Abstract
Bisphenol A (BPA) is an environmental endocrine disruptor that has been associated with cardiovascular outcomes in previous observational studies. We aimed to examine the relationships of urinary BPA levels with hypertension and early macrovascular diseases.
This is a cross-sectional study. From June through August 2009, 3246 participants ages 40 years or older were enrolled from Baoshan District, Shanghai, China. Logistic regression modes were used to estimate the odds ratios (ORs) for prevalent risk of hypertension, elevated carotid intima-media thickness (CIMT), arterial stiffness, and peripheral artery disease (PAD) with multivariable adjustment. We also performed stratification analysis by age and sex.
The median (interquartile range) for BPA was 0.81 (0.48, 1.45) ng/mL, which is notably lower than previously reported in the United States and other Western countries. Urinary BPA concentrations were negatively associated with hypertension (multivariable-adjusted OR for the highest versus lowest BPA quartile = 0.61; 95% confidence interval [CI]: 0.46, 0.80), elevated CIMT (OR = 0.64; 95% CI: 0.47, 0.87), and arterial stiffness (OR = 0.62; 95% CI: 0.44, 0.87). The corresponding OR for PAD (60 cases total) was not significant (OR = 0.89; 95% CI: 0.28, 2.80). The negative associations of BPA with hypertension, elevated CIMT, and arterial stiffness were consistent by age and sex stratifications, and were stronger among participants ≥60 versus <60 years of age, and among women than men.
In contrast with previous investigations, our study suggests negative associations of BPA exposure with hypertension and early macrovascular diseases among middle-aged and elderly Chinese. Future investigations are needed to draw more definite conclusions and generalize to other populations.
INTRODUCTION
Bisphenol A (BPA) is one of the world's most widely manufactured chemicals, with more than 2.2 million tons produced each year.1,2 Human population is widely and continuously exposed to BPA through food, drinking water, dermal exposures and inhalation of dusts. Detectable urinary BPA levels have been found to be present in 93% to 95% of children and adults in national surveys conducted in United States.3,4 BPA is considered as an environmental endocrine disruptor.5–7 Experimental and epidemiological studies have suggested that BPA exposure may be related to diabetes,7–9 cardiovascular disease,8 thyroid disease,10,11 obesity, and insulin resistance.5,6,12,13
Hypertension is a major public health problem worldwide and is a core risk factor for cardiovascular disease. Macrovascular complications are major target organ damages in hypertension.14 The markers of the deterioration of macrovascular structure and function, such as carotid intima-media thickness (CIMT), brachial-ankle pulse wave velocity (ba-PWV), and Ankle-Brachial Index (ABI), are predictors for cardiovascular disease.15–17 Recent epidemiological studies have reported that higher levels of urinary BPA were associated with hypertension,18,19 cardiovascular disease,8 coronary, and peripheral artery disease (PAD).20,21 Urinary BPA levels in Chinese adults are notably lower than those reported in the United States.4,9,22 Until recently, no previous study to our knowledge has examined the relationships of urinary BPA levels with hypertension and early macrovascular diseases in Chinese population. The objective of this study was to examine the relationship between urinary BPA levels, a marker of BPA exposure, and hypertension and early macrovascular diseases in Chinese adults.
METHODS
Study Design and Sample
Study participants were enrolled from Songnan Community in Baoshan District, Shanghai, China, as reported previously.9,11,23 During June and July 2008, 10,185 residents ages 40 years or older participated in a health examination, and were classified into 1 of 3 groups (normal glucose regulation, impaired glucose regulation, and diabetes) according to fasting glucose levels.9 From June to August 2009, we randomly selected 4012 participants from each group, and among these participants, 3455 participants with blood and urine samples available were included in this study. We excluded participants with missing data for urinary BPA concentration (n = 32), hypertension (n = 51), elevated CIMT (n = 14), or arterial stiffness (n = 112), and finally 3246 participants (1295 men and 1951 women) were included in final analysis. All study participants have provided written informed consent. The study protocol has been approved by the Committee on Human Research at Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine.
Clinical and Biochemical Measurements
Interviews about the sociodemographic characteristics, lifestyle factors, medical history, and family history were conducted by trained personnel. We collected the information on hypertension medication by asking whether the participants have taken any hypertension medications (yes or no). Clinical examinations, including measurements of weight, height, waist circumference, and blood pressure were performed by experienced nurses according to a standard protocol. Body mass index was calculated as weight/height2 in kg/m2. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using an electronic BP monitor (OMRON Model HEM-752 FUZZY; Omron Company, Dalian, China), on the nondominant arm in a seated position after resting for 10 min. We used the average value of 3 measurements (taken at 5-min intervals) for analysis.
Consistent with our previously published studies,11 all participants underwent a 75-g oral glucose tolerance test after an overnight fast, and blood samples were collected using BD Vacutainer® vacuum blood-collection tubes (BD Company, Shanghai, China) at 0 and 2 hr during the test. Biochemical measurements were performed at the central laboratory in Shanghai Institute of Endocrine and Metabolic Diseases, which is certified by the College of American Pathologists. Blood glucose was measured on an autoanalyzer (ADVIA-1650 Chemistry System, Bayer, Germany) using the glucose oxidase method. Serum lipids (total cholesterol, high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and triglycerides) and urinary creatinine were measured by an autoanalyzer (ADVIA-1650 Chemistry System). Diabetes was defined as fasting plasma glucose ≥7.0 mmol/L, or 2-hr oral glucose tolerance test plasma glucose ≥11.1 mmol/L, or self-reported physician diagnosis of diabetes or current use of antidiabetic medications. Impaired glucose regulation was defined as fasting plasma glucose ≥6.1 and <7.0 mmol/L, or a 2-hr oral glucose tolerance test plasma glucose ≥7.8 and <11.1 mmol/L, without a history of diabetes.
Morning spot urine samples were collected to measure urinary BPA concentration, using Eppendorf 10 mL centrifuge tubes (Eppendorf Company, Shanghai, China) which are made of polypropylene and free of BPA, delivered at 4°C immediately and frozen at −80°C within 4 hours of collection at the central laboratory.9,11 In order to prevent contaminations, sample processing strictly adhered to the standard operating procedure at the central laboratory in Shanghai Institute of Endocrine and Metabolic Diseases. Urine samples were immediately stored and maintained at −80°C during shipping to the Shanghai Institute of Materia Medica, Chinese Academy of Sciences for BPA measurement. None of the urine samples were thawed, refreezed, or aliquoted before measurement. Total (free and conjugated) urinary BPA concentration was measured by liquid chromatography-tandem mass spectrometry.9,11 The lower limit of detection for BPA was 0.30 ng/mL. The detection rate was 88%, and for analysis purpose, we assigned a value of 0.15 ng/mL for undetectable BPA concentration (N = 386).22
Measurements of Early Macrovascular Diseases
The measurement of CIMT was performed by 1 trained sonographer, using a high-resolution B-mode tomographic ultrasound system (Esaote Biomedica SpA, Genoa, Italy), with a linear 7.5-MHz transducer.24 CIMT was measured on the far wall of the right and left common carotid arteries with 1.5 cm proximal to the bifurcation, and online at the end of diastole as the distance from the leading edge of the first to the second echogenic line, which represent the lumen-intimal interface and the collagen-contained upper layer of tunic adventitia, respectively. We used the greater value of the right and left common CIMT for analysis.
Participants were asked to have a rest of 10 to 15 min before ba-PWV examination. The ba-PWV was determined by Colin VP-1000 (Model BP203RPE II, form PWV/ABI) as reported previously.25 We used the greater value of the right and left common ba-PWV for analysis.
For participants with at least 1 arm and weighting <180 kg, supine SBP was measured using Colin VP-1000 (Model BP203RPE II, form PWV/ABI) after participants resting for 10 to 15 min.25 For participants with conditions precluding the measurement of the right arm, left brachial artery SBP was measured. Left and right ABI values were calculated as the ratio of left and right ankle SBP to arm SBP, respectively. The smallest of the left and right ABI values were used in the analysis. It has been demonstrated that patients with ABIs great equal 1.5 are expected to have severe arterial rigidity,26 and in the present study, all participants had ABIs <1.5.
Definitions
Hypertension was defined as SBP ≥140 mm Hg and/or DBP ≥90 mm Hg and/or taking regular antihypertensive medicine. Participants in the highest 10% of CIMT (≥0.8 mm) were defined as elevated CIMT. Upper quartile of ba-PWV (≥1690 cm/sec) was regarded as arterial stiffness. PAD was defined as ABI < 0.9.27,28
Statistical Analysis
Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC), and P values <0.05 (2-sided) indicated statistical significance. We log10 transformed variables with a skewed distribution to achieve a normal distribution. Basic characteristics were presented as mean ± standard deviation (SD) or median with interquartile range (IQR) for continuous variables depending on normality, or proportions for categorical variables. To test for trend of characteristics across BPA quartiles, BPA was modeled as an ordinal variable coding with regard to its quartiles as 1, 2, 3, and 4. We used linear regression model and logistic regression model to test P values for trend for continuous variables and dichotomous variables, respectively.
To examine the association of BPA levels with hypertension and early macrovascular diseases, the participants were first categorized into quartiles of urinary BPA concentration, and multivariable logistic regression analyses were then performed to calculate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs) of hypertension and early macrovascular diseases for each quartile of BPA with the lowest quartile as the reference group. Potential confounding factors were controlled for in multivariable logistic regression analyses, including age, sex, and urinary creatinine, which are closely related to the exposure levels and metabolism of BPA; and body mass index, waist circumference, smoking and alcohol drinking (never, former, or current), and education attainment (≤6, 6.1–8.9, or ≥9 years), which are known risk factors for the outcomes. A sensitivity analysis was performed with further adjusting for potential intermediates or consequences of the outcome, including total cholesterol, LDL-C, HDL-C, triglycerides, and diabetes status (normal glucose regulation, impaired glucose regulation, or diabetes) and hypertension treatment (yes or no).
RESULTS
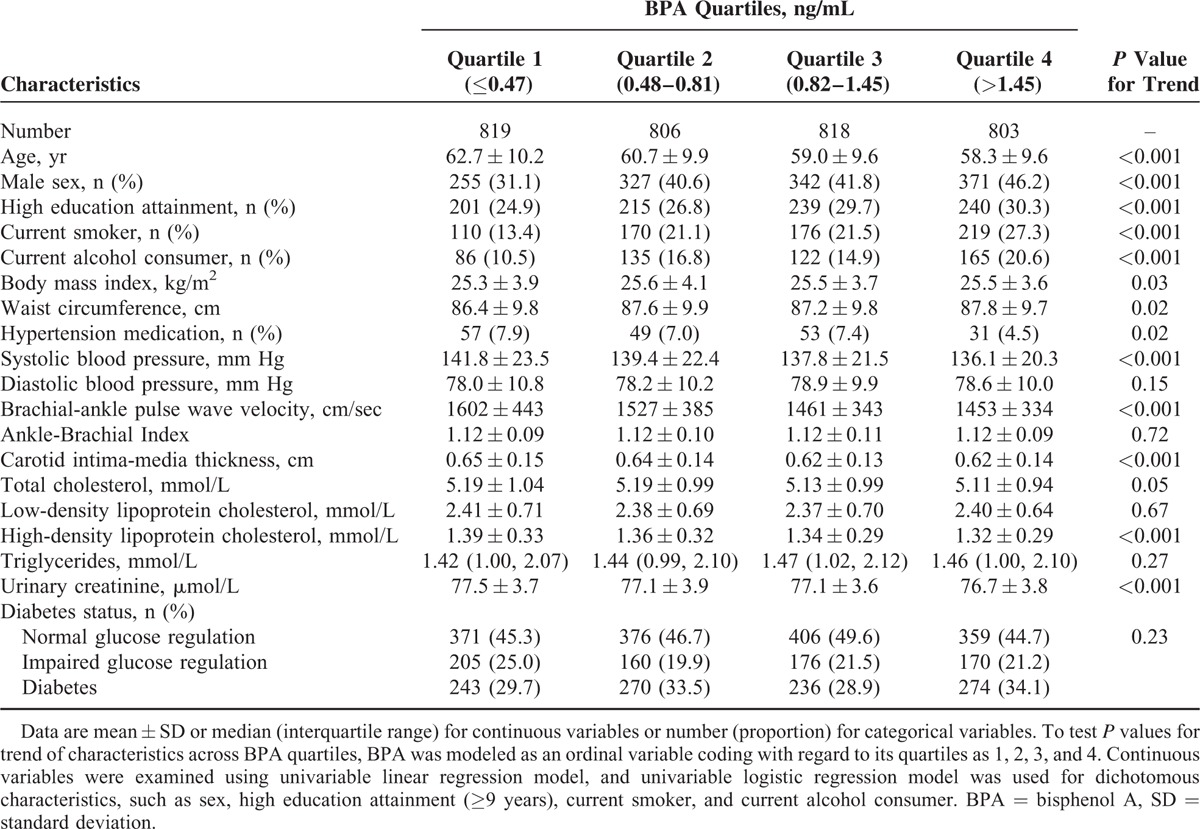
Table 1 shows the basic characteristics of study participants according to BPA quartiles. Overall, median age was 58 years (IQR, 52–68 years), and 40% were men. The median urinary BPA level was 0.81 ng/mL (IQR, 0.48–1.45 ng/mL) (data not shown). Compared with other participants, those in the highest BPA quartile were the youngest (on average) and had the highest proportions of men, current smokers, and current alcohol drinkers, and had the highest proportion with high education attainment (≥9 years). Compared with participants in the lowest BPA quartile, those in higher quartiles had higher levels of body mass index and waist circumference, lower proportion of hypertension medication, and lower levels of SBP, ba-PWV, CIMT, HDL-C, and urinary creatinine.
TABLE 1.
Characteristics of Study Participants
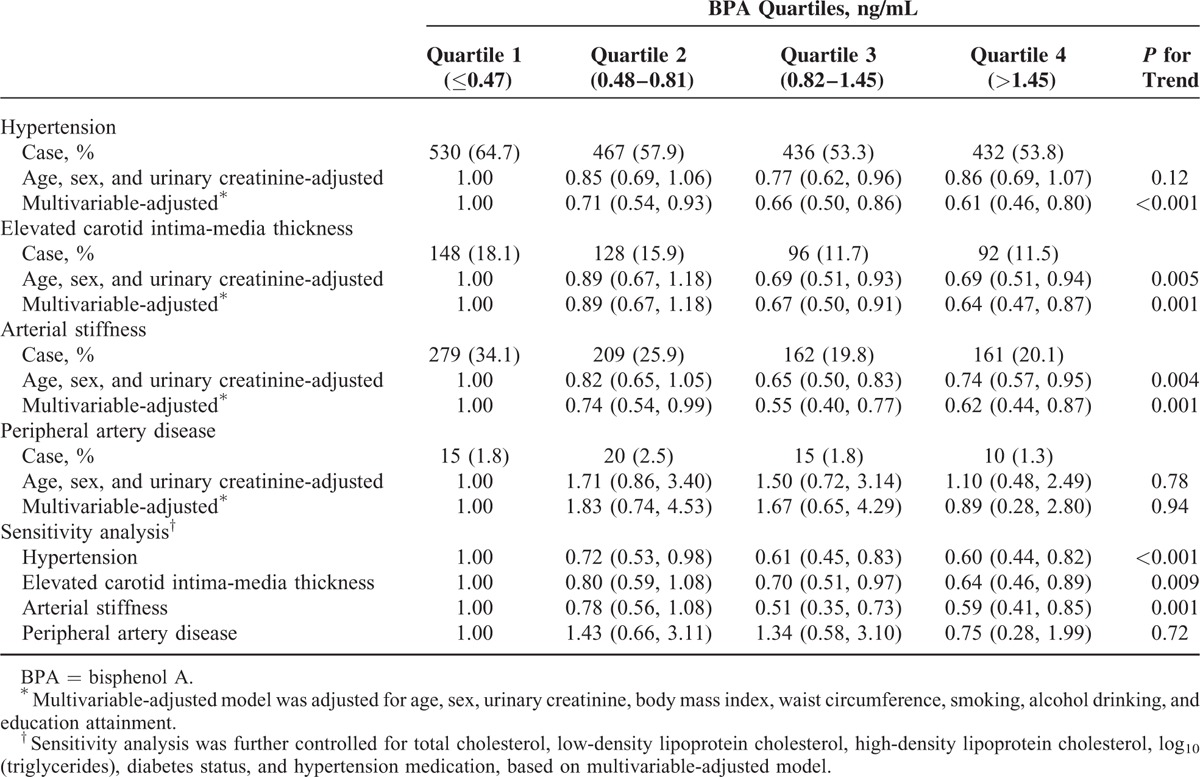
As shown in Figure 1, the prevalence of hypertension, elevated CIMT, and arterial stiffness gradually decreased from the first quartile to the third, but was similar between the third and fourth quartiles. The prevalence of PAD was highest among participants in the second BPA quartile, and no clear linear association was observed for BPA quartiles and PAD. After controlling for potential confounding factors, compared with participants in the lowest quartile, those in the highest quartile had a significantly lower prevalence of hypertension (OR = 0.61; 95% CI: 0.46, 0.80), elevated CIMT (OR = 0.64; 95% CI: 0.47, 0.87), and arterial stiffness (OR = 0.62; 95% CI: 0.44, 0.87) (Table 2). BPA also was negatively associated with PAD, but there were relatively few cases (60 total) and estimates were imprecise (for the fourth vs first quartile, OR = 0.89; 95% CI: 0.28, 2.80). The associations between BPA and hypertension and macrovascular diseases were in dose–response manner (all P for trend <0.05), except for the relationship with PAD. In the sensitivity analysis, we further adjusted potential intermediate variables, including total cholesterol, LDL-C, HDL-C, log10 (triglycerides), diabetes status, and hypertension medication, and the associations were not substantially changed (Table 2).
FIGURE 1.
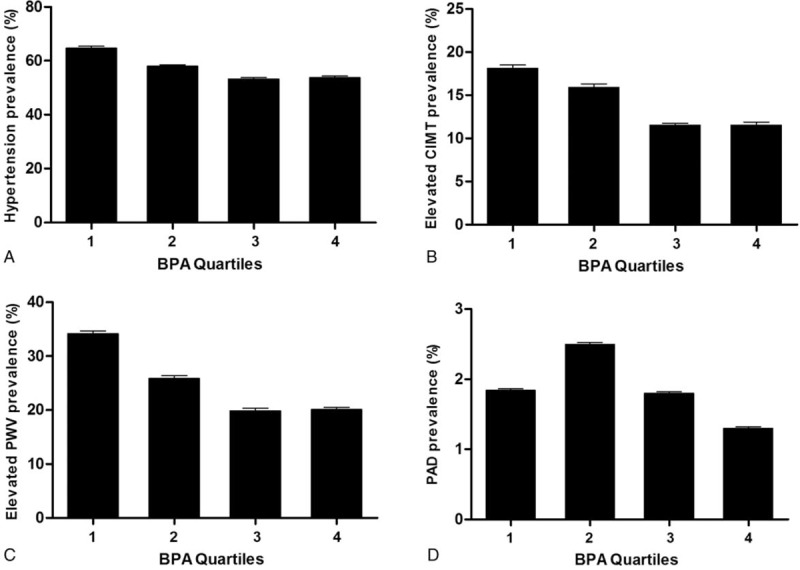
Prevalence (95% confidence interval) of hypertension and early macrovascular diseases across urinary bisphenol A quartiles. (A) Hypertension, P for trend < 0.0001; (B) elevated carotid intima-media thickness (CIMT), P for trend < 0.0001; (C) arterial stiffness, P for trend < 0.0001; and (D) peripheral artery disease (PAD), P for trend = 0.26. Logistic regression model was used to test P values for trend of outcomes across BPA quartiles, and BPA was modeled as an ordinal variable coding with regard to its quartiles as 1, 2, 3, and 4. BPA = bisphenol A.
TABLE 2.
Associations of Urinary BPA With Hypertension and Early Macrovascular Diseases
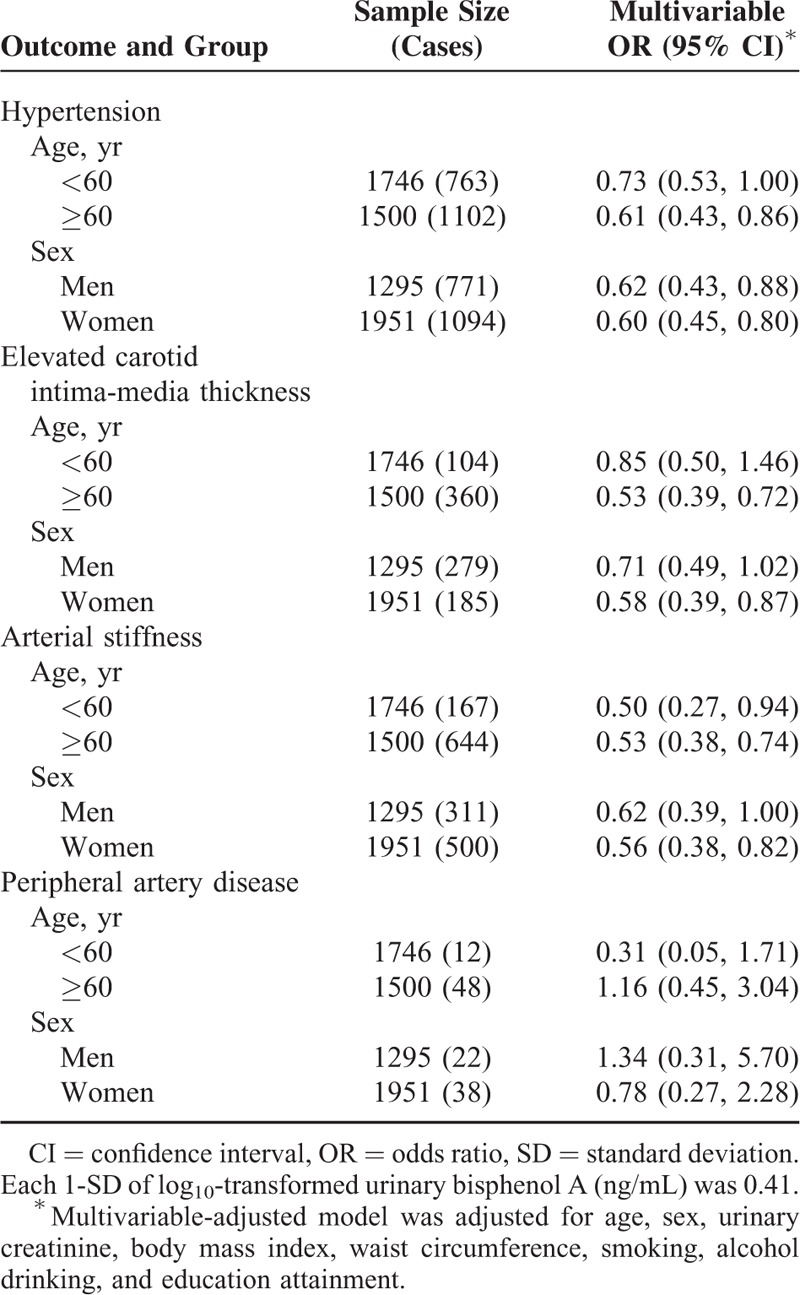
Considering that urinary BPA concentration varied between men and women (median value of urinary BPA concentration: men, 0.90 [IQR, 0.56, 1.61] vs women, 0.76 [0.43, 1.34], P < 0.0001) and according to age (median value of urinary BPA concentration: age <60 years, 0.90 [IQR, 0.55, 1.57] vs age ≥60 years, 0.71 [IQR, 0.41, 1.26], P < 0.0001), we further investigated the associations between BPA and hypertension and early macrovascular diseases by age and sex stratifications. Associations of each 1-SD increase in urinary BPA with hypertension and early macrovascular diseases, stratified by age and sex are presented in Table 3. The negative association of BPA with hypertension and elevated CIMT was stronger in magnitude for older (hypertension: OR = 0.61; 95% CI: 0.43, 0.86; elevated CIMT: OR = 0.53; 95% CI: 0.39, 0.72) versus younger (hypertension: OR = 0.73; 95% CI: 0.53, 1.00; elevated CIMT: OR = 0.85; 95% CI: 0.50, 1.46), and with elevated CIMT and arterial stiffness were stronger in women (elevated CIMT: OR = 0.58; 95% CI: 0.39, 0.87; arterial stiffness: OR = 0.56; 95% CI: 0.38, 0.82) versus men (elevated CIMT: OR = 0.71; 95% CI: 0.49, 1.02; arterial stiffness: OR = 0.62; 95% CI: 0.39, 1.00), but the differences between stratum-specific estimates were not statistically significant (all P for interactions ≥0.05). There were relatively few cases (60 total) of PAD, and the differences between sex or age strata were not statistically significant.
TABLE 3.
Associations of Each 1-SD Increase in Urinary Bisphenol A With Hypertension and Early Macrovascular Diseases, by Age and Sex Stratifications
DISCUSSION
In the present study, we found that urinary BPA levels were negatively associated with the prevalence of hypertension and early macrovascular diseases in 3246 middle-aged and elderly Chinese. The associations remained even after adjusting for potential confounding factors, intermediates, and potential consequences of the outcome.
Previous studies have reported that urinary BPA concentrations were positively associated with self-reported heart disease in National Health and Nutrition Examination Survey (NHANES) 2003 to 2004 and NHANES 2005 to 2006, after adjusting for traditional risk factors.8,20 Recently, several epidemiological studies have suggested positive associations between urinary BPA levels and hypertension,18,19 cardiovascular disease,8 coronary arterial disease, and PAD.20,21 Most of studies above-mentioned were conducted in western populations, except for 1 Korean study.18 Bae et al reported a positive nonlinear dose–response association between urinary BPA and hypertension in 258 participants ≥60 years of age who did not report previous history of hypertension. The concentrations of urinary BPA (median value, 0.81 ng/mL; IQR, 0.48–1.45 ng/mL) in the present study were low relative to concentrations in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort study (median value, 1.3 ng/mL),20 which reported a positive association between BPA and coronary artery disease in a dose–response manner in a longitudinal analysis. Urinary BPA concentrations in our study population also were lower than concentrations reported for other recent study populations (available median values range from 1.20 to 2.30 ng/mL, available mean values range from 1.79 to 6.93 ng/mL).8,18–21,29 Different from the Korean study, in the present study, the associations between BPA and hypertension, elevated CIMT and arterial stiffness were negative and in dose–response relationship. The most current study by Bae and Hong reported that consuming canned beverage and consequent increase of BPA exposure increased blood pressure acutely in 60 participants (56 women and 4 men) ages 60 years or older. It is an interesting study revealing a relationship between short-term BPA exposure and acute effect on blood pressure.30 The association of chronic BPA exposure with cardiovascular risk is still to be evaluated in further longitudinal study.
BPA is suspected to have a variety of effects in humans, and its estrogenic action is considered to be 1 of the major mechanisms.31 Estrogen has several important effects on the cardiovascular system.32 Estrogen alters the expression or activity of ion channels, contractile proteins, and reactive oxygen species production and all of which may contribute to cardiovascular health.32–34 It is well-documented that estrogen improves vascular function and reduces atherosclerosis.32 Experimental studies of mice have indicated that the atheroprotection is mediated by estrogen receptor-α and β,35,36 which have important roles in repairing blood vessels and controlling blood pressure.31,36 Estrogen is also important for maintaining and repairing the endothelium. This process appears to involve an estrogen mediated increase in endothelial nitric oxide synthase and cyclooxygenase.34 In humans, the incidence of cardiovascular disease differs widely among men and women, partly because of the variations in hormones.37 Studies have found that the incidence of cardiovascular disease is usually low in premenopausal women and tends to increase in postmenopausal women, however, in postmenopausal women who receive estrogen therapy, the incidence can be reduced to premenopausal levels.37–39 Thus it is biologically possible that exogenous estrogen may have protective effects on hypertension and early macrovascular diseases.
The exact mechanisms by which BPA exposure may affect the cardiovascular system are not fully understood. Experimental studies of rats and in vitro reported that BPA affects cardiovascular system mainly through inducing liver and oxidative cellular damage,40 disrupting pancreatic β-cell function,5 and promoting obesity and insulin resistance.6 Although hypertension and early macrovascular diseases are risk factors for cardiovascular disease, experimental evidence of mice and in vitro suggested that BPA also acts on cardiovascular system through a series of other ways, including inducing obesity, diabetes, and hormone and lipids dysfunctions.5,6,10 Furthermore, it has been suggested that the actions of estrogen and its receptors in the cardiovascular system differ by sex and age.37,41 In the present study, we observed that negative association of BPA with elevated CIMT were stronger in magnitude among those ≥60 versus <60 years of age. Although it is plausible to speculate that as an exogenous estrogen, BPA may affect the cardiovascular system by mimicking estrogen, future research is needed to give a more clear demonstration on behavior of BPA in humans.
The strength of the present study included the large sample size, and the understudied population. The present study has several limitations. Firstly, due to the cross-sectional nature of the study, we cannot establish that BPA exposure precedes hypertension and early macrovascular diseases. Longitudinal studies are needed to clarify the chronic effect of BPA exposure on health outcomes. Secondly, we did not collect data on diet behavior, and the specific hypertension medication categories or dose. Thus, confounding by diet or medication cannot be ruled out. Thirdly, the BPA measures are from single spot urine samples, repeated measurements over weeks, months, or even years would improve the evaluation of a long-term exposure. Mahalingaiah et al42 measured urinary BPA concentrations in 217 samples collected over weeks to months from 82 participants attending a fertility clinic in the United States. The authors reported that 63% of participants classified into the highest tertile of the geometric mean concentration of all samples would have been correctly classified based on a single urine sample. Fourthly, due to a generally lower level of BPA exposure in this population, it might be less likely to detect any possibly positive association between BPA and cardiovascular risk than in a population with a wider range of possible exposure levels. Fifthly, our study participants were restricted to middle-aged and elderly Chinese population. Therefore, age, race, and the BPA exposure levels should be considered when generalizing the findings to other populations. Further studies in prospective cohorts are warranted to verify our findings in other demographic or ethnic populations.
In contrast with previously published studies, our study suggested negative relationships between BPA exposure and hypertension, elevated CIMT, and arterial stiffness in middle-aged and elderly Chinese adults. Much about the actions of BPA on cardiovascular functions in humans remain unknown, and well-designed longitudinal studies including a wide range of BPA exposure levels, ages, and genders are imperative for confirming associations and understanding the mechanisms.
Footnotes
Abbreviations: ABI = Ankle-Brachial Index, ba-PWV = brachial-ankle pulse wave velocity, BPA = bisphenol A, CI = confidence intervals, CIMT = carotid intima-media thickness, DBP = diastolic blood pressure, HDL-C = high-density lipoprotein cholesterol, IQR = interquartile range, LDL-C = low-density lipoprotein cholesterol, OR = odds ratio, PAD = peripheral artery disease, SBP = systolic blood pressure, SD = standard deviation.
TW and MX contributed equally to this article.
This work was supported by the grants 2013BAI09B13 from the National Clinical Research Center for Metabolic Diseases, 81321001, 81390350, 81222008, 81471059, 81130016, and 81500610 from the National Natural Science Foundation of China, the Shanghai Municipal of Science and Technology (13495810200), Shu Guang Project of Shanghai Municipal Education Commission and Shanghai Education Development Foundation (12SG21), SMC-Chen Xing Young Scholars Program of Shanghai Jiao Tong University (2012), the ‘Yang Fan Project for Young Scientist’ of Shanghai Science and Technology Committee (15YF1410100), and the Youth Scientific Research Project of Shanghai Municipal Health and Family Planning Commission (20144Y0109).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kelland K. Experts demand European action on plastics chemical. Reuters. 2010. Available: http://www.reuters.com/article/2010/06/22/us-chemical-bpa-health-idUSTRE65L6JN20100622?loomia_ow=t0:s0:a49:g43:r3:c0.084942:b35124310:z0 (Accessed November 13, 2014). [Google Scholar]
- 2.European Union. European Union Risk Assessment Report. Bisphenol A, CAS No: 80-05-7. Institute for Health and Consumer Protection, European Chemicals Bureau, European Commission Joint Research Centre, 3rd Priority List, Luxembourg: Office for Official Publications of the European Communities; 2003. [Google Scholar]
- 3.Calafat AM, Kuklenyik Z, Reidy JA, et al. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 2005; 113:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calafat AM, Ye X, Wong LY, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 2008; 116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso-Magdalena P, Morimoto S, Ripoll C, et al. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect 2006; 114:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugo ER, Brandebourg TD, Woo JG, et al. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect 2008; 116:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ropero AB, Alonso-Magdalena P, García-García E, et al. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int J Androl 2008; 31:194–200. [DOI] [PubMed] [Google Scholar]
- 8.Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008; 300:1303–1310. [DOI] [PubMed] [Google Scholar]
- 9.Ning G, Bi Y, Wang T, et al. Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: a cross-sectional analysis. Ann Intern Med 2011; 155:368–374. [DOI] [PubMed] [Google Scholar]
- 10.Moriyama K, Tagami T, Akamizu T, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab 2002; 87:5185–5190. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Lu J, Xu M, et al. Urinary bisphenol A concentration and thyroid function in Chinese adults. Epidemiology 2013; 24:295–302. [DOI] [PubMed] [Google Scholar]
- 12.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res 2011; 111:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, Li M, Chen B, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 2012; 97:E223–E227. [DOI] [PubMed] [Google Scholar]
- 14.Shantsila A, Dwivedi G, Shantsila E, et al. Persistent macrovascular and microvascular dysfunction in patients with malignant hypertension. Hypertension 2011; 57:490–496. [DOI] [PubMed] [Google Scholar]
- 15.Fowkes FG, Murray GD, et al. Ankle Brachial Index Collaboration. Ankle Brachial Index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008; 300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet 2012; 379:2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashina A, Tomiyama H, Arai T, et al. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res 2003; 26:615–622. [DOI] [PubMed] [Google Scholar]
- 18.Bae S, Kim JH, Lim YH, et al. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 2012; 60:786–793. [DOI] [PubMed] [Google Scholar]
- 19.Shankar A, Teppala S. Urinary bisphenol A and hypertension in a multiethnic sample of US adults. J Environ Public Health 2012; 2012:481641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melzer D, Osborne NJ, Henley WE, et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012; 125:1482–1490. [DOI] [PubMed] [Google Scholar]
- 21.Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect 2012; 120:1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y, Miao M, Herrinton LJ, et al. Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ Res 2009; 109:629–633. [DOI] [PubMed] [Google Scholar]
- 23.Wang T, Lu J, Xu Y, et al. Circulating prolactin associates with diabetes and impaired glucose regulation: a population-based study. Diabetes Care 2013; 36:1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Z, Liu Y, Xu Y, et al. Impaired lung function is associated with increased carotid intima-media thickness in middle-aged and elderly Chinese. PLoS ONE 2013; 8:e53153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Bi Y, Xu M, et al. Nonalcoholic fatty liver disease is associated with atherosclerosis in middle-aged and elderly Chinese. Arterioscler Thromb Vasc Biol 2012; 32:2321–2326. [DOI] [PubMed] [Google Scholar]
- 26.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation 1993; 88:837–845. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care 2003; 26:3333–3341. [DOI] [PubMed] [Google Scholar]
- 28.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004; 110:738–743. [DOI] [PubMed] [Google Scholar]
- 29.Melzer D, Rice NE, Lewis C, et al. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS ONE 2010; 5:e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae S, Hong YC. Exposure to bisphenol A from drinking canned beverages increases blood pressure: randomized crossover trial. Hypertension 2015; 65:313–319. [DOI] [PubMed] [Google Scholar]
- 31.Vandenberg LN, Maffini MV, Sonnenschein C, et al. Bisphenol A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 2009; 30:75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res 2011; 109:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnal JF, Fontaine C, Billon-Galés A, et al. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol 2010; 30:1506–1512. [DOI] [PubMed] [Google Scholar]
- 34.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 2005; 308:1583–1587. [DOI] [PubMed] [Google Scholar]
- 35.Hodgin JB, Krege JH, Reddick RL, et al. Estrogen receptor alpha is a major mediator of 17beta-estradiol's atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest 2001; 107:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Bian Z, Lu P, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 2002; 295:505–508. [DOI] [PubMed] [Google Scholar]
- 37.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation 1997; 95:252–264. [DOI] [PubMed] [Google Scholar]
- 38.Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 1992; 117:1016–1037. [DOI] [PubMed] [Google Scholar]
- 39.Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med 1991; 325:756–762. [DOI] [PubMed] [Google Scholar]
- 40.Bindhumol V, Chitra KC, Mathur PP. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 2003; 188:117–124. [DOI] [PubMed] [Google Scholar]
- 41.Stice JP, Lee JS, Pechenino AS, et al. Estrogen, aging and the cardiovascular system. Future Cardiol 2009; 5:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahalingaiah S, Meeker JD, Pearson KR, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect 2008; 116:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]


