Abstract
To evaluate the risk of secondary primary malignancy (SPM) in patients with cervical cancer using a nationwide population-based dataset.
Patients newly diagnosed with cervical cancer between 1997 and 2011 were identified using Taiwan's National Health Insurance database. Patients with antecedent malignancies were excluded. Standardized incidence ratios (SIRs) for SPM were calculated by comparing with the cancer incidence in the general population. Risk factors for cancer development were analyzed using Cox proportional hazard models.
During the 14-year study period (follow-up of 223,062 person-years), 2004 cancers developed in 35,175 patients with cervical cancer. The SIR for all cancers was 1.56 (95% confidence interval, 1.50–1.63, P < 0.001). SIRs for follow-up periods of >10, 5 to 10, 1 to 5, and <1 year were 1.37, 1.51, 1.34, and 2.59, respectively. After the exclusion of SPM occurring within 1 year of cervical cancer diagnosis, SIRs were significantly higher for cancers of the esophagus (2.05), stomach (1.38), colon, rectum, and anus (1.36); lung and mediastinum (2.28), bone and soft tissue (2.23), uterus (3.76), bladder (2.26), and kidneys (1.41). Multivariate analysis showed that age ≥60 was a significant SPM risk factor (hazard ratio [HR] 1.59). Different treatments for cervical cancer, including radiotherapy (HR 1.41) and chemotherapy (HR 1.27), had different impacts on SPM risk. Carboplatin and fluorouracil independently increased SPM risk in cervical cancer patients.
Patients with cervical cancer are at increased risk of SPM development. Age ≥60 years, chemotherapy, and radiotherapy are independent risk factors. Carboplatin and fluorouracil also increased SPM risk independently. Close surveillance of patients at high risk should be considered for the early detection of SPMs.
INTRODUCTION
Cervical cancer is the fourth most common cancer in women worldwide, with about 528,000 new cases diagnosed in 2012.1 Cervical cancer incidence rates are decreasing among women in the United States. However, the incidence of cervical cancer remains high and is a leading cause of death in women in developing countries. Advances in screening, surgery technique, radiotherapy, and chemotherapy have improved survival in recent years. Subsequent secondary primary malignancies (SPMs) are sometimes observed clinically and usually result in inferior outcomes;2 therefore, SPMs are worth discussing alongside cervical cancer.
Several studies have reported SPMs in patients with cervical cancer. Since 1988, several studies have reported a correlation between radiotherapy for cervical cancer and subsequent cancer risk, but they focused mainly on bladder and colorectal cancers, leukemia, and non-Hodgkin's lymphoma.3–5 In addition, a population-based study in the United States that was based on the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) program enrolled 26,290 cervical cancer patients and showed a significantly increased risk in subsequent esophageal cancer, stomach cancer, lung and bronchial cancer, and bladder cancer.6,7 A Japanese cohort, which enrolled 2167 cervical patients who underwent radiotherapy, showed a small but significant risk of SPMs.8 Most of these studies focused on the effect of radiotherapy. Furthermore, few studies have comprehensively coordinated subjects’ medical history and radiation therapy history to evaluate these effects thoroughly. To clarify the incidence of SPMs in cervical cancer, we conducted a nationwide population-based study to examine SPM after the occurrence of cervical cancer.
Taiwan's National Health Insurance Research Dataset (NHIRD) provides nationwide population data for health research. As all malignancies are registered precisely, the NHIRD is proper for the analysis of SPMs. In addition to patients’ age and sex, the NHIRD provides complete information on comorbidities, which were not simultaneously integrated in most previous studies on cervical cancer. The aim of this study is to compare the overall incidence of SPMs among patients with cervical cancer with the expected incidence in an age-, sex-, and calendar year-matched population using the NHIRD, and to calculate the standardized incidence ratios (SIRs). In addition, we investigated the potential predisposition of patients with cervical cancer to SPMs with respect to chemotherapy, radiotherapy, and comorbidities. The different impacts to risk of SPMs in individual chemotherapy agents were also analyzed.
METHODS
Data Sources
The universal National Health Insurance (NHI) program in Taiwan was initiated in 1995. It provides comprehensive medical care to all of Taiwan's residents, with a coverage rate of more than 99% 9. The program provides coverage for outpatient, emergency, inpatient, dental, and traditional Chinese medicine services, as well as prescription drugs.
Based on NHIRD, we also introduced the Registry of Catastrophic Illness, which provides comprehensive information on NHI enrollment and health care resource provisions for patients with serious diseases, whose medical copayments are exempted under the NHI program. The NHIRD integrates several NHI databases, which consists of claims data, NHI enrollment files, and the drug prescription registry. Cervical cancer and all other types of malignancies are categorized as catastrophic illnesses. All information that would potentially identify individual patients is encrypted. The data are confidential, as mandated by the Bureau of NHI and the National Health Research Institutes. Because the NHI dataset contains unidentifiable secondary data for research purposes, the institutional review board of Taipei Veterans General Hospital exempted this study from full review (2013-10-002CE).
Study Population
Newly diagnosed cases of cervical cancer (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 181) during the period January 1, 1997 to December 31, 2011 were identified and enrolled from the Registry of Catastrophic Illness. Patients diagnosed with cervical cancer before January 1, 1997 were not enrolled. Patients aged under 20 at the time of diagnosis and those who had antecedent malignancies were excluded. The main dependent variable in our study was development of SPMs. To avoid misclassification, ill-defined or unspecific cancers were not considered as SPMs. For subjects identified using the Registry of Catastrophic Illness, histological evidence for the malignant diagnosis was required. Every patient was followed until the occurrence of an SPM, death, dropout from the NHI program, or the end of 2011. Information on comorbidities, radiotherapy, and chemotherapeutic agents was also collected from NHIRD for further analysis.
Statistical Analysis
SIRs, defined as the observed number of cancer occurrences divided by the expected number of such occurrences, were used to determine the risk of SPMs in our study cohort. The expected number of cancer occurrences was calculated simply by multiplying the cancer incidence in the general population (retrieved from Taiwan's National Cancer Registry) by the number of patients in the corresponding age group in the study cohort. Each group was stratified according to calendar year in 5-year intervals by the corresponding stratum-specific person-time accrued in the cohort. Ninety-five percent confidence intervals (CIs) of SIRs were estimated by the assumption that the observed number of cancer occurrences followed a Poisson probability distribution. We defined SIRs for subgroups based on age. A subgroup analysis stratified by the period of SPM development was performed to avoid surveillance bias. For the same reason, SIRs for different types of cancer were estimated by excluding SPMs occurring within 1 year after the diagnosis of cervical cancer. Risk factors for SPM development among patients with cervical cancer were analyzed using univariate and multivariate Cox proportional hazard models. These factors included not only age and comorbidities but also surgery, radiotherapy, and chemotherapy. Factors with P values <0.1 in the univariate analysis were entered into the Cox multivariate analysis.
Data were extracted and computed using the Perl programming language (version 5.12.2; Perl Foundation, Walnut, CA). Data linkage, processing, and sampling were conducted using Microsoft SQL Server 2012 (Microsoft Corporation, Redmond, WA). SAS software (version 9.2; SAS Institute Inc, Cary, NC) was used for all statistical analyses. Statistical significance was defined as P <0.05.
RESULTS
Characteristics of the Study Population
We identified 36,399 patients diagnosed with cervical cancer between 1997 and 2011 in the NHIRD's Catastrophic Illness Registry. Of these, 296 patients were misclassified, 8 patients were aged <20, and 920 patients had antecedent malignancies. Thus, the final sample consisted of 35,175 patients, with a median age of 55.32 (interquartile range, 45.49–67.33) at diagnosis. The detail of patients’ enrolment is demonstrated in Figure 1. Overall, this cohort was observed for 223,062 person-years from 1997 to 2011. The characteristics of the cohort are shown in Table 1.
FIGURE 1.
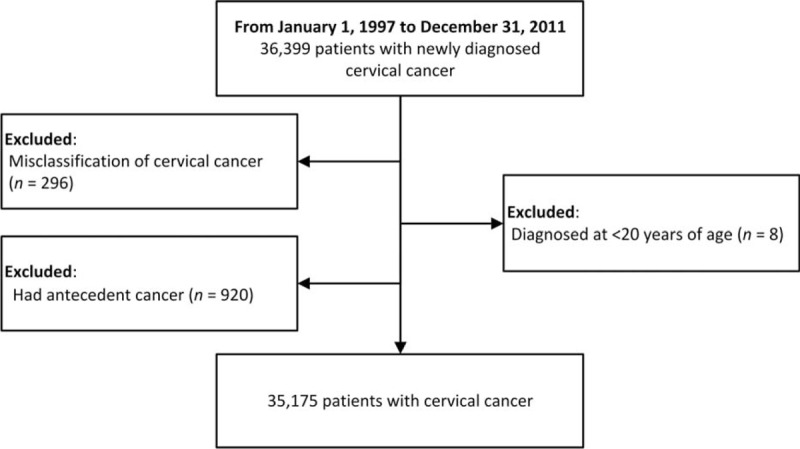
Flowchart.
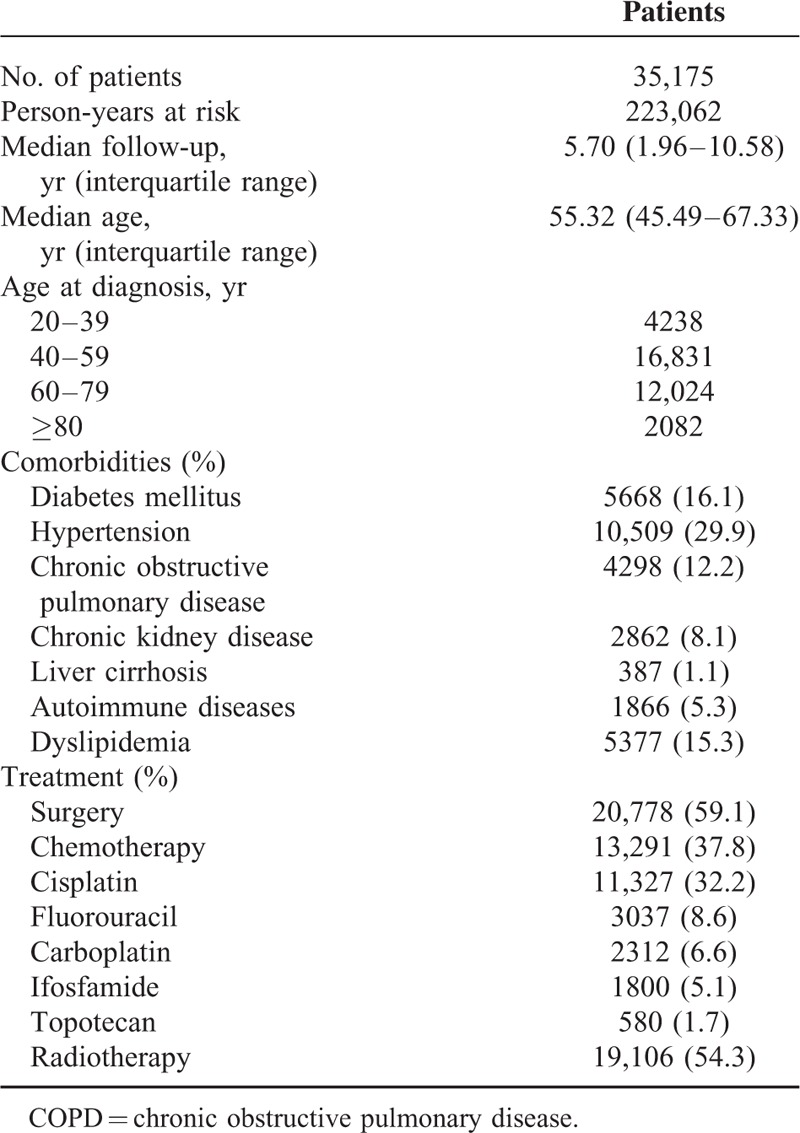
Table 1.
Characteristics of Cervical Cancer Patients
All Cancers
During the observation period, 2004 cancers developed. Compared with the general population, patients with cervical cancer had a significantly increased risk of all cancers (SIR 1.56, 95% CI 1.50–1.63, P < 0.001). Subgroup analysis showed that SIRs for all cancers were highest among patients aged 20 to 39 at the time of diagnosis (SIR 3.99, 95% CI 3.07–5.09, P < 0.001). Subgroup analysis based on the period of SPM development (0–1, 1–5, 5–10, and ≥10 yr) after cervical cancer diagnosis yielded SIRs of 2.59 (95% CI 2.35–2.85, P < 0.001), 1.34 (95% CI 1.24–1.45, P < 0.001), 1.51 (95% CI 1.40–1.63, P < 0.001), and 1.37 (95% CI 1.21–1.56, P < 0.001), respectively. The results of these subgroup analyses are summarized in Table 2. The cumulative incidence of secondary primary malignancies in patients with cervical cancer is demonstrated in Figure 2.
Table 2.
Standardized Incidence Ratios According to Sex, Age at Diagnosis, and Follow-Up Time
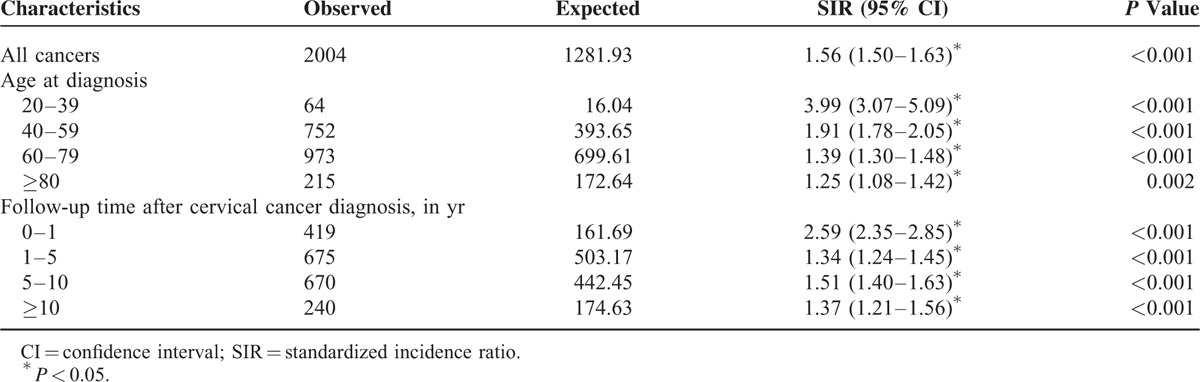
FIGURE 2.
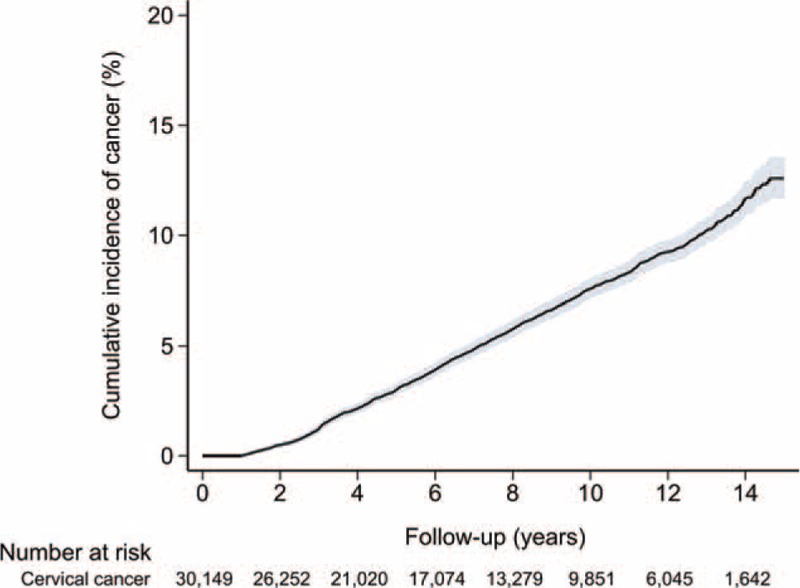
Cumulative incidence of secondary primary malignancy in patients with cervical cancer.
Specific Cancer Types
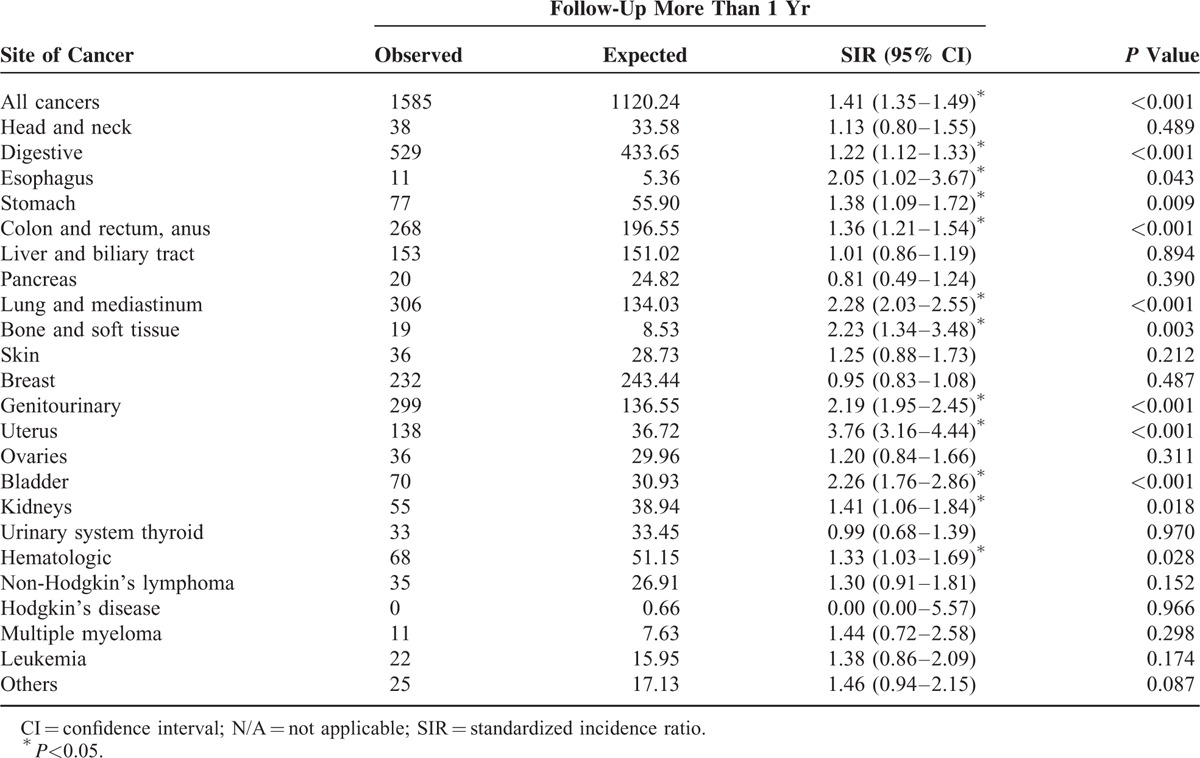
After excluding SPMs developed within 1 year after diagnosis of cervical cancer, significantly higher SIRs were observed for cancers of the esophagus (2.05, 95% CI 1.02–3.67, P = 0.043), stomach (1.38, 95% CI 1.09–1.72, P = 0.009), colon and rectum (1.36, 95% CI 1.21–1.54, P < 0.001), lung and mediastinum (2.28, 95% CI 2.03–2.55, P < 0.001), bone and soft tissue (2.23, 95% CI 1.34–3.48, P = 0.003), uterus (3.76, 95% CI 3.16–4.44, P < 0.001), bladder (2.26, 95% CI 1.76–2.86, P < 0.001), and kidneys (1.41, 95% CI 1.06–1.84, P = 0.018). SIRs for specific cancer types are shown in Table 3.
Table 3.
Standardized Incidence Ratios for Cancer Subtypes Among Cervical Cancer Patients (Follow-Up More Than 1 Yr)
Predictors of Cancer Risk
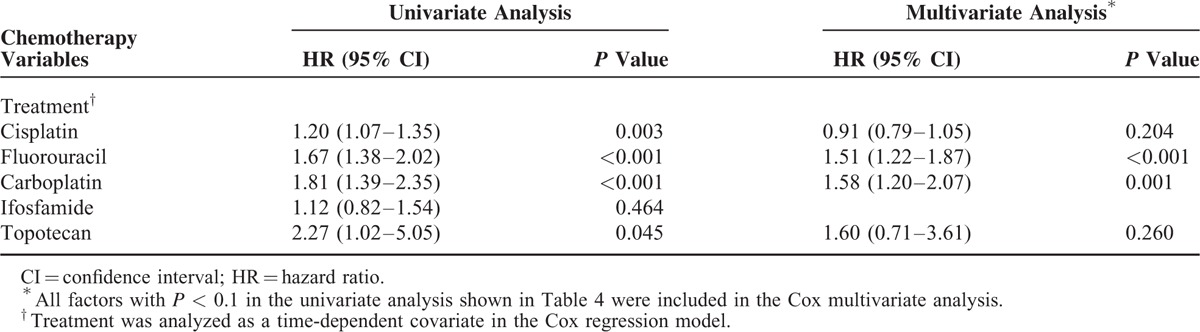
Univariate Cox proportional hazard analysis showed that age ≥60, diabetes mellitus, hypertension, chronic obstructive pulmonary disease (COPD), chronic kidney disease, liver cirrhosis, autoimmune disease, and dyslipidemia were associated significantly with a higher risk of cancer development. Multivariate analysis showed that age ≥60 (hazard ratio [HR] 1.59, 95% CI 1.43–1.77, P < 0.001) remained an independent predictor of SPM development. Furthermore, chemotherapy (hazard ratio [HR] 1.41, 95% CI 1.25–1.59, P < 0.001) and radiotherapy (hazard ratio [HR] 1.27, 95% CI 1.14–1.43, P < 0.001) were independent risk factors in the multivariate analysis. We also analyzed the impact of common chemotherapy agents on SPM occurrence among cervical cancer patients. Multivariate analysis showed that chemotherapy agents with fluorouracil ([HR] 1.51 95% CI 1.22–1.87, P < 0.001) and carboplatin ([HR] 1.58 95% CI 1.20–2.07, P = 0.001) independently increased risk of SPM under the multivariate analysis. These results are itemized in Tables 4 and 5.
Table 4.
Risk Factors for Cancer Development Among Cervical Cancer Patients (Follow-Up More Than 1 Yr) (n = 30,149)
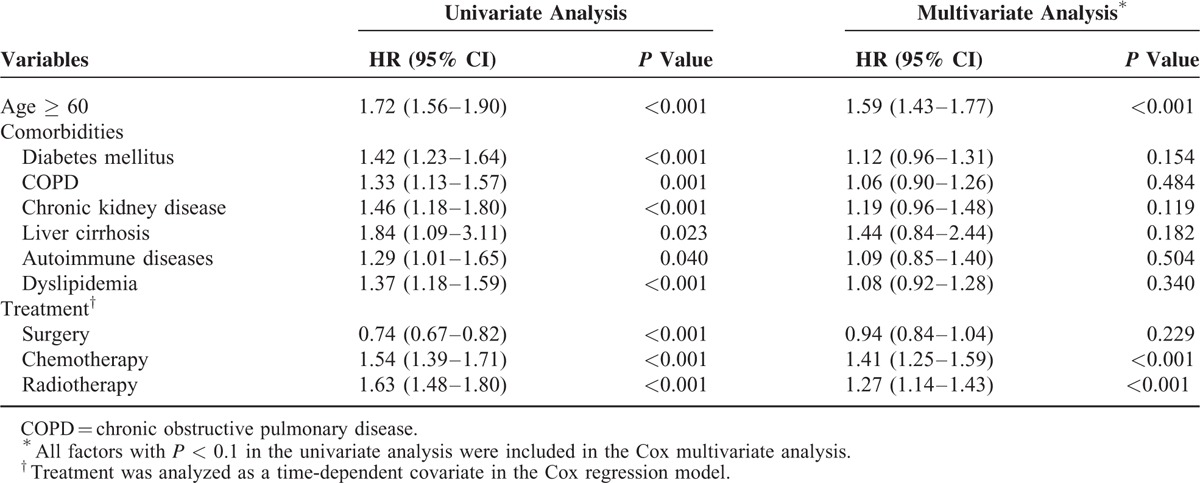
Table 5.
Risk Factors for Cancer Development Among Cervical Cancer Patients (Follow-Up More Than 1 Yr) (n = 30,149)
DISCUSSION
Our study is a nationwide population-based study to clearly demonstrate significantly increased SIRs for metachronous SPMs among patients with cervical cancer. The main findings were that patients with cervical cancer have significantly increased risk of SPMs in the esophagus, stomach, colon and rectum, lung and mediastinum, bone and soft tissue, uterus, bladder, kidneys; SIRs for all cancers were highest among patients aged 20 to 39 at the time of diagnosis; independent risk factors for SPMs include age ≥60, hypertension, chemotherapy, and radiotherapy; the chemotherapy agents carboplatin and fluorouracil are independent risk factors for SPMs in cervical cancer patients.
Several studies have discussed the issue of SPMs after cervical cancer.2–5,10–23 Most of these studies emphasized the effects of radiotherapy4,5,8,11,12,18,21,23–29 and the majority focused on Western patients. Most Asian studies were conducted in or by single institutes and had limited populations.4,8,25,30,31 A study published in 2012 based on the Taiwan Cancer Registry that enrolled 52,972 patients also found increased SIRs in patients with cervical cancer.2 However, only the age of patients, registry date, and sites of cancer were analyzed. In the present study, we enrolled 35,175 cervical cancer patients and not only obtained the full claims data but also corroborated with the cancer registry to confirm the results. By using the NHIRD, we can take patients’ comorbidities and their treatment modalities into consideration. Our results are convincing because our cohort included patients identified by unbiased nationwide selection and reliable diagnostic criteria supported by pathological evidence.
Although our study demonstrated that SIRs for SPMs were highest at 0 to 1 years, the higher SIRs might be confounded by surveillance bias. After excluding SPMs at 0 to 1 years, SIRs showed no difference between those of the 1 to 5, 5 to 10, and ≥10 groups when examined using a Pearson χ2 test (P = 0.476). In the subgroup analysis, we found that patients aged 20 to 39 at diagnosis had the highest SIRs of SPMs. The SEER study reported a similar results.7 Though the absolute risk of SPMs in this age group is modest when compared with those of the elderly, they are at a much higher risk than people without cervical cancer in the same age group. These findings imply that thorough examination is warranted to detect synchronous cancers when cervical cancer is diagnosed. Cautious follow-up is also necessary even after 10 years.
The increased risk of SPMs in cervical cancer patients we found was consistent with most previous studies.2–8,10–23,30,32 Boice et al conducted an international collaborated study that enrolled 182,040 patients from 15 cancer registries in 8 countries and reported that a 9% excess of secondary cancers (5146 observed versus 4736 expected) had occurred 1 or more years after treatment.21 Chen et al reported a significantly greater SIR (1.36) of SPM in cervical cancer patients in the Taiwan Cancer Registry.2 Other studies highlighted an increased risk of gastrointestinal cancer after cervical cancer.10,12,13 Some of these studies observed the correlation of secondary primary malignancies with human papillomavirus (HPV) and tobacco use.6,7 We found an increased SIR for SPMs in the esophagus, stomach, colon and rectum, anus, lungs and mediastinum, bones and soft tissue, uterus, bladder, and kidneys.
Several possible etiologies may be applied for the increased risk of SPMs after cervical cancer. First, cervical cancer is a human papillomavirus (HPV)-associated cancer.33 Patients with cervical cancer share the risk with other HPV-associated cancers, such as anal cancer, oropharyngeal cancer, and vaginal cancer.34,35 Second, patients of some cancers may have socioeconomic status and some lifestyle-related risk factors in common, such as smoking and sexual activity.6,7,36–38 Third, treatment modality, such as chemotherapy or radiotherapy, may induce SPMs.5,8,18,23,27 Further research is needed to determine the possible relationships between these factors and to find the underlying mechanisms.
Increased age was found to be an independent risk factor for SPM after the diagnosis of cervical cancer. Additionally, none of the comorbidities available in our database were independent risk factors for SPMs in cervical cancer patients. However, radiotherapy and chemotherapy, which were considered to be time-dependent variables, increased the risk of SPMs. In 1982, Kleinerman et al found an increased risk of SPM in 5997 cervical cancer patients who had received radiotherapy. No excess was found among the 1130 nonirradiated women. An international study using data from 104,760 1-year survivors of cervical cancer in 13 population-based cancer registries from Denmark, Finland, Norway, Sweden, and the United States found a statistically significant increased SIR (1.3) for the risk of all SPMs in cervical cancer patients who had received radiotherapy.15 Most studies suggest that these SPMs occur mainly in the irradiation sites and gastrointestinal tract.10,12,18,21,23 Our study also found that carboplatin and fluorouracil were independent risk factors of SPMs in cervical cancer patients. As far as we know, there have been no nationwide population-based studies to date that have mentioned the effects of chemotherapy agents on SPMs in cervical cancer. Several studies have also mentioned the possible carcinogenic effects of platinum-based chemotherapy regimens.39,40 The effects of chemotherapy which may result in secondary primary malignancies were mostly discussed in children with hematologic disease and sarcomas.41–47 By the improvement of cancer therapy, this is an emerging issue for adult cancer survivors. Further basic investigation is warranted to clarify the effects.
Our study has several limitations. First, family history and lifestyle factors, such as exercise, sexual activity, tobacco use, and alcohol consumption, which were not recorded in the NHI database, may be potential confounders. Among them, the women tobacco-smoking prevalence is relatively low in Taiwan [3.5% in 2014 according to the annual report from Health Promotion Administration, Ministry of Health and Welfare (http://tobacco.hpa.gov.tw/Show.aspx?MenuId = 581 accessed on 2015/9/7). Therefore, the effects of tobacco smoking may be negligible in our cohort. Second, disease stages and microscopic features (ie, grade) of cervical cancers were not included in the NHI database. However, previous studies have reported a similar risk of SPMs in patients with invasive cervical cancer and carcinomas in situ,17 which may be due to these patients having certain risk factors, such as treatment modality and exposure (ie, HPV infection), in common. Nevertheless, we indeed found an increase of risk in HPV-related cancers in our cohort. Finally, clinical symptoms and detailed laboratory data were not available, which limited further analysis into quality of life and other medical scenarios.
In conclusion, our results demonstrate that the risk of SPMs is significantly higher among patients with cervical cancer. Age, chemotherapy, and radiotherapy are independent risk factors for SPM in this population. Furthermore, among all the common chemotherapy agents, carboplatin and fluorouracil independently increased the risk of SPMs. Postchemotherapy/radiotherapy surveillance is crucial for early detection of SPMs.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, HPV = human papillomavirus, HR = hazard ratio, ICD-9-CM = International Classification of DiseasesNinth RevisionClinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Dataset, SIR = standardized incidence ratio, SPM = secondary primary malignancy.
This study is based on data from the National Health Insurance Research Database, provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Using the largest nationwide population-based Asian dataset examined to date, this study reveals an increased cancer risk in patients with cervical cancer, with a standardized incidence ratio (SIR) of 1.63 (95% confidence interval, 1.56–1.70) among patients aged 20 to 39 years at the time of cervical cancer diagnosis. SIRs for different cancer sites and risk factors were determined. Different treatment modality and chemotherapy agents were also analyzed.
Y-PH and C-JL contributed equally to this manuscript.
This study was supported by grants from Taipei Veterans General Hospital (V104E10-001 and V104B-023), the Taiwan Clinical Oncology Research Foundation, and the Szu-Yuan Research Foundation of Internal Medicine and Chong Hin Loon Memorial Cancer and Biotherapy Research Center, National Yang-Ming University.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cancer IAfRo. Cervical Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer = cervix Accessed May 2, 2015, 2015 [Google Scholar]
- 2.Chen CY, Lai CH, Lee KD, et al. Risk of second primary malignancies in women with cervical cancer: a population-based study in Taiwan over a 30-year period. Gynecol Oncol 2012; 127:625–630. [DOI] [PubMed] [Google Scholar]
- 3.Kleinerman RA, Boice JD, Jr, Storm HH, et al. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer 1995; 76:442–452. [DOI] [PubMed] [Google Scholar]
- 4.Arai T, Nakano T, Fukuhisa K, et al. Second cancer after radiation therapy for cancer of the uterine cervix. Cancer 1991; 67:398–405. [DOI] [PubMed] [Google Scholar]
- 5.Boice JD, Jr, Engholm G, Kleinerman RA, et al. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res 1988; 116:3–55. [PubMed] [Google Scholar]
- 6.Underwood JM, Rim SH, Fairley TL, et al. Cervical cancer survivors at increased risk of subsequent tobacco-related malignancies, United States 1992–2008. Cancer Causes Control 2012; 23:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis REFD, Ron E, Ries LAG, et al. Jr New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute 2006. [Google Scholar]
- 8.Ohno T, Kato S, Sato S, et al. Long-term survival and risk of second cancers after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys 2007; 69:740–745. [DOI] [PubMed] [Google Scholar]
- 9.Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health affairs (Project Hope) 2003; 22:61–76. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez AM, Kuo YF, Goodwin JS. Risk of colorectal cancer among long-term cervical cancer survivors. Med Oncol 2014; 31:943. [DOI] [PubMed] [Google Scholar]
- 11.Arnold M, Liu L, Kenter GG, et al. Second primary cancers in survivors of cervical cancer in The Netherlands: implications for prevention and surveillance. Radiother Oncol 2014; 111:374–381. [DOI] [PubMed] [Google Scholar]
- 12.Kleinerman RA, Smith SA, Holowaty E, et al. Radiation dose and subsequent risk for stomach cancer in long-term survivors of cervical cancer. Int J Radiat Oncol Biol Phys 2013; 86:922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleem AM, Paulus JK, Shapter AP, et al. Risk of anal cancer in a cohort with human papillomavirus-related gynecologic neoplasm. Obstet Gynecol 2011; 117:643–649. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Kleinerman RA, Hildesheim A, et al. Second cancers after squamous cell carcinoma and adenocarcinoma of the cervix. J Clin Oncol 2009; 27:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi AK, Engels EA, Gilbert ES, et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J Natl Cancer Inst 2007; 99:1634–1643. [DOI] [PubMed] [Google Scholar]
- 16.Bjorge T, Hennig EM, Skare GB, et al. Second primary cancers in patients with carcinoma in situ of the uterine cervix. The Norwegian experience 1970–1992. Int J Cancer 1995; 62:29–33. [DOI] [PubMed] [Google Scholar]
- 17.Pettersson F, Ryberg M, Malker B. Second primary cancer after treatment of invasive carcinoma of the uterine cervix, compared with those arising after treatment for in situ carcinomas. An effect of irradiation? A cancer registry study. Acta Obstet Gynecol Scand 1990; 69:161–174. [DOI] [PubMed] [Google Scholar]
- 18.Storm HH. Second primary cancer after treatment for cervical cancer. Late effects after radiotherapy. Cancer 1988; 61:679–688. [DOI] [PubMed] [Google Scholar]
- 19.Rose PG, Herterick EE, Boutselis JG, et al. Multiple primary gynecologic neoplasms. Am J Obstet Gynecol 1987; 157:261–267. [DOI] [PubMed] [Google Scholar]
- 20.Storm HH, Ewertz M. Second cancer following cancer of the female genital system in Denmark, 1943–80. Natl Cancer Inst Monogr 1985; 68:331–340. [PubMed] [Google Scholar]
- 21.Boice JD, Jr, Day NE, Andersen A, et al. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J Natl Cancer Inst 1985; 74:955–975. [PubMed] [Google Scholar]
- 22.Clarke EA, Kreiger N, Spengler RF. Second primary cancer following treatment for cervical cancer. Can Med Assoc J 1984; 131:553–556. [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinerman RA, Curtis RE, Boice JD, Jr, et al. Second cancers following radiotherapy for cervical cancer. J Natl Cancer Inst 1982; 69:1027–1033. [PubMed] [Google Scholar]
- 24.Vistad I, Cvancarova M, Fossa SD, et al. Postradiotherapy morbidity in long-term survivors after locally advanced cervical cancer: how well do physicians’ assessments agree with those of their patients? Int J Radiat Oncol Biol Phys 2008; 71:1335–1342. [DOI] [PubMed] [Google Scholar]
- 25.Ota T, Takeshima N, Tabata T, et al. Treatment of squamous cell carcinoma of the uterine cervix with radiation therapy alone: long-term survival, late complications, and incidence of second cancers. Br J Cancer 2007; 97:1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner-Wasik M, Schmid CH, Bornstein LE, et al. Increased risk of second malignant neoplasms outside radiation fields in patients with cervical carcinoma. Cancer 1995; 75:2281–2285. [DOI] [PubMed] [Google Scholar]
- 27.Boice JD, Jr, Blettner M, Kleinerman RA, et al. Radiation dose and breast cancer risk in patients treated for cancer of the cervix. Int J Cancer 1989; 44:7–16. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson F, Fotiou S, Einhorn N, et al. Cohort study of the long-term effect of irradiation for carcinoma of the uterine cervix. Second primary malignancies in the pelvic organs in women irradiated for cervical carcinoma at Radiumhemmet 1914–1965. Acta Radiol Oncol 1985; 24:145–151. [DOI] [PubMed] [Google Scholar]
- 29.Kapp DS, Fischer D, Grady KJ, et al. Subsequent malignancies associated with carcinoma of the uterine cervix: including an analysis of the effect of patient and treatment parameters on incidence and sites of metachronous malignancies. Int J Radiat Oncol Biol Phys 1982; 8:197–205. [DOI] [PubMed] [Google Scholar]
- 30.Dost F, Ford PJ, Farah CS. Heightened risk of second primary carcinoma of the head and neck following cervical neoplasia. Head Neck 2014; 36:1132–1137. [DOI] [PubMed] [Google Scholar]
- 31.Hiyama T, Fujimoto I, Hanai A, et al. Occurrence of second primary cancers among patients with cervical cancer in Osaka, Japan. Natl Cancer Inst Monogr 1985; 69:181–184. [PubMed] [Google Scholar]
- 32.Evans HS, Newnham A, Hodgson SV, et al. Second primary cancers after cervical intraepithelial neoplasia III and invasive cervical cancer in Southeast England. Gynecol Oncol 2003; 90:131–136. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease C. Prevention Human papillomavirus-associated cancers: United States, 2004–2008. MMWR Morb Mortal Wkly Rep 2012; 61:258–261. [PubMed] [Google Scholar]
- 34.Gillison ML, Shah KV. Human papillomavirus-associated head and neck squamous cell carcinoma: mounting evidence for an etiologic role for human papillomavirus in a subset of head and neck cancers. Curr Opin Oncol 2001; 13:183–188. [DOI] [PubMed] [Google Scholar]
- 35.Mao C, Hughes JP, Kiviat N, et al. Clinical findings among young women with genital human papillomavirus infection. Am J Obstet Gynecol 2003; 188:677–684. [DOI] [PubMed] [Google Scholar]
- 36.Benard VB, Johnson CJ, Thompson TD, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer 2008; 113 (10 Suppl):2910–2918. [DOI] [PubMed] [Google Scholar]
- 37.Coughlan D, Frick KD. Economic impact of human papillomavirus-associated head and neck cancers in the United States. Otolaryngol Clin North Am 2012; 45:899–917. [DOI] [PubMed] [Google Scholar]
- 38.Burk RD, Ho GY, Beardsley L, et al. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infect Dis 1996; 174:679–689. [DOI] [PubMed] [Google Scholar]
- 39.Greene MH. Is cisplatin a human carcinogen? J Natl Cancer Inst 1992; 84:306–312. [DOI] [PubMed] [Google Scholar]
- 40.Sanderson BJ, Ferguson LR, Denny WA. Mutagenic and carcinogenic properties of platinum-based anticancer drugs. Mutat Res 1996; 355:59–70. [DOI] [PubMed] [Google Scholar]
- 41.Morton LM, Onel K, Curtis RE, et al. The rising incidence of second cancers: patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol Educ Book 2014; e57–67. [DOI] [PubMed] [Google Scholar]
- 42.Veiga LH, Bhatti P, Ronckers CM, et al. Chemotherapy and thyroid cancer risk: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev 2012; 21:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morton LM, Dores GM, Curtis RE, et al. Stomach cancer risk after treatment for hodgkin lymphoma. J Clin Oncol 2013; 31:3369–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 2007; 99:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henderson TO, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys 2012; 84:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol 2009; 6:638–647. [DOI] [PubMed] [Google Scholar]
- 47.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Natl Cancer Inst 2003; 95:971–980. [DOI] [PubMed] [Google Scholar]


