Abstract
Radiofrequency ablation (RFA) is indicated for early-stage hepatocellular carcinoma (HCC), but the comparative efficacy between RFA and surgical resection (SR) is inconclusive. We aim to develop a prognostic nomogram for predicting recurrence-free survival (RFS) after RFA. We also evaluate the possibility of using nomogram in improving treatment algorithm.
We retrospectively enrolled 836 patients with Barcelona Clínic Liver Cancer very-early/early-stage HCC receiving SR or RFA. A visually-orientated nomogram was constructed with Cox proportional hazards model, and number and size of tumor, platelet count, albumin level, and model for end-stage liver disease score were included. The concordance index of the nomogram was 0.69.
Radiofrequency ablation patients were stratified into low and high-risk groups by the median of nomogram scores. The RFS and overall survival (OS) of 2 risk groups were compared with SR patients with propensity score matching analysis. SR provided better RFS and OS compared with high-risk (nomogram score ≥9.8) RFA patients in the propensity model. The 5-year RFS rates were 36% versus 11%, whereas the 5-year OS rates were 74% versus 60% for SR and high-risk RFA groups, respectively (both P < 0.05). However, SR was associated with better RFS (5-year RFS rates 41% vs 29%), but similar OS (5-year OS rates 80% vs 81%), compared with low-risk (nomogram score <9.8) RFA patients in the propensity model (P < 0.05 and P > 0.05, respectively).
In conclusion, this user-friendly nomogram offers individualized recurrence risk estimation and stratification for early HCC patients receiving curative RFA. The nomogram can be integrated into current treatment algorithm. SR should be considered the first-line treatment for high-risk patients to achieve better long-term survival.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the major leading cause of cancer-related mortality in the world.1 Implementation of surveillance programs for patients with chronic liver disease combined with refined imaging technologies led to increased number of patients diagnosed at the Barcelona Clínic Liver Cancer (BCLC) very-early or early-stage (BCLC stage 0 or stage A, single tumor ≤5 cm or up to 3 tumors ≤3 cm).2 According to the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) HCC management guidelines, the first-line managements for BCLC stage 0 and BCLC stage A patients are surgical resection (SR), radiofrequency ablation (RFA), and liver transplantation.3,4
Surgical resection can achieve excellent results in BCLC very-early/early-stage HCC patients, and is the treatment of choice in noncirrhotic patients who can tolerate major operations.3 SR offers the possibilities of complete tumor eradication at the cost of more damage to nonmalignant liver parenchyma.5 On the contrary, RFA was related to comparable overall survival (OS) and better tolerability, and is recommended for patients with limited hepatic functional reserve.6 Several strategies had evolved for patients with small tumor burden when both SR and RFA could be applied, including the “ablate and wait” strategy and “surgery preferred” strategy.7,8 Currently, robust evidence to guide decision-making is still lacking.
Nomograms are graphical representations of statistical predictive models that generate numerical probabilities of an event and have been applied to various malignancies.9 The abilities of nomograms in producing personalized predictions allow their use in patient counseling and risk stratification. Nomograms have been proposed to predict recurrence, survival, and distant metastasis after SR for HCC.10–12 To our knowledge, predictive nomogram for HCC recurrence after RFA has not been reported. We aimed to construct a simple and clinically relevant nomogram to predict tumor recurrence for patients with very-early/early-stage HCC undergoing curative RFA. We also explored the possibilities of using the nomogram for risk stratification and treatment guidance by comparing the recurrence-free survival (RFS) and OS in a prospectively followed cohort of very-early/early-stage HCC patients receiving SR or RFA in the propensity score model.
PATIENTS AND METHODS
Patients
Patients with newly diagnosed HCC seen in Taipei Veterans General Hospital between 2002 and 2013 were enrolled. During this timeframe, 3182 patients were screened. Patients with BCLC very-early/early-stage HCC receiving SR or RFA as the definite treatment were enrolled in this analysis. Patients with relatively preserved performance status (performance status 1) who otherwise met the criteria for BCLC stage 0 or stage A were also included. Baseline demographics were collected. The RFS and OS were derived from medical records. The current study was approved by the Institutional Review Board of Taipei Veterans General Hospital.
Diagnosis and Treatment
Patients were diagnosed as HCC based on AASLD and EASL HCC management guidelines.3,4,13,14 Total tumor volume (TTV) was calculated to indicate tumor burden.15 Recurrent tumors were defined as radiological evidence of residual tumors adjacent to the original tumor, as well as residual tumors inside or outside the liver.
In Taipei Veterans General Hospital, a multidisciplinary HCC board was set up for treatment counseling and guidance. Risks and benefits of each therapeutic procedure were explained to individual patients in detail. Written informed consent was obtained before administrating any definite treatment. RFA and SR were performed with standard procedures and had been reported previously.16–18
Development, Validation, and Risk Stratification of the Nomogram
We used tumor recurrence after RFA as the primary endpoint in this study. Factors significant (P < 0.10) in predicting tumor recurrence in the univariate survival analysis were selected into multivariate survival model. The confidence intervals (CIs) and hazard ratios (HRs) were calculated. The β-coefficients from the final Cox model were used to construct the nomogram.
A 2-step validation of nomogram was employed. Firstly, Harrell concordance index was used to determine the discriminating ability.19 Internal validation with 100 sets of full bootstrap samples was performed to evaluate the ability of the nomogram in predicting RFS for RFA patients. Secondly, calibration plot was drawn to compare predicted probabilities of survival and observed survival at 1 and 3-year intervals after RFA, after grouping patients with quartiles of nomogram scores. The median value of nomogram scores was used to stratify RFA patients into 2 (low and high recurrence risk) groups for further analysis.
Propensity Score Matching Analysis
We employed a propensity score matching analysis to minimize potential selection bias. Generated by binary logistic regression, the propensity score estimated the probability that a patient would receive RFA or SR.20 Variables involved in the process of treatment decision, including baseline information, laboratory values, liver dysfunction, tumoral extent, and performance status, were collectively included in the model. To ensure adequate match, a 1:1 nearest-neighbor match with a preset caliber was carried out.21
Statistics
Categorical data were evaluated by chi-square test and by the Fisher exact test. For continuous variables, the Mann–Whitney U test was employed. Kaplan–Meier survival curve was used to examine RFS and OS. Statistical analyses were conducted with IBM SPSS version 20 for Windows (IBM, Armonk, NY) and SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC). Statistical significance was defined as a P value <0.05 in a 2-tailed test.
RESULTS
Identification, Characteristics, and Survival of Study Patients
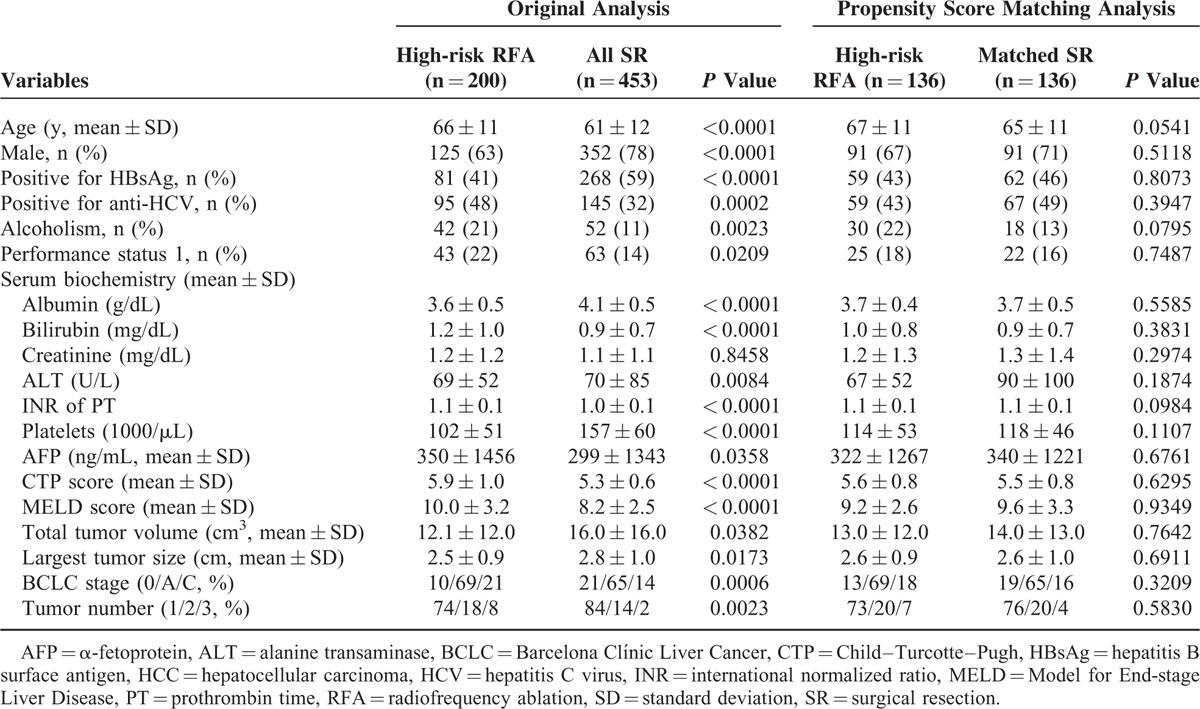
A total of 1165 patients met the enrollment criteria and were included in the study. RFA and SR were the primary treatment modalities in 383 and 453 of patients, respectively (Fig. 1). The median follow-up duration for the 2 treatment groups was 42 and 43 months, respectively. Patients with BCLC very-early/early-stage HCC receiving RFA were significantly older, but had smaller TTV compared with patients undergoing SR (both P < 0.0001; Table 1). The RFA group was also linked with lower serum albumin level, lower platelet count, higher international normalized ratio of prothrombin time, and higher MELD score compared with patients receiving SR (all P < 0.0001). The RFA group had significantly worse RFS and OS compared with the SR group (both P < 0.05; Fig. 2). The estimated 1, 3, and 5-year RFS rates were 85%, 53%, and 40% for the SR group; and 64%, 30%, and 20% for the RFA group. The 1, 3, and 5-year OS rates were 97%, 89%, and 77% versus 97%, 84%, and 70% for the SR and the RFA group, respectively. For the RFA group, 267 (70%) patients developed recurrent HCC, whereas 116 (30%) patients did not show evidence of recurrence at the last follow-up.
FIGURE 1.
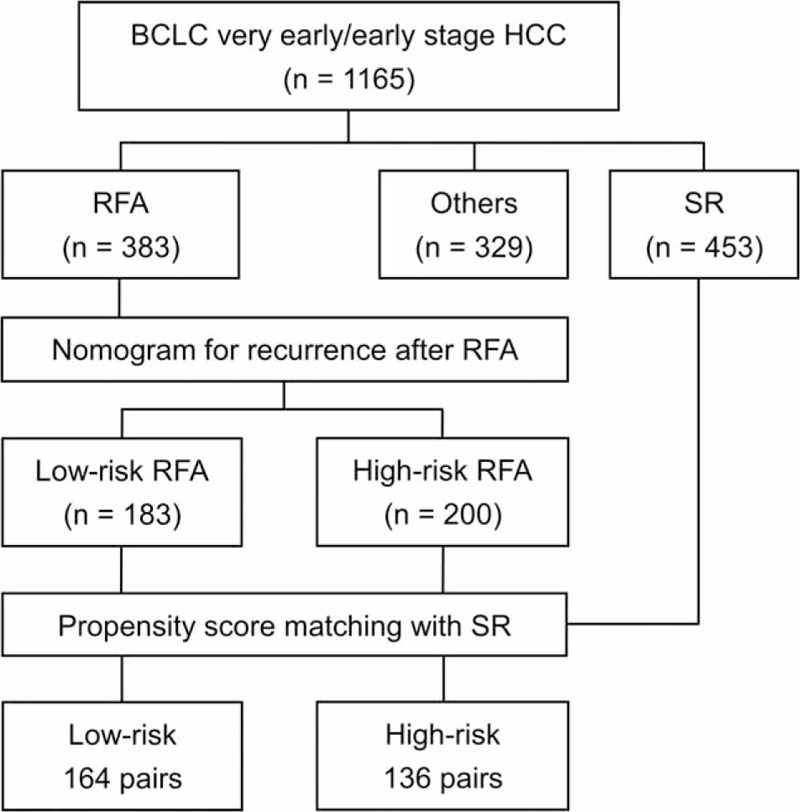
Study flowchart. A total of 1165 patients with BCLC very-early/early-stage hepatocellular carcinoma were enrolled. Among these, 383 and 453 patients received radiofrequency ablation (RFA) and surgical resection (SR), respectively. RFA patients were split into low-risk (183 patients) and high-risk group (200 patients) according to the nomogram score. Propensity score matching analysis was used to match patients receiving RFA to patients receiving SR. In the propensity score model, 164 and 136 matched-pairs of patients were selected from low-risk and high-risk group, respectively. BCLC = Barcelona Clínic Liver Cancer.
TABLE 1.
Baseline Demographics for BCLC Very-early/Early-stage HCC Patients Receiving RFA or SR
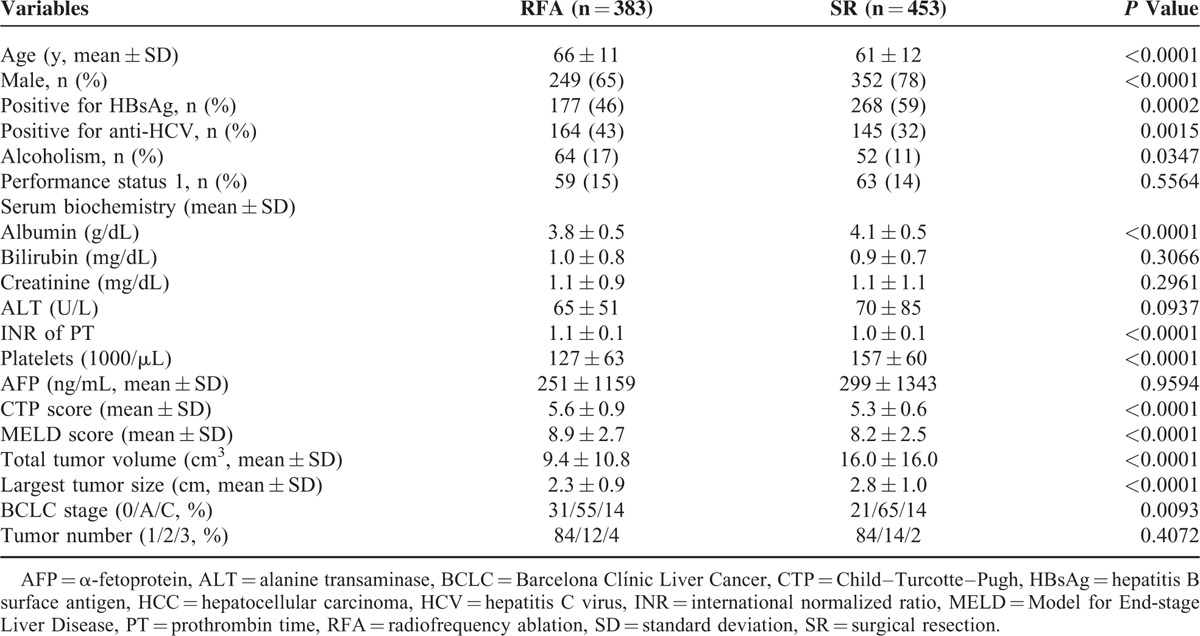
FIGURE 2.
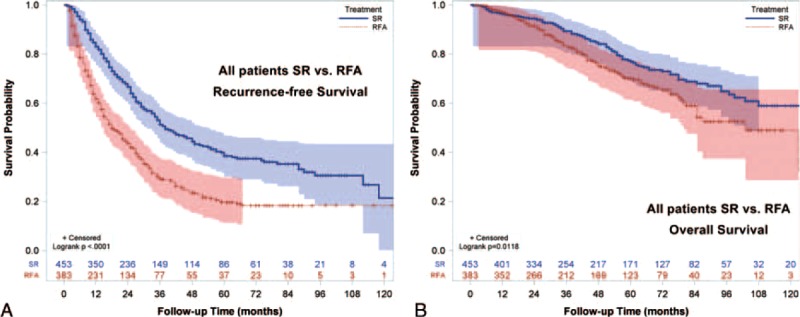
Recurrence-free survival (RFS) and overall survival (OS) for BCLC very-early/early-stage hepatocellular carcinoma receiving radiofrequency ablation (RFA) or surgical resection (SR). SR was associated with better RFS compared with RFA (P < 0.0001). Patients receiving SR had better OS compared with patients receiving RFA (P = 0.0118). BCLC = Barcelona Clínic Liver Cancer.
Construction and Validation of the Nomogram
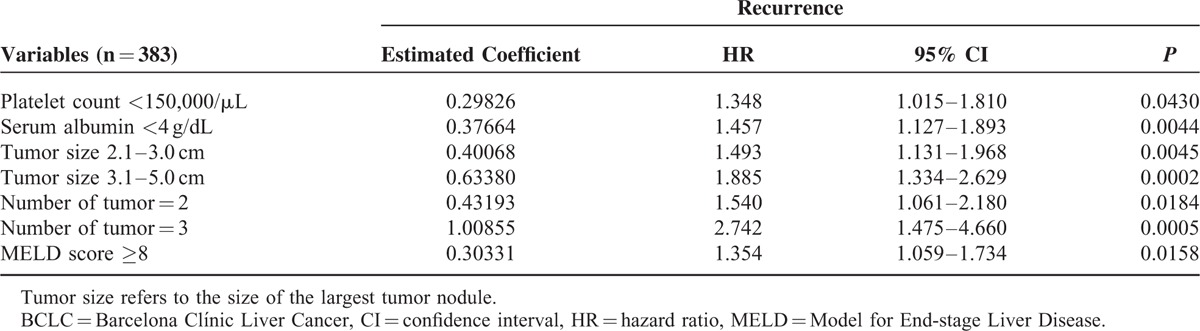
In patients receiving RFA, candidate predictors which may be linked with tumor recurrence were included in survival analysis. These factors included age, sex, etiology of liver disease, serum biochemistries, severity of chronic liver diseases, serum α-fetoprotein (AFP) level, performance status, tumor number, and tumor size. Continuous variables were dichotomized by the median values and were handled as categorical variables. Size of the largest tumor nodule was classified as ≤2 cm, 2.1 to 3.0 cm, and 3.1 to 5.0 cm. Decisions regarding the grouping of variables were made before actual modeling. Factors that were significant in predicting RFS after RFA in the final Cox model were number of tumor nodule (1, 2, and 3 nodules); diameter of the largest tumor (≤2 cm, 2.1–3.0 cm, and 3.1–5.0 cm), serum albumin level (albumin ≥4 and albumin <4 g/dL), Model for End-stage Liver Disease (MELD) score (MELD score ≥8 and MELD score <8), and blood platelet count (platelet ≥150,000/μL and <150,000/μL; Table 2).
TABLE 2.
Multivariate Regression Results For Recurrence in BCLC Very-early/Early-stage Hepatocellular Carcinoma Patients Receiving Radiofrequency Ablation
The nomogram was constructed using β-coefficients from the final Cox multivariate model. Multinodularity with 3 tumor nodules had the highest impact in the model and was given 10 points in the nomogram. The nomogram points for other variables were allocated according to the ratios of β-coefficients between multinodularity with 3 tumors and the selected variables (Fig. 3). The concordance index for the model in predicting recurrence after RFA was 0.69, and with bootstrapping (cycle = 100), the 95% CI of concordance index was 0.671 to 0.723. Calibration curves were plotted at 1 and 3-year intervals (Fig. 4).
FIGURE 3.
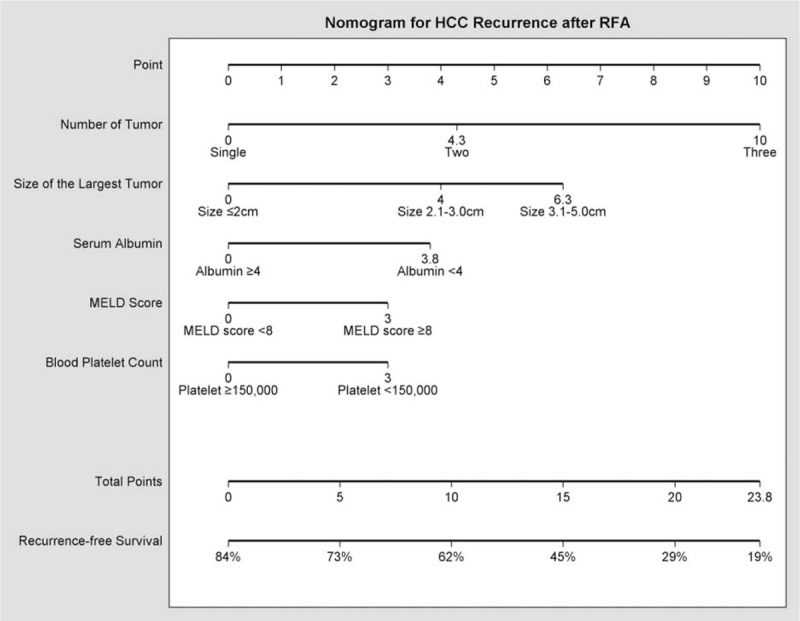
Nomogram predicting recurrence-free survival for BCLC very-early/early-stage hepatocellular carcinoma patients receiving radiofrequency ablation. To calculate the probability of recurrence, sum up the points identified on the scale for the 5 variables and draw a vertical line from the total points scale to the probability scale to obtain an estimate on recurrence-free survival. BCLC = Barcelona Clínic Liver Cancer.
FIGURE 4.
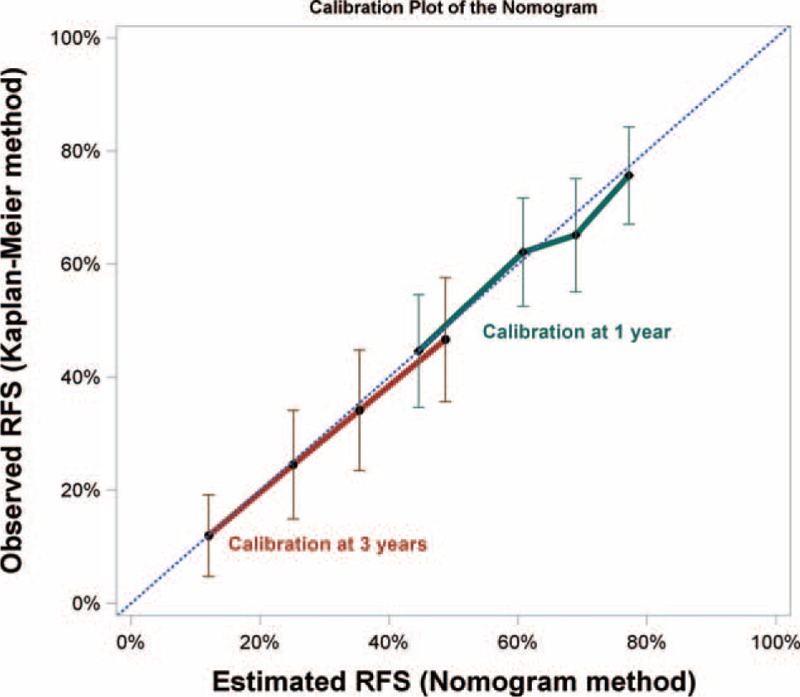
Calibration plot of the nomogram. Calibration curves of the nomogram at 1 and 3-year showed good correlation between predicted and observed outcomes. The calibration curves were close to the 45-degree line.
Risk Group Classification and Propensity Score Matching Analysis
The median of the nomogram scores in patients receiving RFA was 9.8. Patients with nomogram scores <9.8 were defined as low-risk RFA patients (n = 183), whereas patients with nomogram scores ≥9.8 belonged to the high-risk RFA group (n = 200). Propensity score matching analyses were performed between patients receiving SR and RFA in the 2 risk groups. The propensity model identified 164 pairs of SR and low-risk RFA patients, as well as 136 pairs of SR and high-risk RFA patients to compare RFS and OS (Fig. 1).
Characteristics and Survival Analysis Between SR and Low-risk RFA Patients
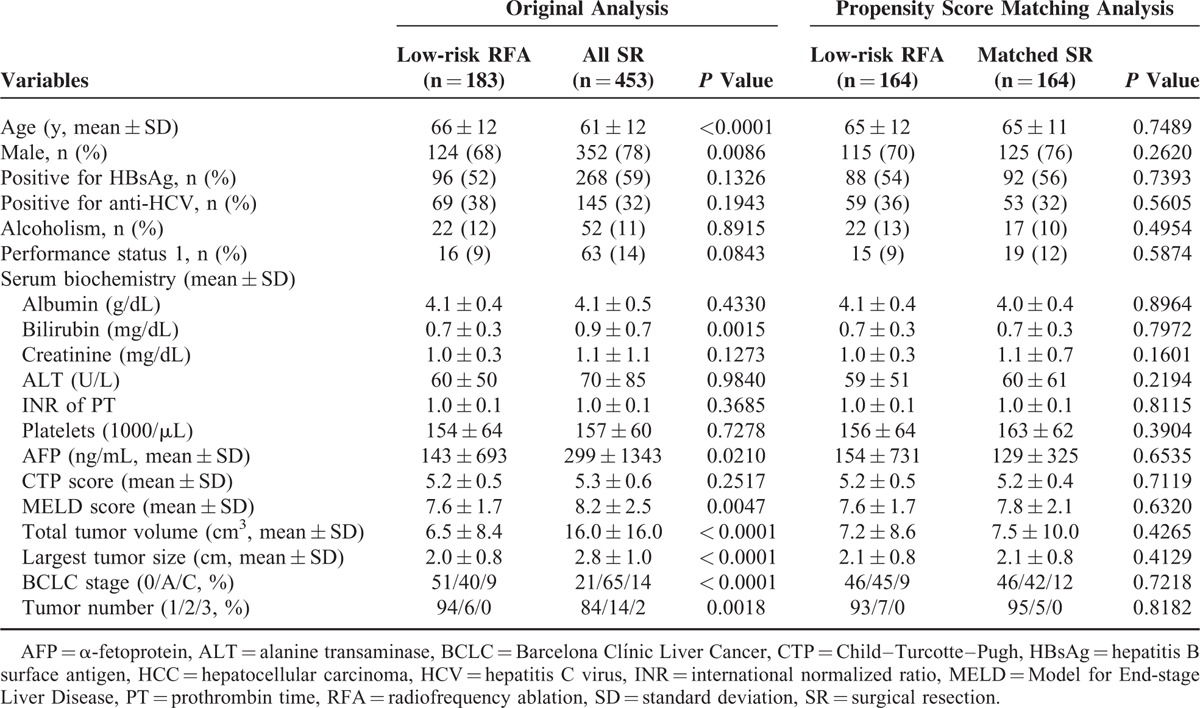
The baseline characteristics of SR patients and low-risk RFA patients are summarized in Table 3. Patients receiving SR were more often younger male and had multinodular lesions (all P < 0.05). The SR group was also associated with higher serum AFP level, higher serum bilirubin level, higher MELD score, larger TTV, and larger tumor size (all P < 0.05).
TABLE 3.
Baseline Demographic For Low-risk RFA Patients and SR Patients With BCLC Very-early/Early-stage HCC in the Original Analysis and in the Propensity Score Matching Analysis
Low-risk patients receiving RFA were associated with a significantly worse RFS compared with patients undergoing SR (P = 0.0063; Fig. 5A). The estimated 1, 3, and 5-year RFS rates were 71%, 41%, and 28% for the low-risk RFA group. On the contrary, the 1, 3, and 5-year RFS rates for SR patients were 85%, 53%, and 40%, respectively. However, the OS was similar between the low-risk RFA and the SR groups (P = 0.5860; Fig. 5B). The 1, 3, and 5-year OS rates were 99% versus 97%, 89% versus 89%, and 81% versus 77% for the low-risk RFA and the SR groups, respectively.
FIGURE 5.
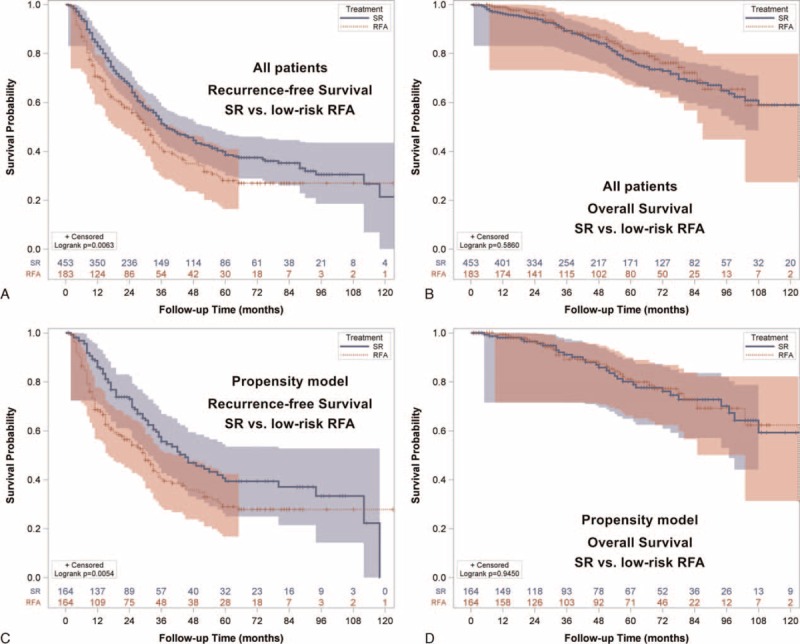
Recurrence-free survival (RFS) and overall survival (OS) for BCLC very early/early stage hepatocellular carcinoma in the low-risk group. (A) Patients undergoing surgical resection (SR) had better RFS compared with low-risk patients receiving radiofrequency ablation (RFA, p = 0.0063). (B) Patients undergoing SR had similar OS compared with low-risk RFA patients (p = 0.5860). (C) In the propensity model, SR patients had better RFS compared with low-risk RFA patients (p = 0.0054). (D) In the propensity model, the OS was similar between SR patients and low-risk RFA patients (p = 0.9450).
The baseline demographics between low-risk RFA and SR patients were not different in propensity matching analysis (all P > 0.05; Table 3). SR was related to better RFS when compared with low-risk RFA patients (P = 0.0054; Fig. 5C). The approximate 1, 3, and 5-year RFS rates were 89%, 57%, and 41% in the SR group; and 69%, 41%, and 29% in the low-risk RFA group. In the propensity model, the OS was similar between SR and low-risk RFA patients (P = 0.9450; Fig. 5D). The estimated 1, 3, and 5- year OS rates were 98% versus 99%, 91% versus 89%, and 80% versus 81% for SR and low-risk RFA group, respectively.
Characteristics and Survival Analysis Between SR and High-risk RFA Patients
Baseline demographics of SR and high-risk RFA patients are shown in Table 4. High-risk RFA patients were older. Patients receiving RFA also had a lower prevalence of chronic hepatitis B, but a higher rate of chronic hepatitis C and alcoholism (all P < 0.001). On the contrary, SR patients were linked with higher blood platelet count, higher serum albumin level, lower INR of PT, lower serum bilirubin level, lower serum AFP level, lower CTP scores, and lower MELD scores (all P < 0.05). Compared with SR patients, high-risk RFA patients had smaller tumor volume, but had more tumor nodules (both P < 0.05).
TABLE 4.
Baseline Demographic For High-risk RFA Patients and SR Patients With BCLC Very-early/Early-stage HCC in the Original Analysis and in the Propensity Score Matching Analysis
Before propensity score matching, SR patients were found to have a significantly lower recurrence rates when compared to high-risk RFA patients (P < 0.0001; Fig. 6A). The estimated 1, 3, and 5-year RFS rates were 85%, 53%, and 40% for SR; and 57%, 19%, and 12% for high-risk RFA patients. Similarly, patients undergoing resection also had a better OS compared with high-risk patients receiving RFA (P < 0.0001; Fig. 6B). The 1, 3, and 5-year OS rates were 97%, 89%, and 77% versus 96%, 79%, and 59% for SR and high-risk RFA groups, respectively.
FIGURE 6.
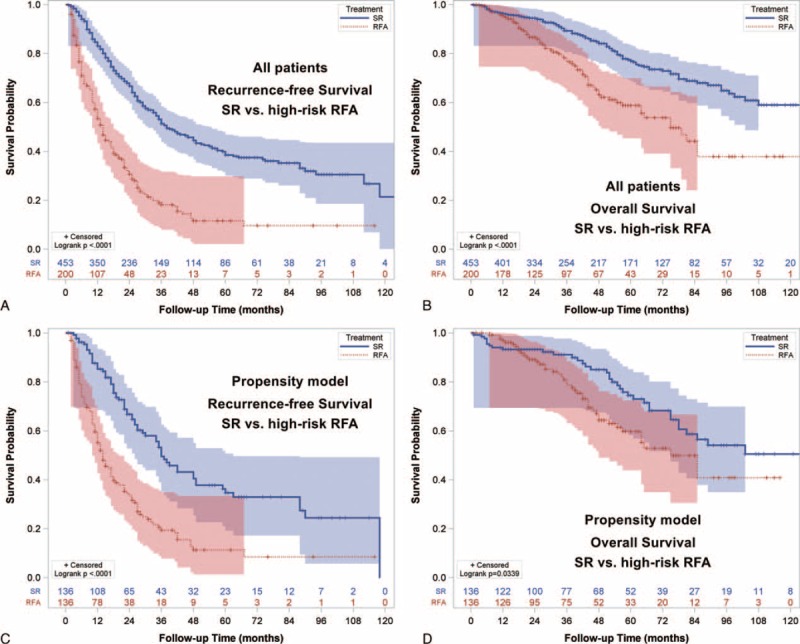
Recurrence-free survival (RFS) and overall survival (OS) for BCLC very early/early stage hepatocellular carcinoma in the high-risk group. (A) Patients undergoing surgical resection (SR) had better RFS compared with high-risk patients receiving radiofrequency ablation (RFA, p < 0.0001). (B) Patients undergoing SR had better OS compared with high-risk RFA patients (p < 0.0001). (C) In the propensity model, SR patients had better RFS compared with high-risk RFA patients (p < 0.0001). (D) In the propensity model, SR patients were associated with better OS compared with high-risk RFA patients (p = 0.0339).
High-risk RFA patients and SR patients were similar in baseline demographics in propensity model (all P > 0.05; Table 4). Patients undergoing SR had both significantly better RFS and OS compared to high-risk patients receiving RFA (P < 0.0001 and P = 0.0039; Fig. 6C and D). The estimated 1, 3, and 5-year RFS rates were 88%, 54%, and 36% for SR group; and 60%, 21%, and 11% for high-risk RFA group. The approximate 1, 3, and 5-year survival rates were 94%, 91%, and 74% for SR patients. The estimated 1, 3, and 5-year survival was 98%, 81%, and 60% for high-risk RFA patients.
DISCUSSION
There is limited evidence supporting the optimal management algorithm for patients with BCLC very-early/early-stage HCC. Therefore, current consensus guidelines are generally based on careful interpretation of retrospective studies.3,4 SR and RFA are both recommended first-line therapies for patients with very-early/early-stage HCC. On the basis of a large cohort of patients receiving RFA as the primary treatment, we constructed a predictive nomogram tailored to individual patients with the ability to predict tumor recurrence after RFA. Two risk groups were defined according to this nomogram. With propensity score matching analyses, we demonstrated that low-risk RFA patients (nomogram score <9.8) had inferior RFS, but similar OS compared with patients receiving SR. However, high-risk RFA patients (nomogram score ≥9.8) were associated with both higher risks of recurrence and mortality compared with patients receiving SR. Importantly, these results suggest that the nomogram may not only serve as a personalized predictor for recurrence but also function as a surrogate marker for treatment allocation. For patients with early HCC and high-risk profile, SR is a superior treatment option with satisfactory recurrence profile and favorable long-term outcome; for patients with low-risk profile, SR was associated superior RFS but similar OS compared with RFA.
Whether SR or RFA should be the priority treatment for BCLC very-early/early-stage HCC remains a matter of heated debate due to lack of adequately powered randomized controlled trials.22 Taking patients receiving RFA as a whole group, we found that SR was associated with superior OS and RFS compared with patients receiving RFA. The superiority of SR over RFA regarding tumor recurrence is in concordance with previously published data.22–24 It has been shown that patients without HCC recurrence had significantly better survival compared with patients with HCC recurrence.25 It could be reasonably postulated that tumor recurrence may crucially determine the prognosis. Therefore, we aimed to develop a clinically relevant method for predicting tumor recurrence in patients undergoing RFA.
With a large patient cohort receiving RFA for very-early/early-stage HCC, we constructed a predictive nomogram that is capable of generating personalized risk estimation for tumor recurrence after RFA. The easy-to-use graphical tool consists of ordinary clinical variables, including tumor number, tumor size, serum albumin level, MELD score, and blood platelet count. These variables include tumoral factors, as well as surrogates for underlying cirrhosis and portal hypertension. More importantly, the nomogram did not incorporate pathological features and were derived solely from pretreatment clinical variables. These features enable the nomogram to be an integral part in pretreatment counseling and decision-making.
The nomogram was developed to predict tumor recurrence after curative RFA for early HCC. We further divided these patients by the median of nomogram scores into 2 distinct risk groups: the low-risk (score <9.8) and high-risk groups (score ≥9.8), and compared their survival with patients receiving SR. After subgrouping RFA patients into 2 distinct risk groups, SR was still consistently superior to both groups in terms of recurrence. Regarding OS, SR was superior to RFA in the high-risk group, but not in the low-risk group. It should be noted that patients in different treatment groups had discrete baseline characteristics and tumor status, which made direct comparison not logically feasible. We therefore conducted propensity score matching analyses between SR patients and low-risk RFA patients and also between SR patients and high-risk RFA patients to minimize potential bias.
In propensity score model between SR and low-risk RFA patients, the 2 treatment groups were well balanced for baseline features. SR was associated with better RFS but similar OS compared with low-risk RFA patients. In agreement with previous reports, RFA has been shown to offer similar OS compared with SR for patients with early-stage HCC.24 RFA has the advantages of repeatability, low complication rate, and acceptable long-term prognosis.26 Moreover, the OS for primary RFA followed by SR after treatment failure was similar to primary SR for BCLC very-early–stage HCC.7 Our results indicate that the acceptable long-term survival, higher tolerability, as well as repeatability of treatment render RFA a favorable treatment option for patients with low-recurrence–risk profiles.
On the contrary, SR was associated with both better RFS and OS compared with high-risk RFA patients in propensity model where the clinical variables were well balanced between the groups. SR is traditionally considered the preferred treatment option for patients with small HCC. SR offers the potential benefits of complete tumor eradication by removing satellite lesions and neoplastic emboli.27–29 For patients with higher risks of HCC recurrence, it may be of prime importance for patients to receive aggressive therapy to achieve better tumor control and therefore the long-term survival can be improved. In this study, for patients with high-risk profiles of recurrence, SR provided better RFS and OS compared with RFA. These results imply that the nomogram could serve as a prognostic marker in treatment planning and allocation.
There are certain important considerations when constructing a nomogram. When the outcome of interest is tumor recurrence, both the numbers of recurrence and nonrecurrence should be greater than 10 times the number of predictors to reduce the expected error in the predicted probabilities to less than 10%.9 Furthermore, validation of the model is required. Data-splitting and resampling with bootstrapping are 2 possible validation methods. Data-splitting is commonly used, but is associated with the disadvantage of reduced accuracy, and that data-splitting does not adequately validate the final model. Resampling with bootstrapping can be used to obtain nearly unbiased estimates of model performance without sacrificing sample size.19 In this study, the number of nonrecurrence was 116, which is 23 times greater than the number of predictors used in the nomogram. We also applied the internal validation with resampling method by bootstrapping the entire cohort to calibrate the nomogram. These approaches further increase the validity and data integrity of our results.
Ideally, a robust nomogram should be validated externally from different patient cohorts. Therefore, a major concern of this study is its dependence on a single institutional cohort of patients from the Asia-Pacific region. There is marked heterogeneity among HCC patients around the globe regarding disease presentation, resource availability, and clinical practice, and external validation is needed from different centers.8
This study has some limitations. Firstly, this retrospective study is prone to certain biases which could not be completely avoided even with meticulous propensity score matching analysis. Secondly, the decision between SR and RFA as the primary treatment was partly dependent on both physicians’ and patients’ preferences. Although the nomogram could be incorporated into the treatment algorithm, prospective study is required to validate its prognostic accuracy. Finally, liver transplantation remains an integral management for small HCC, but could not be assessed adequately in this study due to scarcity of donors in Taiwan.
In conclusion, we have constructed a clinically relevant and easy-to-use nomogram which consistently predicts tumor recurrence after curative treatment with RFA for BCLC very-early/early-stage HCC patients. With this nomogram, investigators are able to provide specific information on individual prognosis and to classify patients receiving RFA into low and high-risk groups. We further demonstrate that SR offers better OS and RFS compared with high-risk patients receiving RFA. However, SR is associated with better RFS, but similar OS, compared with low-risk patients receiving RFA. Due to the heterogeneity of HCC patients within the same clinical stage, this nomogram could serve as a useful instrument to improve treatment selection for BCLC very-early/early-stage HCC. Although these results require external validation and further justification from adequately designed trials, our findings support the use of nomogram for risk stratification and to treat high-risk patients with SR to achieve less recurrence which could translate into better long-term overall survival.
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Diseases, AFP = α-fetoprotein, ALT = alanine transaminase, BCLC = Barcelona Clínic Liver Cancer, CI = confidence interval, CTP = Child-Turcotte-Pugh, EASL = European Association for the Study of the Liver, HBsAg = hepatitis B surface antigen, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HR = hazard ratio, INR = international normalized ratio, MELD = Model for End-stage Liver Disease, OS = overall survival, PT = prothrombin time, RFA = radiofrequency ablation, RFS = recurrence-free survival, SD = standard deviation, SR = surgical resection, TTV = total tumor volume.
Funding: This study was supported by grants from the Center of Excellence for Cancer Research at Taipei Veterans General Hospital (MOHW104-TDU-B-211-124-001), Taiwan, from Taipei Veterans General Hospital (V104C-008, V104A-004), Taipei, Taiwan, and from the Ministry of Education, Aiming for the Top University Plan (103AC-P618), Taiwan.
Authorship statement Guarantor of the article: T-IH.
Specific author contributions: P-HL, C-YH, and T-IH performed the research. Y-HL, C-YH, Y-HH, C-WS, and Y-YC collected and analyzed the data. P-HL and T-IH designed the research study and wrote the paper. H-CL contributed to the design of the study. All authors approved the final version of the manuscript.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr Accessed August 23, 2015. [Google Scholar]
- 2.Kudo M. The 2008 Okuda lecture: management of hepatocellular carcinoma: from surveillance to molecular targeted therapy. J Gastroenterol Hepatol 2010; 25:439–452. [DOI] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver, European Organisation For Research, Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56:908–943. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon RT, Ng IO, Fan ST, et al. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol 2001; 19:3037–3044. [DOI] [PubMed] [Google Scholar]
- 6.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg 2009; 249:20–25. [DOI] [PubMed] [Google Scholar]
- 7.Cho YK, Kim JK, Kim WT, et al. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology 2010; 51:1284–1290. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014; 63:844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008; 26:1364–1370. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Liu Y, Yan Z, et al. A nomogram predicting pulmonary metastasis of hepatocellular carcinoma following partial hepatectomy. Br J Cancer 2014; 110:1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg 2015; 261:939–946. [DOI] [PubMed] [Google Scholar]
- 12.Cho CS, Gonen M, Shia J, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg 2008; 206:281–291. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35:421–430. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CY, Huang YH, Hsia CY, et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei Integrated Scoring System. J Hepatol 2010; 53:108–117. [DOI] [PubMed] [Google Scholar]
- 16.Liu PH, Lee YH, Hsu CY, et al. Survival advantage of radiofrequency ablation over transarterial chemoembolization for patients with hepatocellular carcinoma and good performance status within the Milan criteria. Ann Surg Oncol 2014; 21:3835–3843. [DOI] [PubMed] [Google Scholar]
- 17.Chen JY, Chau GY, Lui WY, et al. Clinicopathologic features and factors related to survival of patients with small hepatocellular carcinoma after hepatic resection. World J Surg 2003; 27:294–298. [DOI] [PubMed] [Google Scholar]
- 18.Liu PH, Lee YH, Hsia CY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol 2014; 21:1825–1833. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE., Jr Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 20.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JH, Wang CC, Hung CH, et al. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol 2012; 56:412–418. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 2013; 58:724–729. [DOI] [PubMed] [Google Scholar]
- 24.Hung HH, Chiou YY, Hsia CY, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol 2011; 9:79–86. [DOI] [PubMed] [Google Scholar]
- 25.Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015; 261:947–955. [DOI] [PubMed] [Google Scholar]
- 26.Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma < / = 3 cm. Results of a multicenter Italian survey. J Hepatol 2013; 59:89–97. [DOI] [PubMed] [Google Scholar]
- 27.Hong SN, Lee SY, Choi MS, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol 2005; 39:247–252. [DOI] [PubMed] [Google Scholar]
- 28.Huo TI, Huang YH, Wu JC. Percutaneous ablation therapy for hepatocellular carcinoma: current practice and look into future. J Chin Med Asso 2005; 68:155–159. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010; 252:903–912. [DOI] [PubMed] [Google Scholar]


