Abstract
The aim of the study was to describe the epidemiologic and clinical characteristics and identify the risk factors of short-term and 1-year mortality in a recent cohort of patients with infective endocarditis (IE).
From January 2008, multidisciplinary teams have prospectively collected all consecutive cases of IE, diagnosed according to the Duke criteria, in 25 Spanish hospitals.
Overall, 1804 patients were diagnosed. The median age was 69 years (interquartile range, 55–77), 68.0% were men, and 37.1% of the cases were nosocomial or health care-related IE. Gram-positive microorganisms accounted for 79.3% of the episodes, followed by Gram-negative (5.2%), fungi (2.4%), anaerobes (0.9%), polymicrobial infections (1.9%), and unknown etiology (9.1%). Heart surgery was performed in 44.2%, and in-hospital mortality was 28.8%. Risk factors for in-hospital mortality were age, previous heart surgery, cerebrovascular disease, atrial fibrillation, Staphylococcus or Candida etiology, intracardiac complications, heart failure, and septic shock. The 1-year independent risk factors for mortality were age (odds ratio [OR], 1.02), neoplasia (OR, 2.46), renal insufficiency (OR, 1.59), and heart failure (OR, 4.42). Surgery was an independent protective factor for 1-year mortality (OR, 0.44).
IE remains a severe disease with a high rate of in-hospital (28.9%) and 1-year mortality (11.2%). Surgery was the only intervention that significantly reduced 1-year mortality.
INTRODUCTION
Infective endocarditis (IE) is a severe disease with high morbidity, and prolonged hospital stay. Mortality during the IE admission ranges from 13% to 25%, and a further 9% to 20% of the patients will die during the first year after discharge.1–5
Owing to the low incidence of IE (1.7–7.9 cases/100,000 inhabitants3,4,6–9), data on clinical presentation, complications, and outcomes are mainly obtained from series collected over prolonged periods, in single centers, or over shorter periods in multicenter, multinational studies from selected centers.3,9–11 Consequently, they do not necessarily represent the current situation of a whole country.
In 2008, in association with the International Collaboration on Endocarditis (ICE),1,12 we created a national cooperative endocarditis study group, The Spanish Collaboration on Endocarditis-Grupo de Apoyo al Manejo de la Endocarditis Infecciosa en España (GAMES), with the objectives of improving the care of IE patients and conducting research in Spain.
The objective of the present study was to define the characteristics of IE in a prospective multicenter, nationwide study, performed in 25 centers of Spain and to identify risk factors of early and late mortality.
MATERIALS AND METHODS
Patients
The study sample comprised all consecutive patients with IE in the 25 centers from January 1, 2008 to December 31, 2012. GAMES is a prospective registry performed by multidisciplinary group dedicated to improve the management of IE. Hospital-based endocarditis groups include microbiologists, infectious disease physicians, heart surgeons, echocardiographers, imaging specialists, and cardiologists. These groups prospectively recorded all consecutive episodes of IE at their institutions and collected the data according to a preestablished clinical form with common standard definitions.13–15 At discharge, the clinical forms were sent to the coordinating center or data were entered directly by the investigators through a secure data entry system. In the coordinating center, specialized clinicians and data managers reviewed the data for accuracy and contacted the referring centers, if necessary, for queries and clarifications. Patients were followed for 1 year.
Episodes were classified into 4 distinct categories representing different populations: native valve IE in intravenous drug users (IVDU), native valve IE in non-IVDU, prosthetic valve IE, and IE involving implantable cardiac devices.
Definitions
IE was defined according to the modified Duke criteria.14,16 Site of IE acquisition was defined following ICE recommendations.13 In brief, community-acquired IE was defined as IE diagnosed within the first 48 hours of admission in a patient who did not fulfill the criteria for nosocomial or health care-associated infection. Nosocomial IE was defined as IE in a patient who had been hospitalized for >48 hours before the onset of signs or symptoms consistent with IE. Health care-associated IE was an IE diagnosed within 48 hours of admission of an outpatient with any of the following criteria17: intravenous therapy, wound care, or specialized nursing care at home within the 30 days before the onset of IE; attendance at a hospital or hemodialysis clinic or receipt of intravenous chemotherapy within the 30 days before the onset of IE; hospitalization in an acute care hospital for 2 or more days during the 90 days before the onset of IE; or residence in a nursing home or long-term care facility. An implantable cardiac device was defined as a permanent pacemaker and/or cardioverter-defibrillator. Perivalvular extension was considered to be substantial when abscesses were present or other echocardiography findings suggested that the infection was invasive (communication between chambers, wall dissection, or large valvular dehiscence). Prosthetic valve IE was defined as an endovascular infection occurring on parts of a valve prosthesis or on reconstructed native heart valves whether a mechanical prosthesis, and/or a bioprosthetic xenograft, stented or unstented, and/or a repaired native valve with implantation of an annular ring. The EuroSCORE20 was used to assess operative risk.18,19 We used the Charlson comorbidity index as a method of categorizing comorbidities of the patients.20
Chronic immunosuppressive therapy was defined as the administration of recognized immunosuppressive agents for >30 days at the time of IE diagnosis.
Central nervous system (CNS) event was defined as an acute neurological deficit of vascular etiology lasting >24 hours.21 Systemic embolization was defined as an embolic event outside of the CNS. Congestive heart failure was defined according to the New York Heart Association classification system.22
Statistical Analysis
The 4 classic types of IE were compared: native valve IE in IVDU and in non-IVDU, prosthetic valve IE, and IE affecting intracardiac devices. Quantitative variables were expressed as mean ± standard deviation or as medians with interquartile range (IQR), as appropriate; qualitative variables were expressed as frequency and percentage. Continuous variables were compared using the t test, and categorical variables were compared using the χ2 test or Fisher exact test when the χ2 test was not appropriate. Adjusted odds ratios (ORs)23 were computed using logistic regression analysis. Stepwise logistic regression analysis was performed including variables with a P value ≤0.1 in the univariate analysis. All statistical analyses were performed using SPSS software version 18 (IBM PASW Statistics 18.0, Armonk, New York, NY).
Ethics
The project and the common case report form were approved by the national and local institutional review boards and ethics committees (E.C. 18/07).
RESULTS
Incidence of IE
The study sample comprises total of 1804 IE cases from 25 centers, located throughout Spain. Those institutions attend an estimated population of 10,218,634 habitants, that is, 21.7% of the Spanish population.24 Therefore, we estimated an annual incidence of at least 3.5 cases of IE per 100,000 inhabitants.
General Characteristics of the Cohort
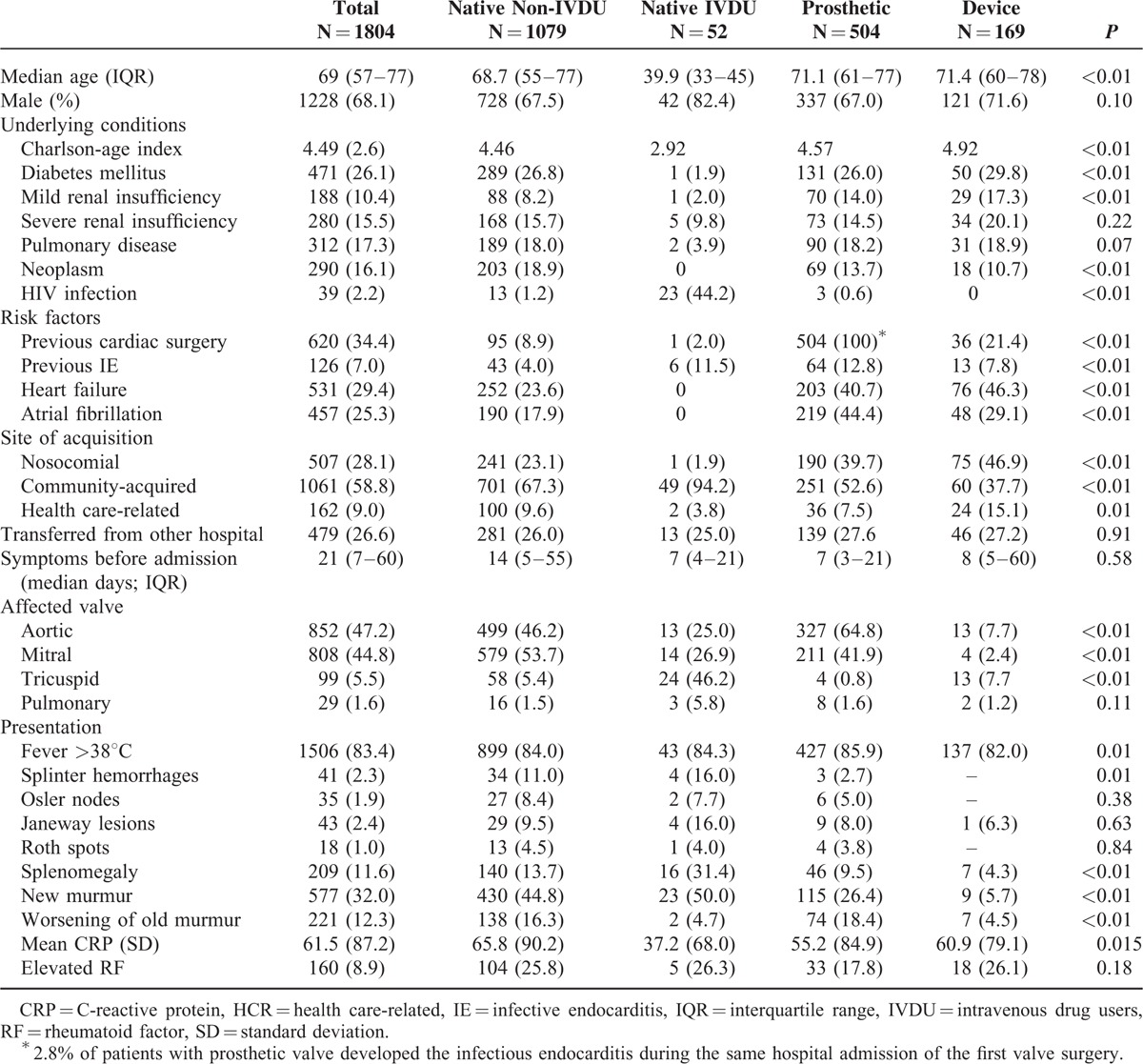
The median age of the cohort was 69 years (IQR, 55–77; mean, 65.1), and 1228 (68.0%) patients were male. The most common “extracardiac underlying conditions” are shown in Table 1. The main comorbidities were diabetes mellitus (471, 26.1%), pulmonary disease (312, 17.3%), and neoplasm (290, 16.1%). Other comorbidities that were not as frequent, but nevertheless had a high impact on clinical course were, hemodialysis (79, 4.4%), HIV infection (39, 2.2%), and transplantation (27, 1.5%). The mean Charlson-age corrected comorbidity index was 4.49 ± 2.6. The most common “predisposing heart conditions” were native valve disease (41.8%) (degenerative [27%]; rheumatic valve disease [8.0%]; and congenital heart disease [3.2%]), followed by previous valve surgery (34.4%) and previous IE episode (6.9%).
TABLE 1.
Epidemiological and Clinical Characteristics of 1804 Episodes of Infective Endocarditis Prospectively Collected in Spain
Affected Valve
Most of the patients (62.7%) had native valve IE, and most episodes were left-sided (mitral 808 [44.8%], aortic 852 [47.2%]). The tricuspid valve was involved in 99 cases (5.5%) and the pulmonary valve in 29 cases (1.6%). Prosthetic valve endocarditis occurred in 504 cases (27.9%) and device-related endocarditis in 169 patients (9.3%).
The “site of acquisition” was determined in 95.9% of patients (Table 1); 28.1% episodes were classified as nosocomial. In the case of community-acquired episodes, most patients (86%) were admitted within 1 month of the initial signs of illness (12.6% at 1–3 months and 6.3% >3 months). The “source of the infection” was suspected in 842 (46.6%) patients (vascular 305; gastrointestinal 127; skin and soft tissue 124; odontogenic 115; urinary 88; respiratory 21; others 62).
“Clinical manifestations” are shown in Table 1. It is noteworthy that the classic signs of IE were uncommon. These included splenomegaly (11.6%), splinter hemorrhages (2.3%), Janeway spots (2.4%), and Osler nodes (1.9%). However, patients with IE had other common manifestations (respiratory [41%], renal [39%], neurological [19.7%], osteoarticular [11.5%], and ocular [6.3%]).
Etiology
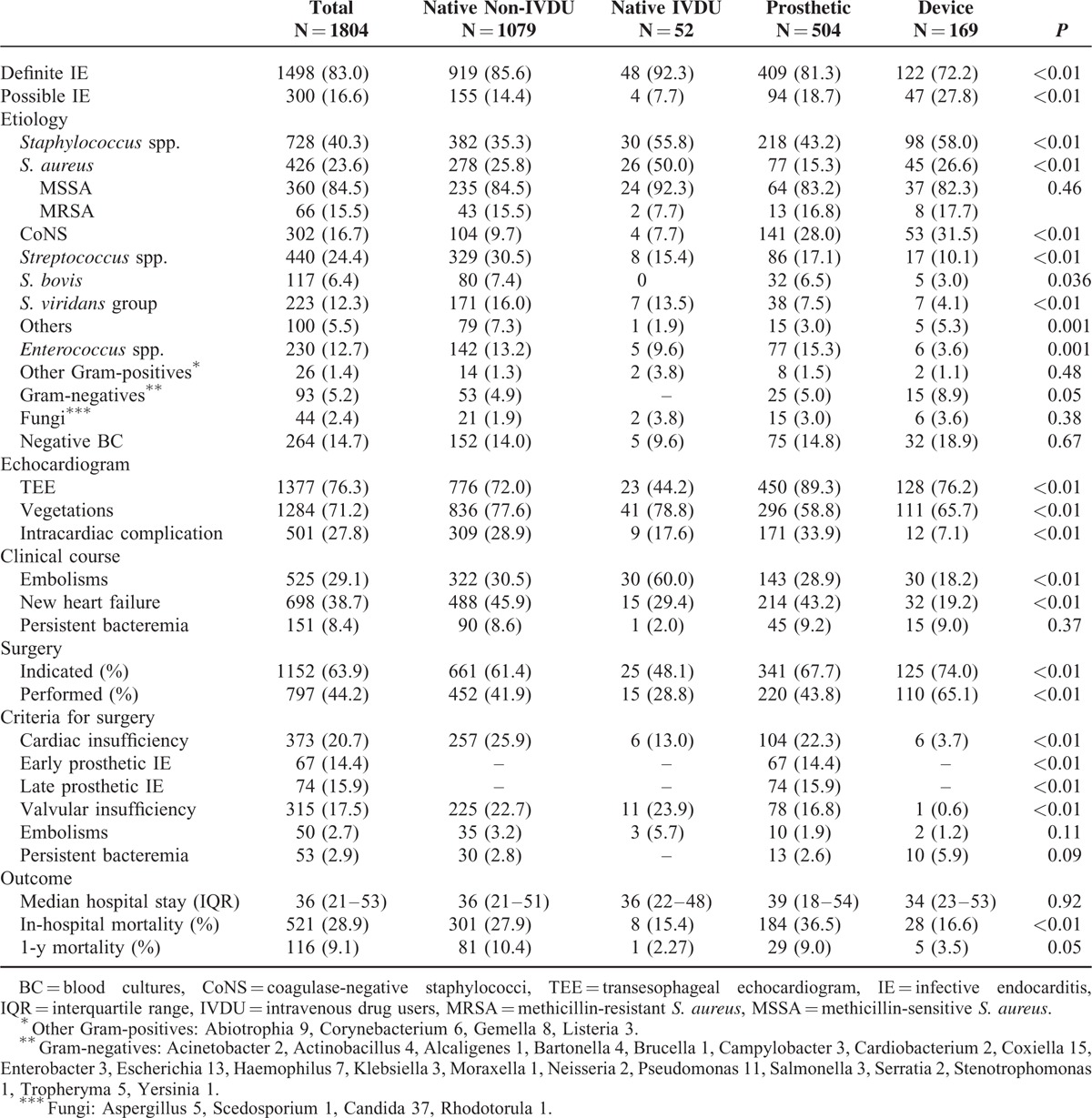
Most episodes (79.3%) were caused by Gram-positive microorganisms, followed by Gram-negative microorganisms (5.2%), fungi (2.4%), anaerobes (0.9%), and polymicrobial infections (1.9%). The distribution of the most common microorganisms is shown in Table 2. Twenty-two episodes were caused by microorganisms of the HACEK group. Other fastidious microorganisms included Coxiella burnetii (15), Listeria monocytogenes (6), Tropheryma whipplei (5), Bartonella spp. (4), and Brucella melitensis (1). Accordingly, the rate of unknown etiology of endocarditis was 9.1%.
TABLE 2.
Etiology, Diagnosis, and Outcome of 1804 Episodes of Infective Endocarditis Prospectively Collected in Spain
Diagnosis
Blood cultures were obtained in 1787 patients (99.1%) and provided the etiology in 1523 (85.3%). Of the 264 patients (14.7%) with negative blood cultures, 34% had received antimicrobial agents in the previous week. An etiologic diagnosis was achieved in 106 cases with a combination of the following techniques: sequence analysis of the 16S ribosomal RNA (rRNA) gene polymerase chain reaction (PCR) 66, 25% (heart valve 56, 21.2%; blood 8, 3.0%; and cardiac device 5, 1.9%); serology 99, 37.5%; and extracardiac cultures 59, 22.3%. Transesophageal echocardiography was done in most patients (76.3%), and 1148 (83.4%) presented vegetations. Abscess was the most common paravalvular complication (27.8%), whereas 26.6% of patients with prosthetic valve IE had evidence of a prosthetic valve complication such as dehiscence or new paravalvular regurgitation.
COMPARISON OF THE 4 TYPES OF IE
Non-IVDU Patients With Native Valve IE
Most of the patients in our series (59.8%) were non-IVDU, which is therefore the most heterogeneous group. Although it is difficult to identify one characteristic that stands out, these patients presented less frequently history of heart failure (23.6%) or renal failure (23.9%). Most cases were community-acquired, and Streptococcus spp. was the most common pathogen involved.
“Native valve IE in IVDU” accounted for the smallest group of our series. Native valve IE affected significantly younger patients with fewer comorbid conditions, except in the case of HIV infection (44.2%). Acquisition was nosocomial in only 1.9% of the cases and, interestingly, half of these patients had left-sided IE. A typical clinical presentation was more evident in this population including splinter hemorrhages (16%) and splenomegaly (31.4%). Staphylococcus aureus predominated as the etiological microorganism (53.8%), and embolisms were frequent (60.0%). Outcome was clearly better in this group.
Prosthetic Valve IE
The highest in-hospital mortality was recorded in patients with prosthetic valve IE (36.5%, P < 0.01); however, it is of even greater concern that infection was nosocomial in 39.7% of these patients. Coagulase-negative staphylococci accounted for 28.0% of the cases. Accordingly, intracardiac complications were significantly more frequent (33.9%).
Cardiac Device IE
Patients with heart devices were older, with the highest comorbidity index and a great part were nosocomial or health care-related IE (62%). The device involved was surgically removed in 65.1% of the cases.
Short-Term and Long-Term Risk Factors for Mortality
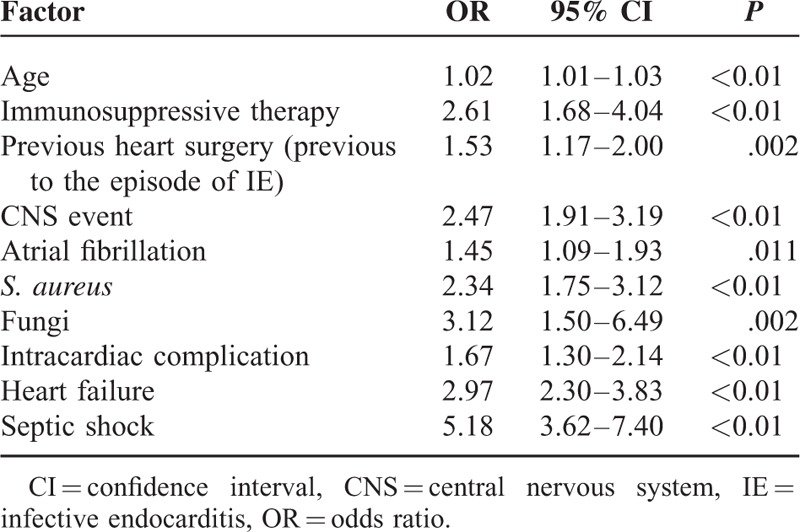
Table 3 shows a comparison of the patients who survived (71.1%) and those who died (28.9%) during admission; Table 4 shows the independent risk factors associated with a higher risk of in-hospital death. Independent mortality risk factors could be grouped as epidemiological characteristics of the patient, endocarditis etiology (Staphylococcus spp. [OR, 2.34], fungi [OR, 3.12]) and complications (intracardiac complication [OR, 1.67], heart failure [OR, 2.97], and septic shock [OR, 5.18]).
TABLE 3.
Risk Factors for In-Hospital Mortality
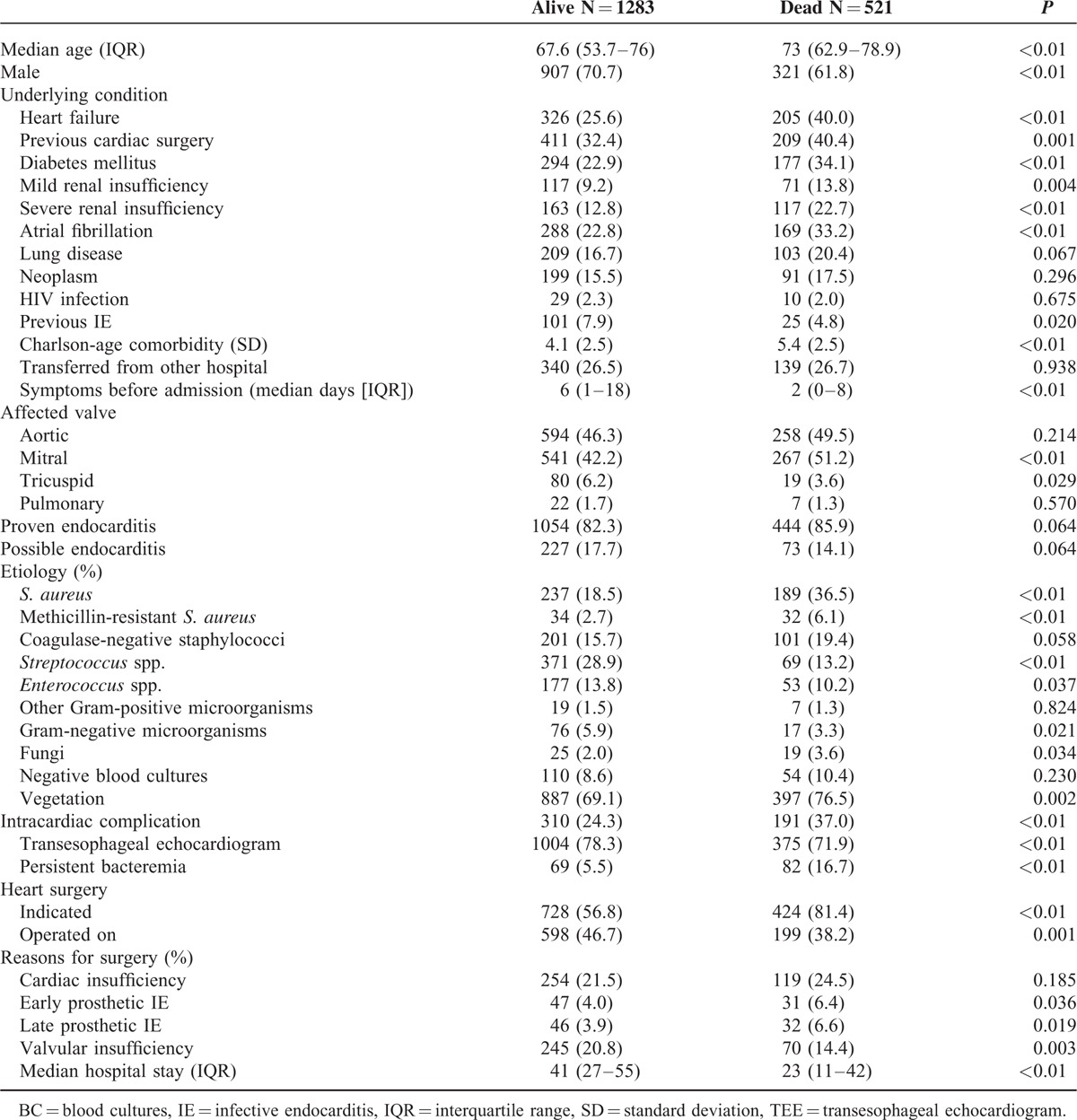
TABLE 4.
Independent Risk Factors for In-Hospital Mortality
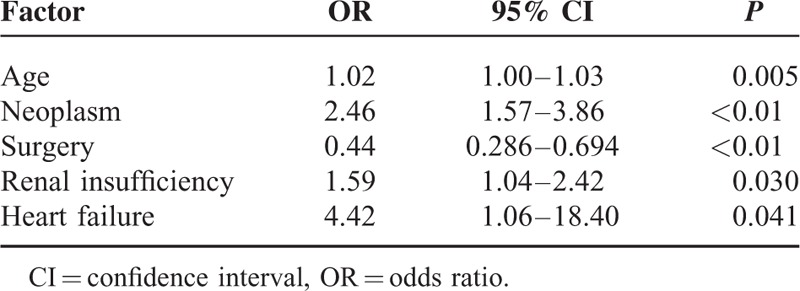
Independent risk factors for 1-year mortality are shown in Table 5, and include increasing age (OR, 1.02), neoplasm (OR, 2.46), renal insufficiency (OR, 1.59), and heart failure (OR, 4.42). Surgery was independently associated with a decreased risk of 1-year mortality (OR, 0.44) and was the only factor amenable of intervention.
TABLE 5.
Independent Risk Factors for 1-Year Mortality
DISCUSSION
Our very large series, collected from different institutions in a single country over a short period of time, shows that IE is mainly a disease of the elderly, with multiple predisposing conditions, frequently nosocomial, and has still a very high mortality, both during admission and during the 1-year follow-up.
Incidence rates of IE have been collected over long periods of time, and data based on population studies are scarce. In our series, including population-based studies of endocarditis recruited from 1960 to 2008, incidence rates range from 3 to 10 cases/100,000 habitants,4,6–9 and our figure of 3.5 IE cases/100,000 habitants is concordant with that. The advantage of our series is its large dimension collected over a short period.
The underlying conditions of patients with IE have also drastically changed,3,4,7 and most of our cases presented with severe comorbid conditions. This population of fragile patients is frequently exposed to health care-related and nosocomial complications. In our series, 28% of the episodes were classified as nosocomial; this percentage is similar to that reported by Fernandez-Hidalgo et al.25
Clinical presentation of the disease has also changed, and the signs that were once typical of IE (splinter hemorrhages, Janeway lesions, and Osler nodes) are now only seen in 2% of the patients. One possible explanation is that IE patients are now diagnosed earlier (86% were admitted ≤1 month of the initial signs of illness), thus reducing the incidence of immunological manifestations.1 On the contrary, we commonly observed complications such as respiratory manifestations (41%), kidney failure (39%), neurological events (19.7%), osteoarticular symptoms (11.5%), and ocular manifestations (6.3%). The rate of embolic events in our series was 29%, and although our results are similar to the ones reported by others,1,26 we believe that this figure could be underestimated, as the extension study depends on the institutional protocol and the technology available in each center. The introduction of newer diagnostic imaging tools such as positron emission tomography–computed tomography as part of the diagnostic algorithm in patients with IE, as suggested by Saby et al,27 should prove to be of great interest in this field.
A shift in the type of patient with IE has been observed: one major change in our series was the very low proportion of IE now occurring in IVDU. In Spain, this is probably related with the programs to control IVDÚs and particularly the methadone maintenance program.28 Although historically native valve IE in IVDU represented an important number of affected patients,29 in our series this population accounted for the smallest group while the number of patients with prosthetic valve and device IE (37%), on the other hand, seems to be increasing.4,9
Microbiological diagnostic tools have changed and improved over recent decades, and although etiology was confirmed by blood culture in most cases (85%), there are still cases in which the etiology is unknown (9%). Molecular techniques such as 16S rRNA PCR of the heart valves enabled us to establish the etiology in 21% of the negative blood culture episodes that would otherwise have been considered IE of unknown etiology. However, even though molecular methods have been used to diagnose IE, are long time, well-known methods,30 this diagnostic approach is still not routinely available in all diagnostic laboratories.
The most common microorganisms in our series were staphylococci, streptococci, and enterococci. Thus, a major change in the microbiology of IE is that Enterococcus spp. has emerged as the third most important group of pathogens and is now causative of 13% of IE cases. Because enterococci have shown the ability to develop antibiotic resistance,31 further studies are needed to evaluate novel approaches to this increasingly frequent problem32 and specially to identify factors that enable the early selection of patients who are at risk for Enterococcal IE.33
As for mortality, IE is a severe disease with a poor outcome. In our series, in-hospital mortality was 29%, and it seems that IE mortality has remained close to 25% since the 1970s3 despite the introduction of broad-spectrum antibiotics and new diagnostic tools. This is probably related with the increase in age and comorbidity. Although it is a complex procedure and it is not free of complications, surgery, when indicated, seems to have a major impact on mortality. In our experience, surgery was independently associated with a decreased risk of 1-year mortality (OR, 0.44); our results agree with those of a recent analysis of published studies34,35 that shows a significant correlation between the rate of early surgery and mortality.
Study Limitations
Our series may not represent the situation of IE in other countries where levels of health care differ from those of Spain (public run universal health care), but in our opinion represents well the situation in many western countries.
In conclusion, well inside the XXI century, IE, collected in a large number of institutions in a western country, remains an uncommon but devastating infectious disease. It commonly affects elderly patients with severe comorbidities, is frequently nosocomially acquired, and may be underdiagnosed if only suspected in the presence of classic clinical signs or typical microorganisms bacteremia. Surgery seems the only clear protective intervention and mortality within 1 year after discharge remains disappointedly high. Multidisciplinary teams are essential to optimize the management and outcome of this severe disease.
Acknowledgment
Members of GAMES: Hospital Costa del Sol (Marbella): Fernando Fernández Sánchez, Marian Noureddine, Gabriel Rosas, Javier de la Torre Lima; Hospital de Cruces (Bilbao): José Aramendi, Elena Bereciartua, María Victoria Boado, Marta Campaña Lázaro, JosuneGoikoetxea, Juan José Goiti, José Luis Hernández, José Ramón Iruretagoyena, Josu IrurzunZuazabal, Inés Martínez, Pedro María Pérez, Regino Rodríguez, Roberto Voces; Hospital Clínico Virgen de la Victoria (Málaga): Mª Victoria García López, RadkaIvanovaGeorgieva, Manuel Márquez Solero, Isabel Rodríguez Bailón, Josefa Ruiz Morales; Hospital Donostia-Policlínica Gipuzkoa (San Sebastián): Ana María Cuende, Pedro Idígoras, José Antonio Iribarren, Alberto Izaguirre Yarza, Carlos Reviejo, Tomás Echeverría, Eduardo Gaminde, MiguerAngelGoenaga, Ana Fuertes; Hospital General Universitario de Alicante (Alicante): Rafael Carrasco, Vicente Climent, Patricio Llamas, Esperanza Merino, Joaquín Plazas, Sergio Reus; Hospital Juan Canalejo (A Coruña): Nemesio Álvarez, José María Bravo-Ferrer, Dolores Sousa Regueiro, and Efrén Sanchez. Laura Castelo, José Cuenca, Enrique Miguez Rey, María Rodríguez Mayo, Pedro Llinares, Efrén Sanchez; Hospital Juan Ramón Jiménez (Huelva): José Manuel Lomas, Francisco Javier Martínez; Hospital Universitario de Canarias (Canarias): Mª del Mar Alonso, Beatriz Castro, Dácil García Marrero, Mª del Carmen Durán, Mª Antonia Miguel Gómez, Juan La Calzada, Ibrahim Nassar; Hospital Regional Universitario Carlos Haya (Málaga): Antonio Plata Ciezar, José Mª Reguera Iglesias; Hospital Universitario Central Asturias (Oviedo): Víctor Asensi Álvarez, Carlos Costas, Jesús de la Hera, Jonnathan Fernández Suárez, José Manuel García Ruiz, Lisardo Iglesias Fraile, José López Menéndez, Pilar Mencia Bajo, Carlos Morales, Alfonso Moreno Torrico, Carmen Palomo, Francisco Pérez, Ángeles Rodríguez, Mauricio Telenti; Hospital Universitario Clínic de Barcelona (Barcelona): Manuel Almela, Yolanda Armero, Manuel Azqueta, Ximena Castañeda, Carlos Cervera, Carlos Falces, Cristina García de la Maria, José M. Gatell, Magda Heras, Jaume Llopis Pérez, Francesc Marco, JoseMaria Miró, Carlos A. Mestres, Asunción Moreno, Salvador Ninot, José Ramírez, Marta Sitges, Carlos Paré, Juan Manuel Pericás; Hospital General Universitario Gregorio Marañón (Madrid): Emilio Bouza, Javier Bermejo, AliaEworo, Ana Fernández Cruz, Marcela González del Vecchio, Víctor González Ramallo, Martha Kestler, Mercedes Marín, Manuel Martínez-Sellés, Mª Cruz Menárguez, Patricia Muñoz, Hugo Rodríguez-Abella, Marta Rodríguez-Créixems, Jorge Rodríguez Roda, Marisol Salas, Antonio Segado, Blanca Pinilla, Ángel Pinto, Maricela Valerio, Eduardo Verde; Hospital Universitario La Paz (Madrid): Isabel Antorrena, José Mª Fraile Vicente, Carlos García Cerrada, Luis García Guereta, Alicia Lorenao Hernández, Alejandro Martín Quirós, Mar Moreno, José Ramón Paño, Mª Angustias Quesada Simón, Mikel Rico, Mª Ángeles Rodríguez Dávila, María Romero, Sandra Rosillo, Alicia Rico Nieto, Mikel Rico, María Romero; Hospital Universitario Marqués de Valdecilla (Santander): Ana Arnaiz, José Berrazueta, Sara Bellisco, Manuel Cobo Belaustegui, Raquel Durán, Concepción Fariñas-Álvarez, Carlos Fernández Mazarrasa, Rubén Gómez Izquierdo, Claudia González Rico, José Gutiérrez Díez, Rafael Martín Durán, Marcos Pajarón, José Antonio Parra, Ramón Teira, Jesús Zarauza; HospitalUniversitario Puerta de Hierro (Madrid): Pablo García Pavía, Jesús González, Beatriz Orden, Antonio Ramos, Elena Rodríguez González; Hospital Universitario Ramón y Cajal (Madrid): Tomasa Centella, José Hermida, José Moya, Pilar Martínez, Enrique Navas, Enrique Oliva, Alejandro del Río, Soledad Ruiz; Complexo Hospitalario Universitario de Vigo (Pontevedra): César Martínez, Andrés Nodar, Roberto Pérez, Francisco José Vasallo; Hospital Universitario Virgen de las Nieves (Granada): Carmen Hidalgo Tenorio; Hospital Universitario Virgen Macarena (Sevilla): Antonio de Castro, Marina de Cueto, Pastora Gallego, Jesús Rodríguez Baño; Hospital Universitario Virgen del Rocío (Sevilla):Arístides de Alarcón, Emilio García, Juan Luis Haro, José Antonio Lepe, Francisco López, Rafael Luque; Hospital San Pedro (Logroño): Luis Javier Alonso, José Ramón Blanco, Lara García, José Antonio Oteo; Hospital de la Santa Creu i Sant Pau (Barcelona): Natividad de Benito, MercéGurguí, Cristina Pacho, Roser Pericas, Guillem Pons; Hospital Santiago de Compostela (A Coruña): M. Álvarez, A. L. Fernández, Amparo Martínez, A. Prieto, Benito Regueiro, E. Tijeira, Marino Vega; Hospital Santiago Apóstol (Vitoria): Andrés Canut Blasco, José Cordo Mollar, Juan Carlos Gainzarain Arana, Oscar García Uriarte, Alejandro Martín López, Zuriñe Ortiz de Zárate, José Antonio Urturi Matos; Hospital SAS Línea (Cádiz): Mª Belén Nacle, Antonio Sánchez, Luis Vallejo; Hospital Clínico Universitario Virgen de la Arrixaca (Murcia): José Mª Arribas Leal, Elisa García Vázquez, Alicia Hernández Torres, Joaquín Ruiz Gómez, Gonzalo de la Morena Valenzuela; Hospital de Pontevedra (Pontevedra): Nicolás Bayón, Mª Dolores Díaz, Darío Durán, Juan Carlos Rodríguez, Antonio Moreno; Hospital de Txagorritxu (Vitoria): Ángel Alonso, Javier Aramburu, Felicitas Elena Calvo, Anai Moreno Rodríguez, Paola Tarabini-Castellani.
Footnotes
Abbreviations: 16S rRNA PCR = 16S ribosomal RNA (rRNA) gene polymerase chain reaction (PCR), CNS = central nervous system, GAMES = The Spanish Collaboration on Endocarditis-Grupo de Apoyo al Manejo de la Endocarditis Infecciosa en España, ICE = International Collaboration on Endocarditis, IE = infective endocarditis, IQR = interquartile range, IVDU = intravenous drug users, OR = odds ratio.
This study was partially financed by the CIBER de Enfermedades Respiratorias (CIBERES) from the ISCIII (CB06/06/0058) and by Fundación BBVA-Fundación Carolina (research fellowship of Maricela Valerio).
The authors have no conflicts of interests to disclose.
REFERENCES
- 1.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bor DH, Woolhandler S, Nardin R, et al. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PLoS One 2013; 8:e60033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slipczuk L, Codolosa JN, Davila CD, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013; 8:e82665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouza E, Menasalvas A, Munoz P, et al. Infective endocarditis—a prospective study at the end of the twentieth century: new predisposing conditions, new etiologic agents, and still a high mortality. Medicine (Baltimore) 2001; 80:298–307. [DOI] [PubMed] [Google Scholar]
- 5.Alagna L, Park LP, Nicholson BP, et al. Repeat endocarditis: analysis of risk factors based on the International Collaboration on Endocarditis–Prospective Cohort Study. Clin Microbiol Infect 2013; 18: [DOI] [PubMed] [Google Scholar]
- 6.Carton JA, Asensi V, Maradona JA, et al. [Infectious endocarditis of the native valve: its epidemiological profile and an analysis of its mortality between the years 1984 and 1993]. Med Clin (Barc) 1995; 104:493–499. [PubMed] [Google Scholar]
- 7.Fernandez-Hidalgo N, Tornos Mas P. Epidemiology of infective endocarditis in Spain in the last 20 years. Rev Esp Cardiol 2013; 66:728–733. [DOI] [PubMed] [Google Scholar]
- 8.Hoen B, Alla F, Selton-Suty C, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA 2002; 288:75–81. [DOI] [PubMed] [Google Scholar]
- 9.Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91:571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Valle H, Farinas-Alvarez C, Garcia-Palomo JD, et al. Clinical course and predictors of death in prosthetic valve endocarditis over a 20-year period. J Thorac Cardiovasc Surg 2010; 139:887–893. [DOI] [PubMed] [Google Scholar]
- 11.Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med 2002; 162:90–94. [DOI] [PubMed] [Google Scholar]
- 12.Cabell CH, Abrutyn E. Progress toward a global understanding of infective endocarditis. Lessons from the International Collaboration on Endocarditis. Cardiol Clin 2003; 21:147–158. [DOI] [PubMed] [Google Scholar]
- 13.Baddour LM. Infective endocarditis: diagnosis antimicrobial therapy and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434. [DOI] [PubMed] [Google Scholar]
- 14.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96:200–209. [DOI] [PubMed] [Google Scholar]
- 15.Gerald L. Mandell, MD, MACP, John E. Bennett, MD, MACP, and Raphael Dolin, MD. Mandell, Douglas and Bennett́s Principles and Practice of Infectious Diseases. 7th Edition pp 4320 Imprint: Churchill Livingstone. ISBN: 978-0-443-06839-3. [Google Scholar]
- 16.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–638. [DOI] [PubMed] [Google Scholar]
- 17.Cockerill FR, Wilson JW, Vetter EA, et al. Optimal testing parameters for blood cultures. Clin Infect Dis 2004; 38:1724–1730. [DOI] [PubMed] [Google Scholar]
- 18.Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999; 15:816–822. [DOI] [PubMed] [Google Scholar]
- 19.Nashef SA, Roques F, Hammill BG, et al. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg 2002; 22:101–105. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 21.Adams HP, Jr, Brott TG, Crowell RM, et al. Guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1994; 25:1901–1914. [DOI] [PubMed] [Google Scholar]
- 22.Yancy CW, Fau-Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 23.Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol 2013; 112:1171–1176. [DOI] [PubMed] [Google Scholar]
- 24.Boletin Oficial del Estado. Spanish National Institute of Health. Order 1016/2013 Official population figures resulting from the review of the national census to January 1, 2013 2013(311):4. BOE-A-2013-13732. [Google Scholar]
- 25.Fernandez-Hidalgo N, Almirante B, Tornos P, et al. Contemporary epidemiology and prognosis of health care-associated infective endocarditis. Clin Infect Dis 2008; 47:1287–1297. [DOI] [PubMed] [Google Scholar]
- 26.Miro JM, Anguera I, Cabell CH, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 2005; 41:507–514. [DOI] [PubMed] [Google Scholar]
- 27.Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditisincreased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61:2374–2382. [DOI] [PubMed] [Google Scholar]
- 28.Brugal MT, Domingo-Salvany A, Puig R, et al. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and aids in a cohort of heroin users in Spain. Addiction 2005; 100:981–989. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Davila P, Navas E, Fortun J, et al. Analysis of mortality and risk factors associated with native valve endocarditis in drug users: the importance of vegetation size. Am Heart J 2005; 150:1099–1106. [DOI] [PubMed] [Google Scholar]
- 30.Lisby G, Gutschik E, Durack DT. Molecular methods for diagnosis of infective endocarditis. Infect Dis Clin North Am 2002; 16:393–412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2015; 10:266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chirouze C, Athan E, Alla F, et al. Enterococcal endocarditis in the beginning of the 21st century: analysis from the International Collaboration on Endocarditis-Prospective Cohort Study. Clin Microbiol Infect 2013; 7: [DOI] [PubMed] [Google Scholar]
- 33.Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis 2015; 60:528–535. [DOI] [PubMed] [Google Scholar]
- 34.Kang D-H, Kim Y-J, Kim S-H, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366:2466–2473. [DOI] [PubMed] [Google Scholar]
- 35.Galvez-Acebal J, Almendro-Delia M, Ruiz J, et al. Influence of early surgical treatment on the prognosis of left-sided infective endocarditis: a multicenter cohort study. Mayo Clin Proc 2014; 89:1397–1405.doi: 10.1016/j.mayocp.2014.06.021. [DOI] [PubMed] [Google Scholar]


