholar]
- 5.Sable-Fourtassou R, Cohen P, Mahr A, et al. Antineutrophil cytoplasmic antibodies and the Churg–Strauss syndrome. Ann Intern Med 2005; 143:632–638. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RH, Grab JD, O’Brien SM, et al. Clinical characteristics and in-hospital outcomes of patients with cardiogenic shock undergoing coronary artery bypass surgery: insights from the Society of Thoracic Surgeons National Cardiac Database. Circulation 2008; 117:876–885. [DOI] [PubMed] [Google Scholar]
- 7.Jeger RV, Harkness SM, Ramanathan K, et al. Emergency revascularization in patients with cardiogenic shock on admission: a report from the SHOCK trial and registry. Eur Heart J 2006; 27:664–670. [DOI] [PubMed] [Google Scholar]
- 8.Thomson D, Chamsi-Pasha H, Hasleton P. Heart transplantation for Churg–Strauss syndrome. Br Heart J 1989; 62:409–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg M, Lorenz HM, Gassler N, et al. Rapid progressive eosinophilic cardiomyopathy in a patient with Churg–Strauss syndrome (CSS). Clin Res Cardiol 2006; 95:289–294. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari M, Pfeifer R, Poerner TC, et al. Bridge to recovery in a patient with Churg–Strauss myocarditis by long-term percutaneous support with microaxial blood pump. Heart 2007; 93:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courand PY, Croisille P, Khouatra C, et al. Churg–Strauss syndrome presenting with acute myocarditis and cardiogenic shock. Heart, Lung Circ 2012; 21:178–181. [DOI] [PubMed] [Google Scholar]
- 12.Shanks M, Ignaszewski AP, Chan SY, et al. Churg–Strauss syndrome with myocarditis manifesting as acute myocardial infarction with cardiogenic shock: case report and review of the literature. Can J Cardiol 2003; 19:1184–1188. [PubMed] [Google Scholar]
- 13.Tsao NW, Shih CM, Yeh JS, et al. Extracorporeal membrane oxygenation-assisted primary percutaneous coronary intervention may improve survival of patients with acute myocardial infarction complicated by profound cardiogenic shock. J Crit Care 2012; 27:530.e531–e511. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto S, Taniguchi N, Nakajima S, et al. Extracorporeal life support for cardiogenic shock or cardiac arrest due to acute coronary syndrome. Ann Thorac Surg 2012; 94:1–7. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Lim SH, Hong J, et al. Efficacy of veno-arterial extracorporeal membrane oxygenation in acute myocardial infarction with cardiogenic shock. Resuscitation 2012; 83:971–975. [DOI] [PubMed] [Google Scholar]
- 16.Esper SA, Bermudez C, Dueweke EJ, et al. Extracorporeal membrane oxygenation support in acute coronary syndromes complicated by cardiogenic shock. Cathet Cardiovasc Interv 2015. [DOI] [PubMed] [Google Scholar]


